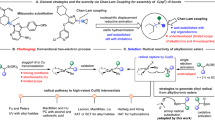
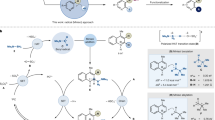
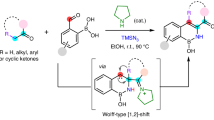
Abstract
Remote boronate rearrangement of boronic acids to C═N bonds is a valuable in synthetic chemistry. Conventional approaches are constrained by the need to pre-install specialized directing groups onto the starting materials. Here, we report a lactam-driven dynamic directing strategy, achieving 1,5- and 1,4-boronate rearrangements. The strategy circumvents the need for substrate pre-activation procedures, successfully overcoming a challenge in the functionalization of inactive C = N bonds to N-alkyl anilines and 3-aryl quinoxalinones. Comprehensive mechanistic investigations unveil three transformative insights: (i) Lactam leverages boron activation to C = N bonds through tetracoordinate boron species; (ii) the 1,5-boronate rearrangement to N-alkyl anilines is favored via an eight-membered boronate complex, as supported by density functional theory (DFT) studies; (iii) a catalyst-free 1,4-boronate rearrangement pathway operates through HFIP-stabilized tetracoordinate boron intermediates. This lactam-enabled boronate rearrangements offers a methodology with transformative potential.

Similar content being viewed by others
Data availability
The data generated in this study are provided in the Supplementary Information file. For the experimental procedures, data of NMR and HRMS analysis and computational details, see the Supplementary Information file. The computational data for each intermediate are provided in Supplementary Data 1. The list of XYZ coordinates for each structure are supplied as. zip files in Supplementary Data 3–6. The X-ray crystallographic coordinates for structures reported in this study are provided in Supplementary Data 2 and 7 and have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers 2441557 and 2341146. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Sharma, H. A., Essman, J. Z. & Jacobsen, E. N. Enantioselective catalytic 1,2-boronate rearrangements. Science 374, 752–757 (2021).
Wang, H., Jing, C. C., Noble, A. & Aggarwal, V. K. Stereospecific 1,2-migrations of boronate complexes induced by electrophiles. Angew. Chem. Int. Ed. 59, 16859–16872 (2020).
Hall, D. G. Boronic acid catalysis. Chem. Soc. Rev. 48, 3475–3496 (2019).
Ma, X. et al. Modular assembly of versatile tetrasubstituted alkenyl monohalides from alkynyl tetracoordinate borons. Chem 9, 1164–1181 (2023).
Guo, Y. et al. Decarboxylation of β-boryl NHPI esters enables radical 1,2-boron shift for the assembly of versatile organoborons. Nat. Commun. 14, 5693 (2023).
Ma, X., An, Y., Li, L., Cai, M. & Song, Q. Interception of alkynyl tetracoordinate borons with sulfur electrophiles beyond the Zweifel pathway. Angew. Chem. Int. Ed. 64, e202416579 (2024).
Wang, D. H., Mück-Lichtenfeld, C. & Studer, A. 1,n-Bisborylalkanes via radical boron migration. J. Am. Chem. Soc. 142, 9119–9123 (2020).
Ma, X. et al. Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift. Nat. Chem. 16, 42–53 (2024).
Yang, K. et al. Construction of C-B axial chirality via dynamic kinetic asymmetric cross-coupling mediated by tetracoordinate boron. Nat. Commun. 14, 4438 (2023).
Li, C. et al. Photo-induced trifunctionalization of bromostyrenes via remote radical migration reactions of tetracoordinate boron species. Nat. Commun. 13, 1784 (2022).
Wei, H., Luo, Y., Ren, J., Yuan, Q. & Zhang, W. Ni(II)-catalyzed asymmetric alkenylation and arylation of aryl ketones with organoborons via 1,5-metalate shift. Nat. Commun. 15, 8775 (2024).
Li, X., Zhang, G. & Song, Q. Recent advances in the construction of tetracoordinate boron compounds. Chem. Commun. 59, 3812–3820 (2023).
Yang, K. & Song, Q. Tetracoordinate boron intermediates enable unconventional transformations. Acc. Chem. Res. 54, 2298–2312 (2021).
Li, C. et al. Remote boryl and alkenyl radical migration of olefin-bearing aryl bromides. CCS Chem. 7, 279–292 (2025).
Liang, J., Chen, X., Chen, J., Ma, X. & Song, Q. Highly stereoselective synthesis of multisubstituted olefins from alkynyl tetracoordinate borons and iodonium ylides via a cyclic intermediate. Org. Lett. 26, 3872–3877 (2024).
Lu, X. et al. Versatile synthesis of α-oxygen organoboron compounds via photo-induced siloxycarbene. Chin. J. Chem. 42, 2712–2716 (2024).
Zhu, F., Jia, Z., Zhang, F. & Wu. X. F. Palladium/copper-catalyzed 1,2-arylboration of styrenes with b2pin2 and indoles. Green Synth. Catal. in press (2025).
Wu, P., Givskov, M. & Nielsen, T. E. Reactivity and synthetic applications of multicomponent Petasis reactions. Chem. Rev. 119, 11245–11290 (2019).
Li, Y., Li, T., Wu, H. & Yang, J. Progress in Petasis reaction. Chin. J. Org. Chem. 32, 1836–1845 (2012).
Petasis, N. A. & Akritopoulou, I. The boronic acid Mannich reaction: a new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 34, 583–586 (1993).
Smith, A. B. III, Fox, R. J. & Razler, T. M. Evolution of the Petasis-Ferrier union/rearrangement tactic: construction of architecturally complex natural products possessing the ubiquitous cis-2,6-substituted tetrahydropyran structural element. Acc. Chem. Res. 41, 675–687 (2008).
Candeias, N. R., Montalbano, F., Cal, P. M. S. D. & Gois, P. M. P. Boronic acids and esters in the Petasis-Borono Mannich multicomponent reaction. Chem. Rev. 110, 6169–6193 (2010).
Pellissier, H. Stereocontrolled domino reactions. Chem. Rev. 113, 442–524 (2013).
Pandit, N. T. & Kamble, S. B. The Petasis reaction: applications and organic synthesis-a comprehensive review. Top. Curr. Chem. 383, 7 (2025).
Krajcovicova, S. Ideas behind the tryptophan-mediated Petasis reaction (TMPR) concept for peptide stapling. ChemMedChem 19, e202400148 (2024).
Petasis, N. A. & Zavialov, I. A. A new and practical synthesis of α-amino acids from alkenyl boronic acids. J. Am. Chem. Soc. 119, 445–446 (1997).
Petasis, N. A. & Zavialov, I. A. Highly stereocontrolled one-step synthesis of anti-β-amino alcohols from organoboronic acids, amines, and α-hydroxy aldehydes. J. Am. Chem. Soc. 120, 11798–11799 (1998).
Batey, R. A., MacKay, D. B. & Santhakumar, V. Alkenyl and aryl boronates-Mild nucleophiles for the stereoselective formation of functionalized N-heterocycles. J. Am. Chem. Soc. 121, 5075–5076 (1999).
Wu, P. et al. Reductive cyclization and Petasis-like reaction for the synthesis of functionalized γ-lactams. Eur. J. Org. Chem. 2015, 2346–2350 (2015).
Wang, Q. & Finn, M. G. 2H-Chromenes from salicylaldehydes by a catalytic Petasis reaction. Org. Lett. 2, 4063–4065 (2000).
Petasis, N. A. & Boral, S. One-step three-component reaction among organoboronic acids, amines and salicylaldehydes. Tetrahedron Lett. 42, 539–542 (2001).
Kumagai, N., Muncipinto, G. & Schreiber, S. L. Short synthesis of skeletally and stereochemically diverse small molecules by coupling Petasis condensation reactions to cyclization reactions. Angew. Chem. Int. Ed. 45, 3635–3638 (2006).
Muncipinto, G., Moquist, P. N., Schreiber, S. L. & Schaus, S. E. Catalytic diastereoselective Petasis reactions. Angew. Chem. Int. Ed. 50, 8172–8175 (2011).
Ascic, E., Le Quement, S. T., Ishoey, M., Daugaard, M. & Nielsen, T. E. Build/couple/pair strategy combining the Petasis 3-component reaction with Ru-catalyzed ring-closing metathesis and isomerization. ACS Comb. Sci. 14, 253–257 (2012).
Mandai, H., Yamada, H., Shimowaki, K., Mitsudo, K. & Suga, S. Petasis Boronic-Mannich reaction of chiral lactol derivatives. Synthesis 46, 2672–2681 (2014).
Mundal, D. A., Lutz, K. E. & Thomson, R. J. A direct synthesis of allenes by a traceless Petasis reaction. J. Am. Chem. Soc. 134, 5782–5785 (2012).
Montalbano, F. et al. Four-component assembly of chiral N-B heterocycles with a natural product-like framework. Org. Lett. 14, 988–991 (2012).
Jiang, Y., Diagne, A. B., Thomson, R. J. & Schaus, S. E. Enantioselective synthesis of allenes by catalytic traceless Petasis reactions. J. Am. Chem. Soc. 139, 1998–2005 (2017).
Bering, L. & Antonchick, A. P. Regioselective metal-free cross-coupling of quinoline N-oxides with boronic acids. Org. Lett. 17, 3134–3137 (2015).
Yang, K., Zhang, F., Fang, T., Zhang, G. & Song, Q. Stereospecific 1,4-metallate shift enables stereoconvergent synthesis of ketoximes. Angew. Chem. Int. Ed. 58, 13421–13426 (2019).
Xu, H., Ye, M., Yang, K. & Song, Q. Regioselective cross-coupling of isatogens with boronic acids to construct 2,2-disubstituted indolin-3-one derivatives. Org. Lett. 23, 7776–7780 (2021).
Zou, P. et al. Photochemical 1,3-boronate rearrangement enables three-component N-alkylation for α-tertiary hydroxybenzylamine synthesis. Nat. Commun. 15, 10234 (2024).
Li, X. et al. (sp²)-C(sp³) cross-coupling of α-(pseudo)halo aliphatic ketones with boronic acids via a 1,4-metallate shift. Nat. Synth. 2, 1211–1221 (2023).
Hao, K. et al. Metal-free 1,3-boronate rearrangement to ketones driven by visible light. Angew. Chem. Int. Ed. 63, e202316481 (2023).
Yoshinaga, Y., Yamamoto, T. & Suginome, M. Stereoinvertive C–C bond formation at the boron-bound stereogenic centers through copper-bipyridine-catalyzed intramolecular coupling of α-aminobenzylboronic esters. Angew. Chem. Int. Ed. 59, 7251–7255 (2020).
Fawcett, A. et al. Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017).
Ohmura, T., Awano, T. & Suginome, M. Stereospecific Suzuki-Miyaura coupling of chiral α-(acylamino)benzylboronic esters with inversion of configuration. J. Am. Chem. Soc. 132, 13191–13193 (2010).
Sandrock, D. L., Jean-Gérard, L., Chen, C. Y., Dreher, S. D. & Molander, G. A. Stereospecific cross-coupling of secondary alkyl -trifluoroboratoamides. J. Am. Chem. Soc. 132, 17108–17110 (2010).
Awano, T., Ohmura, T. & Suginome, M. Inversion or retention? Effects of acidic additives on the stereochemical course in enantiospecific Suzuki-Miyaura coupling of α-(acetylamino)benzylboronic esters. J. Am. Chem. Soc. 133, 20738–20741 (2011).
Lee, J. C. H., McDonald, R. & Hall, D. G. Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nat. Chem. 3, 894–899 (2011).
He, Z., Song, F., Sun, H. & Huang, Y. Transition-metal-free Suzuki-type cross-coupling reaction of benzyl halides and boronic acids via 1,2-metalate shift. J. Am. Chem. Soc. 140, 2693–2699 (2018).
Yang, K. et al. Passerini-type reaction of boronic acids enables α-hydroxy ketones synthesis. Nat. Commun. 12, 441–450 (2021).
Carrër, A., Brion, J.-D., Messaoudi, S. & Alami, M. Palladium(II)-catalyzed oxidative arylation of quinoxalin-2(1H)-ones with arylboronic acids. Org. Lett. 15, 5606–5609 (2013).
Ramesh, B., Reddy, C. R., Kumar, G. R. & Reddy, B. V. S. Mn(OAc)₃·2H₂O promoted addition of arylboronic acids to quinoxalin-2-ones. Tetrahedron Lett. 59, 628–631 (2018).
Yin, K. & Zhang, R. Transition-metal-free direct C–H arylation of quinoxalin-2(1H)-ones with diaryliodonium salts at room temperature. Org. Lett. 19, 1530–1533 (2017).
Paul, S., Ha, J. H., Park, G. E. & Lee, Y. R. Transition metal-free iodosobenzene-promoted direct oxidative 3-arylation of quinoxalin-2(H)-ones with arylhydrazines. Adv. Synth. Catal. 359, 1515–1521 (2017).
Yuan, J., Liu, S. & Qu, L. Transition metal-free direct C-3 arylation of quinoxalin-2(1H)-ones with arylamines under mild conditions. Adv. Synth. Catal. 359, 4197–4207 (2017).
Singh, N., Sharma, A., Singh, J., Pandey, A. P. & Sharma, A. Visible light-induced electron-donor-acceptor-mediated C-3 coupling of quinoxalin-2(1H)-ones with unactivated aryl iodides. Org. Lett. 26, 6471–6476 (2024).
Kwon, S. J., Jung, H. I. & Kim, D. Y. Visible light photoredox-catalyzed arylation of quinoxalin-2(1H)-ones with aryldiazonium salts. ChemistrySelect 3, 5824–5827 (2018).
Acknowledgements
The authors (J.L., X. L., Z.-G.X., Z.-Z.C.) would like to thank Chongqing talents Foundation Project (cstc2022ycjh-bgzxm0170, cstc2022ycjh-bgzxm0170-1 and cstc2024ycjh-bgzxm0134), the Natural Science Foundation Project of CQ CSTSC (CSTB2024NSCQ-LZX0049 and CSTB2024NSCQMSX0917), Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202401347, KJQN202201302, KJZD-K202501310 and KJZD-M202401303), and Chongqing University of Arts and Sciences: Program for Talents Introduction (R2022YX07), the Natural Science Foundation Project of Yongchuan (2023yc-jckx20074 and 2025yc-cxfz10073).
Author information
Authors and Affiliations
Contributions
J.L. conceived the project and designed experiments. J.X. performed experiments and prepared the supplemental information. W.Y. participated in the data collection. J.L., X.L., Z.-G.X., and H. L. prepared this manuscript. Z.-Z.C. and H.L. supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lei, J., Xu, J., Li, X. et al. Lactam enables remote boronate rearrangements to C═N bonds. Commun Chem (2026). https://doi.org/10.1038/s42004-026-01890-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-026-01890-2