Abstract
All-solid-state lithium–sulfur batteries exhibit high energy densities, operate safely, and suppress polysulfide shuttling. However, their electrochemical performance is restricted by the insulating nature of sulfur and Li2S, and by severe cathode-related volumetric changes during cycling. Here, we prepare a Li2S-based cathode composite with high mixed-conductivity and stability, by infiltrating a Li2S–LiI active material solution into a mesoporous carbon replica with ~10-nm-sized pores, followed by mixing with a liquid-phase-synthesized Li6PS5Br solid electrolyte and vapour-grown carbon fibres. Benefiting from a mechanically reinforced, three-dimensional ion/electron-conducting structure, the cathode exhibits high discharge capacity (1009 mAh g−1, 20 cycles, 298 K, 0.05 C) and high reversible capacity (650 mAh g−1, 100 cycles, 298 K, 0.1 C). These findings underscore the feasibility of developing high-performance all-solid-state lithium-sulfur batteries by designing three-dimensional mixed-conducting mechanically robust cathodes.
Similar content being viewed by others
Introduction
All-solid-state lithium–sulfur batteries (ASSLSBs) with solid electrolytes (SEs) are considered promising next-generation energy storage systems owing to their high theoretical specific capacity (S, 1675 mAh g−1; Li2S, 1166 mAh g−1), high energy density (2500 Wh kg−1), non-flammability, and the natural abundance and low toxicity of sulfur1,2,3. Moreover, the shuttle effect caused by the dissolution and diffusion of lithium polysulfide intermediates (Li2Sx, 4 < x < 8) in liquid electrolytes is radically suppressed in solid-state redox reactions4,5. However, three issues prevent ASSLSBs from efficiently utilizing active materials (AMs), resulting in low electrochemical performances: (1) the insulating property of Li2S/S towards both electrons and ions, (2) the severe stress and strain caused by the volumetric change (80%) of the sulfur cathode during lithiation/delithiation, and (3) the poor solid–solid interfacial contact between the AM and the SE6,7. Therefore, the development of a solid-state cathode composite that can simultaneously overcome these three drawbacks is vital.
Typically, a cathode composite of an ASSLSB comprises an SE, AM (S or Li2S), and conductive additives8. Numerous studies have been conducted to optimize each of these three components. Notably, sulfide-based SEs have been used considerably more than other types of SEs (such as polymer-9, oxide-10, hydride-11, and halide-based SEs12) in ASSLSBs, owing to their high ionic conductivity, interface formability, and electrochemical stability windows that suitably match the operating voltages of ASSLSBs13,14. Among them, sulfide SEs with argyrodite-15, Li10GeP2S12-16,17, and Li7P3S11-type18 crystal structures show promise as practically viable SEs because they exhibit remarkably high conductivities (>10−3 S cm−1), comparable to those of liquid electrolytes. However, the conventional mechanical ball-milling method used for synthesizing these SEs is not only time-consuming (>3 days) and energy-inefficient (wearing of the balls and wall armor, friction, heating of the material, etc.) but also yields large (>1 μm), inhomogeneous SE particles19,20. Therefore, introducing sulfide SEs with small particles to the cathode composite is crucial for enhancing the solid–solid interfacial contact between AMs and SEs, thereby facilitating the construction of effective ion-conducting pathways and consequently improving the electrochemical performance21. Specifically, liquid-phase synthesis is a promising alternative to ball milling for developing SEs with small particles; this process, which features relatively short synthesis durations (≈1 day) and is therefore scalable, involves dissolving the original materials into organic solvents and then precipitating the product by heat treatment22,23. For instance, Yubuchi et al.23 reported a liquid-phase technique for preparing argyrodite-type Li6PS5Br with high Li-ion conductivity (3.1 × 10−3 S cm−1) and small particle sizes (~1 μm) using a homogeneous tetrahydrofuran (THF)–ethanol (EtOH) solution. This study provides new insight into the fabrication of sulfide SEs to realize optimal cathode composites.
Several ideal composite preparation strategies originally intended for sulfur can now be applied to Li2S, which is more chemically stable and is the least dense phase of fully lithiated sulfur24,25. Additionally, a lithium-free anode such as graphite, Si, and Sn can be used when Li2S is selected as the AM, thus avoiding the safety issues of the lithium metal anode26. However, other conductive materials are generally doped into Li2S to accelerate sluggish Li2S/S redox reactions, given the insulating properties of Li2S27,28,29. For instance, Hakari et al.29 systematically compared the electrochemical performances of different Li2S–LiX solid solutions (X = Cl, Br, or I) in ASSLSBs, and found that the ASSLSB with the 80Li2S–20LiI cathode composite exhibited optimal electrochemical performance and the highest Li2S utilization. The introduction of LiX evidently enhanced the ionic conductivity of Li2S and provided more electrochemical reaction sites, thereby significantly increasing Li2S utilization. This innovative approach can be leveraged to further improve cathode composites with Li2S as the AM.
In addition to enhancing the ionic conductivity, considerable efforts have been devoted to improving the electronic conductivity of the ASSLSB cathode composite, such as incorporating various electronic conductive additives including copper30, acetylene black31, graphite3, and carbon nanofibers32. Although these additives can moderately boost electron conduction within the cathode, their framework structures can hardly buffer the cathode-associated volumetric change, resulting in poor cycling and rate performances. Nevertheless, Suzuki et al.33 recently employed a carbon replica (CR) with three-dimensional (3D) regularly arranged mesopores as the conductive framework. Loading the AM into the pores of the CR enhanced its electronic conductivity and provided a robust matrix and suitable space for accommodating the volumetric change.
Therefore, the following strategies are key to realizing an optimal Li2S-based cathode composite: (1) reducing the particle size of the cathode SE (<1 μm), (2) enhancing the mixed (ion/electron) conductivity, and (3) constructing a stable cathode framework and 3D conductive pathways. However, approaches that can concurrently achieve all three requirements have rarely been reported. Additionally, alternatives to the commonly used mechanical mixing method, which can lead to poor interfacial contact and disintegration of the porous structure, must be established34,35.
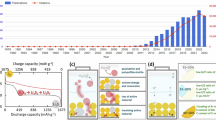
Here, we report a Li2S-based cathode composite featuring (i) a hybrid (AM–CR10) of the AM and a carbon replica with ~10-nm-sized pores (CR10), (ii) a Li6PS5Br SE (SE-liq) obtained by liquid-phase synthesis, and (iii) vapor-grown carbon fibers (VGCFs), exhibiting outstanding mixed conductivity and a stable cathode structure, prepared using liquid-phase methods (Fig. 1). This AM–CR10/SE-liq/VGCF composite is hereafter termed ACSV. Essentially, dissolving Li2S–LiI (the AM) in EtOH in a mass ratio of 3:1 and then precipitating it in the ~10-nm-sized pores of CR10 yields the AM–CR10 hybrid. Notably, modifying Li2S with LiI enhances its ionic conductivity and the redox kinetics of Li2S/S. After the mild liquid-phase procedure, the porous CR10 is sufficiently filled with the AM without undergoing collapse. The highly conductive SE-liq possessing a small particle-size distribution (<1 μm) is successfully synthesized using the THF–EtOH solvent system. The ACSV cathode composite can then be prepared by mixing AM–CR10, high-conductivity SE-liq, and rod-shaped VGCFs. The ACSV cathode exhibits exceptional capacity and reversibility in ASSLSBs, owing to its noteworthy mechanical robustness, lithium storage capability, and 3D ion/electron-conducting structure. This paper reports a fabrication scheme for realizing a composite cathode that can simultaneously suppress its insulating nature, volumetric change, and poor solid–solid interfacial contact, aiding the development of high-performance ASSLSBs.
The ACSV (AM–CR10/SE-liq/VGCF) composite features a LiI-modified Li2S active material (AM), carbon replica with ~10-nm-sized pores (CR10) framework, high-conductivity liquid-phase-synthesized Li6PS5Br solid electrolyte (SE-liq), and vapor-grown carbon fiber (VGCF) additive, in a Li2S:LiI:CR10:SE-liq:VGCF weight ratio of 30:10:10:45:5.
Results and discussion
xLi2S–LiI composites prepared via a liquid-phase process
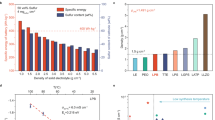
To clarify the manner in which LiI activated the insulating Li2S, three xLi2S–LiI composites with different Li2S weight contents (x = 1, 3, 5) were prepared using Li2S and LiI as the original materials via dissolution and precipitation in anhydrous EtOH, followed by evaporating the solutions at 100 °C for 6 h. Furthermore, Li2S and LiI were also independently subjected to the same liquid-phase treatment for comparison. A minor amount of LiI in the composite could react with a small amount of Li2S to form an amorphous sulfide electrolyte36, resulting in a substantial amount of Li2S with poor ionic conductivity. Therefore, to add sufficient LiI and while maintaining a reasonably low Li2S content, 3Li2S–LiI was selected as the AM as it has relatively high ionic conductivity and a suitable Li2S content according to previous findings37. Morphological analysis of the 3Li2S–LiI AM (Supplementary Fig. 1) indicated that the liquid-phase-synthesized AM exhibited a microstructure with an average particle size of ~1 μm, which was smaller than that of the specimen prepared by ball milling (~10 μm)37; moreover, the transparency of the AM solution suggested that Li2S and LiI were adequately dissolved in EtOH. Furthermore, X-ray diffractometry (XRD) patterns of the AMs were acquired (Fig. 2a) to explore their crystal structures. While the width of the LiI-phase peaks remained comparable to that of the LiI reference (ICSD #414244), the Li2S-related peaks broadened compared with those of the Li2S reference (ICSD #642291) owing to the enhanced amorphousness and smaller primary crystallites of Li2S38. Rietveld refinement was conducted to probe the leftward shifts in the peak positions of the Li2S component, which suggested the existence of larger Li2S-phase parameters (Supplementary Fig. 2; see Fig. 2b for the related lattice parameters). Notably, the lattice parameters of the LiI phase remained almost unchanged from those of the reference and pure LiI (6.025 Å), whereas the lattice parameters of the Li2S phase increased from 5.690 to 5.741 Å after the introduction of LiI. Because both LiI and Li2S exhibited a cubic structure indexed to the Fm−3m space group, the aforementioned increase was probably due to the incorporation of I− into the Li2S lattice, as reported previously39. Additionally, the lattice parameters of the Li2S phase in xLi2S–LiI were independent of x, suggesting that only a limited amount of I− was doped into the Li2S lattice. Therefore, the liquid-phase-synthesized xLi2S–LiI composites comprised both the Li2S + LiI mixture and Li2S–LiI solid solution states.
a X-ray diffraction (XRD) patterns of liquid-phase-synthesized xLi2S–LiI (x = 1, 3, 5), Li2S, and LiI. b Lattice parameters based on Rietveld refinement of data shown in Supplementary Fig. 3. c Arrhenius plots of Li2S, LiI, and 3Li2S–LiI constructed using AC electrochemical impedance spectroscopy (EIS) data at different temperatures. d Total density of states (DOS) and (e) crystal orbital Hamilton population (COHP) analyses of Li2S and xLi2S–LiI based on first-principles density functional theory (DFT) calculations. The perpendicular dashed line at 0 eV denotes the Fermi level.
Additionally, the temperature dependence of the ionic conductivity was investigated for pristine Li2S, LiI, and the liquid-phase-synthesized 3Li2S–LiI AM (Fig. 2c). The calculated activation energies for Li2S, LiI, and 3Li2S–LiI, which were 0.70, 0.34, and 0.47 eV, respectively, indicated that the introduction of LiI into Li2S could reduce the activation energy, thereby significantly enhancing the ionic conductivity of the cathode AM. First-principles density functional theory (DFT) calculations were performed to further investigate the effects of incorporating I− anions into the S2− sites. Pristine Li2S exhibited a density-of-states value of 0 at the Fermi energy level (0 eV) and a bandgap of ~3 eV, highlighting its insulating properties; in contrast, the xLi2S–LiI system did not exhibit a density-of-states value of 0 at the Fermi energy level within the conduction band, indicating its conducting characteristics, which implied that the electronic conductivity was improved by the LiI modification (Fig. 2d). Thus, both the ionic and electronic conductivities of the AM were increased. Furthermore, the catalytic effect of LiI on the redox conversion reaction of Li2S/S was confirmed by crystal orbital Hamilton population (COHP) analysis (Fig. 2e). Essentially, after the LiI modification, the state of the Li–S bonding weakened, and the negative-integral COHP value consequently decreased from 0.76 to 0.63 eV, suggesting that Li2S could be converted more easily to the charge product S using less energy. Thus, incorporating I− effectively promoted the Li2S/S conversion redox kinetics.
AM–CR10 composite obtained via liquid-phase synthesis
The XRD pattern of the AM–CR10 composite pre-synthesized using a liquid-phase process (Supplementary Fig. 3) indicated that the overall crystallinity was relatively poor, and that the main peaks of the composite could be indexed to diffraction from the (111), (200), (220), and (311) planes of Li2S (ICSD #642291) with the Fm−3m space group. The peak shifts of Li2S to smaller angles indicated lattice expansion through the formation of a solid solution with LiI, which had a larger anion in its lattice29. The ambiguity of the LiI component was probably due to the greater amorphousness of LiI and its assimilation into the nano-sized CR10 pores.
The morphological and nanometer-scale structures of CR10 and AM–CR10 were characterized by field-emission scanning electron microscopy (FE-SEM; Fig. 3). The cellular architecture of CR10 featured a uniform porous structure with ~10-nm-sized pores (Fig. 3a, b). After the mild liquid-phase process conducted to implant the AM into the pores, the porous structure disappeared, and a mechanically reinforced AM-containing matrix emerged without undergoing damage (Fig. 3c, d). Elemental mapping analysis (Fig. 3e–g) revealed uniform distributions of S, I, and C in AM–CR10, suggesting an even dispersion of Li2S and LiI within CR10.
N2 adsorption–desorption isotherms of CR10 and AM–CR10 were acquired to further analyze their pore structures (Fig. 4a). Porous CR10 exhibited strong gas adsorption characteristics; in contrast, AM–CR10 was virtually unresponsive to nitrogen. Brunauer–Emmett–Teller (BET) analysis (Table 1) revealed significant decreases in the specific area and total pore volume, suggesting the occupation of the nano-pores by the AM component. Furthermore, the Barrett–Joyner–Halenda pore size distribution analysis (Fig. 4b) directly proved that the 10-nm-sized pores were sufficiently impregnated with the AM after the liquid-phase process.
X-ray photoelectron spectroscopy (XPS) analysis was conducted to evaluate the chemical states of S and I in the 3Li2S–LiI AM before and after it was loaded into CR10. After charge-compensation-based binding energy correction, the peak representing the C–C bond at 284.8 eV in the C 1 s profile (Fig. 5a) adequately matched the reported eigenvalues28. Additionally, the peak representing the O=C–O group at 288.8 eV28 likely originated from the small fraction of organic molecules that remained after vacuum drying in the liquid-phase process. Furthermore, the presence of divalent sulfur (S2−) in Li2S was evidenced by the S 2p3/2 and S 2p1/2 peaks at ~160.4 and ~161.6 eV, respectively (Fig. 5b). Additionally, characteristic peaks of non-bridging sulfur (S−) oxidized from S2− ions in 3Li2S–LiI and AM–CR10 appeared at ~162 eV40,41, indicating that Li2S was partially oxidized during the AM preparation. Moreover, after the addition of CR10, the original peak of AM–CR10 shifted towards a higher binding energy than that of the other sample, implying enhanced surface conductivity42,43. Notably, the I 3d3/2 and I 3d5/2 peaks corresponding to the I 3d orbitals barely shifted and maintained a theoretical difference of 11.5 eV; thus, the unique existence of I− proved that LiI was not the main redox center (Fig. 5c). These results collectively demonstrated that while the chemical state of I− in LiI was not affected, that of S2− in Li2S was changed, probably owing to the incorporation of I− and the increased surface conductivity after the AM was embedded in the CR10 pores.
Li6PS5Br SE obtained via liquid-phase synthesis
Argyrodite-type Li6PS5Br (SE-liq) was obtained via liquid-phase synthesis according to a previously reported method23 to control the cathode SE particle size; a specimen prepared by mechanical ball-milling (SE-bm) was used for comparison (see Fig. 6a for schematics of the two synthesis protocols, which involved different precursor synthesis processes but the same sintering method, and Supplementary Fig. 4 for photographs of the liquid-phase synthesis). Raman spectra of the SE-liq precursor solution in THF–EtOH, raw pre-sintered SE-liq, SE-liq, and SE-bm (Fig. 6b) validated the existence of the PS43− unit, given the emergence of strong peaks centered at ~420 cm−1, which indicated that the PS43− unit was not decomposed by THF or EtOH. Notably, the PS43− peak of the precursor solution shifted to a lower wavenumber than that of the raw-SE-liq-associated peak, implying that the PS43− unit was probably solvated with THF and/or EtOH molecules. After high-temperature sintering, the PS43− peak of SE-liq redshifted again to a lower wavenumber, suggesting that the P–S bond vibration in the PS43− unit weakened and became more kinetically active44. Moreover, no peaks of EtOH or THF appeared in the SE-liq profile between 880 and 920 cm−1, indicating that the organic solvents were predominantly removed. The XRD data corroborated the liquid-phase synthesis of highly crystalline argyrodite-type Li6PS5Br, given the emergence of sharp, well-indexed main peaks (ICSD #122370); however, the appearance of certain minor impurity peaks suggested the existence of residual Li2S and LiBr (Fig. 6c). Synchrotron X-ray Rietveld refinement was performed to gain further insight into the SE components (Supplementary Fig. 5; Supplementary Tables 1 and 2). Argyrodite (F−43m) Li6PS5Br existed as the major crystalline phase in SE-bm (99.6 wt% purity), while the remainder consisted of minor LiBr (0.4 wt%) impurity. The SE-liq product from the liquid-phase synthesis showed a relatively lower purity (92.0 wt% purity; impurities: 1.4 wt% LiBr, 3.1 wt% Li2S, 3.5 wt% Li3PO4), which was possibly caused by some side reactions between PS43- units and EtOH, as previously reported45, or trace moisture in the organic solvents. Moreover, the particle size distribution analysis of SE-liq revealed the presence of 0.1–1-µm-sized particles (Fig. 6d), which were considerably smaller than those of SE-bm (1–30 µm). Micromorphological analysis (Supplementary Fig. 6) revealed that SE-bm and SE-liq possessed coarse and relatively smaller particles, respectively, consistent with the particle size distribution results. For both SEs, Arrhenius plots of the electrochemical impedance spectroscopy (EIS) data recorded at different temperatures and Nyquist plots of the data acquired at room temperature were constructed to examine the differences in ionic conductivity (Fig. 6e, f). SE-liq exhibited a conductivity of 2.23 ×10−3 S cm−1 at 25 °C and an activation energy of 0.29 eV, similar to those of SE-bm (2.26 ×10−3 S cm−1 and 0.28 eV, respectively), indicating that the impurities in the liquid-phase-synthesized SE-liq did not significantly decrease Li+ conduction in the highly crystalline SEs. The semicircles for the two SEs in the high- and mid-frequency regions of Nyquist plots were similar in size, suggesting a negligible difference in grain boundary resistance. Additionally, the linear slope of the Warburg diffusion process in the low-frequency region depended on the roughness of the electrode surface. As SE-bm had larger particles and a more uneven surface at the microscopic scale, part of the diffusion process occurred over more sphere-like surfaces. Therefore, its linear slope deviated further from the theoretical 45° compared with that of SE-liq. Electrochemical performance analysis of SE-liq and SE-bm as cathode SEs in ASSLSBs (Supplementary Fig. 7) indicated that after the activation of insulating Li2S in the first charge–discharge cycle46, the SE-liq specimen exhibited a higher discharge capacity (195 mAh g−1) and lower overvoltage than those of the SE-bm sample. Overall, the characterization results of SE-liq and SE-bm (Table 2) emphasized the viability of obtaining a high-conductivity, nano-sized Li6PS5Br SE through time- and energy-saving liquid-phase synthesis for preparing ASSLSB cathodes.
Characterization of ACSV cathode composite
The cathode composite was prepared by mixing the pre-synthesized AM–CR10, SE-liq, and rod-shaped VGCFs by hand-grinding for 30 min to minimize the capacity contribution of the sulfide SE47. The concentration of the Li2S AM in the entire cathode composite was 30 wt%. In the XRD pattern of ACSV (Supplementary Fig. 8), the main peaks could be indexed to the diffraction of Li6PS5Br (ICSD #122370), whereas those of Li2S and LiI were unclear probably owing to the relatively low crystallinity and interference effects of carbon materials. Additionally, the FE-SEM image of ACSV (Fig. 7) revealed 3D conductive pathways within the cathode composite. Therefore, the electrochemical lithiation/delithiation reactions could occur on the optimized triple-phase boundaries of the composite featuring LiI-modified Li2S, CR10 with high electronic conductivity, and submicron-sized SE-liq with high ionic conductivity, with the VGCFs acting as conductive bridges connecting the separated regions. According to the elemental mapping analysis of ACSV (Supplementary Fig. 9), the signals for P, S, and Br suggested a uniform distribution of SE-liq, whereas the signals for C and I revealed where aggregation occurred in the carbon frameworks (CR10 and VGCF) and the AM (3Li2S–LiI), respectively.
EIS and direct current (DC) polarization of the AM, AM–CR10, and ACSV were performed to calculate and compare their ionic and electronic conductivities at room temperature (Supplementary Figs. 10–12 and Table 348,49,50,51). For 3Li2S–LiI AM, the ionic and electronic conductivities were determined to be 1.06 ×10−6 S cm−1 and 1.77 ×10−7 S cm−1 from its Nyquist plot (Supplementary Fig. 10a) and DC polarization curve (Supplementary Fig. 10b), respectively, based on the Au/AM/Au cell with ion-blocking electrodes composed of Au. As established earlier, the ionic and electronic conductivities of Li2S increased considerably upon the introduction of LiI. For the AM–CR10 composite, the region of the Nyquist plots representing the inductance (Supplementary Fig. 11a) revealed that in AM–CR10, electron conduction was dominant52; thus, the electronic conductivity of 2.46 ×10−2 S cm−1 was obtained directly from the intercept of the arc and the Z’ axis. Subsequently, the ionic conductivity was assessed to be 6.30 ×10−7 S cm−1 using DC polarization on a five-layer cell composed of Li–In/Li3.45Ge0.45P0.55S4(LGPS0.55)/AM–CR10/LGPS0.55/Li–In cell (Supplementary Fig. 11b), where the LGPS0.55 SE layers were used as both ion-conducting and electron-blocking electrodes, and Li–In alloy foils were used as reversible electrodes and Li reservoirs53. AM–CR10 exhibited significantly enhanced electronic conductivity, evidently owing to the construction of the electron-conducting CR10 carbon framework. For the ACSV composite, the Nyquist plot of the Au/ACSV/Au cell exhibited a flat semicircle intersecting the Z’ axis without a visible diagonal line representing the Warburg diffusion process (Supplementary Fig. 12a), suggesting mixed ionic/electronic conductive behavior rather than double-layer capacitance52,53,54. Because the ionic current was mainly shunted by the electronic current, the ion-blocking Au/ACSV/Au and the electron-blocking Li–In/LGPS0.55/AM–CR10/LGPS0.55/Li–In cells were assembled for DC polarization to measure the electronic conductivity (Supplementary Fig. 12b) and ionic conductivity (Supplementary Fig. 12c), respectively. The resistance contributions from the bulk LGPS0.55 and the Li–In/LGPS0.55 interfaces on both sides (Supplementary Fig. 12d) were subtracted to calculate the ionic conductivities of AM–CR10 and ACSV. As a result, ACSV was revealed to be a mixed conductor, a well-balanced electronic and ionic conductivities (1.56 ×10−2 and 2.85 ×10−4 S cm−1, respectively).
Furthermore, in situ cross-sectional FE-SEM images were recorded before and after cycling at a high current density of 0.856 mA cm−2 (0.4 C) at 25 °C (Fig. 8), revealing the structural toughness and stability of the ACSV cathode with the CR10 framework. An ASSLSB cell was assembled primarily with a Li–In alloy anode, the ACSV composite cathode, and a LGPS0.55 SE separator (Fig. 8a). LGPS0.55 was selected as the SE instead of Li6PS5Br owing to its higher Li+ conductivity and greater compatibility with Li2S-based cathodes55, whereas the Li–In alloy anode was anticipated to decrease the resistance and instability of the anode–SE interface by eliminating adverse SE decomposition56. For comparison, a Li2S–LiI/SE-liq/VGCF (CR10-free) cathode was used as a control. The cross-sectional elemental mapping images of the two cathodes recorded by FE-SEM (Supplementary Figs. 13,14) reveal the hierarchical structure of the cathode, where the current collector layer (represented by Al), the cathode composite layer (represented by C and I), and the SE separator layer (represented by Ge) can be clearly distinguished. Before cycling, the three layers were in close contact with each other, although some cracks seemed to be present owing to the pressurization process (Fig. 8a, c). After 20 cycles, the ACSV layer remained intact and undamaged (Fig. 8b), although some new cracks appeared in the SE layer, which were probably attributed to the relatively poor mechanical properties of LGPS0.55. In contrast, some cracks appeared in the CR10-free layer after cycling, and the SE layer was more severely deteriorated (Fig. 8d), suggesting the reinforced structural stability of ACSV and the effectiveness of CR10 in buffering volumetric changes in the cathode.
Electrochemical performance of ASSLSB with ACSV cathode
In terms of the cathode composite, four different compositions were selected for comparison: Li2S–LiI–CR10/SE-liq/VGCF (ACSV), Li2S/CR10/SE-liq/VGCF (LiI-free), Li2S–LiI/SE-liq/VGCF (CR10-free), and Li2S/SE-liq/VGCF (LiI/CR10-free), as illustrated in Fig. 9a.
a Schematics illustrating the four different cathode composites (ACSV and three control groups) prepared for ASSLSB operation. b Galvanostatic charge–discharge profiles of ACSV at 0.107 mA cm−2 (0.05 C). c Differential capacity vs. voltage (dQ/dV) curves of ACSV corresponding to the 1st, 2nd, 10th, and 20th cycles at 0.107 mA cm−2 (0.05 C). d Differential capacity vs. voltage (dQ/dV) of ACSV and LiI-free samples corresponding to the 20th cycle at 0.107 mA cm−2 (0.05 C). e Galvanostatic charge–discharge profiles of ACSV and LiI-free samples at the 20th cycle at 0.107 mA cm−2 (0.05 C). f Cycling abilities of the four cathode composites at 0.214 mA cm−2 (0.1 C). g Rate performance analysis. Nyquist plots of (h) ACSV, (i) CR10-free, and (j) LiI/CR10-free cathode composites before and after 50 cycles at 0.214 mA cm−2 (0.1 C). k Cycling performance of ACSV cathode at 0.214 mA cm−2 (0.1 C).
Galvanostatic charge–discharge profiles of the ACSV cathode were acquired at a current density of 0.107 mA cm−2 (0.05 C) at 25 °C (Fig. 9b). Only one plateau appeared in the curves (1.3 and 1.7 V for discharging and charging, respectively), consistent with the binary solid–solid phase transition between Li2S and S during ASSLSB cycling57. Moreover, the initial charging curve showed a high overvoltage as well as overcharge behavior. Li2S should be activated during the initial charging stage because of the large potential barrier that limits the charging depth and results in low AM utilization46; consequently, activated Li2S exhibits typical Li–S battery performance. The ACSV cathode exhibited a high initial discharge capacity of 841 mAh g−1, which increased to 1009 mAh g−1 at the 20th cycle, reaching 86.6% of the theoretical capacity of the Li2S AM (1166 mAh g−1). The capacity increase was presumably due to the contribution of the decomposition product of the argyrodite-type SE58.
Differential capacity (dQ/dV) curves of ACSV were calculated from the galvanostatic charge–discharge profiles to investigate the redox reactions during the initial cycles (Fig. 9c). For the first cycle, the prominent oxidation peak at ~2.25 V vs. Li–In (peak V) could be attributed to the initial activation process, during which Li2S was converted to S8 while the Li6PS5Br sulfide SE oxidatively decomposed59,60. The reduction peak at ~1.36 V vs. Li–In (peak I) was derived from the one-step reductive conversion of S8 to Li2S2, without the generation of long-chain polysulfides Li2Sn (n = 4–8)61,62,63. Starting at the second cycle, the oxidation peak V disappeared, probably because of the irreversibility of the initial SE decomposition process. In subsequent cycles, however, a new oxidation peak appeared at ~1.79 V vs. Li–In (peak III), which originated from the oxidation reactions of the cathode AM and the redox-active decomposition product of Li6PS5Br59,60,64. Previous studies demonstrated that sulfide SEs could form complicated chemical species with –P–Sn–P– bonding and amorphous sulfur through oxidative decomposition; thus, the increased capacity during the initial cycles could be attributed to the reversible redox activity of the decomposition product of sulfide SEs within the cathode composite55,65,66,67. As cycling proceeded, more AM and SE decomposition products were involved in the redox reaction. As a result, a new reduction peak at ~1.16 V vs. Li–In (peak II) appeared in the 10th cycle, indicating the conversion of Li2S2 to Li2S and the reduction reaction of the oxidized SE decomposition products60,61,62,63. In addition, a weak oxidation peak was observed at ~2.15 V vs. Li–In (peak IV), corresponding to the oxidation reaction from I− to I3− in the ACSV cathode composite68,69. Because the oxidation reaction voltage of LiI was slightly higher than that of Li2S, the oxidation reaction of LiI could be used as an electrochemical redox mediator to promote the redox reaction of Li2S AM68,70.
To elucidate the role of LiI in the redox kinetics for the Li2S cathode material, differential capacity (dQ/dV) curves of ACSV and the LiI-free composites were constructed based on their charge–discharge profiles at the 20th cycle (Fig. 9d). Although both curves exhibited two characteristic reduction peaks (peaks I and II) and one oxidation peak (peak III), the reduction peak for ACSV at ~1.36 V vs. Li–In shifted to a higher potential, while the oxidation peak at ~1.79 V vs. Li–In shifted to a lower potential, signifying accelerated Li2S/S redox kinetics71. Similarly, compared with the LiI-free cathode, ACSV demonstrated a smaller potential polarization (\(\triangle E\)) in its charge–discharge profile (Fig. 9e), implying enhanced Li2S/S redox kinetics72.
Moreover, the cycling capabilities of ACSV and the three other control samples were investigated at a current density of 0.214 mA cm−2 (0.1 C) at 25 °C (Fig. 9f). The ACSV cathode delivered an excellent reversible capacity of 785 mAh g−1 at the first discharge, which increased to 837 mAh g−1 after 50 cycles. In contrast, the LiI-free sample exhibited consistently low absolute capacities (259 and 266 mAh g−1 at the first and 50th cycle, respectively) owing to the lack of Li2S activation, whereas the CR10-free specimen displayed severe capacity decay (552 and 144 mAh g−1 at the first and 50th cycle, respectively) owing to the lack of a supporting framework in the cathode. These results showcase the outstanding capacity and cycling ability of ACSV.
Furthermore, the rate performances of ACSV and the three other samples were evaluated at current densities ranging from 0.107 to 0.856 mA cm−2 (0.05 C to 0.4 C; Fig. 9g). At current densities of 0.107, 0.214, and 0.428 mA cm−2, ACSV delivered the highest capacities, with steady averages of 925, 913, and 788 mAh g−1, respectively. Even when the current density was increased considerably to 0.856 mA cm−2, the ACSV cathode retained an impressive capacity of 584 mAh g−1, whereas the other samples showed extremely low capacities (<50 mAh g−1), indicating the ultrahigh rate capability of ACSV. Upon returning to the current density of 0.214 mA cm−2 at the 26th cycle, the capacity recovered to a value as high as 99.9% of the initial capacity at the 11th cycle, signifying that the ACSV cathode composite showed exceptional structural stability under high-rate cycling.
EIS measurements of ACSV, the CR10-free sample, and the LiI/CR10-free specimen were subsequently compared before and after 50 cycles at a current density of 0.214 mA cm−2 (0.1 C) at 25 °C (Fig. 9h–j). After cycling, the second semicircle seems unclear in the ACSV specimen, whereas both the CR10-free and LiI/CR10-free samples exhibited sizeable second semicircles, as expected, indicating large increases in the resistance. To reach a deeper understanding, an equivalent circuit analysis was performed on the EIS results. The Randles equivalent circuit for the cell (Supplementary Fig. 15) and the relevant fitting parameters (Supplementary Table 3) suggested that in addition to the ohmic resistance of the SEs and cell components (R1, the intercept of the starting point of the Nyquist plots on the x-axis), all three cathodes exhibited charge-transfer resistance (Rct, the recessed semicircle at high frequencies) and interfacial contact resistance for the bulk electrode (R2, the recessed semicircle at intermediate frequencies). Compared with ACSV, the Rct value of the CR10-free sample increased substantially after cycling, probably owing to the deterioration of the contact among the components caused by the volumetric change within the cathode73. This finding was consistent with the increased number of cracks observed by in situ SEM. In addition, without LiI and CR10, the LiI/CR10-free sample exhibited higher Rct and R2 values, indicating that LiI contributed to improving both the charge transfer ability and interfacial contact between the AM and SE. Effectively, the LiI coating around the Li2S phase could lower the energy barrier to diffusion through grain boundaries towards the Li2S and argyrodite-type SE, which helps to decrease the interfacial resistance39.
Consequently, the ACSV cathode demonstrated long-term cycling performance at a current density of 0.214 mA cm−2 (0.1 C) at 25 °C. Furthermore, the ACSV cathode delivered a discharge capacity of 650 mAh g−1 after 100 cycles, which was reduced slightly from that of the first cycle (785 mAh g−1), resulting in an outstanding retention rate of 82.8% and a virtually unchanged Coulombic efficiency of 100% (Fig. 9k). The areal active material density in the electrode composite is directly related to the performance of the battery, such as energy density and cycle characteristics. Notably, the active material loading in all cathode composites examined in this study was above 2 mg cm−2. A comparison of the Li2S-based cathode prepared in this study with previously reported state-of-the-art cathodes (Supplementary Table 4) suggests that the ACSV cathode composite exhibits superior performance in terms of discharge capacity and cycling stability, owing to its mechanical robustness, lithium storage capability, and 3D ion/electron-conducting structure. Overall, these findings illuminate the potential of ACSV-type multi-functional composites in enhancing the mixed conductivity and suppressing the volumetric change within the cathode to realize practically viable ASSLSBs.
Conclusions
A Li2S-based cathode composite (ACSV) with stellar mixed-conductivity and mechanical stability was rationally synthesized using liquid-phase methods. As the cathode AM, Li2S–LiI (3:1 mass ratio) was infiltrated into a mesoporous carbon replica with ~10-nm-sized pores (CR10) through mild liquid-phase precipitation. The LiI activation of Li2S substantially enhanced both the ionic conductivity of the AM and the redox kinetics of Li2S/S; moreover, intimate contact was established between the AM and CR10, enabling mixed conducting properties and reliable accommodation of the cycling-induced stress and strain. To achieve superior solid–solid interfacial contact between the AM and SE, a high-conductivity (2.22 mS cm−1 at 25 °C), nano-sized (0.1–1 µm) Li6PS5Br SE (denoted as SE-liq) was prepared using a time- and energy-saving liquid-phase method instead of conventional ball milling for constructing an optimal ASSLSB cathode. Subsequently, the ACSV cathode composite was fabricated by mixing the AM–CR10 composite, SE-liq, and VGCFs, yielding 3D ion/electron-conducting pathways. Consequently, the ACSV cathode delivered an exceptional discharge capacity (1009 mAh g−1 at the 20th cycle at 0.107 mA cm−2), outstanding cycling stability (retention rate of 82.8% after 100 cycles at 0.214 mA cm−2), and noteworthy rate performance, owing to its mechanically sturdy configuration, high lithium storage capability, and three-dimensional mixed conductive structure. Essentially, this study affirmed the potency of designing a Li2S-based composite cathode using liquid-phase methods to mitigate the insulating property of Li2S, inhibit the volumetric change within the cathode, and improve the solid–solid interfacial contact between Li2S and SE, thereby helping achieve high-performance ASSLSBs.
Methods
Li6PS5Br SE (SE-liq) preparation using a liquid-phase method
Li2S (99.9%, Mitsuwa Chemicals) and P2S5 (99%, Sigma-Aldrich) were mixed in a molar ratio of 3:1. The resulting solid powder was added to a stabilizer-free super-dehydrated THF solvent (99.5 wt%, Wako Pure Chemical Industries) in a glove box under an argon atmosphere (THF, 3.0 wt%), and the suspension was stirred overnight for 6 h. Subsequently, Li2S and LiBr (99.9%, Sigma-Aldrich) were dissolved in a molar ratio of 1:1 in a super-dehydrated EtOH solvent (99.5 wt%, Wako Pure Chemical Industries; EtOH, 3.0 wt%). The THF suspension and EtOH solution were then mixed in a molar ratio of 5:1:2 (Li2S:P2S5:LiBr). The solution was dried at 100 °C under vacuum to remove the organic solvents. The obtained Li6PS5Br precursor powder was pressed in a pellet compactor, and the obtained pellet was sealed in a quartz tube under vacuum. The sample was sintered at 550 °C for 6 h in an argon atmosphere and then cooled to room temperature naturally. The entire synthesis process was adopted from a previous study23. Another specimen was prepared by conventional ball milling for comparison, as described below.
Preparation of carbon replica 10 (CR10)
The CR10 synthesis protocol has been described in our previous report74. Essentially, monodisperse 10-nm-diameter silica nanosphere (SNS) colloidal crystals were synthesized using a hydrothermal method as a template for CR75. To synthesize CR10 (0.2 g), L-arginine (0.35 g; ≥98.0%, Kanto Chemicals) was selected as a base catalyst, mixed in distilled water (348 g), and then stirred at 1000 rpm for ~5 min at room temperature until dissolution. A silicic acid source – tetraethyl orthosilicate (TEOS; 20.9 g, ≥96.0%, Tokyo Kasei Kogyo) – was subsequently added, and the resulting mixture was stirred at 1000 rpm at 70 °C for 24 h in a bath stirrer (EWS-100RD, AS One). The obtained solution was transferred to a ceramic baking dish and then dried in a thermostatic oven (SU241, ESPEC) at 100 °C for 10 h, yielding a white powder of 10-nm-sized SNS particles. Subsequently, furfuryl alcohol (10 g; 97.0%; Wako Pure Chemical Industries) was selected as a carbon source and then mixed with oxalic acid (0.05 g, 98.0%; Wako Pure Chemical Industries), which was used as an acid catalyst. The stirred furfuryl alcohol solution was added dropwise onto the SNS powder, which was subsequently placed in a perfluoroalkoxy fluoropolymer jar until the SNSs became lumpy. The furfuryl alcohol was then polymerized by heating the sample in a muffle furnace at 100 °C for 48 h. The specimen obtained after natural cooling was pulverized in an alumina mortar to obtain a brown powder. The resulting sample was carbonized at 120 °C for 1 h and at 800 °C for 5 h under argon flow in a tube furnace (TF-1300, R-DEC). This yielded a black CR10 powder, which was treated with a 29 wt% aqueous hydrofluoric acid solution to remove the silica template.
Preparation of Li6PS5Br SE by ball milling (SE-bm)
For the ball-milling synthesis, Li2S (99.9%, Mitsuwa Chemicals), P2S5 (99%, Sigma-Aldrich), and LiBr (99.9%, Sigma-Aldrich) were mixed in a molar ratio of 5:1:2 by hand for ~15 min in a glove box under an argon atmosphere. Mechanical ball-milling was performed at 600 rpm for 48 h using a planetary ball mill (Pulverisette 7, Fritsch Japan). The sample and eighteen 10-mm-diameter zirconia balls were sealed in a zirconia (ZrO2) grinding vessel in a glove box to prevent contact with air. The obtained powder was pressed in a pellet compactor, and the obtained pellet was sealed in a quartz tube under vacuum. The sample was sintered at 550 °C for 6 h in an argon atmosphere and then cooled to room temperature naturally. This synthesis process has been reported previously60.
Preparation of Li3.45Ge0.45P0.55S4 SE by vibration milling
The Li3.45Ge0.45P0.55S4 SE (denoted herein as LGPS0.55) was used as the separating layer of the ASSLSBs. The LGPS synthesis protocol adopted in our previous study16 was used. Li2S, P2S5, and GeS2 (99.9%, Kojundo Chemical Lab) were hand-mixed in a molar ratio of 69:11:18 for 15 min in an agate mortar in a glove box under an argon atmosphere. The mixture was transferred into a cylindrical container and subjected to vibration milling (VM) in a high-speed vibratory mill (TI-100, CMT) for 30 min to obtain the precursor powder. The resulting sample was sintered at 550 °C for 8 h in an argon atmosphere and then cooled to room temperature naturally.
ACSV cathode composite preparation
Li2S and LiI (99.9%, Sigma-Aldrich) were dissolved in anhydrous EtOH (5.0 wt%) in a weight ratio of 3:1. Subsequently, a carbon replica with ordered ~10-nm-sized mesopores (CR10) was added to the preceding solution in an AM/CR10 weight ratio of 4:1. After complete dispersion, the AM–CR10 composite powder was obtained by vacuum drying at 100 °C for 6 h. The ASCV cathode composite was then obtained by hand-mixing AM–CR10, the pre-synthesized SE-liq, and the VGCF carbon additive (Showa Denko K.K.) for 30 min, in an AM–CR10/SE-liq/VGCF weight ratio of 5:4.5:0.5.
Material characterization
XRD measurements of the synthesized powders were performed using a Rigaku SmartLab X-ray diffractometer with CuKα1 radiation. To that end, the sample powder was placed in a measurement holder in an Ar atmosphere to prevent it from reacting with moisture. Synchrotron XRD measurements were conducted using the BL02B2 beamline at SPring-8 (λ = 0.5 Å). Essentially, the specimen was sealed in an Ar atmosphere in Lindemann glass capillaries (~0.3 mm inner diameter), and a Debye–Scherrer diffraction camera was employed for the measurements. The structural parameters corresponding to the recorded synchrotron data were refined using Z-Rietveld program76. Raman spectral measurements were performed using a confocal Raman microscope (Raman 11, Nanophoton) at an excitation wavelength of 532 nm to confirm the local structure. A laser scattering particle size distribution analyzer (ParticaLA-960, Horiba) was used to measure the particle sizes of the samples. Structural and morphological analyses were conducted by FE-SEM (Regulus 8230, Hitachi High-Tech) and energy-dispersive X-ray spectroscopy (EDS) (QUANTAX FlatQUAD, Bruker). BET surface area measurements based on nitrogen adsorption–desorption were performed in liquid nitrogen (77 K) using an automated adsorption mass spectrometer (BELSORP-mini, Microtrack Bell). XPS analysis was conducted using a chamber system (ULVAC-PHI). In the XPS data analysis, charge compensation was performed, and the binding energy was corrected by calibrating the data to the C 1 s photoemission peak of adventitious hydrocarbons (C–H) at 284.8 eV. The data were fit using CasaXPS software (version 2.3.24).
Conductivity measurements
Sample pellets with a diameter of 10.2 mm (formed under a pressure of 110 MPa) were sandwiched between blocking electrodes in an Ar atmosphere. The AC EIS measurements were conducted in a thermostatic chamber (SU241, ESPEC) with an applied voltage of 20–30 mV over a frequency range of 0.1 Hz to 7 MHz using a frequency response analyzer (VSP-300, Bio-Logic). The temperature was regulated using a cryogenic controller (Model 336, Lake Shore). Additionally, the DC polarization measurements were conducted at a constant voltage of 1.0 V and a response time limit of 30 min using an electrochemical workstation (Solartron 1260).
Computational calculations
First-principles DFT calculations were performed using the Vienna Ab initio Simulation Package (VASP) with the projector augmented wave method77. The exchange-functional was treated within the generalized gradient approximation using the Perdew–Burke–Ernzerhof functional78. A plane wave basis set with an energy cutoff of 500 eV was employed, and geometry relaxation was performed until the forces on each atom were less than 0.03 eV Å−1. The Brillouin zone was sampled using a 2 × 2 × 1 k-point grid. Self-consistent calculations were conducted with an energy convergence threshold of 10−5 eV. The model was established by expanding the Li2S cell to 2 × 2 × 3. COHP computations were performed using LOBSTER software.
ASSLSB assembly and electrochemical measurements
All ASSLSB cells were fabricated in a vacuum glove box (DBO-1.5-T1000 + MM2-H15S, MIWA MFG) in an argon atmosphere. Li3.45Ge0.45P0.55S4 (LGPS0.55) powder (~70 mg) was pressed into a pellet in a polyethylene terephthalate tube (inner diameter 10.2 mm, outer diameter 30 mm, height 20 mm) at 178 MPa for 1 min. Subsequently, the cathode composite powder (5 mg) was evenly dispersed on one side of the LGPS0.55 pellet, and an aluminum plate and aluminum mesh (diameter 10 mm, thickness 0.1 mm) were placed afterwards on the composite as the current collector; the resulting unit was pressed at 355 MPa for 1 min. For the anode side, an indium plate (diameter 10 mm, thickness 0.1 mm), lithium foil (diameter 5 mm, thickness 0.6 mm), and copper mesh (diameter 10 mm, thickness 0,1 mm) were placed on the opposite side of the LGPS0.55 pellet and then pressed at 178 MPa for 1–2 s. Finally, the entire cell was enveloped in an outer frame (SSBC-2, SUS-303, Premium Glass). The electrochemical performances of the cells were evaluated through galvanostatic charge–discharge measurements conducted using a multi-channel galvanostat (TOSCAT-3100, Toyo System) in the voltage range 0.6–2.5 V vs. Li–In.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Liu, Y., He, P. & Zhou, H. Rechargeable solid‐state Li–Air and Li–S batteries: materials, construction, and challenges. Adv. Energy Mater. 8, 1701602 (2018).
Nagao, M. et al. Reaction mechanism of all-solid-state lithium–sulfur battery with two-dimensional mesoporous carbon electrodes. J. Power Sources 243, 60–64 (2013).
Agostini, M., Aihara, Y., Yamada, T., Scrosati, B. & Hassoun, J. A lithium–sulfur battery using a solid, glass-type P2S5–Li2S electrolyte. Solid State Ionics 244, 48–51 (2013).
Kinoshita, S., Okuda, K., Machida, N., Naito, M. & Sigematsu, T. All-solid-state lithium battery with sulfur/carbon composites as positive electrode materials. Solid State Ionics 256, 97–102 (2014).
Lin, Z. & Liang, C. Lithium–sulfur batteries: from liquid to solid cells. J. Mater. Chem. A 3, 936–958 (2015).
Barai, P., Mistry, A. & Mukherjee, P. P. Poromechanical effect in the lithium–sulfur battery cathode. Extreme Mech. Lett. 9, 359–370 (2016).
Liang, C., Dudney, N. J. & Howe, J. Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery. Chem. Mater. 21, 4724–4730 (2009).
Yang, X., Luo, J. & Sun, X. Towards high-performance solid-state Li-S batteries: from fundamental understanding to engineering design. Chem. Soc. Rev. 49, 2140–2195 (2020).
Liu, M., Jin, B., Zhang, Q., Zhan, X. & Chen, F. High-performance solid polymer electrolytes for lithium ion batteries based on sulfobetaine zwitterion and poly (ethylene oxide) modified polysiloxane. J. Alloys Compd. 742, 619–628 (2018).
Zhang, Z. et al. New horizons for inorganic solid state ion conductors. Energy Environ. Sci. 11, 1945–1976 (2018).
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Li, X. et al. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 13, 1429–1461 (2020).
Wu, J., Liu, S., Han, F., Yao, X. & Wang, C. Lithium/sulfide all-solid-state batteries using sulfide electrolytes. Adv. Mater. 33, 2000751 (2021).
Kudu, Ö. U. et al. A review of structural properties and synthesis methods of solid electrolyte materials in the Li2S − P2S5 binary system. J. Power Sources 407, 31–43 (2018).
Zhou, L., Assoud, A., Zhang, Q., Wu, X. & Nazar, L. F. New family of argyrodite thioantimonate lithium superionic conductors. J. Am. Chem. Soc. 141, 19002–19013 (2019).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Yamane, H. et al. Crystal structure of a superionic conductor, Li7P3S11. Solid State Ionics 178, 1163–1167 (2007).
Choi, S. et al. Application of rod-like Li6PS5Cl directly synthesized by a liquid phase process to sheet-type electrodes for all-solid-state lithium batteries. J. Electrochem. Soc. 166, A5193–A5200 (2019).
Sakuda, A., Takeuchi, T. & Kobayashi, H. Electrode morphology in all-solid-state lithium secondary batteries consisting of LiNi1/3Co1/3Mn1/3O2 and Li2S-P2S5 solid electrolytes. Solid State Ionics 285, 112–117 (2016).
Peng, L. et al. Tuning solid interfaces via varying electrolyte distributions enables high‐performance solid‐state batteries. Energy Environ. Mater. 6, e12308 (2023).
Teragawa, S., Aso, K., Tadanaga, K., Hayashi, A. & Tatsumisago, M. Liquid-phase synthesis of a Li3PS4 solid electrolyte using N-methylformamide for all-solid-state lithium batteries. J. Mater. Chem. A 2, 5095–5099 (2014).
Yubuchi, S. et al. An argyrodite sulfide-based superionic conductor synthesized by a liquid-phase technique with tetrahydrofuran and ethanol. J. Mater. Chem. A 7, 558–566 (2019).
Seh, Z. W. et al. Facile synthesis of Li2S–polypyrrole composite structures for high-performance Li2S cathodes. Energy Environ. Sci. 7, 672–676 (2014).
Yang, Y. et al. High-capacity micrometer-sized Li2S particles as cathode materials for advanced rechargeable lithium-ion batteries. J. Am. Chem. Soc. 134, 15387–15394 (2012).
Li, S. et al. Recent progress in developing Li2S cathodes for Li–S batteries. Energy Storage Mater. 27, 279–296 (2020).
Hayashi, A., Ohtsubo, R., Ohtomo, T., Mizuno, F. & Tatsumisago, M. All-solid-state rechargeable lithium batteries with Li2S as a positive electrode material. J. Power Sources 183, 422–426 (2008).
Fujita, Y. et al. Li2S–LiI solid solutions with ionic conductive domains for enhanced all-solid-state Li/S batteries. ACS Appl. Energy Mater. 5, 9429–9436 (2022).
Hakari, T., Hayashi, A. & Tatsumisago, M. Li2S‐based solid solutions as positive electrodes with full utilization and superlong cycle life in all‐solid‐state Li/S batteries. Adv. Sustain. Syst. 1, 1700017 (2017).
Hayashi, A., Ohtsubo, R. & Tatsumisago, M. Electrochemical performance of all-solid-state lithium batteries with mechanochemically activated Li2S–Cu composite electrodes. Solid State Ionics 179, 1702–1705 (2008).
Kobayashi, T. et al. All solid-state battery with sulfur electrode and thio-LISICON electrolyte. J. Power Sources 182, 621–625 (2008).
Yamada, T. et al. All solid-state lithium–sulfur battery using a glass-type P2S5–Li2S electrolyte: benefits on anode kinetics. J. Electrochem. Soc. 162, A646–A651 (2015).
Suzuki, K. et al. High cycle capability of all-solid-state lithium–sulfur batteries using composite electrodes by liquid-phase and mechanical mixing. ACS Appl. Energy Mater. 1, 2373–2377 (2018).
Yu, C. et al. Unravelling Li-ion transport from picoseconds to seconds: bulk versus interfaces in an argyrodite Li6PS5Cl-Li2S all-solid-state Li-ion battery. J. Am. Chem. Soc. 138, 11192–11201 (2016).
Yao, X. et al. High-energy all-solid-state lithium batteries with ultralong cycle life. Nano Lett. 16, 7148–7154 (2016).
Rao, R. P. & Seshasayee, M. Molecular dynamics simulation of ternary glasses Li2S–P2S5–LiI. J. Non-Cryst. Solids 352, 3310–3314 (2006).
Wei, C. et al. Tuning ionic conductivity to enable all-climate solid-state Li–S batteries with superior performances. Mater. Adv. 3, 1047–1054 (2022).
Holzwarth, U. & Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 6, 534 (2011).
Liu, M. et al. Quantification of the Li-ion diffusion over an interface coating in all-solid-state batteries via NMR measurements. Nat. Commun. 12, 5943 (2021).
Diao, Y., Xie, K., Xiong, S. & Hong, X. Insights into Li-S battery cathode capacity fading mechanisms: irreversible oxidation of active mass during cycling. J. Electrochem. Soc. 159, A1816–A1821 (2012).
Gu, S. et al. A hybrid electrolyte for long-life semi-solid-state lithium sulfur batteries. J. Mater. Chem. A 5, 13971–13975 (2017).
Nisar, A. et al. Quantification of complex protective surface oxide layer formed during plasma jet exposure of multicomponent ultra-high temperature carbides. Appl. Surf. Sci. 592, 153247 (2022).
Krishna, D. N. G. & Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): recent developments and challenges. Appl. Surf. Sci. Adv. 12, 100332 (2022).
Ito, S., Nakakita, M., Aihara, Y., Uehara, T. & Machida, N. A synthesis of crystalline Li7P3S11 solid electrolyte from 1,2-dimethoxyethane solvent. J. Power Sources 271, 342–345 (2014).
Hatz, A.-K. et al. Chemical stability and ionic conductivity of LGPS-type solid electrolyte tetra-Li7SiPS8 after solvent treatment. ACS Appl. Energy Mater. 4, 9932–9943 (2021).
Zhang, L., Sun, D., Feng, J., Cairns, E. J. & Guo, J. Revealing the electrochemical charging mechanism of nanosized Li2S by in situ and operando X-ray absorption spectroscopy. Nano Lett. 17, 5084–5091 (2017).
Hakari, T. et al. Solid electrolyte with oxidation tolerance provides a high‐capacity Li2S‐based positive electrode for all‐solid‐state Li/S batteries. Adv. Funct. Mater. 32, 2106174 (2022).
Yang, Y., Zheng, G. & Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 42, 3018–3032 (2013).
Altorfer, F. et al. Lithium diffusion in the superionic conductor Li2S. Phys. B 180–181, 795–797 (1992).
Maekawa, H. et al. Halide-stabilized LiBH4, a room-temperature lithium fast-ion conductor. J. Am. Chem. Soc. 131, 894–895 (2009).
Miyazaki, R., Hiroi, S., Miyazaki, H. & Hihara, T. Improvement of Li+ conductivity in ball-milled LiI and the local structure analysis by pair distribution function based on X-ray total scattering. J. Solid State Electrochem. 26, 1577–1583 (2022).
Wang, Z. et al. Lithium anode interlayer design for all-solid-state lithium-metal batteries. Nat. Energy 9, 251–262 (2024).
Li, J. et al. Mixed ion‐electron conducting Li3P for efficient cathode prelithiation of all‐solid‐state Li‐ion batteries. SmartMat. 4, e1200 (2023).
Wang, M. J., Wolfenstine, J. B. & Sakamoto, J. Mixed electronic and ionic conduction properties of lithium lanthanum titanate. Adv. Funct. Mater. 30, 1909140 (2020).
Dewald, G. F. et al. Experimental assessment of the practical oxidative stability of lithium thiophosphate solid electrolytes. Chem. Mater. 31, 8328–8337 (2019).
Han, F., Zhu, Y., He, X., Mo, Y. & Wang, C. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 6, 1501590 (2016).
Lin, Z., Liu, Z., Fu, W., Dudney, N. J. & Liang, C. Lithium polysulfidophosphates: a family of lithium-conducting sulfur-rich compounds for lithium–sulfur batteries. Angew. Chem. Int. Ed. 52, 7460–7463 (2013).
Tan, D. H. S. et al. Elucidating reversible electrochemical redox of Li6PS5Cl solid electrolyte. ACS Energy Lett. 4, 2418–2427 (2019).
Gamo, H., Hikima, K. & Matsuda, A. Understanding decomposition of electrolytes in all-solid-state lithium–sulfur batteries. Chem. Mater. 34, 10952–10963 (2022).
Yu, C. et al. Tailoring Li6PS5Br ionic conductivity and understanding of its role in cathode mixtures for high performance all-solid-state Li–S batteries. J. Mater. Chem. A 7, 10412–10421 (2019).
Cao, D. et al. Understanding electrochemical reaction mechanisms of sulfur in all-solid-state batteries through operando and theoretical studies. Angew. Chem. Int. Ed. 62, e202302363 (2023).
Kim, J. T. et al. Manipulating Li2S2/Li2S mixed discharge products of all-solid-state lithium sulfur batteries for improved cycle life. Nat. Commun. 14, 6404 (2023).
Zha, C. et al. Single-atom tailoring of Li2S to form Li2S2 for building better lithium-sulfur batteries. Energy Storage Mater. 47, 79–86 (2022).
Gamo, H., Hikima, K. & Matsuda, A. Transition-metal sulfides for high-performance lithium sulfide cathodes in all-solid-state lithium-sulfur batteries. ACS Omega 8, 45557–45565 (2023).
Hakari, T. et al. Structural and electronic-state changes of a sulfide solid electrolyte during the Li deinsertion–insertion processes. Chem. Mater. 29, 4768–4774 (2017).
Wang, S. et al. Lithium argyrodite as solid electrolyte and cathode precursor for solid‐state batteries with long cycle life. Adv. Energy Mater. 11, 2101370 (2021).
Schwietert, T. K. et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020).
Gamo, H. et al. Electrochemical redox of Li2S–CaS and –CaX2 (X = Cl, Br, and I) cathode materials for all-solid-state lithium-sulfur batteries. Electrochim. Acta 431, 141149 (2022).
Zhao, Q., Lu, Y., Zhu, Z., Tao, Z. & Chen, J. Rechargeable lithium-iodine batteries with iodine/nanoporous carbon cathode. Nano. Lett. 15, 5982–5987 (2015).
Li, M. et al. Electrochemically primed functional redox mediator generator from the decomposition of solid state electrolyte. Nat. Commun. 10, 1890 (2019).
Dai, C. et al. Honeycomb-like spherical cathode host constructed from hollow metallic and polar Co9S8 tubules for advanced lithium–sulfur batteries. Adv. Funct. Mater. 28, 1704443 (2018).
Lu, K. et al. Manipulating polysulfide conversion with strongly coupled Fe3O4 and nitrogen doped carbon for stable and high capacity lithium–sulfur batteries. Adv. Funct. Mater. 29, 1807309 (2018).
Song, Y.-X. et al. Direct tracking of the polysulfide shuttling and interfacial evolution in all-solid-state lithium–sulfur batteries: a degradation mechanism study. Energy Environ. Sci. 12, 2496–2506 (2019).
Nagao, M. et al. All-solid-state lithium–sulfur batteries with three-dimensional mesoporous electrode structures. J. Power Sources 330, 120–126 (2016).
Watanabe, R. et al. Extension of size of monodisperse silica nanospheres and their well-ordered assembly. J. Colloid Interface Sci. 360, 1–7 (2011).
Oishi, R. et al. Rietveld analysis software for J-PARC. Nucl. Instrum. Methods Phys. Res. A 600, 94–96 (2009).
Blöchl, P. E., Jepsen, O. & Andersen, O. K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B Condens. Matter 49, 16223–16233 (1994).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B Condens. Matter 46, 6671–6687 (1992).
Acknowledgements
This study was partly supported by Grants-in-Aid for Scientific Research on Innovative Areas (19H05785) and Scientific Research (C) (nos. 20K05683 and 23K04907) from the Japan Society for the Promotion of Science; the Open Innovation Platform with Enterprises, Research Institute, and Academia (OPERA) Program (JPMJOP1862) established by the Japan Science and Technology Agency (JST); and JST SPRING (JPMJSP2106). Synchrotron radiation experiments were performed using the BL02B2 and BL19B2 beamlines at the SPring-8 facility with the approval of the Japan Synchrotron Radiation Research Institute (JASRI; proposal nos. 2021B1776 and 2022B2093).
Author information
Authors and Affiliations
Contributions
P.J. and K.S. conceived the study. K.S., M.H., S.H., and R.K. supervised the project. P.J. and Y.Y. synthesized the samples. P.J. and H.Z. characterized the samples and analyzed the data. N.M. and S.S. helped perform the synchrotron X-ray diffraction measurements. P.J. conducted the theoretical simulations and calculations. The manuscript was drafted by P.J. and then revised by K.S., K.W., and M.H. All authors have contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks Feifei Shi, Guang Yang and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editors: Andrew Westover and Jet-Sing Lee. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, P., Zhou, H., Song, S. et al. A composite cathode with a three-dimensional ion/electron-conducting structure for all-solid-state lithium–sulfur batteries. Commun Mater 5, 105 (2024). https://doi.org/10.1038/s43246-024-00537-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43246-024-00537-w