Abstract
Predicting the migration of marine life due to climate change is important not only ecologically but also for the management of valuable living marine resources. Anticipating how the habitable area of species will change on decision-relevant time scales, however, remains a challenge. Here we assess the predictability of a normalized metabolic index that expresses habitat-relevant anomalies in metabolic constraints using a coupled physical-biogeochemical prediction system based on an Earth system model. The normalized metabolic index was generally more predictable than temperature, especially in the subsurface tropics where persistent lateral oxygen advection anomalies at the boundary of oxygen minimum zones extended the predictability horizons. Further investigations suggest that interannual catch variations of bigeye tuna in the tropical exclusive economic zones can be anticipated from the predicted metabolic constraints a year in advance, supporting the potential utility of Earth system model-based physiological prediction for the proactive climate-informed management of living marine resources.
Similar content being viewed by others
Introduction
Marine ecosystems have been facing unprecedented climate changes marked by elevated temperatures, declines in dissolved oxygen levels, and rapid ocean acidification1,2,3,4. These environmental disturbances have led to a decrease in species richness, changes in phenology, and dynamic shifts in marine organisms2,5,6,7,8,9. Given the substantial influence of climate-driven fluctuations on marine ecological communities, anticipating marine ecosystem variations holds considerable importance for the sustainable management of living marine resources10,11,12,13. This is further underscored by declining future global fisheries production14,15, accentuating the urgency of comprehending future changes in marine life for a range of stakeholders.
Marine species exhibit distinct capacities to tolerate temperature and oxygen changes. Warmer ocean temperatures due to climate change increase metabolic demands and generally occur with a decrease in dissolved oxygen, further limiting biological aerobic scope16,17,18,19. Notably, hypoxic conditions can limit suitable habitats for species by inducing physiological adjustments in their life cycles20,21,22,23,24. The effort to develop a metabolic index that mechanistically integrates both temperature and oxygen factors into a combined constraint has shown promise in determining viable habitat limits for marine organisms17,25.
With the advent of global Earth system models (ESMs) capable of simulating both physical and biogeochemical processes in the ocean, many efforts have been undertaken to elucidate the primary drivers determining present and future changes in marine ecosystems. These initiatives encompass the reconstruction of historical ocean biogeochemical variables26,27, the development of marine biogeochemical prediction and environmentally informed resource decision systems25,28,29,30,31, and the future projection of metabolically viable habitats and species ranges under anthropogenic warming17,18,32. Such efforts show the potential of integrating ESMs into management frameworks for marine resources, although unraveling the effects of climate-driven environmental forcing on marine ecosystems is complex.
In this study, we broadly assess the predictability of a simple normalized metabolic index (\({{\Phi }}_{N}\)) and explore its potential utility for fish catch prediction by using a recently developed ESM-based prediction system29. The metabolic index, which represents metabolically viable habitats, has been previously defined as the ratio between the ambient oxygen supply and the resting oxygen demand of marine species17. Here we introduce a \({{\Phi }}_{N}\) derived from dividing the current metabolic index by its monthly climatological value (see Methods), providing a succinct index of metabolic anomalies relative to typical conditions. This approach also isolates the effect of species-specific contrasts in the temperature sensitivity of metabolism, allowing for an efficient exploration of relative predictability.
We pursue this with an extensive set of seasonal to multiannual reforecast experiments from a physical climate prediction system developed at the Geophysical Fluid Dynamics Laboratory (GFDL) coupled with a comprehensive biogeochemical model33,34. Predictions are initialized with both physical and biogeochemical variables from a data-assimilative physical simulation with integrated biogeochemical model27, and predictability is evaluated against the reconstructed historical physical and biogeochemical data. We focus on the 1–2-year prediction horizons, which are highly relevant to fisheries harvest controls12, and conclude by examining the potential utility of \({{\Phi }}_{N}\) for tropical Pacific tuna fisheries.
Results
Predictability of metabolic index
The predictability of the \({{\Phi }}_{N}\) is evaluated by defining the index across three different depths (0-100 m, 100-300 m, and 300-800 m) with three different values of the temperature-sensitivity of metabolic demands, \({E}_{o}\) (0.2, 0.7, and 1.2 eV). The selection of different depths aims to reflect three species categories, i.e., pelagic, demersal, and mesopelagic species, and the \({E}_{o}\) range was chosen to encompass the range of temperature/hypoxia sensitivities. Note that a lower \({E}_{o}\) in the metabolic index implies a steeper increase in metabolic demand with warming, indicating reduced sensitivity of the metabolic index to temperature. Consequently, with a lower \({E}_{o}\), the variability of the metabolic index is more influenced by oxygen relative to temperature (see Eq. (2) in the Method section). The three \({E}_{o}\,\) cases will thus be referred to as the high, medium, and low hypoxia sensitivity cases, respectively.
The predictability of the metabolic index is pronounced in many ocean regions 1 year in advance (Fig. 1). Overall, the subsurface ocean tends to exhibit greater predictability compared to the surface, primarily due to the susceptibility of the surface ocean to comparatively unpredictable high-frequency air-sea interaction35,36. The root mean square error (RMSE) analysis also showed that the predictability error decreases with increasing depth (Supplementary Fig. 1). The skillful prediction of the metabolic index extends to a 2-year lead time across many global oceans, particularly within the subsurface layers. Nevertheless, the predictability is generally reduced compared to that at a 1-year lead time (cf. Fig. 1A–I, J–R), except for the North Atlantic and Southern Oceans where low-frequency fluctuations linked to the Atlantic meridional overturning circulation and deep ocean convections contributes to enhanced potential predictability of climate variables35,37,38,39. A notable decrease in 2-year predictive skill above 300 m depth appears in the tropical Pacific. This limited skill is attributed to the inherent challenges dynamic models encounter in predicting interannual climate variability, such as El Niño-Southern Oscillation (ENSO), beyond a 1-year horizon40.
Prediction skill of the \({{\Phi }}_{N}\) at 0–100 m depth, with \({E}_{o}\) values of A high (\({E}_{o}\) = 0.2 eV), B medium (\({E}_{o}\) = 0.7 eV), and C low (\({E}_{o}\) = 1.2 eV) hypoxia sensitivities. D–F, G–I are similar to A–C but at 100–300 m and 300–800 m depth, respectively. The prediction skill is measured by the annual anomaly correlation coefficient between reanalyzed data and 1-year lead-time forecasts during 1991–2017. J–R show similar analyses to A–I but for 2-year lead-time forecasts. Gray hatchings indicate areas with statistically significant values at the 95% confidence level.
Improved predictability for the \({{\Phi }}_{N}\) relative to temperature emerges during the first year of the prediction in the equatorial Pacific (Fig. 2) and becomes particularly prominent by year 2. This is especially true for species at 100–300 m depths whose hypoxia tolerance is highly sensitive to temperature, indicating increased predictability due to oxygen. The enhanced predictability by oxygen within the tropics is also evident when comparing the predictability of oxygen and temperature at lead times of 1 and 2 years (Supplementary Figs. 2 and 3).
A–C Differences in prediction skill, calculated as the correlation coefficient of temperature subtracted from that of the metabolic index \(({{\Phi }}_{N})\), at 0–100 m with \({E}_{o}\) values for different hypoxia sensitivities at a 1-year lead-time: A high (\({E}_{o}\) = 0.2 eV), B medium (\({E}_{o}\) = 0.7 eV), and C low (\({E}_{o}\) = 1.2 eV) sensitivities. D–F, G–I are similar to A–C, but at 100–300 m and 300–800 m depth, respectively. J–R show similar analyses to A–I but for 2-year lead-time forecasts. Positive values indicate that the metabolic index prediction skill is higher than that of temperature.
Mechanisms underlying high predictability of metabolic index in the tropical Pacific
A budget analysis is conducted to elucidate the detailed mechanism underlying the higher oxygen predictability relative to temperature predictability, given that the enhanced oxygen predictability contributes to the prolonged and enhanced predictability of the metabolic index. We analyzed the respective roles of lateral and vertical advection in controlling the temporal evolution of oxygen and temperature by focusing on the tropical Pacific (20°S–20°N and 150°W–90°E) at depths of 100–300 m where oxygen substantially contributes to metabolic index predictability. In the case of oxygen, lateral advection exhibits the strongest correlation with overall oxygen changes (Fig. 3A). Vertical advection and residual (including diffusion and biological source/sink) terms have only a weak or negligible influence on oxygen variability. In contrast, the budget analysis for temperature reveals that vertical advection predominates in influencing temperature variability, while other terms contribute minimally (Fig. 3B).
Timeseries of reanalyzed subsurface (100–300 m) A oxygen and B temperature advections over the tropical region (150°E–90°W, 20°S–20°N) for 1991–2017. Red lines show the lateral advections which are the sum of anomalous zonal and meridional advections. Blue lines indicate the anomalous vertical advections. Black, purple, and yellow lines indicate the anomalous time tendencies, the sum of lateral and vertical advections, and the residual, respectively. The numbers in the brackets denote the correlation coefficient between the time tendency term and each term. Asterisks mean statistically significant at the 95% confidence level. Persistency of the advections in subsurface C oxygen and D temperature. Red and blue bars indicate the persistency of the lateral and the vertical advection terms, respectively. Significance at 95% level confidence is indicated by black dashed lines.
The difference in the main drivers of oxygen and temperature variability arises from distinct characteristics in the spatial patterns of their climatology. The areas of higher predictability of oxygen relative to temperature are located along the periphery of the oxygen minimum zone (OMZ) in the eastern Pacific, defined by deficient oxygen concentrations (<20–45 μmol kg−1) (Supplementary Fig. 4). Consequently, horizontal inflow across the strong horizontal gradient of oxygen concentration plays a dominant role in influencing oxygen variations compared to vertical advection. In contrast to the oxygen, interannual variability of temperature in the tropical Pacific is primarily affected by the vertical fluctuations in thermocline depth, largely caused by phenomena like the ENSO41,42. During El Niño events, for example, westerly winds initiate the eastward propagation of downwelling Kelvin waves, subsequently deepening the thermocline and further reinforcing the dominant role of vertical advection in controlling tropical Pacific temperature dynamics.
The extended predictability of oxygen relative to temperature in the tropical Pacific can be attributed to the prolonged persistence of lateral advection (Fig. 3C). The anomalies of lateral oxygen advection maintain their persistence for up to 3 years, whereas the persistence of vertical advection diminishes after 1 year. The longer persistence of lateral oxygen advection leads to its higher and longer predictability compared to vertical advection (Supplementary Fig. 5A). In contrast, temperature anomalies persist for less than 1 year for both lateral and vertical advections, leading to a diminished predictability of both advection terms after 1 year (Fig. 3D and Supplementary Fig. 5B). This suggests that the high oxygen predictability in this area results from the sustained nature of the dominant oxygen budget term, i.e., lateral advection, while the lower temperature predictability can be attributed to more ephemeral vertical advection effects that persist for less than 1 year. This result aligns with previous studies demonstrating that marine biogeochemical variables can be highly predictable due to their long-lasting memory, which contrasts with physical variables that interact with the atmosphere36,43,44.
The long-lasting lateral oxygen advection appears to be linked to Pacific Decadal Oscillation (PDO). The effects of PDO on subsurface oxygen concentration in the tropical Pacific have been previously demonstrated by modeling45,46 and observational47 studies. These studies showed that the physical processes associated with PDO primarily involve the lateral transport of oxygen. During a negative PDO phase, there is an increase in equatorward meridional transport due to the intensified subtropical cells, connecting the high oxygen levels in the subtropics with the lower oxygen levels in the tropics. Thus, the subsurface oxygen levels in the tropical Pacific rise during a cold PDO regime, while the opposite occurs during a positive PDO phase45,46,48. Consistent with these previous works, we also found a noteworthy correlation between the lateral advection of oxygen and the PDO index, with a correlation coefficient of 0.58 (p < 0.1).
Potential utility of metabolic index for anticipating bigeye tuna catch
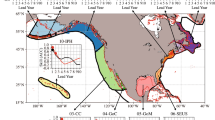
The high predictability of the \({{\Phi }}_{N}\) in the tropical Pacific regions, which is attributed to the longer persistence of oxygen, suggests a potential utility of the \({{\Phi }}_{N}\) prediction to inform interannual fish-catch fluctuations. This is particularly relevant for large pelagic fish whose migratory behaviors are shaped by physiological constraints on habitat availability. In this study, we focus on the bigeye tuna, a commercially important species with a wide distribution across tropical and sub-tropical Pacific and extensive historical catch records49. We used the aggregated bigeye tuna catches from 6 exclusive economic zones (EEZs) and the \({{\Phi }}_{N}\) calculated at 100-300 m within the region where \({{\Phi }}_{N}\) predictability is higher than temperature predictability (hatched area in Fig. 4A). The bigeye tuna catch was linearly detrended to mitigate the potential misattribution of fishing effort trends to environmental factors.
A Hatched area is a region where oxygen is well predicted, which means the difference between metabolic Index and temperature predictability of more than 0.25. The six EEZs shown in the hatched area represent the full set of EEZs within the high predictability region. The EEZs include the Galapagos Islands (219), Kiribati (942), Palmyra Atoll & Kingman Reef (844), Jarvis Islands (845), Marshall Islands (584), and Micronesia (583). B Black line shows reported annual catches of Bigeye tuna in EEZs where oxygen is well predicted. Green, red, and blue lines indicate the reanalyzed, the 1-year lead-time, and 2-year lead-time forecasts of metabolic Index, respectively, calculated for the depth range of 100–300 m. Both the catch data and the metabolic index are detrended. Asterisks denote the significant (p < 0.05) correlation between reported catches and metabolic indexes.
The interannual variability of \({{\Phi }}_{N}\), derived from reconstructed historical temperature and oxygen data, exhibits a significant correlation with detrended bigeye tuna catch (Fig. 4B). This suggests a potential influence of environmental conditions—reflected through physiological constraints—on tuna catch variability in the analyzed tropical region. The 1-year lead forecast of the metabolic index also captures these interannual variations, implying its potential as a predictive tool for linking ESM-based environmental forecasts with fishery-relevant population dynamics. However, at a 2-year lead time, the forecast shows relatively limited prediction skill of tuna catch presumably due to less pronounced interannual signals and increased uncertainty in environmental variability at longer timescales.
Given the limitation of fishery-dependent catch data in interpreting habitat suitability and environmental drivers of fish abundance, we additionally compared the normalized metabolic index with the catch per unit effort (CPUE) data derived from the Western and Central Pacific Fisheries Commission (WCPFC) grid-based dataset for the whole tropical Pacific. The CPUE time series also exhibited notable correlations with interannual variability of metabolic index derived from both reconstructed data and the 1-year lead forecast (Supplementary Fig. 6). This consistency across datasets suggests the potential of the metabolic index as a meaningful predictor for anticipating fishery-relevant outcomes in this region.
Discussion
The metabolic index has been used to explore the potential distribution of marine species based on temperature and oxygen data from ESMs. This approach offers notable advantages, including the ability to make predictions across broad temporal and spatial scales, such as long-term climate change and oceanic environmental shifts, while maintaining computational efficiency for large-scale data analysis. However, recent studies have shown that the predictive performance of the metabolic index can vary depending on species traits and environmental context. In some cases, models based solely on oxygen or other environmental factors outperform the metabolic index, particularly for deep-dwelling species or populations near the cold edge of their range50,51. These findings highlight the importance of carefully selecting modeling frameworks based on the species’ ecology and regional ocean conditions.
The current framework shown in this study does not account for interspecies interactions or complex ecosystem dynamics that can notably influence fish populations and catches. To address this limitation, our future research will integrate ESM-derived variables into high trophic models. This integration will enable us to simulate ecosystem interactions, including predator-prey relationships and competition, providing a more comprehensive understanding of the factors shaping marine ecosystem dynamics and improving the accuracy of fishery predictions. By bridging the strengths of ESM and fish models, this approach aims to enhance the utility of the metabolic index for sustainable fisheries management.
The fish catch data used in this study are fishery-dependent catch data and nominal CPUE, which do not directly reflect habitat suitability or true abundance. Due to the lack of operational details such as vessel identity and set-level effort, formal CPUE standardization was not feasible in the area analyzed here. Our analysis instead focused on interannual variability and its covariation with the metabolic index, a proxy for physiological constraints. While this approach offers initial insights into environmental influences on tuna catch fluctuations, future assessments would benefit from fishery-independent data or more detailed fishery-dependent records to enable robust standardization and strengthen catch–environment relationships.
While this study focused on applying the metabolic index to annual mean predictions of fish catch, this approach also holds strong potential for short-term predictions on monthly or seasonal timescales. These shorter timescales have physiological significance for marine organisms, particularly those inhabiting regions with high environmental variability, making them directly relevant to fisheries management. The short-term predictions can account for species’ seasonal migration and reproduction patterns, supporting timely and effective decision-making. However, a key challenge here is the lack of reliable monthly fisheries data, largely due to difficulties in data collection, reporting inconsistencies, and the absence of standardized data protocols across countries. In many regions, particularly in developing nations and areas dominated by small-scale fisheries, systematic monitoring and reporting systems remains inadequate, making accurate data collection challenging. If reliable monthly data became available, the metabolic index approach could be applied to develop seasonal predictions, providing more actionable insights for effective marine resource management.
In contrast to physical variables, marine biogeochemical factors, such as oxygen and primary productivity, tend to exhibit long-lasting characteristics, offering enhanced predictive capabilities over extended lead times35,36,52. This increased and longer predictability of biogeochemical variables, as observed with the metabolic index when compared to temperature, provides optimism in the potential of dynamic prediction systems based on Earth system models for managing and exploiting fisheries resources. Moreover, refining the metabolic index or developing supplementary metrics that incorporate other biogeochemical variables at the species level53 can provide valuable insights to inform optimal harvest guidelines and future migration patterns, ultimately promoting the sustainable use of marine living resources.
Materials and methods
Normalized metabolic index
The metabolic index is a metric used to estimate a metabolically sustainable environment for marine life, defined as the ratio of ambient oxygen supply to the resting oxygen demand of marine species17. The index can serve as a tool for estimating changes in marine habitats. In this study, to focus on the interannual variability of the metabolic constraints, we used the normalized metabolic index, denoted \({{\Phi }}_{N}\), obtained by dividing the previously defined metabolic index by its corresponding monthly climatology:
where \({A}_{0}\) is the ratio of coefficients for oxygen supply and metabolic rate at a reference temperature, indicating how effectively an organism can meet its oxygen needs in a given environment, and \({B}^{n}\) is the scaling term that reflects the impact of body size on the balance between oxygen supply and metabolic demand. \({{pO}}_{2}\) is the oxygen concentration within the environment, \({E}_{o}\) is the temperature sensitivity of hypoxia vulnerability, \({K}_{B}\) is Boltzmann constant, and T is the absolute temperature. In the present study, monthly mean ocean dissolved oxygen and temperature data are used to calculate \({{\Phi }}_{N}\).
The parameter \({E}_{o}\) controls the temperature scaling of metabolic demands and, consequently, amplifies hypoxia vulnerability as temperatures rise. A smaller \({E}_{o}\) indicates a greater dependence of the normalized metabolic index on oxygen compared to temperature. In the normalized formulation, the constant species-dependent parameters \({A}_{0}\) and \({B}^{n}\) are removed by dividing by the monthly climatology. However, \({E}_{o}\) remains in the expression and continues to reflect species-specific hypoxia tolerance against inverse temperature. Thus, the normalized metabolic index retains biologically meaningful differences across species through \({E}_{o}\), even without explicitly including other physiological constants. In this study, three \({E}_{o}\) values were chosen, 0.2, 0.7, and 1.2 eV, to encompass a range of hypoxia sensitivities across different species, from high to low.
Reconstructed metabolic index and its retrospective forecasts
The predictability of the \({{\Phi }}_{N}\) is assessed using reconstructed data generated by the Earth System Model (ESM) developed by the Geophysical Fluid Dynamics Laboratory (GFDL). The ESM is a fully coupled climate model, including atmosphere, land, sea-ice, and ocean models. A marine biogeochemical model, the Carbon, Ocean Biogeochemistry and Lower Trophics (COBALT) version 2 is coupled to the ocean model of the ESM. The COBALT model employs 33 tracers to simulate global cycles of dissolved organic and inorganic substances, with bacteria, three explicit phytoplankton, and three zooplankton groups. The atmospheric and land components of the model exhibit a horizontal resolution of 2.5° × 2° (longitude-latitude), while the ocean, sea-ice, and marine biogeochemistry components have a 1° × 1° resolution33.
The ESM is coupled to GFDL’s Ensemble Coupled Data Assimilation system (ECDA), employing an ensemble Kalman filter to assimilate observational fields54. For the ocean, observations of ocean temperature and salinity, including profiles from the World Ocean Database (WOD), Argo floats, Global Temperature-Salinity Profile Program (GTSPP) data, and NOAA Optimum Interpolation Sea Surface Temperature v2, are assimilated into the ECDA. For the atmosphere, 6-hourly atmospheric temperature and winds from the National Centers for Environmental Prediction, Department of Energy (NCEP-DOE) are assimilated into the ECDA system. Due to the scarcity of global observations and the complexity between observed and inferred variables in the biogeochemical process, marine biogeochemical fields are not directly assimilated. The biogeochemical model, however, does benefit from integration within data-assimilative physical fields27. This reconstructed data is monthly data, comprising 12 ensemble members, and the mean of these 12 ensembles is used here.
The retrospective forecast of the metabolic index is initialized on the first month of every year during the period 1991–2017. This forecast dataset incorporates monthly predictions covering 2 years and has a total of 12 ensemble members. Note that once the prediction begins, the model runs freely to forecast future states without assimilating real-time observations, mimicking real-world forecasting conditions. As the ECDA system adopts full-field physical assimilation, the forecast data needs to be adjusted to correct model drift, a phenomenon characterized by the model’s gradual movement from its data assimilative state toward its preferred state as the prediction progresses. Thus, the predicted anomalies are calculated relative to the lead-dependent monthly climatology of the predictions29,54.
Bigeye tuna catch data
The catch data for bigeye tuna (Thunnus obesus) was provided by the Sea Around Us project (https://www.seaaroundus.org/). The SAU has compiled reported fisheries data documented by the Food and Agriculture Organization of the United Nations, the International Council for the Exploration of the Seas, and individual countries55,56. This study employs exclusively reported fish catch data, excluding unreported and unregulated catches to improve consistency across different fisheries production datasets. Our analysis focuses on the catch data from six Exclusive Economic Zones (EEZs) in tropical regions (Fig. 4A). The six EEZs were selected in regions exhibiting a difference of more than 0.25 between metabolic index and temperature predictability, where oxygen contributes added values to metabolic index predictability. The EEZs encompassed the Galapagos Islands, Kiribati, Palmyra Atoll & Kingman Reef, Jarvis Islands, Marshall Islands, and Micronesia. To remove long-term non-environmental trends, the aggregated catch data were linearly detrended. In addition, CPUE data for bigeye tuna were obtained from the publicly available grid-based dataset provided by the Western and Central Pacific Fisheries Commission (WCPFC; https://www.wcpfc.int/public-domain). These data were used in a supplementary analysis to compare CPUE variability with both the reconstructed catch data and the normalized metabolic index.
Prediction skill and persistency analysis
Prediction skill is evaluated by determining the anomaly correlation coefficient between annual anomalies from the reconstructed data and the retrospective forecast dataset, which can be calculated as follows:
where \({R}^{{\prime} }\) and \({F}^{{\prime} }\) indicate annual anomalies of the reconstructed data and the forecast data, respectively. The anomaly correlation coefficient was statistically examined at the 95% confidence level using a Student’s t test, accounting for the degree of freedom to address the autocorrelation between the two timeseries.
To investigate the factors contributing to extended predictability, we conducted a persistency analysis using the autocorrelation function. The autocorrelation function quantifies the correlation between a variable and its lagged values over time, allowing us to assess the system’s memory and its influence on predictability. High autocorrelation values indicate strong temporal persistence, suggesting that the variable retains its previous state over time. In this study, the statistical significance of autocorrelation was determined using a 95% confidence interval, accounting for the length of the time series.
Budget analysis
The budget analysis is conducted to examine the mechanisms influencing the predictability of oxygen and temperature in the tropical Pacific, allowing us to identify dominant components driving their variations. Note that the temperature budget analysis serves as a proxy for heat content, while oxygen refers to dissolved oxygen concentration. The budget equation is expressed as:
where C represents either ocean temperature or dissolved oxygen concentration, and u, v, and w denote the zonal, meridional, and vertical velocities, respectively. Res is the residual term accounting for diffusion and biological source/sink. Variables are decomposed into climatology and anomalous components, where overbars denote monthly time averages and primes indicate deviations from the mean. Lateral advection is defined as the sum of meridional and zonal advection components.
Data availability
The processed datasets used in this study are archived on Zenodo and can be accessed at: https://doi.org/10.5281/zenodo.15800874.
Code availability
The analysis code used in this study is publicly available on GitHub at: https://github.com/HwajinChoi/Metabolic_index.
References
Doney, S. C. et al. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 4, 11–37 (2012).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925 (2013).
Hoegh-Guldberg, O. & Bruno, J. F. The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528 (2010).
Penn, J. L. & Deutsch, C. Avoiding ocean mass extinction from climate warming. Science 376, 524–526 (2022).
Ottersen, G., Kim, S., Huse, G., Polovina, J. J. & Stenseth, N. C. Major pathways by which climate may force marine fish populations. J. Mar. Syst. 79, 343–360 (2010).
Cheung, W. W. L. et al. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 3, 254–258 (2013).
Drinkwater, K. F. et al. On the processes linking climate to ecosystem changes. J. Mar. Syst. 79, 374–388 (2010).
Brander, K. M. Global fish production and climate change. Proc. Natl. Acad. Sci. USA 104, 19709–19714 (2007).
Liu, O. R. et al. Species redistribution creates unequal outcomes for multispecies fisheries under projected climate change. Sci. Adv. 9, eadg5468 (2023).
Hobday, A. J., Spillman, C. M., Paige Eveson, J. & Hartog, J. R. Seasonal forecasting for decision support in marine fisheries and aquaculture. Fish. Oceanogr. 25, 45–56 (2016).
Payne, M. R. et al. Lessons from the first generation of marine ecological forecast products. Front. Mar. Sci. 4, 289 (2017).
Tommasi, D. et al. Managing living marine resources in a dynamic environment: the role of seasonal to decadal climate forecasts. Prog. Oceanogr. 152, 15–49 (2017).
Payne, M. R. et al. Skilful decadal-scale prediction of fish habitat and distribution shifts. Nat. Commun. 13, 2660 (2022).
Lotze, H. K. et al. Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change. Proc. Natl. Acad. Sci. USA 116, 12907–12912 (2019).
Tittensor, D. P. et al. Next-generation ensemble projections reveal higher climate risks for marine ecosystems. Nat. Clim. Change 11, 973–981 (2021).
Pörtner, H. O., Bock, C. & Mark, F. C. Oxygen- & capacity-limited thermal tolerance: bridging ecology & physiology. J. Exp. Biol. 220, 2685–2696 (2017).
Deutsch, C., Ferrel, A., Seibel, B., Pörtner, H. O. & Huey, R. B. Climate change tightens a metabolic constraint on marine habitats. Science 348, 1132–1135 (2015).
Deutsch, C., Penn, J. L. & Seibel, B. Metabolic trait diversity shapes marine biogeography. Nature 585, 557–562 (2020).
Pörtner, H. O. & Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 (2007).
Ekau, W., Auel, H., Pörtner, H. O. & Gilbert, D. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 7, 1669–11699 (2010).
Stramma, L. et al. Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nat. Clim. Change 2, 33–37 (2012).
Leung, S., Thompson, L. A., McPhaden, M. J. & Mislan, K. A. S. ENSO drives near-surface oxygen and vertical habitat variability in the tropical Pacific. Environ. Res. Lett. 14, 064020 (2019).
Rubalcaba, J. G. et al. Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proc. Natl. Acad. Sci. USA117, 31963–31968 (2020).
Howard, E. M. et al. Climate-driven aerobic habitat loss in the California Current System. Sci. Adv. 6, eaay3188 (2020).
Chen, Z. et al. Skillful multiyear prediction of marine habitat shifts jointly constrained by ocean temperature and dissolved oxygen. Nat. Commun. 15, 1–13 (2024).
Clarke, T. M. et al. Linking observed changes in pelagic catches to temperature and oxygen in the Eastern Tropical Pacific. Fish. Fish. 23, 1371–1382 (2022).
Park, J. Y. et al. Modeling global ocean biogeochemistry with physical data assimilation: a pragmatic solution to the equatorial instability. J. Adv. Model Earth Syst. 10, 891–906 (2018).
Miesner, A. K. et al. Exploring the potential of forecasting fish distributions in the North East Atlantic with a dynamic earth system model, exemplified by the suitable spawning habitat of blue whiting. Front. Mar. Sci. 8, 777427 (2022).
Park, J., Stock, C. A., Dunne, J. P., Yang, X. & Rosati, A. Seasonal to multiannual marine ecosystem prediction with a global Earth system model. Science 365, 284–288 (2019).
Koul, V. et al. Skilful prediction of cod stocks in the North and Barents Sea a decade in advance. Commun. Earth Environ. 2, 140 (2021).
Tommasi, D. et al. Improved management of small pelagic fisheries through seasonal climate prediction. Ecol. Appl. 27, 378–388 (2017).
Cheung, W. W. L., Brodeur, R. D., Okey, T. A. & Pauly, D. Projecting future changes in distributions of pelagic fish species of Northeast Pacific shelf seas. Prog. Oceanogr. 130, 19–31 (2015).
Stock, C. A., Dunne, J. P. & John, J. G. Global-scale carbon and energy flows through the marine planktonic food web: an analysis with a coupled physical-biological model. Prog. Oceanogr. 120, 1–28 (2014).
Dunne, J. P. et al. GFDL’s ESM2 global coupled climate-carbon earth system models. Part II: carbon system formulation and baseline simulation characteristics. J. Clim. 26, 2247–2267 (2013).
Frölicher, T. L., Ramseyer, L., Raible, C. C., Rodgers, K. B. & Dunne, J. Potential predictability of marine ecosystem drivers. Biogeosciences 17, 2061–2083 (2020).
Séférian, R. et al. Multiyear predictability of tropical marine productivity. Proc. Natl. Acad. Sci. USA111, 11646–11651 (2014).
Buckley, M. W. & Marshall, J. Observations, inferences, and mechanisms of the Atlantic meridional overturning circulation: a review. Rev. Geophys. 54, 5–63 (2016).
Marchi, S. et al. Reemergence of Antarctic sea ice predictability and its link to deep ocean mixing in global climate models. Clim. Dyn. 52, 2775–2797 (2019).
Zhang, L. et al. Estimating decadal predictability for the Southern Ocean using the GFDL CM2.1 model. J. Clim. 30, 5187–5203 (2017).
Meehl, G. A. et al. Initialized Earth System prediction from subseasonal to decadal timescales. Nat. Rev. Earth Environ. 2, 340–357 (2021).
Wang, H., Kumar, A. & Wang, W. Characteristics of subsurface ocean response to ENSO assessed from simulations with the NCEP climate forecast system. J. Clim. 26, 8065–8083 (2013).
Hui, Y., Zhang, L., Wang, F. & Yan, X. Revisit of seasonal variability of subsurface temperature in the tropical Pacific with Argo data. J. Mar. Syst. 204, 103312 (2020).
Taboada, F. G. et al. Anticipating fluctuations of bigeye tuna in the Pacific Ocean from three-dimensional ocean biogeochemistry. J. Appl. Ecol. https://doi.org/10.1111/1365-2664.14346 (2023).
Chikamoto, M. O., Timmermann, A., Chikamoto, Y., Tokinaga, H. & Harada, N. Mechanisms and predictability of multiyear ecosystem variability in the North Pacific. Glob. Biogeochem. Cycles 29, 2001–2019 (2015).
Duteil, O., Böning, C. W. & Oschlies, A. Variability in subtropical-tropical cells drives oxygen levels in the tropical Pacific Ocean. Geophys. Res. Lett. 41, 8926–8934 (2014).
Duteil, O., Oschlies, A. & Böning, C. W. Pacific decadal oscillation and recent oxygen decline in the eastern tropical Pacific Ocean. Biogeosciences 15, 7111–7126 (2018).
Stramma, L. et al. Trends and decadal oscillations of oxygen and nutrients at 50 to 300 m depth in the equatorial and North Pacific. Biogeosciences 17, 813–831 (2020).
Hong, L., Zhang, L., Chen, Z. & Wu, L. Linkage between the Pacific Decadal Oscillation and the low frequency variability of the Pacific Subtropical Cell. J. Geophys. Res. Oceans 119, 3464–3477 (2014).
Bigelow, K. A., Hampton, J. & Miyabe, N. Application of a habitat-based model to estimate effective longline fishing effort and relative abundance of Pacific bigeye tuna (Thunnus obesus). Fish. Oceanogr. 11, 143–155 (2002).
Essington, T. E. et al. Advancing statistical models to reveal the effect of dissolved oxygen on the spatial distribution of marine taxa using thresholds and a physiologically based index. Ecography 2022, e06249 (2022).
Bandara, R. M. W. J., Curchitser, E. & Pinsky, M. L. The importance of oxygen for explaining rapid shifts in a marine fish. Glob. Chang. Biol. 30, e17008 (2024).
Krumhardt, K. M. et al. Potential predictability of net primary production in the ocean. Glob. Biogeochem. Cycles. 34, e2020GB006531 (2020).
Clarke, T. M. et al. Aerobic growth index (AGI): an index to understand the impacts of ocean warming and deoxygenation on global marine fisheries resources. Prog. Oceanogr. 195, 102588 (2021).
Chang, Y. S., Zhang, S., Rosati, A., Delworth, T. L. & Stern, W. F. An assessment of oceanic variability for 1960-2010 from the GFDL ensemble coupled data assimilation. Clim. Dyn. 40, 775–803 (2013).
Pauly, D. The Sea around Us Project: documenting and communicating global fisheries impacts on marine ecosystems. R. Swed. Acad. Sci. 36, 290–295 (2007).
Pauly, D. & Zeller, D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 7, 10244 (2016).
Acknowledgements
This work was supported by the National Research Foundation of Korea (RS-2023-00207866 and 2022M3I6A1085990). H.-J.C. and J.-Y.P. were supported by the Korea Institute of Marine Science & Technology Promotion grant (RS-2021-KS211500) funded by the Ministry of Oceans and Fisheries. H.-G.L. was supported by the project titled “Ocean Circulation and ecosystem variability and predictability research in the earth system model (PEA0175)”, funded by the Korea Institute of Ocean Science and Technology.
Author information
Authors and Affiliations
Contributions
H.-J.C. collected, analyzed and interpreted the data, and wrote the first draft of the manuscript. C.A.S. conceived the research idea, and J.-Y.P. conducted the model experiments. J.-Y.P., C.A.S., H.-G.L. contributed to revising the manuscript. All authors discussed the results and provided comments on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Precious Mongwe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Mengjie Wang. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, HJ., Park, JY., Stock, C.A. et al. Extended predictability of metabolic constraints on fish habitat. Commun Earth Environ 6, 617 (2025). https://doi.org/10.1038/s43247-025-02600-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02600-x