Abstract
Background
The benefits of mRNA-based platforms, such as rapid response and simplified manufacturing, may be overshadowed by lack of durable protective immunity compared to traditional vaccine technologies targeting certain pathogens. Self-replicating RNA has the potential to induce durable immune responses at lower doses than traditional mRNA. A recent Phase 1 clinical trial showed that a self-replicating RNA vaccine encoding rabies, RBI-4000, was able to show de novo immunogenicity at all doses tested, specifically 0.1, 1, and 10 micrograms in a prime-boost regimen or a single 10 microgram dose (NCT06048770).
Methods
Here, we report the secondary outcome of the Phase 1 study, durability of immune responses elicited by RBI-4000, as assessed by the presence of the rabies virus neutralizing antibody response, up to 8 months post immunization. We compare long term immunogenicity of RBI-4000 to a commercial comparator, an inactivated viral vaccine RabAvert, using several statistical models with a post-hoc analysis. The trial was performed at two sites in the United States enrolling 89 healthy volunteers aged 18-45.
Results
Individual rabies virus neutralizing antibody titers, above the benchmark seropositivity, were detected out to 8 months in all study cohorts. Statistical decay modeling showed that RBI-4000 induces rabies virus neutralizing antibodies with similar or improved durability compared to RabAvert.
Conclusions
We report the first durability data from a head-to-head study of an optimized self-replicating RNA vaccine for rabies that elicits sustained immune responses compared to a commercial comparator that uses a traditional vaccine technology.
Plain language summary
New vaccine types, like mRNA vaccines, can be made quickly and are helpful during outbreaks. But sometimes, they don’t give long-lasting protection, as seen with some COVID-19, flu, and common cold vaccines. We studied a new kind of vaccine called a self-replicating RNA (srRNA) vaccine, which may help the body build stronger and longer-lasting immunity. We tested an srRNA rabies vaccine, called RBI-4000, and compared it to a commonly used rabies vaccine called RabAvert. Over 8 months, we find that RBI-4000 worked as well or even better than RabAvert at keeping the immune response strong. This suggests that srRNA vaccines could be a useful new way to protect people from diseases for longer periods of time.
Similar content being viewed by others
Introduction
mRNA-based vaccines have appreciably changed the landscape of vaccine design with their success during the COVID-19 pandemic. Since their approval, the FDA has authorized eight viral vaccines, seven of which are non-RNA based1. One challenge limiting the wide adoption of mRNA technology for vaccines is the waning of immune responses over time. Longitudinal analysis of immune responses over the following four years has shown that antibody titers to the encoded SARS-CoV-2 Spike antigen in mRNA vaccines decline rapidly following primary vaccination (2 doses) before stabilizing2,3. Moreover, comparison of approved mRNA vaccines, SPIKEVAX (mRNA-1273) and COMIRNATY (BNT162b2) to Ad26.COV2.S, a viral vector approach, showed differential kinetics, where mRNA vaccines showed a more rapid decline in antibody titers, albeit based on a small sample set4. This limitation has now become apparent for additional targets, such as Respiratory syncytial virus (RSV). RSV vaccines, AREXVY and ABRYSVO are approved adjuvanted Fusion (F) protein subunit vaccines and were shown to elicit superior durability of immune responses compared to mRESVIA, the approved mRNA vaccine encoding F. The 2-season efficacy after a single dose was 50, 81 and 67–79% and for mRESVIA, ABRYSVO and AREXVY, respectively5,6,7,8. Thus, for two distinct viral antigens, SARS-CoV-2 Spike and RSV F, mRNA as a class may not be advantaged as a vaccine platform compared to traditional technologies for generation of long-lasting immunity.
In contrast, vaccines using self-replicating RNA (srRNA) technology, which share beneficial attributes with conventional mRNA, such as simplified manufacturing and rapid deployability, have shown to be superior in generation of sustained immunity. Recent studies have shown that antibody titers from SARS-CoV-2 srRNA vaccine KOSTAIVE (ARCT-154), approved in Japan and the EU, have better durability compared to mRNA (BNT162b2) in a head-to-head Phase 3 clinical trial9. In addition, srRNA SARS-CoV-2 vaccine candidate GRT-R910, showed 6 month durability of neutralizing antibody titers when administered as a boost to adenoviral primary vaccination series10, whereas antibody titers from mRNA vaccines (BNT162b2 and mRNA-1273) declined after just 3 months in a similar boost study11. Thus, srRNA vaccines, as a class, are differentiated from conventional mRNA in their ability to elicit more durable immune responses, likely based on extended antigen expression from this platform.
However, a direct head-to-head comparison of srRNA to a traditional (i.e., protein or whole virus) vaccine technology is needed to show the full promise of this platform as a transformative vaccine technology. To unequivocally demonstrate this, we evaluated immunogenicity of an optimized, next generation srRNA vaccine for rabies, RBI-4000, to a commercial inactivated viral vaccine RabAvert in a Phase 1 study (NCT06048770)12.
Rabies virus is endemic in over 150 countries causing 59,000 deaths annually due to its 99% mortality rate if left untreated13. As such, it has been designated an NIAID priority antigen14. Current rabies vaccines are effective for both pre- and post-exposure prophylaxis, however these suffer supply shortages due to complex manufacturing processes which increases the difficulties of administration in resource limited settings. Importantly, in contrast to influenza or SARS-CoV-2 where pre-existing immunity may confound vaccine responses, no previous exposure to rabies is expected, allowing for clear interpretation of vaccine-elicited immune responses in naïve individuals. Lastly, a standardized WHO immune metric exists to benchmark efficacy of rabies vaccines, where titers of rabies virus neutralizing antibodies (RVNA) at or above 0.5 IU/mL are determined to be proof of seroconversion in humans and protective based on animal challenge studies and provide a clear immunogenicity readout15,16,17 In this Phase I study, RBI-4000 was able to prime de novo RVNA responses above the defined indirect measure of protection, while demonstrating a favorable safety profile12.
We have since tracked the durability of these RVNA responses further for an eight month period. Here, we present findings showing that an optimized srRNA elicits durable RVNA responses with equal or superior durability to RabAvert, a traditional whole virus vaccine, using decay model analysis.
Methods
Clinical trial
RBI-4000-101 was a multicenter, open label, randomized, active control Phase I clinical trial (NCT06048770) to assess the safety and immunogenicity of RBI-4000. The study enrolled 89 healthy volunteers, age 18–45 years with no prior exposure or vaccination to rabies. Details of the trial, including demographics, safety, and an interim analysis of immunogenicity, have been previously published12. Participants were enrolled between September 1st 2023 and December 21st 2023. The study was terminated at the Sponsor’s request based on completion of study data analysis on July 31, 2024. The study was conducted in accordance with the protocol, applicable laws, and regulatory requirements, as well as International Council for Harmonization Good Clinical Practice guidelines, and the consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines. The protocol was approved by the central institutional review board (Pro00072358, Advarra, Inc., Columbia, MD, USA) prior to study initiation, and written informed consent was obtained from all participants before enrollment.
Immunogenicity analysis
Immunogenicity was assessed at predefined time points prior and post-immunization.
Sera were used to perform rapid fluorescent foci inhibition tests (RFFIT) to quantify RVNA titers using a validated assay by the Rabies Laboratory, Kansas State University. The lower limit of detection for this assay is <0.1 IU/mL.
The AIM assay was performed by stimulating PBMCs, collected at the indicated timepoints, with an overlapping peptide library spanning the length of the RABV-G protein (CellCarta, Montreal, Canada). DMSO was used as a negative control for mock stimulation. Samples with viability below 70% prior to stimulation (after thawing and rest) were excluded from the analysis. Testing was conducted in accordance with the applicable regulatory agencies as stated in the clinical protocol RBI-4000-101. Applicable portions of the Good Clinical Practices (GCP) and Good Clinical Laboratory Practices (GCLP) guidelines were followed. Data were acquired on an LSR Fortessa flow cytometer (BD biosciences) and analyzed using FlowJo (BD Biosciences). Antibodies used in this assay are shown in Supplementary Table 1.
Data collection and statistical analysis
RFFIT and AIM data were collected, analyzed and presented using GraphPad Prism, version 10.1.1. Statistical modeling for RVNA was performed by Stat One LLC (Morrisville, NC, USA). Any statistical comparisons are noted and called out in the figure legend and main text.
Decay rate modeling
We performed post-hoc, mixed model regression to evaluate the change in the geometric mean of the rabies titer response value (antibodies) in IU/mL (Stat One LLC, Morrisville, NC, USA). Each regression model evaluated log(antibody) values as a function of months from the starting date in the period, the dose cohort, the interaction term of month x cohort and with subject included as a random effect. For all models the LLOQ were included as the log of ½ the LLOQ (0.05). In total, eight models were created to evaluate change in slope of the antibody level. Four of the models evaluated the change from the group peak to some endpoint while the other four models evaluated the change from the individual subject peaks to some endpoint. The endpoints analyzed across the different models were start of stable decay, study Day 240, individual subject values below 0.1 IU/mL, and the half-life of the peak antibody level. The reported statistics for each model included the slope and intercept estimates by cohort, F-statistics for the main effects and day-cohort interaction, exponential values of the slopes and slope 95% CIs, and exponential values of the differences in RBI-4000 slopes relative to control. For the models evaluating half-life, the day of half-life occurrence was also approximated based on the slope of the model.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results and discussion
RBI-4000 primes rabies virus neutralizing antibody responses with distinct kinetics to an inactivated viral vaccine comparator
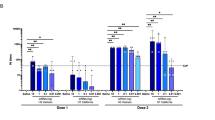
Longitudinal analysis of RVNA responses shows distinct kinetics over time between RabAvert, single dose, and two dose RBI-4000 cohorts, due to the differences in the immunization regimen and platform technologies. RVNA responses peak at three distinct time points, with maximal RabAvert-induced RVNA titers observed at Day 15 (7 days post-boost), followed by a sharp decline prior to entering stable decay, defined by a flattening of the RVNA titers, at Day 70 (Fig. 1a, Supplementary Data 1 Cohort 5). During stable decay, gradual decline in RVNA titers is observed until the end of study (Day 240). For RBI-4000 two-dose cohorts, a clear dose response is observed with a peak at Day 71 (14 days post-boost), while the single dose cohort peaked at Day 29 (Fig. 1a, Supplementary Data 1). Cohorts 3 and 4 that received the highest dose of RBI-4000 as a single or two-dose schedule, respectively, had a more gradual decline in RVNA titers and entered stable decay at Day 180 compared to lower dose cohorts. Importantly, across all cohorts, individuals with RVNA titers above the defined protective threshold were still observed at the end of study.
a Longitudinal measurements of serum geometric mean titers of RVNA as measured by RFFIT assay. Shaded area shows the peak of the response and subsequent decay from this peak. Individual values represented by dots. Dashed gray line indicates 0.5 IU/mL threshold. Number of participants at each time point are shown in Supplementary Table 2. b RVNA responses at six months per cohort. Frequency of subjects within each cohort that maintain detectable RVNA titers but below (light blue) or above (dark blue) the WHO indirect immune measure of protection. Frequency of subjects with no detectable RVNA shown in gray (Cohort 1 n = 17, Cohort 2 n = 18, Cohort 3 n = 18, Cohort 4 n = 17, Cohort 5 n = 12).
Evaluation of RVNA titers at Day 180 of the study showed a clear dose response with the majority of individuals with sustained RVNA titers in all cohorts (Fig. 1b). A two-dose regimen of RBI-4000 at 1 or 10 mcg results in 100% of individuals with detectable RVNA titers and 61 and 88% being at or above the 0.5 IU/mL threshold, respectively for Cohort 2 and 4. Similarly, 100% of individuals in Cohort 5 that received RabAvert also had detectable RVNA titers with 58% achieving this threshold. RVNA levels for Day 240 from an incomplete dataset are shown in Supplementary Fig 2. Cohort 4 shows 100% detection of RVNA at Day 240, 58% of which are above the 0.5 IU/ml threshold.
RBI-4000 primes rabies virus neutralizing antibody responses with equal or superior durability to an inactivated viral vaccine
To assess durability of RVNA responses, post-hoc analysis using eight separate decay analysis models were used to compare RBI-4000 cohorts to RabAvert (Supplementary Fig 1 for details on each model). Of these models, we selected to focus more in depth on Models 6 and 8, as the analysis utilized mathematically quantifiable endpoints of individual responses. Specifically, Model 6 analyzed the decay rate based on each individual participant in the cohort until the first individual reached a RVNA titer below the LLOQ (defined as 0.1 IU/mL). This model showed that RVNA responses, elicited by RBI-4000 in all cohorts, showed equal durability to RabAvert (Fig. 2a). Alternatively, Model 8, which determines the half-life of the RVNA response post-peak, durability of RVNA titers from RBI-4000 cohorts showed significantly lower decay rates, i.e., better durability, compared to RabAvert (Fig. 2b). Specifically, half-lives of RVNA responses in RBI-4000 administered cohorts were extended by at least 6-fold compared to those that received RabAvert (Fig. 2c). One limitation of this study is the different immunization schedules employed for RBI-4000 and RabAvert. RabAvert was administered as per its recommended schedule for pre-exposure prophylaxis and for use as an active comparator arm for this study. Although the outcome of administration of RabAvert in an extended interval is not known, it is likely that the RVNA peak would shift and affect the outcome of the decay analysis. Importantly, although the longer prime-boost immunization interval for RBI-4000 may favor the durability of immune responses for Cohorts 1, 2, and 4, durable RVNA responses were also observed for Cohort 3 where participants received a single administration of RBI-4000.
RVNA decay rate modeling based on either a Model 6 that measured individual subject peak RVNA titers until the first individual reaches lower limit of quantification (LLOQ) of the assay; or b Model 8 where individual RVNA titer peak to individual corresponding RVNA titer half-life. Slopes calculated based on these models are shown as a ratio compared to RabAvert for all RBI-4000 groups. Symbols show ratio and error bars show 95% CI for each group (CI ranges labeled next to each group). c Table shows half-life values for each cohort from the peak of the RVNA response. Number of participants at each time point are shown in Supplementary Table 2.
Additional analysis, such as slope and intercept values for each decay curves are provided in the Supplementary Information (Supplementary Table 3 and Supplementary Fig 3). Complex modeling of decay rate, such as multi-phase decay analysis, was not performed due to the small sample size.
RBI-4000 induces anti-rabies CD4 T helper cell responses
Quality and durability of vaccine-elicited antibody responses relies on engagement of the cellular arm of the immune system to potentiate B cell function. We have previously shown that RBI-4000 can elicit rabies-specific T cells in a dose-specific manner12. We further phenotyped the T cell response to assess the induction of CD4 T helper cells, specifically assessing activation-induced markers (AIM) CD69 and CD40L that are descriptive of antigen-primed T cells associated with potentiating of antibody responses by cognate B cells18,19,20 (Supplementary Fig 4A). PBMCs stimulated with rabies glycoprotein led to significant expansion of antigen-specific AIM+ CD4 T cells in individuals that received RBI-4000. Although some AIM+ CD4 T cells were also observed with RabAvert immunization, the levels were not different from resting (mock-treated) conditions and thus not deemed to be antigen-specific (Supplementary Fig 4B). These data show that RBI-4000 is able to induce antigen-specific CD4 T helper cells that can, in turn, bolster B cell function and antibody production.
Conclusion
Our results demonstrate for the first time that srRNA can elicit protective immunity with comparable durability to an approved traditional vaccine platform. This is in contrast to conventional mRNA technology where, in head-to-head clinical studies, inferior durability of humoral immunity is observed compared to protein subunit vaccines, suggesting that mRNA may be of limited use outside of the SARS-CoV-2 pandemic5. In more complex infectious diseases in which the virus uses periods of latency to evade immune detection, such as Epstein-Barr virus or cytomegalovirus, vaccine platforms will need sufficient antibody durability to combat viral reactivation. Similarly in the case of travel vaccination, durability needs to match existing approved vaccines before the benefits of replacing these existing vaccines with newer RNA technology become apparent. RBI-4000 shows equal or increased durability in RVNA titers to RabAvert with levels remaining above the defined indirect measure of protection for over six months. The advantages in manufacturing cost, speed and dose sparing of srRNA, compared to cell-based virus production, positions RBI-4000 for continued clinical development with the aim to replace existing rabies vaccines.
Data availability
The Clinical Study Protocol is provided with the Supplementary Information. Source Data is added as a supplementary file and was used to generate all figures related to RVNA including the statistical modeling (Supplementary Data 1). Clinical data will be available upon request to the corresponding author once the study is formally completed, and a Clinical Study Report is prepared.
References
Research, C. for B. E. and. Biological approvals by Year. (FDA, 2025).
Pegu, A. et al. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science 373, 1372–1377 (2021).
Srivastava, K. et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 57, 587–599 (2024).
Collier, A. Y. et al. Differential kinetics of immune responses elicited by covid-19 Vaccines. N. Engl. J. Med. 385, 2010–2012 (2021).
Linnane, C. Moderna’s stock falls after biotech’s RSV vaccine trails rivals after 18 months. MarketWatch 18, ae4e61b0 (2024).
Walsh, E. E. et al. RENOIR Trial — RSVpreF vaccine efficacy over two seasons. N. Engl. J. Med. 391, 1459–1460 (2024).
Ison, M. G. et al. Efficacy and safety of respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 78, 1732–1744 (2024).
CDC. ACIP Presentation Slides: June 26-28, 2024 Meeting. Advisory Committee on Immunization Practices (ACIP) https://www.cdc.gov/acip/meetings/presentation-slides-june-26-28-2024.html (ACIP, 2024).
Oda, Y. et al. Persistence of immune responses of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2. Lancet Infect. Dis. 24, 341–343 (2024).
Palmer, C. D. et al. GRT-R910: a self-amplifying mRNA SARS-CoV-2 vaccine boosts immunity for ≥6 months in previously-vaccinated older adults. Nat. Commun. 14, 3274 (2023).
Liu, X. et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: Three month analyses of the COV-BOOST trial. J. Infect. 84, 795–813 (2022).
Maine, C. J. et al. Safety and immunogenicity of an optimized self-replicating RNA platform for low dose or single dose vaccine applications: a randomized, open label Phase I study in healthy volunteers. Nat. Commun. 16, 456 (2025).
Hampson, K. et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 9, e0003709 (2015).
NIAID Biodefense Pathogens | NIAID: National Institute of Allergy and Infectious Diseases. https://www.niaid.nih.gov/research/niaid-biodefense-pathogens (NAID, 2024).
WHO Expert Consultation on Rabies: WHO TRS N°1012 Third report. https://www.who.int/publications/i/item/WHO-TRS-1012.
Moore, S. M. Challenges of rabies serology: defining context of interpretation. Viruses 13, 1516 (2021).
Rao, A. K. Use of a modified preexposure prophylaxis vaccination schedule to prevent human rabies: recommendations of the advisory committee on immunization practices — United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 71, 619–627 (2022).
Reiss, S. et al. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS ONE 12, e0186998 (2017).
Zhang, Z. et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 185, 2434–2451 (2022).
Pallikkuth, S., Williams, E., Pahwa, R., Hoffer, M. & Pahwa, S. Association of Flu specific and SARS-CoV-2 specific CD4 T cell responses in SARS-CoV-2 infected asymptomatic heath care workers. Vaccine 39, 6019–6024 (2021).
Acknowledgements
We thank Stat One LLC for statistical modeling and Halloran Consulting Group for trial oversight and management.
Author information
Authors and Affiliations
Contributions
Clinical trial design, execution and/or analysis: C.J.M., G.P., N.S.W, Z.G., P.A., B.E. and G.S. Production of clinical test article: S.J.M-S. and A.J.G. Project management: J.S. Manuscript writing: C.J.M. and P.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: C.J.M., S.J.M-S., G.P., N.S.W., A.J.G., Z.G., and P.A. are employees of Replicate Bioscience Inc. All other authors declare no competing interests. The sponsor was involved in all stages of the study including design, analysis and manuscript writing.
Peer review
Peer review information
Communications Medicine thanks Leo Visser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Maine, C.J., Picarda, G., Miyake-Stoner, S.J. et al. Durability of next-generation self-replicating RNA vaccine RBI-4000: a phase 1, randomized open label clinical trial. Commun Med 5, 392 (2025). https://doi.org/10.1038/s43856-025-01147-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-01147-4