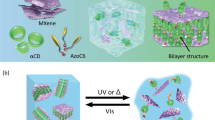
Abstract
Despite being the most abundant sustainable energy resource, solar energy still faces major challenges in efficient capture and long-term storage. Molecular Solar Thermal Energy Storage (MOST) systems address this issue by employing photoswitchable molecules that absorb sunlight and store energy through reversible isomerization, cyclization or other intramolecular rearrangements. Azobenzenes are attractive due to their well-characterized photoresponsive behavior; however, conventional systems are hindered by low energy density, limited energy storage duration, and a reliance on organic solvents. Here, we present the Micellar Solar Thermal Energy Storage system (MIST) approach based on micellar aggregates that operate effectively across aqueous dispersions and gel states. These systems exhibit progressively enhanced energy storage lifetimes with increasing degrees of self-assembly, while delivering competitive energy densities. The thermal stability arises from restricted molecular mobility within the self-assembled structures and is enhanced on gelation, extending the calculated thermal half-life of the cis isomer from 148 days in dimethyl sulfoxide (DMSO), to 233 days in water, and to 12.8 years in the gel state. Compared to previous azobenzene-based MOST systems, our MIST approach offers significantly extended energy storage durations and improved material processability, including water-compatible formulations and, macroscopic heat release in the gel state (up to 5.7 °C).
Similar content being viewed by others
Introduction
Molecular Solar Thermal Energy Storage (MOST) is a means of capturing and storing solar energy through chemical bonds1,2,3. The absorption of sunlight by photoswitchable molecules induces a transition from the ground state to a metastable, high-energy isomer. If the isomer remains stable over a measurable timescale before reverting to its original form, it can serve as an effective medium for energy storage. When energy is needed, back-conversion to the original form can be triggered (typically by heat4, catalyst5,6, electrical potential3,7, or, more efficiently, by direct irradiation with a different wavelength of light8,9,10,11) releasing the stored energy as latent heat. This process has the potential to allow energy to be stored without losses and retrieved on demand, offering an efficient and sustainable method for harnessing solar power.
Azobenzenes have been widely examined for MOST due to their ability to undergo reversible photoisomerization12,13. However, there are many reasons why this class of materials is generally not efficient14. For instance, the stability of the cis isomer over time is critical for long-term energy storage. However, in most azobenzene-based systems, thermal relaxation to the trans isomer occurs readily, especially under elevated temperatures, leading to significant energy loss over time. Therefore, minimizing undesired thermal back-conversion is essential for effective long-term energy storage15. From a practical perspective, the dependence on organic solvents further restricts scalability and real-world applicability, especially when integration with water-based heat transfer fluids is desired. Designing water-soluble azobenzenes, therefore, requires tailored molecular structures to ensure compatibility and functionality in aqueous environments.
As one promising strategy for modifying azobenzenes, the development of azobenzene-based gels has attracted considerable attention, with numerous examples reported in recent years16,17,18. For instance, dipeptide-functionalized azobenzenes have been shown to form gels that transform into solutions upon ultraviolet (UV) irradiation, driven by trans-to-cis isomerization of the azobenzene unit19. Gels were reformed on irradiation with visible light as the trans isomer is reformed. However, whether such systems are suitable for MOST applications remains unexplored.
Previously, we have shown that a range of related functionalized dipeptides can form micellar states at high pH where the carboxylic acid at the C-terminus is deprotonated20,21. The micellar state formed is controlled by the chemical structure of the functionalized dipeptide as well as the counterion and the presence of additives. Micellar aggregates can be formed at concentrations as high as 200 mg mL-1 and are stable for extended periods of time (on the timescale of years). For other azobenzene-based photoswitchable molecules, self-assembly into defined nanostructures has been shown to dramatically enhance the activation barrier to cis-to-trans isomerization22, ascribed to the non-covalent interactions (specifically hydrogen bonding and π − π stacking) between molecules in the aggregated state. Phase changes on photoisomerization have been shown to be effective in improving MOST systems9,10,23,24. With this in mind, we hypothesized that assembling a micellar phase from functionalized azobenzenes to construct a Micellar Solar Thermal Energy Storage (MIST) system could reduce the rate of cis-to-trans isomerization and enable the development of materials suited for the next generation of MOST technologies.
Here, we present a series of azobenzene-functionalized dipeptide amphiphiles designed to self-assemble into micellar structures in aqueous environments at high pH. The systems were engineered to introduce a strategy using self-assembled gels to extend the half-life of photoisomers. Notably, this gel-based approach has not been reported in the previously cited studies. Our MIST methodology does not conflict with established principles for prolonging the thermal lifetime of MOST systems; instead, it builds upon them by offering a different dimension of control. This approach provides a straightforward and efficient means of stabilizing photoisomers, with fewer synthetic demands compared to the development of new photoactive molecular frameworks. We demonstrate that the formation of distinct micellar morphologies, ranging from spherical to worm-like and elliptical cylinder aggregates, can be precisely modulated by the dipeptide sequence, as confirmed by comprehensive small-angle X-ray scattering (SAXS) measurements. Importantly, we further show that transitioning from an organic solution to micellar dispersion and ultimately to gel states, achieved through calcium-induced crosslinking, leads to a progressive enhancement of the energy storage lifetime, reaching up to 4674 days (12.8 years, extrapolated) in the gel state. For a practical demonstration of MIST functionality, we establish that these systems allow multiple photoswitching cycles with structural reversibility, and that macroscopic energy release can be effectively triggered by light in gel films for the first time. Together, these findings validate our hypothesis and offer a different design for next-generation energy storage systems that are water-processable, highly stable, and suitable for scalable solar thermal energy conversion and storage applications.
Results and discussion
Here, we describe a series of functionalized dipeptides with the N-terminus modified by an azobenzene moiety (Fig. 1a), while retaining a free C-terminus. At high pH, where the C-terminus is deprotonated, micellar aggregates are formed. For such systems, it is difficult to predict which micellar structures will be formed, but the use of hydrophobic amino acids favors persistent micelles20,25. Azo-FF forms a liquid crystalline phase at 10 mg mL−1 (Fig. S31), while Azo-FI, Azo-FV, and Azo-FL form free-flowing solutions with varying degrees of turbidity (Fig. 1b). The small angle X-ray scattering (SAXS) data for Azo-FF are best fit to a power law combined with a flexible elliptical cylinder model, showing the presence of persistent worm-like structures with a radius of 9.1 nm and an axis ratio of 2.35 (Fig. 1c and Table S1). For Azo-FI and Azo-FV, the data are best fit to a power law combined with a sphere model, showing the presence of spherical micelles with a radius of 2.4 nm in each case (Fig. 1c and Table S2 and S3, respectively). The data for Azo-FL are best fit to a power law combined with a cylinder model, showing the presence of cylindrical micelles with a radius of 3.5 nm (Fig. 1c and Table S4). Hence, as for other functionalized dipeptides at high pH21,25, different micellar aggregates exist depending on the dipeptide sequence used. The viscosity of each solution correlates with the SAXS data, with the cylindrical structures resulting in higher viscosity solutions than the spherical micelles (Fig. 1d).
a Chemical structures of the four azobenzene-dipeptides used here. The amino acids used are named using one letter codes; b photographs of solutions formed from each azobenzene-dipeptide in water at pH 10.5 at a concentration of 10 mg mL−1; c SAXS data for each azobenzene-dipeptide in water at pH 10.5 at a concentration of 10 mg mL−1 (black, Azo-FF; blue, Azo-FI; orange, Azo-FV; green, Azo-FL) with the fits to the data shown as red lines; d viscosity data for each azobenzene-dipeptide in water at pH 10.5 at a concentration of 10 mg mL−1 (black, Azo-FF; blue, Azo-FI; orange, Azo-FV; green, Azo-FL). The data are plotted as the average of three measurements, with the error bars representing the standard deviation.
All examples show the expected trans-to-cis isomerization when irradiated with 365 nm light (Fig. 2a and Fig. S32). Irradiation with 505 nm light reverses the azobenzene back to the trans state (Fig. 2a and Fig. S32). As for most examples, full reversal to the trans isomer does not occur (a 95% recovery is observed), but this cycle can be carried out many times (Fig. 2b and Fig. S33). The isomerization process also induces a notable change in viscosity (Fig.2c and Fig. S34), which is attributed to a reorganization of the self-assembled structures (Fig. 2d and Fig. S34); for Azo-FV, like the trans isomer, the data for the cis isomer fit best to a sphere combined with a power law model, with the spheres having a higher radius of 3.6 nm. On reversal back to the trans isomer, the SAXS data (all four samples was prepared in water at pH 10.5 at concentration of 10 mg mL−1) show that the original self-assembled structures are reformed, with the spheres having a similar radius of 2.6 nm (Fig. 2d, Fig. S35 and Table S1–S4). Slight differences in the viscosity after reisomerization can be attributed to the history of the sample, driven by stirring and relaxation times. Considering these values are all low, we would not read too much into this. All the above data were collected using LEDs as opposed to using solar energy. Due to the spectral overlap for the two isomerization processes, use of a solar simulator does enable the trans-to-cis and also cis-to-trans isomerization, but not effectively (Fig. S36). The conversion rate reaches a plateau at approximately 50% of the trans-isomer. However, using a 390 nm short-pass filter lens for the trans-to-cis step and a 496 nm long-pass filter lens for the cis-to-trans isomerization shows significant isomerization (Fig. S37), showing the potential here for direct use of sunlight.
All data were collected at pH 10.5 at a concentration of 10 mg mL−1. a UV-vis spectra for the initial trans-isomer (orange squares), after isomerization to the cis-isomer with 365 nm irradiation (purple circles), and after reversal back to the trans-isomer with 505 nm irradiation (pale blue triangles). The inset shows a photograph of the initial trans-isomer (left), the cis-isomer (middle) and the trans-isomer after reversal (right) and the data in (a) were recorded from these solutions; b Change in absorbance at 340 nm over multiple trans-to-cis-to-trans isomerizations; c Change in viscosity form the initial trans-isomer (orange), after isomerization to the cis-isomer with 365 nm irradiation (purple), and after reversal back to the trans-isomer after 505 nm irradiation (pale blue); d SAXS data for the initial trans-isomer (orange), after isomerization to the cis-isomer with 365 nm irradiation (purple), and after reversal back to the trans-isomer after 505 nm irradiation (pale blue). All isomerizations were carried out by placing two LEDs 5 cm away from the sample and irradiating for 10 minutes.
From the perspective of potential candidates for energy storage, we investigated the energy released from the bulk solids for each of the functionalized dipeptides after irradiation to the cis-isomers. There was a significant energy release on heating as expected as cis-to-trans isomerization occurred. By maintaining a constant heating rate (at 10 °C min−1) during differential scanning calorimetry (DSC) for energy storage capacity determination, it was found that the energy densities of the four molecules were comparable to those of other azobenzene-based systems24. Among them, Azo-FV exhibited the highest energy release (151 J g−1, Figs. S38 and S39), and was therefore selected for further investigation. All the molecules are stable to at least 250 °C under an inert atmosphere (Fig. S40). As noted above, we hypothesized that preparing a micellar phase formed by functionalized azobenzenes would reduce the rate of the cis-to-trans back conversion. Specifically, the half-life of the cis-to-trans isomerization at 20 °C was 148 days as a solution at a concentration of 3 mg mL−1 in DMSO (this concentration was chosen due to the absorbance; Fig. 3b and Fig. S41). As a micellar dispersion in water at high pH at the same concentration, the half-life was 233 days (Fig. 3a and Fig. S42 for calculations). We ascribe this increase in half-life to the formation of the micelles; for the isomerization to occur, we presume that there is a need for multiple isomerizations to occur concurrently to ensure packing within a micellar structure is effective. On top of this, association between molecules within the self-assembled aggregates driven by non-covalent interactions such as hydrogen bonding and stacking will also likely enhance the activation barrier for cis-to-trans isomerization as suggested elsewhere22, leading to an increase in the half-life.
a Calculated half-lives for the Azo-FV cis-to-trans isomerization at 20 °C (data were determined from solutions at 3 mg mL−1); (b) photographs of Azo-FV gels formed from the trans-isomer (left), the cis-isomer (middle) and after reversal back to the trans-isomer (right). All data were collected at pH 10.5 at a concentration of 5 mg mL−1; (c) SAXS data for Azo-FV gels formed from the trans-isomer (orange) and cis-isomer (purple) and after reversal back to the trans-isomer (light blue) with fits to the data shown as red lines. All data were collected at pH 10.5 at a concentration of 5 mg mL−1; (d) rheology of Azo-FV gels formed from the trans-isomer (orange), the cis-isomer (purple) and after reversal back to the trans-isomer (light blue). All data were collected at pH 10.5 at a concentration of 5 mg/mL. All isomerizations were carried out by placing two LEDs 5 cm away from the sample and irradiating for 10 min.
To further increase the half-life, we formed a gel by the addition of a calcium salt to the micellar dispersion at high pH at a concentration of 5 mg mL−1. As for related systems20, the divalent salt crosslinks the micelles present at high pH leading to a self-supporting gel (Fig. 3b). Both the trans-isomer and the cis-isomer form gels on addition of a calcium salt. Interestingly, if the trans-gel is irradiated, the gel falls apart but adding a calcium salt to a preformed solution of the cis-isomer results in a gel that is then stable when irradiated to the trans-isomer. Using SAXS to understand the underpinning network, for the gel formed from the trans isomer of Azo-FV, the data fit best to a power law combined with an elliptical cylinder model with a radius of 2.6 nm and an axis ratio of 1.9. Hence, the addition of the calcium salt leads to a morphological change from spheres to elliptical cylinders; the gel is formed by the entangled network of these structures. The data for the gel formed from the cis isomer also fit best to a power law combined with an elliptical cylinder model, with the cylinders having a higher radius of 3.2 nm and an axis ratio of 3.2. On reversal back to the trans isomer, the SAXS data show that the original self-assembled structures are reformed, with the cylinders having a similar radius of 2.8 nm (Fig. 3c and Table S5). All gels have a storage modulus (G′) that is significantly higher than the loss modulus (G″) (Fig. 3d). The cis-gel is mechanically weaker than the trans-gel, and the original trans-gel is stiffer than the trans-gel reformed after irradiation with a 505 nm LED. Again, this shows the process dependency of such systems and may also be related to the fact that the steady state after irradiation is not quite the same as that of the original system (Fig. 2a). An important result of the gelation is that the half-life for the cis-to-trans isomerization is increased significantly as compared to the starting micellar solution to 4674 days (Fig. 3a and Fig S43). This substantial increase in half-life can be attributed to additional interactions from non-covalent crosslinking, as well as increased molecular rigidity within the gel network, which collectively raise the activation barrier for cis-to-trans isomerization. Indeed, the activation energies (Eₐ) for the cis-to-trans isomerization of Azo-FV were calculated to be 85.4 kJ mol⁻¹ in DMSO, 100.3 kJ mol⁻¹ in the micellar state, and 166.1 kJ mol⁻¹ in the gel state (see Supporting Information, page S48). It is worth noting that gels formed through the self-assembly of small molecules are often metastable. However, they can remain stable for extended periods. In fact, this has been experimentally confirmed, as we have gel samples in our laboratory that have maintained their structure and properties for several years.
From a practical application standpoint, whilst the use of water as a medium is particularly appealing, especially for future industrial-scale MOST systems due to its safety compared to organic solvents and its high heat capacity for heat transfer purposes, there have been very few demonstrations of azobenzene derivatives in aqueous solutions for real-world applications. With our materials, micellar aggregates can be formed at a concentration of 200 mg mL−1 (Fig. 4a, left), resulting in an extremely viscous solution (Fig. 4b). Irradiation with 365 nm light results in a clear change in color and turbidity (Fig. 4a, middle) with a significantly lower viscosity (Fig. 4b); irradiation with 505 nm light results in a solution with similar color and turbidity to the original solution (Fig. 4a, right) and a higher viscosity. This higher viscosity likely reflects the process of forming the trans-isomer from the cis-isomer as compared to direct dissolution. SAXS data for these solutions shows that the initially formed solution contains sharp Bragg diffraction peaks (Fig. 4c), indicating the formation of a lyotropic liquid crystal (LLC) phase; the peaks have a q ratio of 1:√3:2:√7, characteristic of a reverse hexagonal phase (Fig. 4c)26. In agreement with this assignment, we observe a birefringent, densely-packed fan pattern by cross-polarized microscope (CPM, Fig. S44). On irradiation, this order is lost, and the data show the presence of a Bragg peak at 56.8 Å (Fig. 4c). After re-isomerization to the trans-isomer using irradiation from a 505 nm LED, the original scattering data are not recovered but shows the presence of a Bragg peak at a similar position to that present in the cis-isomer (Fig. 4c). These data therefore agree with the viscosity data showing that there is a process dependency in the solutions formed. We note that fitting these SAXS data as we were able to do for the low concentration samples is highly challenging due to the high concentration of structures present that renders the models invalid.
a Photographs of solutions formed from the initial Azo-FV trans-isomer (left), the cis-isomer (middle) and the trans-isomer after reversal (right), all at 200 mg mL−1; b viscosity data for solutions formed from the trans-isomer (orange), the cis-isomer (purple) and after reversal back to the trans-isomer (light blue); c SAXS data for the solutions formed from initial trans-isomer (orange), the cis-isomer (purple) and the trans-isomer after reversal (light blue), all at 200 mg mL−1; d photographs of gels formed from initial trans-isomer (left), the cis-isomer (middle) and the trans-isomer after reversal (right), all at 200 mg mL−1, on addition of a calcium salt; e rheology of gels formed at 200 mg mL−1 from the trans-isomer (orange), the cis-isomer (purple) and after reversal back to the trans-isomer (light blue); f SAXS data for the gels formed from initial trans-isomer (orange), the cis-isomer (purple) and the trans-isomer after reversal (light blue), all at 200 mg mL−1. All isomerizations were carried out by placing two LEDs 5 cm away from the sample and irradiating for 10 min.
Gelation can again be achieved by the addition of a calcium salt (Fig. 4d), with extremely stiff gels being formed at this concentration (Fig. 4e). In all cases, G′ is again significantly higher than the loss modulus (G″) and, importantly for real applications, at this concentration the gel state persists when the trans-gel is isomerized to the cis-gel with irradiation from a 365 nm LED. The SAXS data are complicated and (due to the high concentration) difficult to fit, but again it is clear that the data for the initial trans-gel and the resulting cis-gel are different to one another (Fig. 4f). Re-isomerization to the trans-gel does not fully restore the original SAXS profile; instead, the data more closely resemble those of the cis-gel (Fig. 4f), again indicating a strong dependence on the isomerization history and process pathway. We highlight here that many of our systems show such a process dependence where the structures formed depend on the route used to form them.
To further demonstrate that the system’s macroscopic heat release can be effectively harnessed for energy storage applications, a highly concentrated Azo-FV gel (200 mg mL−1) was employed in an on-demand heat release experiment. The cis-gel was found to be stable over extended periods (note that half-life calculations are not possible due to the high concentrations and resulting absorbance but visually the color of the sample is that of the cis-isomer and UV-vis spectroscopy of a small sample removed from the bulk and diluted shows the cis-isomer even after 3 months, Fig. S45). On irradiation of the cis-gel in a glass vial with two 505 nm LEDs, isomerization to the trans-gel occurs (Fig. 5a). Using a thermal camera, we find that the energy released leads to a significant increase in the temperature (up to 5.7 °C, with a theoretical value of 7.2 °C, calculation details in Supporting Information, page S51) of the vial (Fig. 5b and Fig. S46) as compared to a control with just water. Similarly, using the trans-solution or trans-gel results in a low temperature increase (Figs. S53 and S54). Further, as expected, the energy stored is concentration dependent, with a 2.9 °C increase in temperature when a lower concentration of 100 mg mL−1 of Azo-FV was used (Fig. S55 and S56).
a Experimental setup used to monitor heat release. 400 μL of a gel formed from the Azo-FV cis-isomer was placed in a 3.5 mL glass vial and irradiated with two 505 nm LEDs. A thermal camera was used to monitor the temperature; b Heat release from cis-gel of Azo-FV at a concentration of 200 mg mL−1 (red) as compared to water (black). The gel was kept in the dark for 1 week before the release experiment was carried out, with the data being very similar to that from a gel where heat release was measured immediately after isomerization. Inset are photographs from the thermal camera showing the raw data at specific time points (expanded versions are available in the Supporting Information to clearly see the scale bars). c Partial 1H NMR spectra for repeat cycles of irradiation on the same sample showing the repeated transition from trans to cis; d Heat release from the cis-gel and trans-gel over multiple cycles from the same gel. Note that the absolute temperature released in (b) and (d) are different due to different ambient conditions in our laboratory.
Importantly, the system at 200 mg mL−1 can be cycled multiple times with no loss of efficiency. The degree of isomerization is essentially unchanged over 5 cycles (Fig. 5c), with very similar heat released (Fig. 5d) in each of the cycles. Note, the absolute heat released here is slightly lower than in the data shown in Fig. 5b); this can be ascribed to a difference in the room temperature when the experiments were carried out. The micellar structures underpinning these gels are similar at each point, although there are slight changes in the scattering (Fig S60). However, the isomerization from trans-to-cis is unaffected by this (Fig. 5c) allowing the effective use for energy storage and release.
Conclusions
Here, we have introduced a MIST molecular design strategy for azobenzene-based energy storage systems by coupling the photoresponsive functionality with dipeptide-driven self-assembly. By precisely controlling molecular architecture and phase state, we achieve tunable energy storage lifetimes, culminating in exceptionally long storage durations, and reversible, light-triggered energy release across organic, aqueous, and gel environments. In future studies, density functional theory (DFT) calculations could be employed to elucidate the mechanisms by which self-assembled structures significantly extend the half-life of the cis-isomer, as well as to provide insight into the structural transition from the pre-gel to the gel state. It is generally accepted that the half-life of the photoisomer increases with concentration; however, a key limitation in our current system is the solubility of the azobenzene dipeptide. At higher concentrations, the material becomes increasingly viscous, thereby impeding light penetration and affecting the photoconversion process. To address this, future molecular design of MIST compounds will focus on improving solubility, particularly by introducing structural modifications to the azobenzene core to reduce π–π stacking interactions or by incorporating steric hindrance to further stabilize the cis-isomer and hinder its thermal back-conversion. Another promising strategy involves exploring solvents with lower polarity and specific heat capacity, such as ethylene glycol, which may improve solubility and enhance system performance. With these optimizations, we anticipate that the heat release performance could be elevated to the average range of current MOST systems. The formation of highly stable, water-compatible materials that retain functionality at high concentrations and enable macroscopic heat release marks a significant advancement toward scalable, deployable energy storage technologies. These findings expand the design landscape for molecular solar thermal materials and establish a versatile platform for developing next-generation energy storage systems that unify responsiveness, durability, and processability within a single molecular framework.
Methods
General experimental
All chemicals were used as received. Deionized water (Avidity Pico10T2) was used in all experiments. 1H and 13C NMR spectra were recorded on a Bruker AvanceIII HD or Bruker AvanceIII NEO 400 MHz spectrometer. Chemical shifts (δ) are reported in parts per million (ppm) to the residual solvent peak (CDCl3: 7.26 ppm for 1H and 77.0 ppm for 13C; DMSO–d6: 2.50 ppm for 1H and 39.52 ppm for 13C). Absorption spectra were measured on an Agilent Cary 60 UV–vis spectrophotometer. Thermogravimetric analysis (TGA) was performed on the TA Instruments TGA 5500 under N2. Microscope images were taken on a Nikon Eclipse LV100ND with a 5× magnification. Images were collected under cross polarized light at 90° and non-polarized light. Scale bars were added to images using the software, ImageJ. All heat release pictures were carried out using a Teledyne FLIR thermal camera i7. The commercial 365 nm LED (829–0841) was positioned to give approximately 80 mW cm−2 and commercial 505 nm LED (358–3121) was positioned to give approximately 20 mW cm−2 for the experiments.
Materials
Full synthetic details and characterization are described in the Supporting Information.
Solution preparation
The general preparative procedure is as follows: azobenzene dipeptide solutions were prepared by weighing azobenzene dipeptide into a vial. The required volume of water was added to reach a concentration of 5, 10, or 200 mg mL−1. Following this, 2 equivalents of sodium hydroxide aqueous solution (2 M) was added. This mixture was stirred continuously for approximately 17 hours at ambient temperature (around 20 °C) using a 25 × 8 mm stirrer bar within a 50 mL centrifuge tube at a speed of 1000 RPM. The pH of the resulting solution was adjusted to 10.5 ± 0.1 using aqueous sodium hydroxide (1 M). The cis-isomer solutions were prepared by irradiating the fresh solutions with a 365 nm LED for 10 minutes, and the re-trans-isomer solutions were prepared by irradiating the cis-isomer solutions with a 505 nm LED for 10 minutes.
Gel preparation
Gels were prepared from the previously described pre–gelation solutions. Subsequently, 8 μL of an aqueous calcium chloride solution (5.0 M) was added to 2.0 mL of the pre-gelation solution to trigger gelation by depositing the drop on top of the solution. The samples were left to stand for 24 h incubation period at ambient temperature (around 20 °C). The cis-isomer gel and re–trans-isomer gel can be obtained by adding the calcium chloride trigger to cis-isomer solutions and re–trans-isomer solutions respectively.
Rheology
All rheological measurements were carried out using an Anton Paar Physica MCR101 rheometer. Viscosity measurements were performed using a 50 mm cone geometry (CP50) with gap distance between the geometry and the plate set to 0.10 mm and temperature set to 25 °C. Samples were poured onto the rheometer flat plate for measurement and fresh sample used for each run. For the gels, strain and frequency–sweep measurements were conducted in a vane–cup measurement system (Anton Paar ST10–4V–8.8/97.5) and samples were prepared in a plastic Sterlin cup (7 mL volume) and incubated at ambient temperature (around 20 °C) for 24 h before each measurement. Rheology measurements were performed in triplicate, and values averaged with error bars representing the standard deviation between the repeats.
Small–angle X–ray scattering
Small–angle X–ray scattering (SAXS) measurements were conducted at the Diamond Light Source, Didcot, on the I22 beamline. The beamline operated at an energy of 12.4 keV, with a detector distance of 9.750 m, providing a final Q range of 0.0016 to 0.177 Å⁻¹. Samples were prepared as previously described and transferred into borosilicate glass capillaries (1.55 mm internal diameter) using a 1 mL syringe fitted with a 21 G needle. For all samples, 10 × 100 ms frames exposure were collected and averaged. The raw data was processed using Dawn Science software (version 2.27)27 following the standard I22 pipeline28. During processing, background subtraction was performed on the raw 2D SAXS data, followed by full azimuthal integration to generate an I vs q plot. The resulting plots were then fitted to structural models using SasView software (version 5.0.4)29.
Differential scanning calorimetry and thermogravimetric analysis
Differential scanning calorimetry (DSC) measurements were conducted on a TA Instruments DSC 25. Azobenzene dipeptides, (about 100 mg) were dissolved in deuterated acetone (about 7 mL). After irradiation for 10 min using the LED (emitting at 365 nm) and evaporation of the solvent, 94% conversion to cis-isomer was confirmed by 1H NMR spectroscopy. A pre-weighed amount (~3 mg) of the cis-isomer azobenzene dipeptides were sealed and inserted in the DSC at 20 °C. The DSC method involved running two consecutive cycles, each consisting of heating at 10 °C min−1 from 20 °C to 150 °C with 50 mL min−1 nitrogen and in between cooling (at 10 °C min−1 from 150 °C to 20 °C with 50 mL min−1 nitrogen), conversion to the trans-isomer was confirmed by 1H NMR spectroscopy after the DSC measurements. The first heating cycle showed the characteristic exothermic feature associated with back isomerization for Azo-FF and Azo-FV, and the second heating cycle did not show any evidence of any thermal transitions, confirming that the full back isomerization occurred in the first cycle. However, Azo-FI and Azo-FL still have exothermic peaks in the second heating cycle, a part of cis-isomers are not converted to trans-isomers was confirmed by 1H NMR spectroscopy after DSC measurements. Integration of the exothermic peak and normalization respect to the amount of cis-iosmer azobenzene dipeptides present in the neat material gave an enthalpy difference (\(\Delta {H}_{{storage}}\)) between cis- and trans-iosmer azobenzene dipeptides.
Heat release experiments
Heat release experiments were measured using a Teledyne FLIR thermal camera i7. The sample was placed between two LEDs (LedEngin Inc 505 nm, Ultraviolet LED, 350 mA), 5 cm away for 10 minutes, with data being collected every second.
For the cycling experiments, the 200 mg mL−1 Azo-FV trans-gel was placed between two LEDs (LedEngin Inc 365 nm, Ultraviolet LED, 700 mA), 5 cm away for 10 min. A small amount (sampling was performed using a 1.1 × 38 mm needle from TERUMO) was removed and diluted in 3 mL DMSO and DMSO–d6 to collect UV–vis spectra and 1H NMR spectra for comparison. Then the sample was placed between two LEDs (LedEngin Inc 505 nm, Ultraviolet LED, 350 mA), 5 cm away for 10 min and a small sample was again collected for UV–vis spectra and 1H NMR spectra as above. This was repeated three times in total. Throughout, a Teledyne FLIR thermal camera i7 was used to monitor temperature changes during the entire process.
Using a solar simulator
An Abet Technologies’ model 11002 SunLite™ Solar Simulator was used to irradiate at a distance of 5 cm from solutions of 3 mg mL−1 Azo-FV in DMSO, with UV-vis spectra collected every 2 min. A control experiment was also conducted using a solution of the cis-isomer solution pre-formed using direct irradiation with a 365 nm LED. Where noted, we used a THOR LABS 390 nm short-pass filter lens and a 496 nm long-pass filter lens above the sample to block specific wavelengths of light.
Data availability
All data underlying the graphs have been uploaded as Supplementary Data. All primary images and other data are available from the authors on request.
References
Jones, G., Chiang, S.-H. & Xuan, P. T. Energy storage in organic photoisomers. J. Photochem. 10, 1–18 (1979).
Sun, C.-L., Wang, C. & Boulatov, R. Applications of photoswitches in the storage of solar energy. ChemPhotoChem 3, 268–283 (2019).
Wang, Z. et al. Storing energy with molecular photoisomers. Joule 5, 3116–3136 (2021).
Philippopoulos, C., Economou, D., Economou, C. & Marangozis, J. Norbornadiene-quadricyclane system in the photochemical conversion and storage of solar energy. Ind. Eng. Chem. Prod. Res. Dev. 22, 627–633 (1983).
Wang, Z. et al. Macroscopic heat release in a molecular solar thermal energy storage system. Energy Environ. Sci. 12, 187–193 (2019).
Wang, Z. et al. Demonstration of an azobenzene derivative based solar thermal energy storage system. J. Mater. Chem. A 7, 15042–15047 (2019).
Franz, E. et al. Electrochemically triggered energy release from an azothiophene-based molecular solar thermal system. ChemSusChem. 15, e202200958 (2022).
Li, X., Cho, S. & Han, G. G. D. LigHt-responsive Solid–solid Phase Change Materials For Photon And Thermal Energy Storage. ACS Mater. Au 3, 37–42 (2023).
Gerkman, M. A. & Han, G. G. D. Toward controlled thermal energy storage and release in organic phase change materials. Joule 4, 1621–1625 (2020).
Wang, Z. et al. Chip-scale solar thermal electrical power generation. Cell Rep. Phys. Sci. 3, 100789 (2022).
Zhang, Z.-Y. et al. Photochemical phase transitions enable coharvesting of photon energy and ambient heat for energetic molecular solar thermal batteries that upgrade thermal energy. J. Am. Chem. Soc. 142, 12256–12264 (2020).
Dong, L., Feng, Y., Wang, L. & Feng, W. Azobenzene-based solar thermal fuels: design, properties, and applications. Chem. Soc. Rev. 47, 7339–7368 (2018).
Dong, L. et al. Azobenzene-based solar thermal energy storage enhanced by gold nanoparticles for rapid, optically-triggered heat release at room temperature. J. Mater. Chem. A 8, 18668–18676 (2020).
Lennartson, A., Roffey, A. & Moth-Poulsen, K. Designing photoswitches for molecular solar thermal energy storage. Tetrahedron Lett. 56, 1457–1465 (2015).
Xu, X. et al. Azobenzene-containing polymer for solar thermal energy storage and release: advances, challenges, and opportunities. Prog. Polym. Sci. 149, 101782 (2024).
Pramanik, B. & Ahmed, S. Peptide-based low molecular weight photosensitive supramolecular gelators. Gels 8, 533 (2022).
Jiao, T., Wang, Y., Gao, F., Zhou, J. & Gao, F. Photoresponsive organogel and organized nanostructures of cholesterol imide derivatives with azobenzene substituent groups. Prog. Nat. Sci. Mater. Int. 22, 64–70 (2012).
Sahoo, J. K., Nalluri, S. K. M., Javid, N., Webb, H. & Ulijn, R. V. Biocatalytic amide condensation and gelation controlled by light. Chem. Commun. 50, 5462–5464 (2014).
Huang, Y. et al. Supramolecular hydrogels based on short peptides linked with conformational switch. Org. Biomol. Chem. 9, 2149–2155 (2011).
Chen, L., McDonald, T. O. & Adams, D. J. Salt-induced hydrogels from functionalised-dipeptides. RSC Adv. 3, 8714–8720 (2013).
Thomson, L. et al. Transferring micellar changes to bulk properties via tunable self-assembly and hierarchical ordering. ACS Nano 16, 20497–20509 (2022).
Kim, D.-Y. et al. Morphological transitions of a photoswitchable aramid amphiphile nanostructure. Nano Lett. 21, 2912–2918 (2021).
Jones, B. E., Kelly, E. A., Cowieson, N., Divitini, G. & Evans, R. C. Light-responsive molecular release from cubosomes using swell-squeeze lattice control. J. Am. Chem. Soc. 144, 19532–19541 (2022).
Qiu, Q., Shi, Y. & Han, G. G. D. Solar energy conversion and storage by photoswitchable organic materials in solution, liquid, solid, and changing phases. J. Mater. Chem. C 9, 11444–11463 (2021).
McAulay, K. et al. Using Rheo-small-angle neutron scattering to understand how functionalised dipeptides form gels. Org. Mater. 02, 108–115 (2020).
Jones, B. E., Greenfield, J. L., Cowieson, N., Fuchter, M. J. & Evans, R. C. Light-driven hexagonal-to-cubic phase switching in arylazopyrazole lyotropic liquid crystals. J. Am. Chem. Soc. 146, 12315–12319 (2024).
Filik, J. et al. Processing two-dimensional X-ray diffraction and small-angle scattering data in DAWN 2. Appl. Crystallogr. 50, 959–966 (2017).
Pauw, B. R. Everything SAXS: small-angle scattering pattern collection and correction. J. Phys.: Condens. Matter 25, 383201 (2013).
www.sasview.org, accessed 21st June 2025.
Acknowledgements
We thank the EPSRC for funding (EP/Z534109/1). This work benefited from the SasView software, originally developed by the DANSE project under NSF award DMR-0520547. The Ganesha X-ray scattering apparatus was purchased under EPSRC Grant “Atoms to Applications” (EP/K035746/1). ASL’s research project, New Preconcentrators for Improved Portable Detection of Low-Volatility Threats is supported by the Government Office for Science and the Royal Academy of Engineering under the UK Intelligence Community Postdoctoral Research Fellowships scheme.
Author information
Authors and Affiliations
Contributions
Conceptualization: D.J.A.; Methodology: R.H., A.L., A.P., A.J.S., A.S., Z.W., D.J.A.; Data curation: R.H., A.P., A.S. A.J.S; Validation: A.L., A.P.; Formal analysis: R.H., A.L., A.P., A.J.S., A.S., Z.W.; Investigation: R.H, A.P., A.S., Z.W.; Funding acquisition: A.S., D.J.A.; Project administration: A.S., Z.W., D.J.A.; Resources: A.S., D.J.A.; Supervision: A.S. Z.W., D.J.A.; Visualization: R.H., D.J.A.; Writing – original draft: R.H., D.J.A.; Writing – review & editing: All.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, R., Loch, A.S., Pincham, A. et al. Functionalized azobenzenes for micellar solar thermal energy storage as a next-generation MOST system. Commun Chem 8, 369 (2025). https://doi.org/10.1038/s42004-025-01750-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-025-01750-5