Abstract
Background
Acute Kidney Injury (AKI) can adversely affect multiple organ systems, including the heart, brain, and immune system. Stage 1 AKI (AKI-1), although mild in clinical presentation, constitutes a substantial subset of AKI patients with heterogeneous outcomes, warranting further investigation into its subphenotypes.
Methods
We performed clustering analysis on seven-day serum creatinine (SCr) trajectories preceding AKI-1 onset in 53,565 AKI-1 patients (aged 18–89 years; 55.57% male) across eight academic hospitals. Each AKI-1 patient was matched to a non-AKI counterpart to evaluate how different AKI-1 subphenotypes influence clinical indicators and outcomes.
Results
Three distinct AKI-1 subphenotypes are identified. Patients in Subphenotype C (n = 5,378; 10.0%) exhibit a higher proportion of abnormal values across clinical indicators compared to those in Subphenotypes A (n = 27,049; 50.5%) and B (n = 21,138; 39.5%). Subphenotype C is associated with significantly higher odds ratios (ORs) for in-hospital, 30-day, and one-year all-cause mortality relative to Subphenotypes A and B. Conversely, Subphenotype B exhibits a higher susceptibility to developing chronic kidney disease (CKD) within one year after discharge following AKI-1, compared to both Subphenotypes A and C, after adjustment for baseline SCr levels. All AKI-1 subphenotypes are associated with significantly elevated risks of all-cause mortality and the need for dialysis or renal replacement therapy (RRT) compared to their respective non-AKI counterparts.
Conclusions
This study reveals substantial heterogeneity in clinical indicators and outcomes within AKI-1. Future research focusing on these subphenotypes may pave the way for more personalized and targeted interventions for patients with AKI-1.
Plain language summary
Stage 1 acute kidney injury (AKI-1) is the mildest form of sudden kidney damage, usually identified by a small rise in a blood waste marker called creatinine or a slight drop in urine output. Although AKI-1 may seem mild at first, it can still lead to serious consequences. This study investigated whether there are subtypes of AKI-1 that carry different health risks. Using hospital data from 53,565 patients, we grouped patients based on how their serum creatinine (kidney function biomarker) change over time, and found three distinct subtypes of AKI-1, each linked to different health outcomes. One subtype had a significantly higher risk of death, while another subtype was more likely to develop chronic kidney disease (CKD) after hospital discharge. These findings could help doctors provide more personalized care to patients with AKI-1.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI), characterized by a sudden decline in kidney function, is a global health challenge affecting diverse populations. AKI has been reported in 20.0–31.7% of patients across various levels of in-hospital care, with an associated mortality rate of ~23.0%1. While AKI primarily affects the kidneys, it can also cause complications across multiple organ systems, including the cardiovascular, respiratory, and nervous systems2,3,4,5, leading to prolonged hospital stays, persistent kidney dysfunction, and reduced survival6,7,8,9,10.
The Kidney Disease: Improving Global Outcomes (KDIGO) guideline categorizes AKI severity into three stages—1, 2, and 3—based on serum creatinine (SCr) levels and urine output11. AKI-1 represents the mildest form of the severity spectrum of AKI and accounts for a large proportion of AKI cases. According to the ASSESS-AKI study, nearly 80% of patients with AKI either had AKI-1 or initially presented with AKI-1 before any progression12. Despite its mild presentation, AKI-1 outcomes range from rapid renal recovery to prolonged kidney dysfunction13,14,15,16,17. For instance, in a cohort of AKI patients where 74% had AKI-1, 62% had a resolving pattern but still faced a two-fold higher risk of long-term major adverse kidney events (MAKE)17. AKI-1 is also associated with higher mortality (independent of comorbidities) and an increased risk of chronic kidney disease (CKD)16,18,19. Further stratification of AKI-1 patients into subcategories (AKI-1a and AKI-1b) based on SCr levels has revealed significant differences in outcomes, such as length of hospital stay and mortality20, highlighting the heterogeneous nature of AKI-1.
The variability in AKI-1 outcomes is influenced by factors such as renal function differences across populations21, varying sensitivity to changes in SCr16,22, variable rates of progression to advanced stages23,24,25, and complicating factors in patients with preexisting CKD26,27,28. Studies have shown that even small increases in SCr are associated with short-term adverse clinical outcomes, emphasizing the need for closer monitoring of AKI-1 patients9,29,30,31,32.
Despite the substantial prevalence and notable heterogeneity of AKI-1, many studies treat AKI as a single condition, without distinguishing patients who present with only AKI-112,32,33,34,35,36,37. The inclusion of patients who progress to more severe stages of AKI may introduce confounding factors, making it more difficult to isolate and characterize the heterogeneous clinical features and outcomes associated with AKI-1. Consequently, the number of studies specifically focused on subphenotyping AKI-1 to elucidate its early presentation and prognostic significance remains limited20. Additionally, much of the existing literature focuses on intensive care unit (ICU) patients, overlooking the substantial number of AKI cases occurring outside the ICU, many of which are AKI-133,34,36,38. Furthermore, many studies are single-center studies that rely on biomarkers rarely measured in routine clinical practice, making their findings less applicable to broader populations and more difficult to incorporate into care guidelines12,33,34,39. These research gaps hinder the development of personalized care for AKI-1 patients.
In this study, we aim to identify and characterize subphenotypes of AKI-1 patients using consensus k-means clustering of seven-day SCr trajectories preceding AKI onset. The study cohort includes 53,565 AKI-1 patients from eight academic hospitals across seven US states, all of whom experienced AKI-1 during hospitalization without progressing to more severe stages (AKI-2 or AKI-3). We also match each AKI-1 patient with a clinically similar non-AKI patient based on demographics, baseline SCr, and comorbidities to evaluate how the presence of each AKI-1 subphenotype influences clinical indicators and outcomes. Both AKI-1 patients and their non-AKI counterparts are characterized using demographics, comorbidity profiles, and laboratory test results. Additionally, we analyze four adverse clinical outcomes associated with each subphenotype: acute kidney disease (AKD), all-cause mortality, the need for dialysis or renal replacement therapy (RRT), and the incidence of chronic kidney disease (CKD). These outcomes are selected to capture both short-term and long-term renal and survival trajectories following AKI-1.
Our findings reveal significant heterogeneity among the three identified AKI-1 subphenotypes. Overall, Subphenotype A represents the mildest form of AKI-1. In contrast, Subphenotype C is associated with greater abnormalities in clinical indicators and exhibits the highest odds ratio (OR) for all-cause mortality compared to the other subphenotypes. Subphenotype B represents an intermediate profile and is characterized by a greater susceptibility to CKD incidence after adjustment for baseline SCr levels.
Methods
AKI-1 and baseline SCr definition
The study workflow is outlined in Fig. 1. Following the AKI clinical guideline established by KDIGO11, we categorized AKI severity based on the SCr-based criteria. AKI-1 was defined by a 1.5-fold increase in baseline SCr within 7 days or an increase in SCr by 0.3 mg/dL within 48 h. Since there is no standard definition for estimating baseline SCr, the baseline SCr level was determined by a three-step approach described in Supplementary Fig. 1, where the SCr measurements documented 1 week prior to admission had the strongest level of evidence, followed by records from 365 days to seven days before admission, and then the baseline was inferred using the modification of diet in renal disease (MDRD) formula39.
This study comprises four main steps. First, we compute baseline SCr levels for each patient and identify cases of AKI-1. Second, we apply consensus k-means clustering to the SCr trajectories of AKI-1 patients to derive novel AKI-1 subphenotypes. Third, each AKI-1 patient is matched to a clinically similar non-AKI patient to form a control group. Finally, we perform a comprehensive analysis of clinical outcomes for both AKI-1 and non-AKI patients. SCr serum creatinine, AKI acute kidney injury, KDIGO kidney disease: improving global outcomes.
Study population
This retrospective study included adult patients who were hospitalized between February 1, 2009, and February 1, 2022, across eight academic hospitals in seven US states. The study population comprised individuals who either experienced an AKI-1 onset episode or did not during their hospital stay. Participating institutions included the University of Pittsburgh/University of Pittsburgh Medical Center (UPMC), University of Texas Health Science Center at San Antonio (UTHSCSA), University of Iowa (UIOWA), University of Texas Southwestern Medical Center (UTSW), Medical College of Wisconsin (MCW), University of Missouri Health Care (MUHC), University of Utah (UofU), and University of Kansas Medical Center (KUMC).
The detailed eligibility criteria for patient selection were as follows: (1) Exclusion of patients with preexisting end-stage renal disease (ESRD); (2) Exclusion of patients with a history of dialysis or RRT; (3) Exclusion of patients whose estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 or baseline SCr >3.5 mg/dL; (4) A minimum hospital stay of two days; (5) The encounter must involve an AKI-1 as the only AKI stage (i.e., no AKI stage progression during hospitalization); (6) At least one SCr measurement on each of the three days: 2 days before AKI-1 onset, 1 day before AKI-1 onset, and the day of AKI-1 onset; (7) No more than two missing SCr measurements within the seven-day observation window. (8) Only the earliest encounter of each patient was kept, making sure each patient enrolled in the study was unique. A graphical depiction of the cohort entry process is provided in Supplementary Fig. 2.
To minimize confounding variables, we devised a three-step matching framework to pair each AKI-1 patient with a non-AKI counterpart. The matching process was based on demographics, baseline SCr, and comorbidities, as detailed in Supplementary Data 1. Before the matching began, all AKI-1 patients’ encounters were excluded from the non-AKI candidate pool to prevent them from being matched with their own encounters that did not have an AKI onset. In step one, we applied rule-based matching based on demographics and baseline SCr levels to identify all eligible non-AKI candidates for each AKI-1 patient. These variables were matched first because they are closely associated with kidney function and influence all kidney-related clinical outcomes40.
In step two, each eligible non-AKI candidate was assigned a score based on their comorbidities. Different comorbidities were weighted according to their respective impact on kidney-related clinical outcomes2. In step three, the most suitable non-AKI counterpart was then selected based on these comorbidity-based score rankings for each AKI-1 patient. The matching was done without replacement. In this way, each AKI-1 patient was matched with a unique, similar non-AKI patient.
This results in a cohort of AKI-1 patients aged 18–89 years (55.57% male) and a matched cohort of non-AKI patients aged 18–90 years (55.57% male).
Data processing
We collected 7-day SCr measurements preceding AKI-1 onset. Notably, we did not distinguish between the sources of SCr measurements. Both pre-admission and post-admission SCr values were considered equally valid. Multiple measurements of SCr on the same day were averaged. We employed linear interpolation to impute data for days without SCr measurements, assuming constant values outside of the first and last measurements. This approach has been shown to be effective for handling short, unevenly sampled time series data41.
To characterize the identified AKI-1 subphenotypes and quantify their differences and similarities, we extracted the comorbidities of interest, family history, laboratory test results, mortalities, and post-hospitalization dialysis, RRT, and CKD diagnoses from the EHR data of the study population. For comorbidities of interest and family history, data collection covered records prior to the patient’s index hospitalization admission date. The medical codes used to extract comorbidities of interest and family history are presented in Supplementary Data 2. For laboratory test results, we collected results from a seven-day window before AKI-1 onset. For each non-AKI patient, we used the date of the last SCr measurement as the alternative to the AKI-1 onset date to collect laboratory test results. Variables with a missing rate > 30% were excluded. For the remaining variables, missing values were imputed using the Multivariate Imputation by Chained Equations (MICE) algorithm42. For mortalities, dialysis, RRT, and CKD diagnoses, data collection spanned 1 year after the patient’s discharge date from the index hospitalization.
Clustering
Rather than using the raw SCr trajectories as inputs for clustering, we derived four trajectory-based features. Based on our preliminary experiments, these features produced clearer cluster separation compared to using the raw SCr time series directly. The four features were defined as follows: (1) SCr level at AKI-1 onset, measuring the absolute kidney impairment level at that point; (2) change in SCr level from baseline SCr to AKI-1 onset, measuring the absolute reduction in kidney function; (3) change in SCr level from 48-hours prior to AKI-1 onset, measuring the short-period reduction in kidney function; and (4) the difference between the average SCr level of the first four days within the seven-day window and the baseline SCr level, measuring preexisting kidney impairment before the onset of AKI-1. Min-max normalization was performed for each feature so that each feature’s value is between 0 and 1, preventing any single feature from dominating the distance measure between SCr trajectories when performing clustering.
The objective of the clustering was to partition patients into a smaller number of groups or subphenotypes solely based on their derived trajectory features, without incorporating laboratory test results or subsequent clinical outcomes. We employed consensus k-means clustering, an advanced variant of the traditional Euclidean-distance-based k-means algorithm, which aligns with its statistical assumptions because the input trajectory features were outlier-free, normalized, low-dimensional, and independent. It involves running k-means multiple times with different initializations and aggregating the results to find a consensus clustering. We ran a total of 100 iterations on the four SCr trajectory features, sampling 80% of the patients and 3 features each time. This approach addresses the sensitivity of traditional k-means to initial conditions, ensuring more stable and reliable clustering results.
To ascertain the optimal number of clusters, we considered four metrics: the Silhouette score, the Bayesian information criterion, the Davies-Bouldin, and the Calinski-Harabasz index. Hierarchical clustering was further employed to assess clustering robustness. We evaluated the clustering robustness using a confusion matrix and visual checks of t-distributed stochastic neighbor embedding (t-SNE) plots43 to examine overlap between results from consensus k-means and hierarchical clustering. The optimal number of clusters was determined by cluster sizes, clustering quality, clear separation in the consensus-matrix heatmaps, and the structure of the dendrogram produced by hierarchical clustering.
Statistics and reproducibility
Once the optimal number of clusters was determined, AKI-1 patients were clustered accordingly. A matching non-AKI patient was then assigned to each AKI-1 patient in the corresponding group. For each AKI-1 subphenotype, comparisons were made against other subphenotypes and their non-AKI counterparts across demographics, comorbidities, laboratory test results, and short-term and long-term clinical outcomes.
We further estimated the odds ratios (ORs) of adverse clinical outcomes for AKI-1 subphenotypes and their non-AKI counterparts using logistic regression (LR) models. The outcomes evaluated included AKD, post-discharge 30-day and 1-year all-cause mortality, the need for dialysis or RRT within 1 year, and the incidence of CKD within 1 year. AKD was defined by the Acute Dialysis Quality Initiative as a condition wherein criteria for AKI-1 or greater persist ≥7 days after an exposure44. Accordingly, in this study, we defined AKD as a SCr level not returning to below 1.5 times the SCr baseline within 7 days after AKI-1 onset.
The AKI-1 subphenotype associated with more favorable outcomes was used as the reference group for comparisons among subphenotypes, while each AKI-1 subphenotype’s corresponding non-AKI group served as the reference for comparisons between AKI-1 and non-AKI individuals. Comparisons among the remaining AKI-1 subphenotypes (i.e., those not involving the designated AKI-1 reference group) were derived through contrasts of estimated coefficients within the same fitted model, rather than by refitting separate models. All LR models were fitted under four levels of adjustment: (1) Unadjusted; (2) Model 1: adjusted for age and sex; (3) Model 2: further adjusted for the most severe CKD stage, cardiovascular diseases, chronic liver diseases, and diabetes mellitus; (4) Model 3: further adjusted for baseline SCr levels. In addition to the LR models, we also employed Cox proportional hazards models to independently analyze risk. This parallel approach allowed us to verify the robustness of the conclusions derived from the LR analyses by assessing their consistency with the results from the Cox models.
Statistical comparisons of demographics, comorbidities, and laboratory test results were performed using the chi-squared test for categorical variables and the Mann–Whitney U-test for continuous variables. These statistical tests were conducted in a pairwise manner, i.e., between AKI-1 subphenotypes and between AKI-1 subphenotypes and the corresponding non-AKI counterparts. p < 0.05 was considered statistically significant. Variations in laboratory test results were summarized using a table reporting the percentage of patients with abnormalities for each laboratory test, alongside ranked plots of variables based on the mean standardized differences between pairs of subphenotypes.
Ethics statement
This study was determined by the institutional review boards of the University of Florida, the University of Pittsburgh Medical Center, and the University of Missouri as nonhuman subject research because it only involved the collection of existing and deidentified patient medical data. Data use agreements have been executed with both the Greater Plains Collaborative (GPC) and the University of Pittsburgh. Informed written consent was waived due to the retrospective nature of the study. As this study was approved under an exempt/nonhuman subjects research determination, no formal Institutional Review Board (IRB) protocol number was assigned.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Patients included in the study
We extracted deidentified EHR data from eight academic hospitals within the Greater Plains Collaborative (GPC) and PaTH clinical research networks, both part of PCORnet, the National Patient-Centered Outcomes Research Network. The dataset consists of 1,752,438 inpatient encounters that can be accurately labeled with a SCr baseline (see Supplementary Fig. 1, third branch), between February 1, 2009, and February 1, 2022, with lengths of hospitalization at least 2 days.
After applying the eligibility criteria for patient screening, the final study cohort consists of 53,565 unique AKI-1 patients, along with an equal number of matched patients without any AKI, resulting in a total of 107,130 unique patients. The results of AKI-1 patient screening from the eight academic hospitals are provided in Supplementary Data 3.
Subphenotype identification in AKI-1
Clustering metrics and the separation of consensus heatmaps suggest n = 3 to be the optimal cluster number (Supplementary Figs. 3, 4). Hierarchical clustering also confirms that three clusters are optimal. The overlap between the consensus k-means and hierarchical clustering results is 79.50, 99.94, and 72.07% for each cluster, respectively, indicating the robustness of the clustering (Supplementary Fig. 5 b).
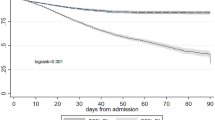
Three distinct AKI-1 subphenotypes are identified: Subphenotype A (n = 27,049; 50.5%), Subphenotype B (n = 21,138; 39.5%), and Subphenotype C (n = 5,378; 10.0%). The distribution of AKI-1 subphenotypes and non-AKI cohorts across the eight participating academic hospitals is shown in Supplementary Fig. 6. The 7-day SCr trajectories preceding and after AKI-1 onset for these subphenotypes are presented in Fig. 2, and are referred to as Classes A, B, and C herein.
Only the 7-day SCr trajectories before AKI-1 onset were used to derive clustering features. The solid lines represent the median SCr values for each class. The shaded areas indicate variability, where the upper and lower bounds correspond to the 99th and first percentiles of SCr, respectively. SCr serum creatinine. Sample sizes: Class A (n = 27,049); Class B (n = 21,138); Class C (n = 5378).
Although 48.61% of Class B trajectories overlap with Class A and 53.56% with Class C during the first 4 days of the 7-day window before AKI-1 onset, Class B is distinguished by a higher SCr level at onset and a larger absolute 48-h SCr change compared to Class A (both p < 0.001). Similarly, Class C shows higher values in both aspects compared to Class B (both p < 0.001).
Distinct clinical characteristics of AKI-1 subphenotypes
The three AKI-1 subphenotypes exhibit distinct clinical characteristics across demographics and comorbidities. Class A is the youngest group, with a significantly higher proportion of individuals aged 18–30 (all p < 0.001) and fewer individuals over 60 (all p < 0.001) compared to the other two subphenotypes. Classes B and C have a higher percentage of males, while Class A has an approximately equal male-to-female ratio. Additionally, Classes B and C have a higher percentage of Black individuals than Class A (all p < 0.001), whereas Class A has a higher percentage of White individuals compared to Class C (p < 0.001).
There is a significant increasing trend across Classes A, B, and C in the prevalence of CKD (stages 2 to 5), diabetes, cardiovascular conditions (circulatory system diseases, hypertension, ischemic heart diseases, and congestive heart failure), coagulopathies, stroke, and peripheral vascular diseases (all p < 0.001). There is no significant difference between Classes A and B in chronic liver disease (p > 0.05). However, Class A have a higher rate of metastatic cancer than Classes B and C (all p < 0.001).
When comparing each AKI-1 subphenotype (Classes A, B, and C) to their matched non-AKI counterparts (designated as Classes A’, B’, and C’), no significant differences are observed in many variables. Detailed patient characteristics of demographics, BMI, comorbidities, and medical histories, along with p values for subphenotype pair comparisons, are presented in Supplementary Data 4, 5.
Comparisons of laboratory test results and the corresponding p values are presented in Supplementary Data 6 and Supplementary Table 1. Overall, Class C shows the most significant laboratory test abnormalities before AKI-1 onset, particularly in blood urea nitrogen (BUN) levels and blood examinations such as erythrocytes, hematocrit, hemoglobin (Hb), mean corpuscular hemoglobin concentration (MCHC) and red blood cell distribution width (RDW) (all p < 0.001, Fig. 3b, c). Compared to its non-AKI counterpart, Class C shows significantly higher rates of abnormalities in all these tests, except for calcium, chloride, glucose, potassium and monocytes (all p > 0.05). Additionally, Classes A and B show greater increases in abnormalities in chloride, hematocrit, Hb, platelets, and erythrocytes compared to their non-AKI counterparts, more so than Class C.
The horizontal distance between two nodes on the same row reflects the magnitude of differences between two classes for each laboratory test. Panels (a–c) compare each AKI-1 subphenotype against the other two AKI-1 subphenotypes. Panels (d–f) compare each AKI-1 subphenotype against its non-AKI counterpart. MCHC mean corpuscular hemoglobin concentration, MCH mean corpuscular hemoglobin, MCV mean corpuscular volume, RDW red cell distribution width, BUN blood urea nitrogen. Sample sizes: Class A (n = 27,049); Class B (n = 21,138); Class C (n = 5,378); Class A’ (n = 27,049); Class B’ (n = 21,138); Class C’ (n = 5378).
Associations of AKI-1 subphenotypes with clinical outcomes
Using Class A as the reference, there are no significant differences in the OR of AKD between Class B and Class A after adjusting for demographics (Model 1) and major comorbidities (Model 2). A slightly elevated OR is observed in Class C compared to Class A (p < 0.05). However, in-hospital mortality rates are higher in Class C compared to Classes A and B, with rates of 6.72, 7.09, and 9.15% for Classes A, B, and C, respectively (all p < 0.001). All AKI-1 classes demonstrate significantly higher in-hospital mortality rates than their respective non-AKI counterparts (all p < 0.001).
After adjusting for demographics (Model 1), major comorbidities (Model 2), and baseline SCr (Model 3), Class C exhibits significantly higher ORs for both 30-day and 1-year all-cause mortality compared to Classes A and B (all p < 0.05). In contrast, no statistically significant differences in these all-cause mortality outcomes are found between Classes A and B (all p > 0.05). Regarding the need for dialysis or RRT within 1 year, Class C does not show a significantly higher OR compared to Classes A and B after adjustment for baseline SCr levels (p > 0.05).
Compared to Classes A and C, Class B has a significantly higher OR of 1-year CKD incidence after adjustment for baseline SCr levels (Model 3). All AKI-1 subphenotypes have increased risks for adverse outcomes compared to their non-AKI counterparts (all p < 0.001), except for 1-year CKD incidence, where Class C does not show a significantly higher risk, both before and after adjustment (p > 0.05). Details of these outcomes with four levels of adjustment are presented in Supplementary Data 7. The results of the Cox models are presented in Supplementary Data 8 and demonstrate trends highly consistent with those observed in the LR analyses, further supporting the robustness of our conclusions. Cumulative rates of all-cause mortality, need for dialysis or RRT, and CKD incidence are shown in Fig. 4.
a Cumulative 30-day all-cause mortality rates for the three AKI-1 subphenotypes and their non-AKI counterparts after discharge. The mini bar chart shows their in-hospital mortality rates. b Cumulative 1-year all-cause mortality rates for the subphenotypes. c Cumulative 1-year rates of dialysis or RRT for the subphenotypes. d Cumulative 1-year rates of CKD incidence among patients without preexisting CKD before the index hospitalization. CKD chronic kidney disease. Sample sizes: Class A (n = 27,049); Class B (n = 21,138); Class C (n = 5378); Class A’ (n = 27,049); Class B’ (n = 21,138); Class C’ (n = 5378).
Discussion
In this multi-center retrospective study, we used consensus k-means clustering to identify distinct AKI-1 subphenotypes based on four features of seven-day SCr trajectories before AKI-1 onset. Class C patients are older, have larger BMI, more comorbidities, and higher abnormality rates in laboratory test results. Conversely, Class A exhibits opposite characteristics, while Class B represents an intermediate subphenotype. Regarding clinical outcomes, Class C patients have higher all-cause mortality rates at 30-day and 1-year. Classes A and B do not have a statistically significant difference in these risks. After adjustment for baseline SCr levels, Class B exhibits a higher susceptibility to developing CKD within 1 year after discharge.
An interesting observation is the difference in sex ratio among subphenotypes. Classes B and C have significantly more males than females, whereas Class A has a nearly equal male-to-female ratio. This aligns with the current literature that men have a higher risk of AKI45. Studies suggest the possible reasons include the effects of gonadal hormones, sex differences in inflammation, and body temperature46.
All three AKI-1 subphenotypes differ from their non-AKI counterparts in several blood parameters, including hematocrit, hemoglobin, platelets, MCH, MCHC, MCV, RDW, and Erythrocytes, within the 7-day window before AKI-1 onset. Specifically, AKI-1 patients have higher RDW but lower levels in all other aforementioned blood test indicators compared to their non-AKI counterparts. This pattern is consistent across the three AKI-1 subphenotypes. Previous studies have demonstrated that in the general population, the incidence of AKI is much higher in the high RDW group than in the low RDW group: for each 1% increase in RDW, the incidence of AKI increased by 5.9%47. A higher RDW value in AKI patients is linked to shorter survival times and higher mortality rates48,49. Additionally, lower levels of hemoglobin and platelets have been observed in AKI patients compared to those without AKI49. Our findings suggest a potential connection between AKI and abnormalities in blood cell parameters, highlighting the need for further research to understand these relationships better.
We also observe that Class C exhibits a consistently higher cumulative incidence of CKD than Class B (Fig. 4d). However, after adjusting for baseline SCr levels, the LR model indicates a lower odds ratio for CKD incidence in Class C compared to Class B (Supplementary Data 7). A possible explanation is that patients in Class C has higher baseline SCr levels prior to hospitalization—despite not being formally diagnosed with CKD. Baseline SCr is a strong predictor of future CKD incidence, with a stronger association than that observed for other outcomes such as short- or long-term mortality50. Compared to Class B, Class C patients are older and have more severe preexisting comorbidities, including cardiovascular disease, hypertension, and diabetes mellitus. These conditions may have already caused structural damage to the kidneys, leading to diminished renal reserve and a gradual, ongoing decline in kidney function. As a result, the marginal impact of AKI-1 may be less pronounced in Class C. In contrast, kidney injury in Class B patients may be primarily attributable to the AKI-1 episode itself in the long term. Therefore, after adjusting for baseline SCr level, Class B patients appear to be more adversely affected by AKI-1 onset than those in Class C.
At the time of in-hospital AKI-1 onset, patients in Class C may be more sensitive to kidney injury, as structural damage to their kidneys was likely already present. As shown in Fig. 2, 53.56% of Class B patients have SCr trajectories that fall within the range observed in Class C during the first four days of the seven-day observation window prior to AKI-1 onset. Nevertheless, the “SCr level jump” at the time of AKI-1 onset in Class B are lower than those in Class C. This may indicate that Class C is less responsive to treatment and intervention compared to Class B.
Previous studies have also utilized SCr trajectories to subphenotype general AKI. For example, Andrew et al. used latent class mixed modeling (LCMM) to identify 12 AKI subphenotypes based on SCr trajectories following cardiac surgery51. Takkavatakarn et al. applied the same algorithm to identify 8 subphenotypes from the SCr trajectories of AKI patients with sepsis38. However, these studies have several limitations. First, their clinical applicability is limited, as the clustering was based solely on SCr trajectories without incorporating clinically interpretable timeseries features to distinguish between subphenotypes, thereby making it challenging to classify ambiguous cases. Second, they identified too many subphenotypes, some of which had very similar characteristics and could have been merged. In contrast, our study uses specific trajectory features to differentiate AKI-1 subphenotypes, allowing for clearer distinction between subphenotypes and addressing the issue of the relatively narrow range of pre-onset SCr values. Specifically, raw SCr data alone often cannot reveal distinct trajectory patterns due to this narrow range. Normalizing pre-onset SCr values using baseline SCr (i.e., [SCr - baseline SCr]/baseline SCr) would cluster most values around zero, complicating the identification of distinct patterns. By extracting clinically meaningful trajectory features, we enhance the ability to distinguish between SCr trajectories, effectively overcoming the limitations posed by the narrow range of pre-AKI-1 SCr values.
However, our study has some limitations. First, the current set of feature combinations may not be optimal for partitioning AKI-1. Exploring alternative combinations could unveil more unique subphenotypes with enhanced clinical significance. For instance, clustering based on characteristics like the number of peaks, variance, and average slope could be used to analyze the fluctuation degree of SCr trajectories, thereby revealing subphenotypes with greater renal function fluctuation. Second, our approach involves several subjective decisions, including (1) the length of SCr trajectory for clustering; (2) the metrics for evaluating cluster quality; (3) the optimal number of clusters; and (4) the time window for capturing laboratory test results. Changing these assumptions may impact the results. Better methods to subphenotype AKI-1 based on SCr trajectories should be developed in future studies. Third, due to measurement missingness, some biomarkers, such as liver function indicators and inflammatory markers, were discarded. Inclusion of these biomarkers could have provided a more comprehensive understanding of the characteristics of the three subphenotypes. Fourth, due to the lack of high-quality medication administration data, we did not include an analysis of nephrotoxic drugs, which may have influenced both the onset and outcomes of AKI-1. Future studies should address this gap by systematically evaluating the relationship between nephrotoxic drug exposure and the development and outcomes of AKI-1. At last, AKI-1 patients who progressed to AKI-2 or AKI-3 were excluded from this study to isolate and characterize the heterogeneity within AKI-1 without the confounding influence. However, this restriction may limit the ability to fully distinguish whether certain AKI-1 subphenotypes–such as Subphenotype C–represent the earliest manifestation of a trajectory toward more severe AKI stages. Future research should aim to compare the AKI-1 subphenotypes identified in this study with AKI-1 patients who experienced stage progression to elucidate the distinguishing risk factors between non-progressive and progressive AKI-1 trajectories.
In summary, our study identifies three distinct AKI-1 subphenotypes by clustering seven-day SCr trajectories preceding AKI-1 onset, revealing substantial heterogeneity and providing a foundation for improved clinical management of patients with AKI-1. Preliminary conclusions can be drawn from the SCr trajectory trends of the three subphenotypes (Fig. 2) to aid physicians in patient management. First, patients with pre-onset SCr fluctuating within 1.0-3.5 mg/dL and onset SCr >2.0 mg/dL generally belong to Class C. Physicians may need to pay closer attention to this group of patients, cautiously using or avoiding nephrotoxic drugs and monitoring SCr levels frequently to prevent AKI progression. Conversely, patients with pre-onset SCr <1.3 mg/dL and onset SCr <1.2 mg/dL generally belong to AKI-1 Class A and represent a lower risk subgroup.
Data availability
The clinical data used for training and validation in this study is not publicly available, and restrictions apply to its use. The deidentified multi-center EHR datasets in PCORnet Common Data Model may be made available by the Greater Plains Collaborative and PaTH clinical research networks, subjective to individual institution’s and network-wide data governance and ethical approvals. The source data for Figs. 2, 3, and 4 are provided in Supplementary Data 9, 10, and 11, respectively.
Code availability
A GitHub repository containing the code used in the analysis is available at https://github.com/GatorAIM/AKI-1_Subphenotyping52.
References
Susantitaphong, P. et al. World incidence of AKI: a meta-analysis. Clin. J. Am. Soc. Nephrol. 8, 1482–1493 (2013).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Prim. 7, 52 (2021).
Odutayo, A. et al. AKI and long-term risk for cardiovascular events and mortality. J. Am. Soc. Nephrol. 28, 377–387 (2017).
McNicholas, B. A. et al. Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: a secondary analysis of a multicenter observational study. Crit. Care Med. 47, 1216–1225 (2019).
Storm, C. et al. Impact of acute kidney injury on neurological outcome and long-term survival after cardiac arrest - a 10 year observational follow up. J. Crit. Care 47, 254–259 (2018).
Ruiz-Criado, J. et al. Long-term mortality among hospitalized non-ICU patients with acute kidney injury referred to nephrology. Nephron 131, 23–33 (2015).
Novo-Veleiro, I. et al. Acute kidney injury is linked to higher mortality in elderly hospitalized patients with non-valvular atrial fibrillation. Aging Clin. Exp. Res. 31, 455–461 (2019).
Lu, R. et al. Survey of acute kidney injury and related risk factors of mortality in hospitalized patients in a third-level urban hospital of Shanghai. Blood Purif. 38, 140–148 (2014).
Chertow, G. M., Burdick, E., Honour, M., Bonventre, J. V. & Bates, D. W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 16, 3365–3370 (2005).
Karagozian, R., Bhardwaj, G., Wakefield, D. B. & Verna, E. C. Acute kidney injury is associated with higher mortality and healthcare costs in hospitalized patients with cirrhosis. Ann. Hepatol. 18, 730–735 (2019).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pr. 120, c179–c184 (2012).
Bhatraju, P. K. et al. Integrated analysis of blood and urine biomarkers to identify acute kidney injury subphenotypes and associations with long-term outcomes. Am. J. Kidney Dis. 82, 311–321.e311 (2023).
Li, Q. et al. Missed diagnosis of acute kidney injury in older patients with invasive mechanical ventilation: a multicenter retrospective study. Aging Clin. Exp. Res. 34, 2887–2895 (2022).
Li, Q., Hu, P., Kang, H. & Zhou, F. Clinical characteristics and short-term outcomes of acute kidney injury missed diagnosis in older patients with severe COVID-19 in intensive care unit. J. Nutr. Health Aging 25, 492–500 (2021).
Griffin, B. R. et al. Stage 1 acute kidney injury is independently associated with infection following cardiac surgery. J. Thorac. Cardiovasc Surg. 161, 1346–1355.e1343 (2021).
Long, T. E. et al. Mild Stage 1 post-operative acute kidney injury: association with chronic kidney disease and long-term survival. Clin. Kidney J. 14, 237–244 (2021).
Mehta, R. L. Renal recovery after acute kidney injury and long-term outcomes: is time of the essence? JAMA Netw. Open 3, e202676 (2020).
Aitken, E. et al. Acute kidney injury: outcomes and quality of care. QJM 106, 323–332 (2013).
Thakar, C. V., Christianson, A., Freyberg, R., Almenoff, P. & Render, M. L. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit. Care Med. 37, 2552–2558 (2009).
Sparrow, H. G., Swan, J. T., Moore, L. W., Gaber, A. O. & Suki, W. N. Disparate outcomes observed within kidney disease: improving global outcomes (KDIGO) acute kidney injury stage 1. Kidney Int. 95, 905–913 (2019).
Jones, C. A. et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination survey. Am. J. Kidney Dis. 32, 992–999 (1998).
Testani, J. M., McCauley, B. D., Chen, J., Shumski, M. & Shannon, R. P. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology 116, 206–212 (2010).
Kister, T. S. et al. Acute kidney injury and its progression in hospitalized patients-results from a retrospective multicentre cohort study with a digital decision support system. PLoS ONE 16, e0254608 (2021).
Koyner, J. L. et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J. Am. Soc. Nephrol. 23, 905–914 (2012).
Mizuguchi, K. A. et al. Predicting kidney disease progression in patients with acute kidney injury after cardiac surgery. J. Thorac. Cardiovasc Surg. 155, 2455–2463.e2455 (2018).
Pannu, N. Bidirectional relationships between acute kidney injury and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 22, 351–356 (2013).
Chawla, L. S. & Kimmel, P. L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82, 516–524 (2012).
Heung, M. & Chawla, L. S. Acute kidney injury: gateway to chronic kidney disease. Nephron Clin. Pr. 127, 30–34 (2014).
Lassnigg, A. et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J. Am. Soc. Nephrol. 15, 1597–1605 (2004).
Ishani, A. et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch. Intern. Med. 171, 226–233 (2011).
Newsome, B. B. et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch. Intern. Med. 168, 609–616 (2008).
Bhatraju, P. K. et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit. Care 20, 372 (2016).
Bhatraju, P. K. et al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am. J. Respir. Crit. Care Med. 199, 863–872 (2019).
Chaudhary, K. et al. Utilization of deep learning for subphenotype identification in sepsis-associated acute kidney injury. Clin. J. Am. Soc. Nephrol. 15, 1557–1565 (2020).
Tan, Y. et al. Identifying acute kidney injury subphenotypes using an outcome-driven deep-learning approach. J. Biomed. Inf. 143, 104393 (2023).
Wiersema, R. et al. Two subphenotypes of septic acute kidney injury are associated with different 90-day mortality and renal recovery. Crit. Care 24, 150 (2020).
Vasquez-Rios, G. et al. Molecular and clinical signatures in acute kidney injury define distinct subphenotypes that associate with death, kidney, and cardiovascular events. Preprint at medRxiv https://doi.org/10.1101/2021.12.14.21267738 (2021).
Takkavatakarn, K. et al. Machine learning derived serum creatinine trajectories in acute kidney injury in critically ill patients with sepsis. Crit. Care 28, 156 (2024).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann. Intern. Med. 130, 461–470 (1999).
Wong, F. et al. Acute kidney injury in cirrhosis: baseline serum creatinine predicts patient outcomes. Am. J. Gastroenterol. 112, 1103–1110 (2017).
Nave Jr, G. K. et al. Clustering of pain dynamics in sickle cell disease from sparse, uneven samples. Preprint at arXiv:2108.13963 (2021).
Azur, M. J., Stuart, E. A., Frangakis, C. & Leaf, P. J. Multiple imputation by chained equations: what is it and how does it work? Int. J. Methods Psychiatr. Res. 20, 40–49 (2011).
Van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Chawla, L. S. et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 13, 241–257 (2017).
Neugarten, J. & Golestaneh, L. Female sex reduces the risk of hospital-associated acute kidney injury: a meta-analysis. BMC Nephrol. 19, 314 (2018).
Curtis, L. M. Sex and gender differences in AKI. Kidney360 5, 160–167 (2024).
Hu, Y., Liu, H., Fu, S., Wan, J. & Li, X. Red blood cell distribution width is an independent predictor of AKI and mortality in patients in the coronary care unit. Kidney Blood Press. Res. 42, 1193–1204 (2017).
Jia, L. et al. Red blood cell distribution width predicts long-term mortality in critically ill patients with acute kidney injury: a retrospective database study. Sci. Rep. 10, 4563 (2020).
Ramires, M. L. V. et al. Relation between red blood cell distribution width and acute kidney injury in patients with sepsis. Einstein 20, eAO6828 (2022).
Bouchard, J. Estimating baseline serum creatinine for assessing acute kidney injury: not a one size fits all approach. Kidney Int. Rep. 6, 562–564 (2021).
Andrew, B. Y. et al. Identification of trajectory-based acute kidney injury phenotypes among cardiac surgery patients. Ann. Thorac. Surg. 114, 2235–2243 (2022).
Li, D. deyili1997/AKI-1_Subphenotyping: v1.0.0. Zenodo https://doi.org/10.5281/zenodo.15700882 (2025).
Acknowledgements
This project was supported by grants R01DK116986 from NIDDK and 2444044 from NSF Smart and Connected Health. Data were extracted from the PaTH and GPC Clinical Research Networks. PaTH and GPC are network partners in PCORnet® which has been developed with funding from the Patient-Centered Outcomes Research Institute® (PCORI®). Their participation in PCORnet is funded through PCORI Award RI-PITT-01-PS1 (PaTH) and RI-MISSOURI-01-PS1 (GPC). YH is supported by the National Natural Science Foundation of China (Grant 72371116) and Major Research Plan of the National Natural Science Foundation of China (Key Program, Grant 91746204).
Author information
Authors and Affiliations
Contributions
M.L. initiated the project and the collaboration. M.L., D.L. and H.Y.C. designed the overall study. D.L. extracted the study cohort, cleaned up the data and performed all experiments. A.S.L.Y., J.A.K. and D.Y.F. contributed their clinical expertise in analyzing results. M.L. and D.L. wrote the paper with critical revision advice provided by A.S.L.Y., J.A.K., D.Y.F., E.A.C., L.C., S.C., J.K., K.M.M., A.M., B.T., L.R.W., M.S. and Y.H.
Corresponding author
Ethics declarations
Competing interests
J.A.K. discloses paid consulting from BioMérieux, AstraZeneca, Bayer, Chugai Pharma, Mitsubishi Tanabe, and Novartis, and is a full-time employee of Spectral Medical, all unrelated to the present work. K.M.M. has led and participated in research studies funded by Pfizer, Amgen, and Eli Lilly, all unrelated to the present work. A.S.L.Y. has served as consultant or on advisory boards for Regulus, Calico, Janssen and Sarepta, none of which are related to the current work. The remaining authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Zhongyu Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, D., Chan, H.Y., Yu, A.S.L. et al. Clustering analysis of multi-site electronic health records reveals distinct subphenotypes in stage-1 acute kidney injury. Commun Med 5, 274 (2025). https://doi.org/10.1038/s43856-025-00993-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-00993-6