Abstract
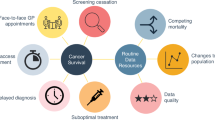
The coronavirus disease 2019 pandemic substantially impacted the delivery of cancer services and programs. Here we reviewed and synthesized the global scale and impact of pandemic-related delays and disruptions on cancer services, including diagnosis, diagnostic procedures, screening, treatment and supportive and palliative care. Based on data from 245 articles in 46 countries, we observed declines in the number of cancer screening participation (39.0%), diagnoses (23.0%), diagnostic procedures (24.0%) and treatment (28.0%), ranging from a 15.0% decline for radiotherapy to a 35.0% decline for systemic treatment during the pandemic compared to during the prepandemic period. Medium-human development index (HDI) category countries experienced greater reductions than high- and very-high-HDI countries. Missing data from low-HDI countries emphasize the need for increased investments in cancer surveillance and research in these settings. PROSPERO registration: CRD42022301816
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
We declare that the data supporting the findings of this study are available within the paper and its supplementary information files. All the studies included in this study were obtained from the WHO COVID-19 global research database. Source data are provided with this paper.
Code availability
No new algorithms were developed for this paper. All code generated for the analysis is available from the authors upon request.
References
The Economist. Estimated cumulative excess deaths per 100,000 people during COVID. Our World in Data https://ourworldindata.org/grapher/excess-deaths-cumulative-per-100k-economist (2023).
World Health Organization. COVID-19 pandemic fuels largest continued backslide in vaccinations in three decades. WHO https://www.who.int/news/item/15-07-2022-covid-19-pandemic-fuels-largest-continued-backslide-in-vaccinations-in-three-decades (2022).
Riera R. et al. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob. Oncol. 7, 311–323 (2021).
Majeed, A. et al. The global impact of COVID-19 on childhood cancer outcomes and care delivery—a systematic review. Front. Oncol. 12, 869752 (2022).
Moynihan, R. et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open 11, e045343 (2021).
Arsenault, C. et al. COVID-19 and resilience of healthcare systems in ten countries. Nat. Med. 28, 1314–1324 (2022).
Cucinotta, D. & Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 91, 157–160 (2020).
Gandhi, M. et al. Impact of COVID-19 on hepatocellular carcinoma management: a multicountry and region study. J. Hepatocell. Carcinoma 8, 1159–1167 (2021).
Monroy-Iglesias, M. J. et al. Continuity of cancer care: the surgical experience of two large cancer hubs in London and Milan. Cancers 13, 1597 (2021).
Bogani, G. et al. Characteristics and patterns of care of endometrial cancer before and during COVID-19 pandemic. J. Gynecol. Oncol. 33, e10 (2022).
Eklöv, K. et al. Colon cancer treatment in Sweden during the COVID-19 pandemic: a nationwide register-based study. Colorectal Dis. 24, 925–932 (2022).
Al-Sukhun, S., de Lima Lopes, G., Gospodarowicz, M., Ginsburg, O. & Yu, P. P. Global health initiatives of the International Oncology Community. Am. Soc. Clin. Oncol. Educ. Book 37, 395–402 (2017).
Lombe, D., Phiri, M. & Msadabwe, S. Negative impact of the COVID-19 pandemic on the management of cervical cancer patients in Zambia. Ecancermedicalscience 14, ed103 (2020).
Kugbey, N., Ohene-Oti, N. & Vanderpuye, V. COVID-19 and its ramifications for cancer patients in low-resource settings: Ghana as a case study. Ecancermedicalscience 14, ed99 (2020).
Vanderpuye, V., Elhassan, M. M. A. & Simonds, H. Preparedness for COVID-19 in the oncology community in Africa. Lancet Oncol. 21, 621–622 (2020).
Salako, O. et al. Upheaval in cancer care during the COVID-19 outbreak. Ecancermedicalscience 14, ed97 (2020).
Van Hemelrijck, M. et al. Global cancer research in the era of COVID-19: a bibliometric analysis. Ecancermedicalscience 15, 1264 (2021).
Patt, D. et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin. Cancer Inform. 4, 1059–1071 (2020).
Solis, R. N. et al. The impact of COVID-19 on head and neck cancer treatment: before and during the pandemic. OTO Open 5, 2473974X211068075 (2021).
London, J. W., Fazio-Eynullayeva, E., Palchuk, M. B., Sankey, P. & McNair, C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin. Cancer Inform. 4, 657–665 (2020).
Bakouny, Z. et al. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 7, 458–460 (2021).
Liu, Y. A. et al. Hospital visiting policies in the time of coronavirus disease 2019: a nationwide website survey in Taiwan. J. Chin. Med. Assoc. 83, 566–570 (2020).
Villain, P. et al. Cross‐sectional survey of the impact of the COVID‐19 pandemic on cancer screening programs in selected low‐ and middle‐income countries: study from the IARC COVID‐19 impact study group. Int. J. Cancer 149, 97–107 (2021).
Allahqoli, L., Mazidimoradi, A., Salehiniya, H. & Alkatout, I. Impact of COVID-19 on cancer screening: a global perspective. Curr. Opin. Support. Palliat. Care 16, 102–109 (2022).
Skovlund, C. W., Friis, S., Dehlendorff, C., Nilbert, M. C. & Mørch, L. S. Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta Oncol. 60, 20–23 (2021).
Nogami, Y. et al. Impact of COVID-19 on gynecologic cancer treatment in Japan: a nationwide survey by the Japan Society of Gynecologic Oncology (JSGO). J. Gynecol. Oncol. 33, e8 (2021).
Tsai, H. Y. et al. Effects of the COVID-19 pandemic on breast cancer screening in Taiwan. Breast 54, 52–55 (2020).
Vanni, G. et al. Breast cancer and COVID-19: the effect of fear on patients’ decision-making process. In Vivo 34, 1651–1659 (2020).
Llanwarne, N., Newbould, J., Burt, J., Campbell, J. L. & Roland, M. Wasting the doctor’s time? A video-elicitation interview study with patients in primary care. Soc. Sci. Med. 176, 113–122 (2017).
Jones, D. et al. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 21, 748–750 (2020).
Black, G. B., Boswell, L., Harris, J. & Whitaker, K. L. What causes delays in diagnosing blood cancers? A rapid review of the evidence. Prim. Health Care Res. Dev. 24, e26 (2023).
Funston, G., O’Flynn, H., Ryan, N. A. J., Hamilton, W. & Crosbie, E. J. Recognizing gynecological cancer in primary care: risk factors, red flags, and referrals. Adv. Ther. 35, 577–589 (2018).
Low, E. L., Whitaker, K. L., Simon, A. E., Sekhon, M. & Waller, J. Women’s interpretation of and responses to potential gynaecological cancer symptoms: a qualitative interview study. BMJ Open 5, e008082 (2015).
Schoonbeek, R. C. et al. Fewer head and neck cancer diagnoses and faster treatment initiation during COVID-19 in 2020: a nationwide population-based analysis. Radiother. Oncol. 167, 42–48 (2022).
Nicholson, B. D. et al. Consultations for clinical features of possible cancer and associated urgent referrals before and during the COVID-19 pandemic: an observational cohort study from English primary care. Br. J. Cancer 126, 948–956 (2022).
Venables, Z. C. et al. The impact of the COVID-19 pandemic on skin cancer incidence and treatment in England, 2020. Br. J. Dermatol. 185, 460–462 (2021).
Makaranka, S., Scutt, F. & Rahman, K. The impact of the COVID-19 pandemic on diagnosis of skin cancer cases in North Cancer Alliance and Scotland. Cureus 14, e25019 (2022).
Koysombat, K., Plonczak, A. M. & West, C. A. The role of teleconsultation in the management of suspected skin malignancy in plastic surgery during COVID-19 outbreak: a single centre experience. J. Plast. Reconstr. Aesthet. Surg. 74, 1931–1971 (2021).
Chuchu, N. et al. Teledermatology for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 12, CD013193 (2018).
Malagón, T., Yong, J. H. E., Tope, P., Miller, W. H. & Franco, E. L. McGill Task Force on the impact of COVID-19 on cancer control and care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int. J. Cancer 150, 1244–1254 (2022).
Safayet Ullah Prodhan, A. H. M. et al. Breast cancer management in the era of COVID-19; key issues, contemporary strategies, and future implications. Breast Cancer 15, 51–89 (2023).
COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br. J. Surg. 107, 1440–1449 (2020).
van Doremalen, N. et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382, 1564–1567 (2020).
Givi, B. et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol. Head Neck Surg. 146, 579–584 (2020).
De Luca, P. et al. Nasal, pharyngeal and laryngeal endoscopy procedures during COVID-19 pandemic: available recommendations from national and international societies. Eur. Arch. Otorhinolaryngol. 277, 2151–2153 (2020).
Wu, V. et al. Considerations for head and neck oncology practices during the coronavirus disease 2019 (COVID-19) pandemic: Wuhan and Toronto experience. Head Neck 42, 1202–1208 (2020).
Zou, L. et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 382, 1177–1179 (2020).
Kochbati, L. et al. Cancer care and COVID-19: tailoring recommendations for the African radiation oncology context. Ecancermedicalscience 14, 1144 (2020).
de Andrade Carvalho, H., Vasconcelos, K. G. M. C., Gomes, H. C. & Salvajoli, J. V. Impact of COVID-19 pandemic on a daily-based outpatient treatment routine: experience of a radiotherapy department of a tertiary public/university hospital in Brazil. Clinics 75, e2298 (2020).
Roberge, D., Delouya, G., Bohigas, A. & Michalowski, S. Catching the wave: quantifying the impact of COVID on radiotherapy delivery. Curr. Oncol. 28, 152–158 (2020).
De Felice, F. et al. A snapshot on radiotherapy for head and neck cancer patients during the COVID-19 pandemic: a survey of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) head and neck working group. Radiol. Med. 126, 343–347 (2021).
Venkatasai, J. et al. Impact of COVID-19 pandemic on patterns of care and outcome of head and neck cancer: real-world experience from a tertiary care cancer center in India. JCO Glob. Oncol. 8, e2100339 (2022).
Araujo, S. E. A. et al. Impact of COVID-19 pandemic on care of oncological patients: experience of a cancer center in a Latin American pandemic epicenter. Einstein 19, eAO6282 (2020).
Alagoz, O. et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J. Natl Cancer Inst. 113, 1484–1494 (2021).
Maringe, C. et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 21, 1023–1034 (2020).
Guével, E. et al. Impact of the COVID‐19 pandemic on clinical presentation, treatments, and outcomes of new breast cancer patients: a retrospective multicenter cohort study. Cancer Med. 12, 20918–20929 (2023).
Fu, R. et al. Early survival for patients newly diagnosed with cancer during COVID-19 in Ontario, Canada: a population-based cohort study. Cancer Med. 12, 11849–11859 (2023).
Resende Souza de, B., Dias, R. M., Ferrari, G. & Rezende, L. F. M. Excess mortality in adults from Sao Paulo during the COVID-19 pandemic in 2020: analyses of all-cause and noncommunicable diseases mortality. Sci. Rep. 13, 23006 (2023).
Starkey, T. et al. A population-scale temporal case–control evaluation of COVID-19 disease phenotype and related outcome rates in patients with cancer in England (UKCCP). Sci. Rep. 13, 11327 (2023).
Bungaro, M., Passiglia, F. & Scagliotti, G. V. COVID-19 and lung cancer: a comprehensive overview from outbreak to recovery. Biomedicines 10, 776 (2022).
Cheng, D. et al. Trends in oncological imaging during the COVID‐19 pandemic through the vaccination era. Cancer Med. 12, 9902–9911 (2023).
Kunyenje, C. A. et al. COVID-19 vaccine inequity in African low-income countries. Front. Public Health 11, 1087662 (2023).
Mathieu, E. et al. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 5, 947–953 (2021).
Sinopoli, A., Baccolini, V. & Di Rosa, E. Killing two birds with one stone: is the COVID-19 vaccination campaign an opportunity to improve adherence to cancer screening programmes? The challenge of a pilot project in a large local health authority in Rome. Vaccines 11, 523 (2023).
World Health Organization. WHO COVID-19 research database. https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/ (2023).
National Cancer Institute. Definition of diagnostic test. NCI Dictionary of Cancer Terms. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/diagnostic-test (2011).
Kreuter, M. & Herth, F. J. F. Supportive and palliative care of advanced nonmalignant lung disease. Respiration 82, 307–316 (2011).
United Nations. Human development index. Human development reports. https://hdr.undp.org/data-center/human-development-index (2023).
United Nations. World population prospects—population division. https://population.un.org/wpp/DefinitionOfRegions/ (2024).
Walters, S. et al. Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int. J. Cancer 132, 676–685 (2013).
National Heart, Lung, and Blood Institute. Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (2023).
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Brit. Med. J. 327, 557–560 (2003).
Sterne, J. A. & Egger, M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol. 54, 1046–1055 (2001).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Brit. Med. J. 315, 629–634 (1997).
StataCorp. Stata Statistical Software: Release 18 (StataCorp, 2023).
Acknowledgements
This study was partly funded by the WHO (2021/1187438-0). The funder had no role in study design, data collection and analysis, decision to publish or preparation of this manuscript. R. Shah was funded by a fellowship from the International Agency for Research on Cancer during the duration of this study.
Author information
Authors and Affiliations
Contributions
R. Shah, I.S., J.S., S.H., M.C., H.H., A.M.I. and K.C. conceptualized the study and developed the study protocol and methodology. R. Shah, N.M.H., C.E.L., A.M., H.F., E.M., M.G., R.G., S.A., J.N., O.L., C.F., N.L., C.E.K. and C.L.G. implemented the method, performed screenings, collected data and performed quality assessments. M.D., S.E., J.V. and R. Shah performed data analyses. R. Shah, N.M.H. and I.S. wrote the original draft. All authors, including R. Sullivan, F.B. and O.G., were involved in interpretation of the results, participated in the review and editing of the paper and approved the final version. K.C. helped with funding acquisition. Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policies or views of the International Agency for Research on Cancer/WHO.
Corresponding author
Ethics declarations
Competing interests
K.C. is co-principal investigator of an investigator-initiated trial of cervical screening, ‘Compass’, run by the VCS Foundation Australia, which is a government-funded not-for-profit charity. She is also co-principal investigator on a major implementation program ‘Elimination of Cervical Cancer in the Western Pacific’, which will receive support from the Minderoo Foundation and the Frazer Family Foundation and equipment donations from Cepheid. M.C. is an investigator on an investigator-initiated trial of cytology and primary human papillomavirus screening in Australia (Compass; ACTRN12613001207707 and NCT02328872), which is also conducted and funded by the VCS Foundation. The VCS Foundation has received equipment and a funding contribution for the Compass trial from Roche Molecular Systems and Ventana. However, K.C., M.C. and their institution on their behalf (the Daffodil Centre, a joint venture between Cancer Council NSW and The University of Sydney) do not receive direct funding from the industry for these or any other research project. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Timothy Hanna and Felicia Knaul for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 PRISMA flow diagram for selection of included studies.
PRISMA flowchart of the systematic search and selection process. From a total of 9,702 studies identified, we screened 9,458 studies for eligibility, removed 244 duplicates, and excluded 8,547 studies. We finally included a total of 245 eligible study for this systematic review and meta-analysis.
Extended Data Fig. 2 Forest plot of pooled rate ratios for number of individuals screened for breast, cervical, and colorectal cancers before and during the COVID-19 pandemic.
Forest plot of pooled rate ratios for individuals screened for breast, cervical, and colorectal cancers. Two-tailed Z-scores, following a natural logarithmic transformation were used to calculate p-values, with the DerSimonian and Laird method being used to pool effect estimates. The square box represents rate ratio (RR) and the size of the box represents study weight. Diamond represents pooled RR. Dotted vertical line represents line of overall effect. Horizontal bars indicate 95% CIs of two-sided random effects meta-analyses. Vertical solid black line represents the line of no effect. Pooled analysis of 48 studies with a combined cohort of approximately 35.2 million individuals pre-COVID-19 and 25.1 million individuals during COVID-19 showed that: a In the sub-group analysis comparing the HDI groups, the greatest reduction was seen in the number of individuals screened for breast cancer (50.0% in very high and 46.0% in high HDI countries), followed by cervical cancer (37.0% in very high and 39.0% in high HDI countries). b In the sub-group analysis comparing the continents, the highest relative decline in the number of individuals screened for breast cancer reported in studies was in the Americas (60.0%), followed by Oceania (51.0%) and Asia (43.0%). Similarly, the decline in cervical cancer screening participation was greatest in the Americas (34.0%) followed by Asia (22.0%). For colorectal cancer screening, the largest proportional decline was seen in Oceania (66.0%), followed by Asia (43.0%) and the Americas (29.0%). c The greatest reduction was seen in the number of individuals screened for breast cancer (49.0%).
Extended Data Fig. 3 Forest plot of pooled rate ratios for individuals diagnosed with cancer before and during the COVID-19.
Two-tailed Z-scores, following a natural logarithmic transformation were used to calculate p-values, with the DerSimonian and Laird method being used to pool effect estimates. The square box represents rate ratio (RR) and the size of the box represents study weight. Diamond represents pooled RR. Dotted vertical line represents line of overall effect. Horizontal bars indicate 95% CIs of two-sided random effects meta-analyses. Vertical solid black line represents the line of no effect. a Pooled analysis of 99 studies with a combined cohort of approximately 2 million individuals pre-COVID-19 and 1.2 million individuals during COVID-19 showed that the overall cancer diagnosis decreased by 23.0% (RR = 0.77; 95% CI: 0.74 to 0.80) with significant heterogeneity between studies (I2 = 99.4%, P < 0.01). Of all world regions with data included, the greatest reduction was reported across African countries (36.0%). b Pooled analysis of 92 studies with a combined cohort of approximately 1 million individuals pre-COVID-19 and 579,581 individuals during COVID-19 showed that overall cancer diagnosis decreased by 24.0% (RR = 0.76; 95% CI: 0.74 to 0.78) with significant heterogeneity between studies (I2 = 97.4%, P < 0.01). The reduction was highest for gynaecological (RR = 0.68; 95% CI: 0.60 to 0.77), skin (melanomas and non-melanomas) (Rate Ratio (RR) = 0.68; 95% CI: 0.57 to 0.81), and urogenital cancers (RR = 0.69, 95% CI: 0.65 to 0.74). c) Pooled analysis of 92 studies with a combined cohort of approximately 1 million individuals pre-COVID-19 and 580,937 individuals during COVID-19 showed that the overall cancer diagnostic procedures decreased by 24.0% (RR = 0.76; 95% CI: 0.75 to 0.78) with significant heterogeneity between studies (I2 = 96%, P < 0.01). For specific cancer types, large decreases were seen for cervical cancer (RR = 0.52, 95% CI: 0.41 to 0.66), vulva cancers (RR = 0.53;95% CI: 0.34 to 0.83), and lymphomas (RR = 0.56; 95% CI: 0.40 to 0.78).
Extended Data Fig. 4 Forest plot of pooled rate ratios for individuals who underwent cancer diagnostic procedures before and during the COVID-19 pandemic.
Two-tailed Z-scores, following a natural logarithmic transformation were used to calculate p-values, with the DerSimonian and Laird method being used to pool effect estimates. The square box represents rate ratio (RR) and the size of the box represents study weight. Diamond represents pooled RR. Dotted vertical line represents line of overall effect. Horizontal bars indicate 95% CIs of two-sided random effects meta-analyses. Vertical solid black line represents the line of no effect. The results are pooled analyses of 55 studies with a combined cohort of approximately 10 million individuals pre-COVID-19 and 7 million individuals during COVID-19. a Overall cancer diagnostic procedures decreased by 24.0% (RR = 0.76; 95% CI: 0.75 to 0.78) with significant heterogeneity between studies (I2 = 96%, P < 0.01). The largest reduction in diagnostic procedures was for haematological cancers (RR = 0.31; 95% CI: 0.24 to 0.40) in medium HDI countries and gastrointestinal cancers in high HDI countries (RR = 0.71; 95% CI: 0.63 to 0.81), and gynaecological cancers in very high HDI countries (RR = 0.66, 95% CI: 0.59 to 0.74). b In Africa, the largest decline in diagnostic procedure was for urogenital cancers (RR = 0.42; 95% CI: 0.30 to 0.58), in the Americas it was for breast cancer (RR = 0.70; 95% CI: 0.62 to 0.80), in Asia, for haematological cancers (RR = 0.37; 95% CI: 0.28 to 0.49), while in Europe, gynaecological cancers exhibited the greatest decrease (RR = 0.53; 95% CI: 0.39 to 0.72). c In the sub-group analysis, the greatest reduction was seen in the number of individuals who underwent procedures for diagnosis of gynaecologic (RR = 0.68; 95% CI: 0.61 to 0.75) and haematological (RR = 0.69; 95% CI: 0.57 to 0.83) cancers.
Extended Data Fig. 5 Forest plots of pooled rate ratios for number of cancer treatment deliveries before and during the COVID-19 pandemic.
Two-tailed Z-scores, following a natural logarithmic transformation were used to calculate p-values, with the DerSimonian and Laird method pooling effect estimates. The square box represents rate ratio (RR) and the size of the box represents study weight. Diamond represents pooled RR. Dotted vertical line represents overall effect. Horizontal bars indicate 95% CIs. Vertical solid black line represents no effect. a–c Analyses of 122 studies showed Asia had the highest reduction in overall cancer treatment (RR = 0.63; 95% CI: 0.57 to 0.70) (between 652,459 and 15,498,757 participants). The highest reductions were for musculoskeletal cancers (RR = 0.47; 95% CI: 0.36 to 0.61) (332,251 pre-COVID-19 and 285,261 during COVID-19), and for oral cavity cancers (RR = 0.13; 95% CI: 0.10 to 0.17) (319,916 pre-COVID-19 and 275,562 during COVID-19). d-f Analyses of 90 studies (616,101 participants) showed the highest decrease in surgical treatment in medium HDI countries (RR = 0.54; 95% CI: 0.47 to 0.63) and for head and neck cancers (RR = 0.45; 95% CI: 0.26 to 0.78). Africa had the highest reduction for head and neck cancers (RR = 0.21; 95% CI: 0.14 to 0.32). The highest reduction by cancer group was for musculoskeletal cancers (RR = 0.50; 95% CI: 0.38 to 0.66). g–i Analyses of 18 studies (33,994 participants) showed the greatest reduction in medium HDI countries and for head and neck cancers (RR = 0.13, 95% CI: 0.08 to 0.22). The largest reduction by site was for neurological cancers (RR = 0.78, 95% CI: 0.66 to 0.92). j-l Analyses of 25 studies (24,897 participants) showed the largest decrease for breast cancer in very high HDI (RR = 0.65; 95% CI: 0.49 to 0.87) and high HDI countries (RR = 0.38; 95% CI: 0.31 to 0.47), and for haematological cancers in medium HDI countries (RR = 0.16; 95% CI: 0.15 to 0.18). The highest decline was in Asia for musculoskeletal cancers (RR = 0.32; 95% CI: 0.16 to 0.64). m) The increase in time from diagnosis to treatment was not statistically significant (P = 0.13).
Extended Data Fig. 6 Forest plot of pooled rate ratios for individuals who received supportive and palliative care before and during the COVID-19 pandemic.
Two-tailed Z-scores, following a natural logarithmic transformation were used to calculate p-values, with the DerSimonian and Laird method being used to pool effect estimates. The square box represents rate ratio (RR) and the size of the box represents study weight. Diamond represents pooled RR. Dotted vertical line represents line of overall effect. Horizontal bars indicate 95% CIs of two-sided random effects meta-analyses. Vertical solid black line represents the line of no effect. Analyses of 12 studies with a combined cohort of 44,414 individuals showed an overall decrease of 70.0% (RR = 0.30; 95% CI: 0.11 to 0.83) in supportive and palliative care during the pandemic with significant heterogeneity between studies (I2 = 99.5%, P < 0.01).
Extended Data Fig. 7 Forest plot of pooled rate ratios for the stage at diagnosis of cancer before and during the COVID-19 pandemic.
Two-tailed Z-scores, following a natural logarithmic transformation were used to calculate p-values, with the DerSimonian and Laird method being used to pool effect estimates. The square box represents rate ratio (RR) and the size of the box represents study weight. Diamond represents pooled RR. Dotted vertical line represents line of overall effect. Horizontal bars indicate 95% CIs of two-sided random effects meta-analyses. Vertical solid black line represents the line of no effect. Results are from pooled analyses from 80 studies with a combined cohort of 174,343 individuals. a The results showed decline in patients diagnosed with cancer at any stage in all continents. This decrease was lowest in medium HDI countries (65.0% in non-metastatic cancers vs 67.0% in metastatic cancers) compared to high HDI (45.0% in non-metastatic cancers vs 25.0% in metastatic cancers) and very high HDI countries (12.0% in non-metastatic cancers vs 10.0% in metastatic cancers). b There was an overall decrease in the number of patients newly diagnosed with both non-metastatic and metastatic cancer (RR = 0.80; 95% CI: 0.77 to 0.83) with significant heterogeneity between studies (I2 = 86.9%, P < 0.01). In the sub-group analysis comparing between continents, significant reductions in newly diagnosed non-metastatic cancers were seen in Americas (RR = 0.75; 95% CI: 0.66 to 0.85), Asia (RR = 0.82; 95% CI: 0.72 to 0.93), and Europe (RR = 0.80; 95% CI: 0.77 to 0.84). The decrease in newly diagnosed metastatic cancers was significant only in Europe (RR = 0.86; 95% CI: 0.77 to 0.96). c The decrease in the number of patients newly diagnosed with non-metastatic cancer (RR = 0.79; 95% CI: 0.75 to 0.82) was higher than the number of patients newly diagnosed with metastatic cancer (RR = 0.86; 95% CI: 0.79 to 0.94) with significant heterogeneity between studies (I2 = 74.5%, P = 0.05).
Extended Data Fig. 8 Funnel Plots for publication bias.
Funnel plots derived from Egger’s linear regression test for publication bias. Statistical test used was two-sided. All funnel plots showed evidence of publication bias as evidenced by substantial number of points outside each funnel and points not symmetrically distributed in each funnel. However, on Egger’s test, publication bias was present in eight of the meta-analyses (b–d,f–i,j,m), with effect sizes tending to decrease as standard errors increased. Publication bias was not present in figures a and e based on the results of Egger's test.
Supplementary information
Supplementary Tables 1–8
Supplementary Table 1. List of countries, geographical regions and HDI level. Supplementary Table 2. Summary of included studies. Supplementary Table 3. PRISMA checklist. Supplementary Table 4. Search strategy. Supplementary Table 5. List of studies excluded at the full-text screening stage, with brief reasons. Supplementary Table 6. Quality assessment tool. Supplementary Table 7. Quality assessments of included studies. Supplementary Table 8. References.
Source data
Source Data Figs. 1–5 and Extended Data Figs. 2–8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, R., Hanna, N.M., Loo, C.E. et al. The global impact of the COVID-19 pandemic on delays and disruptions in cancer care services: a systematic review and meta-analysis. Nat Cancer 6, 194–204 (2025). https://doi.org/10.1038/s43018-024-00880-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-024-00880-4
This article is cited by
-
Optimal timing of cancer treatments: a call for emerging evidence from clinical trials and real-world studies
British Journal of Cancer (2025)
-
COVID-19-Pandemie – verdrängt und wenig gelernt?
Die Gynäkologie (2025)
-
Impact of COVID-19 vaccination on cancer patients: safety, efficacy, and long-term effects
Supportive Care in Cancer (2025)
-
Global burden of acute lymphoblastic leukemia following the COVID-19 pandemic
Annals of Hematology (2025)