Abstract
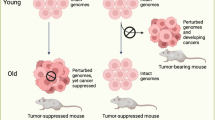
Investigators traditionally use randomized designs and corresponding analysis procedures to make causal inferences about the effects of interventions, assuming independence between an individual’s outcome and treatment assignment and the outcomes of other individuals in the study. Often, such independence may not hold. We provide examples of interdependency in model organism studies and human trials and group effects in aging research and then discuss methodologic issues and solutions. We group methodologic issues as they pertain to (1) single-stage individually randomized trials; (2) cluster-randomized controlled trials; (3) pseudo-cluster-randomized trials; (4) individually randomized group treatment; and (5) two-stage randomized designs. Although we present possible strategies for design and analysis to improve the rigor, accuracy and reproducibility of the science, we also acknowledge real-world constraints. Consequences of nonadherence, differential attrition or missing data, unintended exposure to multiple treatments and other practical realities can be reduced with careful planning, proper study designs and best practices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
24 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s43587-023-00367-4
References
Tchetgen, E. J. T. & VanderWeele, T. J. On causal inference in the presence of interference. Stat. Methods Med. Res. 21, 55–75 (2012).
Razzoli, M. et al. Social stress shortens lifespan in mice. Aging Cell 17, e12778 (2018).
Islam, M. et al. Effect of the resveratrol rice DJ526 on longevity. Nutrients 11, 1804 (2019).
Manski, C. F. Identification of treatment response with social interactions. Econom. J. 16, S1–S23 (2013).
Hong, G. & Raudenbush, S. W. Evaluating kindergarten retention policy: a case study of causal inference for multilevel observational data. J. Am. Stat. Assoc. 101, 901–910 (2006).
Hudgens, M. G. & Halloran, M. E. Toward causal inference with interference. J. Am. Stat. Assoc. 103, 832–842 (2008).
Kerr, J. et al. Cluster randomized controlled trial of a multilevel physical activity intervention for older adults. Int. J. Behav. Nutr. Phys. Act. 15, 32 (2018).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
National Institute on Aging. Strategic directions for research, 2020–2025. https://www.nia.nih.gov/ (2020).
Lucanic, M. et al. Impact of genetic background and experimental reproducibility on identifying chemical compounds with robust longevity effects. Nat. Commun. 8, 14256 (2017).
Bansal, A., Zhu, L. J., Yen, K. & Tissenbaum, H. A. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl Acad. Sci. USA 112, E277–E286 (2015).
Ayyadevara, S., Alla, R., Thaden, J. J. & Shmookler Reis, R. J. Remarkable longevity and stress resistance of nematode PI3K‐null mutants. Aging Cell 7, 13–22 (2008).
Hoffman, J. M., Dudeck, S. K., Patterson, H. K. & Austad, S. N. Sex, mating and repeatability of Drosophila melanogaster longevity. R. Soc. Open Sci. 8, 210273 (2021).
Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F. & Partridge, L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244 (1995).
Prowse, N. & Partridge, L. The effects of reproduction on longevity and fertility in male Drosophila melanogaster. J. Insect Physiol. 43, 501–512 (1997).
Yamamoto, R., Palmer, M., Koski, H., Curtis-Joseph, N. & Tatar, M. Aging modulated by the Drosophila insulin receptor through distinct structure-defined mechanisms. Genetics 217, iyaa037 (2021).
Paigen, B. et al. Physiological effects of housing density on C57BL/6J mice over a 9-month period. J. Anim. Sci. 90, 5182–5192 (2012).
Miller, R. A. et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell 6, 565–575 (2007).
Overton, J. M. & Williams, T. D. Behavioral and physiologic responses to caloric restriction in mice. Physiol. Behav. 81, 749–754 (2004).
Rikke, B. A. et al. Strain variation in the response of body temperature to dietary restriction. Mechanisms Ageing Dev. 124, 663–678 (2003).
Speakman, J. R. & Keijer, J. Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans. Mol. Metab. 2, 5–9 (2012).
Ikeno, Y. et al. Housing density does not influence the longevity effect of calorie restriction. J. Gerontol. A Biol. Sci. Med. Sci. 60, 1510–1517 (2005).
Smith, D. L. Jr., Yang, Y., Hu, H. H., Zhai, G. & Nagy, T. R. Measurement of interscapular brown adipose tissue of mice in differentially housed temperatures by chemical-shift-encoded water-fat MRI. J. Magn. Reson. Imaging 38, 1425–1433 (2013).
Koisumi, A. et al. A tumor preventive effect of dietary restriction is antagonized by a high housing temperature through deprivation of torpor. Mechanisms Ageing Dev. 92, 67–82 (1996).
Lipman, R. D., Gaillard, E. T., Harrison, D. E. & Bronson, R. T. Husbandry factors and the prevalence of age-related amyloidosis in mice. Lab. Anim. Sci. 43, 439–444 (1993).
Nagy, T. R., Krzywanski, D., Li, J., Meleth, S. & Desmond, R. Effect of group vs. single housing on phenotypic variance in C57BL/6J mice. Obes. Res. 10, 412–415 (2002).
Asch, S. E. In Groups, Leadership and Men: Research in Human Relations (ed. H. Guetzkow) 177–190 (Carnegie Press, 1951).
Pasupathi, M. Age differences in response to conformity pressure for emotional and nonemotional material. Psychol. Aging 14, 170–174 (1999).
Snyder-Mackler, N. et al. Social determinants of health and survival in humans and other animals. Science 368, eaax9553 (2020).
Epel, E. S. & Lithgow, G. J. Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 69, S10–S16 (2014).
Egami, N. Identification of causal diffusion effects under structural stationarity. Preprint at https://doi.org/10.48550/arXiv.1810.07858 (2018).
Manski, C. F. Identification of endogenous social effects: the reflection problem. Rev. Econ. Stud. 60, 531–542 (1993).
Lemaitre, M. et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster‐randomized trial. J. Am. Geriatrics Soc. 57, 1580–1586 (2009).
Sandvik, R. K. et al. Impact of a stepwise protocol for treating pain on pain intensity in nursing home patients with dementia: a cluster randomized trial. Eur. J. Pain. 18, 1490–1500 (2014).
Teerenstra, S., Melis, R. J. F., Peer, P. G. M. & Borm, G. F. Pseudo cluster randomization dealt with selection bias and contamination in clinical trials. J. Clin. Epidemiol. 59, 381–386 (2006).
Vu, T., Harris, A., Duncan, G. & Sussman, G. Cost-effectiveness of multidisciplinary wound care in nursing homes: a pseudo-randomized pragmatic cluster trial. Fam. Pract. 24, 372–379 (2007).
Beauchamp, M. R. et al. Group-based physical activity for older adults (GOAL) randomized controlled trial: exercise adherence outcomes. Health Psychol. 37, 451–461 (2018).
Haas, M. C. et al. Calorie restriction in overweight seniors: response of older adults to a dieting study: the CROSSROADS randomized controlled clinical trial. J. Nutr. Gerontol. Geriatrics 33, 376–400 (2014).
Tong, G. et al. Impact of complex, partially nested clustering in a three-arm individually randomized group treatment trial: a case study with the wHOPE trial. Clin. Trials 19, 3–13 (2021).
Fine, P., Eames, K. & Heymann, D. L. ‘Herd Immunity’: a rough guide. Clin. Infect. Dis. 52, 911–916 (2011).
Spatola, C. A. et al. The ACTonHEART study: rationale and design of a randomized controlled clinical trial comparing a brief intervention based on Acceptance and Commitment Therapy to usual secondary prevention care of coronary heart disease. Health Qual. Life Outcomes 12, 22 (2014).
Ogburn, E. L. Challenges to estimating contagion effects from observational data. In Complex Spreading Phenomena in Social Systems (eds Lehmann, S. & Ahn, Y.-Y.) 47–64 (Springer, 2017).
Caselli, G. et al. Family clustering in Sardinian longevity: a genealogical approach. Exp. Gerontol. 41, 727–736 (2006).
Atzmon, G. et al. Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl Acad. Sci. USA 107, 1710–1717 (2009).
Rasmussen, S. H. et al. Improved cardiovascular profile in Danish centenarians? A comparative study of two birth cohorts born 20 years apart. Eur. Geriatr. Med. 13, 977–986 (2022).
Poulain, M., Chambre, D. & Pes, G. M. Centenarians exposed to the Spanish flu in their early life better survived to COVID-19. Aging 13, 21855–21865 (2021).
Basse, G., Ding, P., Feller, A. & Toulis, P. Randomization tests for peer effects in group formation experiments. Preprint at https://arxiv.org/abs/1904.02308 (2019).
Pavela, G. et al. Packet randomized experiments for eliminating classes of confounders. Eur. J. Clin. Invest. 45, 45–55 (2015).
Vazquez-Bare, G. Identification and estimation of spillover effects in randomized experiments. J. Econometrics, https://doi.org/10.1016/j.jeconom.2021.10.014 (2022)
Sacerdote, B. Experimental and quasi-experimental analysis of peer effects: two steps forward. Annu. Rev. Econ. 6, 253–272 (2014).
Gadbury, G., Coffey, C. & Allison, D. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes. Rev. 4, 175–184 (2003).
Escoffery, C. et al. Internet use for health information among college students. J. Am. Coll. Health 53, 183–188 (2005).
Noonan, D. & Simmons, L. A. Navigating nonessential research trials during COVID19: the push we needed for using digital technology to increase access for rural participants? J. Rural Health. 37, 185–187 (2021).
Charness, N. & Boot, W. R. A grand challenge for psychology: reducing the age-related digital divide. Curr. Dir. Psychol. Sci. 31, 187–193 (2022).
Newell, D. J. Intention-to-treat analysis: implications for quantitative and qualitative research. Int. J. Epidemiol. 21, 837–841 (1992).
Taguchi, A., Wartschow, L. M. & White, M. F. Brain IRS2 signaling coordinates lifespan and nutrient homeostasis. Science 317, 369–372 (2007).
Kernan, W. N., Viscoli, C. M., Makuch, R. W., Brass, L. M. & Horwitz, R. I. Stratified randomization for clinical trials. J. Clin. Epidemiol. 52, 19–26 (1999).
Lachin, J. M. Biostatistical Methods: the Assessment of Relative Risks. Vol. 509 (John Wiley & Sons, 2009).
Koch, G. G., Amara, I. A., Davis, G. W. & Gillings, D. B. A review of some statistical methods for covariance analysis of categorical data. Biometrics 38, 563–595 (1982).
Wang, R., Lagakos, S. W., Ware, J. H., Hunter, D. J. & Drazen, J. M. Statistics in medicine–reporting of subgroup analyses in clinical trials. N. Engl. J. Med. 357, 2189–2194 (2007).
Downie, L. E. et al. Appraising the quality of systematic reviews for age-related macular degeneration interventions: a systematic review. JAMA Ophthalmol. 136, 1051–1061 (2018).
Kalache, A. et al. Nutrition interventions for healthy ageing across the lifespan: a conference report. Eur. J. Nutr. 58, 1–11 (2019).
Montgomery, J. M., Nyhan, B. & Torres, M. How conditioning on posttreatment variables can ruin your experiment and what to do about it. Am. J. Political Sci. 62, 760–775 (2018).
Robins, J. A graphical approach to the identification and estimation of causal parameters in mortality studies with sustained exposure periods. J. Chronic Dis. 40, 139S–161S (1987).
Almirall, D., Ten Have, T. & Murphy, S. A. Structural nested mean models for assessing time‐varying effect moderation. Biometrics 66, 131–139 (2010).
Westreich, D. et al. The parametric g‐formula to estimate the effect of highly active antiretroviral therapy on incident AIDS or death. Stat. Med. 31, 2000–2009 (2012).
List, E. O. et al. The effects of weight cycling on lifespan in male C57BL/6J mice. Int. J. Obes. 37, 1088–1094 (2013).
Murray, D. M. Design and Analysis of Group-Randomized Trials. Vol. 29 (Oxford University Press, 1998).
National Institutes of Health. Parallel Group- or Cluster-Randomized Trials (GRTs). https://researchmethodsresources.nih.gov/methods/grt (accessed 14 April 2021).
Campbell, M. K., Piaggio, G., Elbourne, D. R. & Altman, D. G. Consort 2010 statement: extension to cluster randomised trials. BMJ 345, e5661 (2012).
Brown, A. W. et al. Best (but oft-forgotten) practices: designing, analyzing, and reporting cluster randomized controlled trials. Am. J. Clin. Nutr. 102, 241–248 (2015).
Kimura, M. et al. Community-based intervention to improve dietary habits and promote physical activity among older adults: a cluster randomized trial. BMC Geriatr. 13, 8 (2013).
Bolzern, J., Mnyama, N., Bosanquet, K. & Torgerson, D. J. A review of cluster randomized trials found statistical evidence of selection bias. J. Clin. Epidemiol. 99, 106–112 (2018).
Campbell, M. K., Grimshaw, J. M. & Elbourne, D. R. Intracluster correlation coefficients in cluster randomized trials: empirical insights into how should they be reported. BMC Med. Res. Methodol. 4, 9 (2004).
Li, F., Tian, Z., Bobb, J. & Papadogeorgou, G. Clarifying selection bias in cluster randomized trials: estimands and estimation. Clin. Trials 19, 33–41 (2022).
Eldridge, S. M., Ashby, D. & Kerry, S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int. J. Epidemiol. 35, 1292–1300 (2006).
Kahan, B. C., Li, F., Copas, A. J. & Harhay, M. O. Estimands in cluster-randomized trials: choosing analyses that answer the right question. Int. J. Epidemiol. https://doi.org/10.1093/ije/dyac131 (2022).
Hitchings, M. D. T., Lipsitch, M., Wang, R. & Bellan, S. E. Competing effects of indirect protection and clustering on the power of cluster-randomized controlled vaccine trials. Am. J. Epidemiol. 187, 1763–1771 (2018).
Hemming, K., Taljaard, M., Moerbeek, M. & Forbes, A. Contamination: how much can an individually randomized trial tolerate. Stat. Med. 40, 3329–3351 (2021).
Jamshidi-Naeini, Y. et al. A practical decision tree to support editorial adjudication of submitted parallel cluster randomized controlled trials. Obesity 30, 565–570 (2022).
Borm, G. F., Melis, R. J. F., Teerenstra, S. & Peer, P. G. Pseudo cluster randomization: a treatment allocation method to minimize contamination and selection bias. Stat. Med. 24, 3535–3547 (2005).
Melis, R. J. F., Teerenstra, S., Olde Rikkert, M. G. M. & Borm, G. F. Pseudo cluster randomization: balancing the disadvantages of cluster and individual randomization. Eval. Health Prof. 34, 151–163 (2010).
Pence, B. W. et al. Balancing contamination and referral bias in a randomized clinical trial: an application of pseudo-cluster randomization. Am. J. Epidemiol. 182, 1039–1046 (2015).
National Institutes of Health. Individually Randomized Group-Treatment (IRGT) Trials. https://researchmethodsresources.nih.gov/methods/irgt (accessed 1 July 2022).
Candlish, J. et al. Appropriate statistical methods for analysing partially nested randomised controlled trials with continuous outcomes: a simulation study. BMC Med. Res. Method. 18, 105 (2018).
Ravussin, E. et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of healthspan and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1097–1104 (2015).
Andridge, R. R., Shoben, A. B., Muller, K. E. & Murray, D. M. Analytic methods for individually randomized group treatment trials and group-randomized trials when subjects belong to multiple groups. Stat. Med. 33, 2178–2190 (2014).
Halloran, M. E. & Hudgens, M. G. Dependent happenings: a recent methodological review. Curr. Epidemiol. Rep. 3, 297–305 (2016).
Philipson, T. External treatment effects and program implementation bias. NBER working paper no. T0250 https://www.nber.org/papers/t0250 (2000).
Ali, M. et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet 366, 44–49 (2005).
Basse, G. & Feller, A. Analyzing two-stage experiments in the presence of interference. J. Am. Stat. Assoc. 113, 41–55 (2018).
George, B. J. et al. Randomization to randomization probability: estimating treatment effects under actual conditions of use. Psychol. Methods 23, 337–350 (2018).
Chow, S. -C. & Liu, J. -p. Design and Analysis of Clinical Trials: Concepts and Methodologies. Vol. 507 (John Wiley & Sons, 2008).
Klar, N. & Donner, A. Design effects. Wiley StatsRef: Statistics Reference Online (2014).
Plewis, I. & Hurry, J. A multilevel perspective on the design and analysis of intervention studies. Educational Res. Eval. 4, 13–26 (1998).
Bloom, H. S. Randomizing groups to evaluate place-based programs. In Learning More from Social Experiments: Evolving Analytic Approaches (ed. Bloom, H. S.) 115–172 (Russell Sage Foundation, 2005).
Shadish, W. R., Cook, T. D. & Campbell, D. T. Experimental and Quasi-experimental Designs for Generalized Causal Inference (Houghton Mifflin, 2002).
Rhoads, C. H. The implications of ‘contamination’ for experimental design in education. J. Educ. Behav. Stat. 36, 76–104 (2011).
National Institutes of Health. Research Methods Resources: Group- or Cluster-Randomized Trials (GRTs). https://researchmethodsresources.nih.gov/methods/grt (accessed 1 July 2022).
Rubin, D. B. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat. Med. 26, 20–36 (2007).
Cox, D. R. Planning of Experiments (Wiley, 1958).
Neyman, J. & Iwaszkiewicz, K. Statistical problems in agricultural experimentation. Suppl. J. R. Stat. Soc. 2, 107–180 (1935).
Rubin, D. B. Randomization analysis of experimental data: the Fisher randomization test comment. J. Am. Stat. Assoc. 75, 591–593 (1980).
Cohen, M. S. et al. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA 326, 46–55 (2021).
Allee, W. C. Co-operation among animals. Am. J. Sociol. 37, 386–398 (1931).
Balthazart, J. et al. Molecular and Cellular Basis of Social Behavior in Vertebrates. Vol. 3 (Springer Science & Business Media, 2012).
Ludewig, A. H. et al. Larval crowding accelerates C. elegans development and reduces lifespan. PLoS Genet. 13, e1006717 (2017).
Carey, I. M. et al. Increased risk of acute cardiovascular events after partner bereavement: a matched cohort study. JAMA Intern. Med. 174, 598–605 (2014).
Racine, E., Troyer, J. L., Warren-Findlow, J. & McAuley, W. J. The effect of medical nutrition therapy on changes in dietary knowledge and DASH diet adherence in older adults with cardiovascular disease. J. Nutr. Health Aging 15, 868–876 (2011).
Acknowledgements
We thank N. Baidwan for contributions to an early version of the paper. This work was supported in part by the National Institute on Aging (grants P30 AG050886; U24 AG056053, K01 AG072615), the Gordon and Betty Moore Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases (grant P30 DK056336).
Author information
Authors and Affiliations
Contributions
D.B.A. conceived the original idea. D.E.C. managed and coordinated contributions from all co-authors. All co-authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.X. is currently an employee and shareholder of Atara Biotherapeutics at submission. D.B.A. holds equity in one company (Big Sky) and he and his institutions (Indiana University and the Indiana University Foundation) have received grants, contracts, in-kind donations and consulting fees from numerous governmental agencies, non-profit organizations and for-profit organizations including litigators and dietary supplement, food, pharmaceutical, medical device and publishing companies; however, not funded nor are directly relevant to the topic herein. All other authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chusyd, D.E., Austad, S.N., Dickinson, S.L. et al. Randomization, design and analysis for interdependency in aging research: no person or mouse is an island. Nat Aging 2, 1101–1111 (2022). https://doi.org/10.1038/s43587-022-00333-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-022-00333-6
This article is cited by
-
A brief guide to statistical analysis of grouped data in preclinical research
Nature Metabolism (2025)
-
The impact of co-housing on murine aging studies
GeroScience (2025)