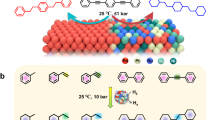
Abstract
Hydrogen-assisted dehydrochlorination process (HaDHC) is an attractive route for the production of fluorine-containing olefins under relatively mild conditions, so far lacking the highly efficient metal-based catalysts and their design strategy. Here, we report a nano-MgF2 supported Pd-Ag catalyst with a tunable Pd dispersion, which is optimized by the Pd-Ag alloy degree in the fresh catalyst and subsequently in situ chlorination during the induction period of reaction. After the in situ restructuring process, the resulting Pd-Ag/nano-MgF2 catalysts with atomically dispersed Pd sites exhibited an excellent catalytic performance for HaDHC of 1,1,1,2-tetrafluoro-2-chloropropane (HCFC-244bb) to 2,3,3,3-tetrafluoropropene (HFO-1234yf), the new-generation refrigerant, with a conversion of ca. 60% and HFO-1234yf selectivity of ca. 82% at 270 °C. Characterization results reveal that the Pd-Ag alloy degree in the fresh catalyst can be facilely tuned by changing the impregnation sequences for bimetallic precursors during catalyst preparation due to different metal-support interactions. Constructing a high Pd-Ag alloy degree offers a high in situ chlorination degree of the catalyst surface to finally get highly isolated Pd sites. Adsorption and computational results demonstrate that the chemisorbed hydrogen species on the single atom Pd sites (Pd-F and Pd-F3 sites) boost the HFO-1234yf formation, while the spillover hydrogen species derived from the large Pd ensembles (Pd6, Pd7, and Pd8 clusters) contribute to the formation of deep hydrogeneration product, 1,1,1,2-tetrafluoropropane (HFC-254eb).

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The raw data for the theoretical calculations during this study are included in the Supplementary Data 1 file. All the raw data for the figures during this study are included in Supplementary Data 2 file.
References
Wang, J. et al. Potential reduction in emissions after replacement of automobile air conditioning refrigerants in China. Energy Rep. 8, 141–151 (2022).
Thomson, J. D. et al. Fluoroform (CHF3) production from CF3CHO photolysis and implications for the decomposition of hydrofluoroolefins and hydrochlorofluoroolefins in the atmosphere. J. Am. Chem. Soc. 147, 33–38 (2024).
Omclinden, M. et al. Limited options for low-global-warming-potential refrigerants. Nat. Commun. 8, 14476 (2017).
Mclinden, M. O., Seeton, C. J. & Pearson, A. New refrigerants and system configurations for vapor-compression refrigeration. Science 370, 791–796 (2020).
van Renssen, S. The greenhouse-gas gang. Nat. Clim. Change 2, 143–144 (2012).
Zhang, J.-J. & Wang, C. China’s hydrofluorocarbon challenge. Nat. Clim. Change 4, 943–945 (2014).
Dreveton, A. Overview of the fluorochemicals industrial sectors. Procedia Eng. 138, 240–247 (2016).
Sicard, A. J. & Baker, R. T. Fluorocarbon refrigerants and their syntheses: Past to present. Chem. Rev. 120, 9164–9303 (2020).
Bellabarba, R. M. Catalysts for modern fluorinated refrigerants. J. Fluor. Chem. 244, 109741 (2021).
Nappa, M., Peng, S. & Sun, X. Industrial syntheses of hydrohaloolefins and related products. Mod. Synth. Processes React. Fluorinated Compd. Elsevier, 2017, pp. 27–69.
Kovalchuk, V. I. & d’Itri, J. L. Catalytic chemistry of chloro- and chlorofluorocarbon dehalogenation: from macroscopic observations to molecular level understanding. Appl. Catal. A 271, 13–25 (2004).
Śrębowata, A. et al. Remarkable effect of post synthesis preparation procedures on catalytic properties of Ni-loaded BEA zeolites in hydrodechlorination of 1,2-dichloroethane. Appl. Catal. B 147, 208–220 (2014).
Gregori, M. et al. Hydrogen-assisted dechlorination of CF3OCFCl-CF2Cl to CF3OCF=CF2 over different metal-supported catalysts. Appl. Catal. A 470, 123–131 (2014).
Śrębowata, A. et al. Hydrodechlorination of 1,2-dichloroethane on active carbon supported palladium-nickel catalysts. Catal. Today 124, 28–35 (2007).
Śrębowata, A. et al. Hydrogen-assisted dechlorination of 1,2-dichloroethane on active carbon supported palladium-copper catalysts. Catal. Today 175, 576–584 (2011).
Xu, L. et al. Mechanistic study of 1,2-dichloroethane hydrodechlorination on Cu-rich Pt-Cu alloys: Combining reaction kinetics experiments with DFT calculations and microkinetic modeling. ACS Sustain. Chem. Eng. 10, 1509–1523 (2022).
Wei, X. et al. Synthesis of Pt-Cu/SiO2 catalysts with different structures and their application in hydrodechlorination of 1,2-dichloroethane. Appl. Catal. B. 121-122, 105–114 (2012).
Ball, M. R. et al. Hydrodechlorination of 1,2-dichloroethane on supported AgPd catalysts. J. Catal. 370, 241–250 (2019).
Sun, J.-Y. et al. Selective hydrodechlorination of 1,2-dichloroethane catalyzed by trace Pd decorated Ag/Al2O3 catalysts prepared by galvanic replacement. Appl. Surf. Sci. 428, 703–709 (2018).
Han, Y.-X. et al. Highly selective hydrodechlorination of 1,2-dichloroethane to ethylene over Ag-Pd/ZrO2 catalysts with trace Pd. Appl. Catal. A 519, 1–6 (2016).
Lambert, S. et al. Pd-Ag/SiO2 and Pd-Cu/SiO2 cogelled xerogel catalysts for selective hydrodechlorination of 1,2-dichloroethane into ethylene. Catal. Today 100, 283–289 (2005).
Job, N. et al. Hydrodechlorination of 1,2-dichloroethane on Pd-Ag catalysts supported on tailored texture carbon xerogels. Catal. Today 102-103, 234–241 (2005).
Xu, L., Stangland, E. E. & Mavrikakis, M. Ethylene versus ethane: a DFT-based selectivity descriptor for efficient catalyst screening. J. Catal. 362, 18–24 (2018).
Heinrichs, B., Schoebrechts, J. P. & Pirard, J. P. Palladium-silver sol-gel catalysts for selective hydrodechlorination of 1,2-dichloroethane into ethylene. III. Kinetics and reaction mechanism. J. Catal. 200, 309–320 (2001).
Tian, S. et al. Breakthrough synthesis of 2,3,3,3-tetrafluoropropene via hydrogen assisted selective dehydrochlorination of 1,1,1,2-tetrafluoro-2-chloropropane over nickel phosphides. J. Catal. 391, 366–377 (2020).
Jia, Z.-H. et al. Hollow nano-MgF2 supported catalysts: Highly active and stable in gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane. Appl. Catal. B 238, 599–608 (2018).
Mao, W. et al. Highly selective dehydrochlorination of 1,1,1,2-tetrafluoro-2-chloropropane to 2,3,3,3-tetrafluoropropeneover alkali metal fluoride modified MgO catalysts. ChemCatChem. 9, 824–832 (2017).
Teinz, K. et al. Catalytic formation of 2,3,3,3-tetrafluoropropene from 2-chloro-3,3,3-trifluoropropene at fluorinated chromia: A study of reaction pathways. Appl. Catal. B 165, 200–208 (2015).
Early, K. O. et al. Hydrogen-assisted 1,2,3-trichloropropane dechlorination on supported Pt-Sn catalysts. Appl. Catal. B 26, 257–263 (2000).
Li, X. et al. Constructing a highly active Pd atomically dispersed catalyst for cinnamaldehyde hydrogenation: Synergistic catalysis between Pd-N3 single Atoms and fully exposed Pd clusters. ACS Catal. 14, 2369–2379 (2024).
Miao, C.-L. et al. Array modified molded alumina supported PdAg catalyst for selective acetylene hydrogenation: Intrinsic kinetics enhancement and thermal effect optimization. Ind. Eng. Chem. Res. 60, 8362–8374 (2021).
Muravev, V. et al. Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts. Science 380, 1174–1178 (2023).
Li, Z.-W. et al. Decoupling active sites enables low-temperature semihydrogenation of acetylene. ACS Catal. 14, 1514–1524 (2024).
Mottaghi, N. et al. Ag/Pd core-shell nanoparticles by a successive method: Pulsed laser ablation of Ag in water and reduction reaction of PdCl2. Appl. Surf. Sci. 292, 892–897 (2014).
Singh, K. B. et al. Sonication-assisted synthesis of Ag@AgCl and Ag@AgCl-GO and their photocatalytic performances. J. Mol. Struct. 1269, 133756 (2022).
Hu, Z.-X. et al. High-rate and selective C2H6- to -C2H4 photodehydrogenation enabled by partially oxidized Pdδ+ species anchored on ZnO nanosheets under mild conditions. J. Am. Chem. Soc. 146, 16490–16498 (2024).
Yang, Y. et al. Breaking scaling relationships in alkynol semihydrogenation by manipulating interstitial atoms in Pd with d-electron gain. Nat. Commun. 13, 2754 (2022).
Mori, K. et al. Surface engineering of a supported PdAg catalyst for hydrogenation of CO2 to formic acid: Elucidating the active Pd atoms in alloy nanoparticles. J. Am. Chem. Soc. 140, 8902–8909 (2018).
Aich, P. et al. Single-atom alloy Pd–Ag catalyst for selective hydrogenation of acrolein. J. Phys. Chem. C. 119, 18140–18148 (2015).
Cao, Y.-Q. et al. Selective hydrogenation of acetylene over Pd-In/Al2O3 catalyst: promotional effect of indium and composition-dependent performance. ACS Catal. 7, 7835–7846 (2017).
Bai, R.-S. et al. Encapsulation of palladium carbide subnanometric species in zeolite boosts highly selective semihydrogenation of alkynes. Angew. Chem. Int. Ed. 62, e202313101 (2023).
Zou, S.-H. et al. Grafting nanometer metal/oxide interface towards enhanced low-temperature acetylene semihydrogenation. Nat. Commun. 12, 5770 (2021).
Yang, T.-X. et al. Improvement of selectivity in acetylene hydrogenation with comparable activity over ordered PdCu catalysts induced by posttreatment. ACS Appl. Mater. Interfaces 13, 706–716 (2021).
Xu, X.-J. et al. Thermal effect optimization endows a selective and stable PdCu single atom alloy catalyst for acetylene hydrogenation. AIChE J. 69, e18042 (2023).
Amorim, C. & Keane, M. A. Catalytic hydrodechlorination of chloroaromatic gas streams promoted by Pd and Ni: The role of hydrogen spillover. J. Hazard. Mater. 211-212, 208–217 (2012).
Sun, M.-S. et al. N-containing silane coupling agent-assisted synthesis of highly dispersed and stable PdC phase for semi-hydrogenation of acetylene. Chem. Eng. Sci. 247, 116939 (2022).
Simone, E. D. et al. Decoding the role of adsorbates entropy in the reactivity of single-atom catalysts. ACS Catal. 15, 447–456 (2025).
Saptal, V. B. et al. An adaptive palladium single-atom catalyst enabling reactivity switching between borylation and C−C coupling. J. Am. Chem. Soc. 147, 18524–18540 (2025).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B. 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Pack, J. D. & Monkhorst, H. J. “Special points for Brillouin-zone integrations”-a reply. Phys. Rev. B. 16, 1748–1749 (1977).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Acknowledgements
We are grateful to be financially supported by the Central-guided local Sci-Tech Development Fund (No. 2022ZY2-JCYJ-02), Smart Grid-National Science and Technology Major Project of China (No. 2025ZD0807500), and Basic Research Program in Natural Science of Shanxi Province (No. 2025JC-YBQN-216, 2025SYS-SYSZD-017). Erhard Kemnitz is dedicating this paper to Prof. Dr. Christian Limberg on the occasion of his 60th birthday.
Author information
Authors and Affiliations
Contributions
C.Y.: Investigation, Methodology, and Experiments. W.M.: Conception, Resources, Methodology, and Writing - Reviewing & Editing. Xingzong Dong: Formal analysis. S.T.: Formal analysis. J.S.: DFT calculation. Zhaotie Liu: Writing - Review & Editing and Funding acquisition. W.Z.: Writing - Review & Editing and Funding acquisition. Jian Lu: Writing - Review & Editing and Funding acquisition. E.K.: Writing - Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, C., Mao, W., Dong, X. et al. Hydrogen-assisted dehydrochlorination of 1,1,1,2-tetrafluoro-2-chloropropane to 2,3,3,3-tetrafluoropropene over Pd-Ag/nano-MgF2 with optimized Pd isolated sites. Commun Chem (2026). https://doi.org/10.1038/s42004-026-01896-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-026-01896-w