Abstract
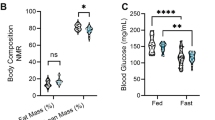
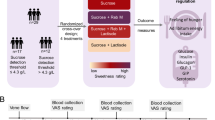
Sucralose, a widely used non-caloric sweetener, provides sweet taste without calories. Some studies suggest that non-caloric sweeteners stimulate appetite, possibly owing to the delivery of a sweet taste without the post-ingestive metabolic signals that normally communicate with the hypothalamus to suppress hunger. In a randomized crossover trial (ClinicalTrials.gov identifier: NCT02945475), 75 young adults (healthy weight, overweight or with obesity) consumed a drink containing sucralose, sweetness-matched sucrose or water. We show that acute consumption of sucralose versus sucrose stimulates hypothalamic blood flow (P < 0.018) and greater hunger responses (P < 0.001). Sucralose versus water also increases hypothalamic blood flow (P < 0.019) but produces no difference in hunger ratings. Sucrose, but not sucralose, increases peripheral glucose levels, which are associated with reductions in medial hypothalamic blood flow (P < 0.007). Sucralose, compared to sucrose and water, results in increased functional connections between the hypothalamus and brain regions involved in motivation and somatosensory processing. These findings suggest that non-caloric sweeteners could affect key mechanisms in the hypothalamus responsible for appetite regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the corresponding author (K.A.P.) on reasonable request, and all brain imaging data are available in the Open Science Framework repository (https://osf.io/tuw93). Access to individual-level data is restricted owing to ethical and legal concerns. However, data may be shared for scientific collaborations upon request, contingent on the execution of appropriate data-sharing agreements. All requests will undergo review and approval by investigators, and will be in compliance with relevant local and national regulations and data-sharing policies. To request access, please contact the corresponding author. An initial response to requests will be provided within four weeks. Source data are provided with this paper.
Code availability
Computer codes used for data analyses are published in the following Open Science Framework repository: https://osf.io/tuw93/.
References
Hales, C. M., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 288, 1–8 (2017).
Hu, F. B. & Malik, V. S. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol. Behav. 100, 47–54 (2010).
Malik, V. S., Pan, A., Willett, W. C. & Hu, F. B. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 98, 1084–1102 (2013).
Bray, G. A. & Popkin, B. M. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes? Health be damned! Pour on the sugar. Diabetes Care 37, 950–956 (2014).
Aguayo-Guerrero, J. A., Méndez-García, L. A., Solleiro-Villavicencio, H., Viurcos-Sanabria, R. & Escobedo, G. Sucralose: from sweet success to metabolic controversies—unraveling the global health implications of a pervasive non-caloric artificial sweetener. Life 14, 323 (2024).
Andrade, L., Lee, K. M., Sylvetsky, A. C. & Kirkpatrick, S. I. Low-calorie sweeteners and human health: a rapid review of systematic reviews. Nutr. Rev. 79, 1145–1164 (2021).
Azad, M. B. et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 189, E929–E939 (2017).
Liauchonak, I., Qorri, B., Dawoud, F., Riat, Y. & Szewczuk, M. R. Non-nutritive sweeteners and their implications on the development of metabolic syndrome. Nutrients 11, 644 (2019).
Sylvetsky, A. C. & Rother, K. I. Nonnutritive sweeteners in weight management and chronic disease: a review. Obesity (Silver Spring) 26, 635–640 (2018).
Toews, I., Lohner, S., de Gaudry, D. K., Sommer, H. & Meerpohl, J. J. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. Brit. Med. J. 364, k4718 (2019).
Van Opstal, A. et al. Dietary sugars and non-caloric sweeteners elicit different homeostatic and hedonic responses in the brain. Nutrition 60, 80–86 (2019).
Tobiassen, P. A. S. & Køster-Rasmussen, R. Substitution of sugar-sweetened beverages with non-caloric alternatives and weight change: a systematic review of randomized trials and meta-analysis. Obesity Rev. 25, e13652 (2024).
Wilk, K., Korytek, W., Pelczyńska, M., Moszak, M. & Bogdański, P. The effect of artificial sweeteners use on sweet taste perception and weight loss efficacy: a review. Nutrients 14, 1261 (2022).
Higgins, K. A. & Mattes, R. D. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am. J Clin. Nutr. 109, 1288–1301 (2019).
Laviada-Molina, H. et al. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: systematic review and meta-analysis. Obesity Rev. 21, e13020 (2020).
Swithers, S. E., Sample, C. H. & Davidson, T. L. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behav. Neurosci. 127, 262 (2013).
Smeets, P. A., de Graaf, C., Stafleu, A., van Osch, M. J. & van der Grond, J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am. J. Clin. Nutr. 82, 1011–1016 (2005).
Van Opstal, A. M. et al. Brain activity and connectivity changes in response to nutritive natural sugars, non-nutritive natural sugar replacements and artificial sweeteners. Nutr. Neurosci. 24, 395–405 (2021).
Frank, G. K. et al. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 39, 1559–1569 (2008).
Smeets, P. A., Weijzen, P., de Graaf, C. & Viergever, M. A. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. Neuroimage 54, 1367–1374 (2011).
Zhang, X. et al. Impacts of acute sucralose and glucose on brain activity during food decisions in humans. Nutrients 12, 3283 (2020).
Sylvetsky, A. C. & Rother, K. I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 164, 446–450 (2016).
Yunker, A. G. et al. Obesity and sex-related associations with differential effects of sucralose vs sucrose on appetite and reward processing: a randomized crossover trial. JAMA Netw. Open 4, e2126313 (2021).
Schiffman, S. S. & Rother, K. I. Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J. Toxicol. Environ. Health B Crit. Rev. 16, 399–451 (2013).
Roger, C. et al. The role of the human hypothalamus in food intake networks: an MRI perspective. Front. Nutr. 8, 760914 (2022).
Osada, T. et al. Functional subdivisions of the hypothalamus using areal parcellation and their signal changes related to glucose metabolism. Neuroimage 162, 1–12 (2017).
Wright, H. et al. Differential effects of hunger and satiety on insular cortex and hypothalamic functional connectivity. Eur. J. Neurosci. 43, 1181–1189 (2016).
Kullmann, S. et al. The effect of hunger state on hypothalamic functional connectivity in response to food cues. Hum. Brain Mapp. 44, 418–428 (2023).
Matsuda, M. et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 48, 1801–1806 (1999).
Liu, Y., Gao, J.-H., Liu, H.-L. & Fox, P. T. The temporal response of the brain after eating revealed by functional MRI. Nature 405, 1058–1062 (2000).
Smeets, P. A., de Graaf, C., Stafleu, A., van Osch, M. J. & van der Grond, J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage 24, 363–368 (2005).
Smeets, P. A. et al. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am. J. Physiol. Endocrinol. Metabol. 293, E754–E758 (2007).
Page, K. A. et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 309, 63–70 (2013).
Luo, S. et al. Resting state hypothalamic response to glucose predicts glucose-induced attenuation in the ventral striatal response to food cues. Appetite 116, 464–470 (2017).
Page, K. A. et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes care 42, 1473–1480 (2019).
Page, K. A. et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J. Clin. Invest. 121, 4161–4169 (2011).
Page, K. A. et al. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes 58, 448–452 (2009).
Neudorfer, C. et al. A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region. Sci. Data 7, 305 (2020).
Heni, M. et al. Insulin action in the hypothalamus increases second-phase insulin secretion in humans. Neuroendocrinology 110, 929–937 (2020).
Hummel, J. et al. Brain insulin action on peripheral insulin sensitivity in women depends on menstrual cycle phase. Nat. Metab. 5, 1475–1482 (2023).
Swithers, S. E., Baker, C. R. & Davidson, T. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav. Neurosci. 123, 772 (2009).
Kohno, D. et al. Sweet taste receptor serves to activate glucose-and leptin-responsive neurons in the hypothalamic arcuate nucleus and participates in glucose responsiveness. Front. Neurosci. 10, 502 (2016).
Kohno, D. Sweet taste receptor in the hypothalamus: a potential new player in glucose sensing in the hypothalamus. J. Physiol. Sci. 67, 459–465 (2017).
Ren, X., Zhou, L., Terwilliger, R., Newton, S. & De Araujo, I. E. Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci. 3, 666 (2009).
Harrold, J. A. et al. Effects of non-nutritive sweetened beverages versus water after a 12-week weight-loss program: a randomized controlled trial. Obesity (Silver Spring) 31, 1996–2008 (2023).
Chao, A. M. et al. Sex/gender differences in neural correlates of food stimuli: a systematic review of functional neuroimaging studies. Obesity Rev. 18, 687–699 (2017).
Yeung, A. Sex differences in brain responses to food stimuli: a meta-analysis on neuroimaging studies. Obesity Rev. 19, 1110–1115 (2018).
Kullmann, S. et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care 38, 1044–1050 (2015).
Hayes, M. R., Skibicka, K. P., Bence, K. K. & Grill, H. J. Dorsal hindbrain 5′-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology 150, 2175–2182 (2009).
Secher, A. et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 124, 4473–4488 (2014).
Bruning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Schwartz, M. W. et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503, 59–66 (2013).
Hetherington, A. & Ranson, S. Hypothalamic lesions and adiposity in the rat. Anat. Rec. 78, 149–172 (1940).
Apps, M. A., Rushworth, M. F. & Chang, S. W. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 90, 692–707 (2016).
Wang, J. et al. Convergent functional architecture of the superior parietal lobule unraveled with multimodal neuroimaging approaches. Hum. Brain Mapp. 36, 238–257 (2015).
Dalenberg, J. R. et al. Short-term consumption of sucralose with, but not without, carbohydrate impairs neural and metabolic sensitivity to sugar in humans. Cell Metab. 31, 493–502.e7 (2020).
Sylvetsky, A. C. et al. Consumption of low-calorie sweeteners among children and adults in the United States. J. Acad. Nutr. Diet. 117, 441–448.e442 (2017).
Martin, C. B., Herrick, K. A., Sarafrazi, N. & Ogden, C. L. Attempts to lose weight among adults in the United States, 2013–2016. NCHS Data Brief 313, 1–8 (2018).
Yunker, A. G., Patel, R. & Page, K. A. Effects of non-nutritive sweeteners on sweet taste processing and neuroendocrine regulation of eating behavior. Current Nutr. Rep. 9, 278–289 (2020).
Contreras-Chavez, G. G., Estrada, J. A. & Contreras, I. Changes in appetite regulation-related signaling pathways in the brain of mice supplemented with non-nutritive sweeteners. J. Mol. Neurosci. 71, 1144–1155 (2021).
Page, K., Luo, S. & Dorton, H. Neural mechanisms for appetitive response for high reward foods. Open Science Framework https://doi.org/10.17605/OSF.IO/E7B9F (2020).
Dye, L. & Blundell, J. Menstrual cycle and appetite control: implications for weight regulation. Hum. Reprod. 12, 1142–1151 (1997).
Krishnan, S., Tryon, R. R., Horn, W. F., Welch, L. & Keim, N. L. Estradiol, SHBG and leptin interplay with food craving and intake across the menstrual cycle. Physiol. Behav. 165, 304–312 (2016).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Aguirre, G. K., Detre, J. A. & Wang, J. Perfusion fMRI for functional neuroimaging. Int. Rev. Neurobiol. 66, 213–236 (2005).
Detre, J. A., Wang, J., Wang, Z. & Rao, H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Current Opin. Neurol. 22, 348–355 (2009).
Wang, Y. et al. Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. Neuroimage 54, 1188–1195 (2011).
Baroncini, M. et al. MRI atlas of the human hypothalamus. Neuroimage 59, 168–180 (2012).
Kullmann, S. et al. Resting-state functional connectivity of the human hypothalamus. Hum. Brain Mapp. 35, 6088–6096 (2014).
Hoang, H. et al. Low-calorie diet-induced weight loss is associated with altered brain connectivity and food desire in obesity. Obesity (Silver Spring) 32, 1362–1372 (2024).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141 (2012).
Nieto-Castanon, A. & Whitfield-Gabrieli, S. CONN functional connectivity toolbox (RRID: SCR_009550), release 21 (Hilbert Press, 2021).
Penny, W. D., Friston, K. J., Ashburner, J. T., Kiebel, S. J. & Nichols, T. E. Statistical parametric mapping: the analysis of functional brain images (Elsevier, 2011).
Nieto-Castanon, A. Handbook of functional connectivity magnetic resonance imaging methods in CONN (Hilbert Press, 2020).
Worsley, K. J. et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 4, 58–73 (1996).
Chumbley, J., Worsley, K., Flandin, G. & Friston, K. Topological FDR for neuroimaging. Neuroimage 49, 3057–3064 (2010).
Flint, A., Raben, A., Blundell, J. & Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obesity 24, 38–48 (2000).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (R01DK102794 to K.A.P, F31DK137584 to S.P.C). A Research Electronic Data Capture, REDCap, database was used for this study, which is supported by the Southern California Clinical and Translational Science Institute through NIH grant UL1TR001855. We thank the volunteers who participated in this study and the staff at the Dornsife Cognitive Neuroimaging Center and Diabetes and Obesity Research Institute of the University of Southern California. A. Romero, E. Trigo, R. Maniego, H. Dorton, E. Jahng, B. Ge, L. N. Overholtzer, J. Hislop, M. Erdstein and P. Dave (all from University of Southern California) assisted with study visits and recruiting volunteers.
Author information
Authors and Affiliations
Contributions
K.A.P. conceived and designed the study. All authors were involved with the acquisition, analysis or interpretation of data. S.P.C. and K.A.P. drafted the manuscript; S.P.C., K.A.P., S.K., R.V., K.J., J.R.M., A.H.X. and A.G.Y. critically reviewed the manuscript for important intellectual content. S.P.C. performed statistical analysis. S.P.C., H.L. and B.A. visualized the project. H.L., A.G.Y., B.A., K.J. and R.V. provided administrative, technical or material support. K.A.P. obtained funding and supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Jean Nakhle and Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Visual Display of Hypothalamic ROI.
Hypothalamic Region of interest (ROIs) and corresponding coordinates. (a) Lateral hypothalamus (green), (b) Medial hypothalamus (blue), (c) Neudorfer (yellow). The images are displayed in neurological convention.
Supplementary information
Supplementary Information
Supplementary Tables 1–10; Supplementary File 1, Study Protocol, Statistical Analysis Plan
Source data
Source Data Fig. 3
Statistical source data
Source Data Fig. 4
Statistical source data
Source Data Fig. 6
Statistical source data
Source Data Fig. 7
Statistical source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chakravartti, S.P., Jann, K., Veit, R. et al. Non-caloric sweetener effects on brain appetite regulation in individuals across varying body weights. Nat Metab 7, 574–585 (2025). https://doi.org/10.1038/s42255-025-01227-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01227-8