Abstract
Background
Although air pollution has been recognized as a risk factor for chronic obstructive pulmonary disease (COPD), little is known regarding the role of air pollutant exposure, specifically particulate matter (PM), in the dynamic transitions among normal spirometry (lung function), pre-COPD status, and COPD status.
Methods
Pulmonary function observations from the UK Biobank cohort were categorized as normal spirometry, preserved ratio impaired spirometry (PRISm), defined as forced expiratory volume in one second (FEV1) < 80% predicted and FEV1/ forced vital capacity (FVC) > 0.70, or spirometry-defined COPD. Multi-state Markov models were used to characterize the association between air pollution exposure (PM2.5, PM coarse, PM10, NO2, and NOx) and transitional pathways among three COPD statuses.
Results
We include 33,217 participants (71,287 visits) and find that each interquartile range increase in exposure of PM2.5, NO2, and NOx is associated with a lower probability of reverting from PRISm to normal spirometry [HR (95% CI): 0.88 (0.81, 0.96) for PM2.5; 0.86 (0.79, 0.93) for NO2 and 0.89 (0.83, 0.96) for NOx]. Progression from PRISm to COPD shows PM10 exposure is positively associated with a significant risk increase [1.16 (1.02, 1.31)]. In a counterfactual scenario where we reduce the exposure level of PM2.5 from 9.92 μg/m3 to 5 μg/m3, the study participants could have been 4.42% less likely to progress from PRISm to COPD.
Conclusions
Findings reveal that higher levels of PM10 exposure are linked to an increased risk of PRISm progression, while exposure to PM2.5, NO2, and NOx is associated with a reduced likelihood of spirometry recovery to normal levels. These findings offer new insights into potential health benefits resulting from interventions targeting air pollution.
Plain language summary
Mild impairment in lung function, known as PRISm, can either progress to chronic lung disease such as COPD or return to normal. While air pollution is known to harm lung health, its impact on the transitional direction of the PRISm stage remains unclear. We studied more than 33,000 adults in the UK Biobank who had repeated lung function tests. We found that people exposed to higher levels of fine particles (PM2.5) and traffic-related pollutants (NO2 and NOx) were less likely to recover to normal lung function. In addition, greater exposure to PM10 increased the risk of PRISm progressing to COPD. These findings highlight the importance of reducing air pollution to protect people at risk from developing serious lung diseases in the future.
Similar content being viewed by others
Introduction
Preserved ratio impaired spirometry (PRISm) is characterized by a forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) ratio ≥ 0.7 with FEV1 less than 80% of the predicted value1. PRISm has been defined as a precursor condition of the chronic obstructive respiratory disease (COPD)2,3,4, which represents significant disease and financial burdens worldwide. Individuals with PRISm are found to have an elevated risk of progressing to COPD (“forward”); however, as an intermediate status and an unstable phase, PRISm can also revert back to normal spirometry (“backward”), or remain in the PRISm status5,6. These “forward” and “backward” transitions of PRISm shape the pathway of the aggravation or alleviation of airway obstruction. Pinpointing the factors influencing these transition processes is essential for creating prevention and intervention strategies for COPD7: in terms of identifying both potential interventions, and the time windows and targeted populations to intervene.
Air pollution is a well-established risk factor for COPD1, nevertheless, little is known about its role in the dynamic transitions of the pre-COPD states. While there has been evidence on the association between air pollutants (particulate matter with an aerodynamic diameter <2.5 µm (PM2.5) or <10 µm (PM10), and nitrogen dioxide (NO2), etc.) and the incidence, prevalence, admission and mortality of COPD8,9, no previous study have quantified or compared their associations with both the progression to and regression from PRISm. In terms of “forward” transitions, although ozone and NOx had been found to accelerate the progression of emphysema10,11, it remains unclear how air pollution contributes to the deterioration from normal spirometry to COPD via the intermediate PRISm stage. Conversely, for the “backward” transitions, evidence suggests that 15% to 50% of individuals with PRISm reverted to normal spirometry4,6, indicating the potential for lung function recovery. Yet, the role of air pollution in impeding this recovery process remains unknown. Estimating the effect of air pollution along the trajectory of lung function variation, not only deterioration but also recovery, can provide unique understandings on the potential pathway underlying the adverse respiratory effects of air pollutants, while quantifying the health benefit and political urgency of air pollution regulation.
Compelled by the clinical and public health needs, this study aims to investigate the association of air pollution with the dynamic transitions amongst the disease-free, pre-disease, and disease status of COPD. We find that exposure to higher levels of air pollutants is not only associated with increased risk of progressing from PRISm to COPD but also relates to reduced likelihood of recovery from pre-COPD condition to normal spirometry.
Methods
Study populations
The UK Biobank is a large prospective cohort study covering more than 500,000 UK residents aged 40 to 69 years old12. Participants were invited to 22 assessment centres located in Scotland, England and Wales for the initial assessment visit during 2006–2010, and information on sociodemographic characteristics, lifestyle, and health-related factors was collected. Since 2012, several repeated investigations (the first repeat assessment visit (2012–13), the imaging visit (2014+) and the first repeat imaging visit (2019+)) have been conducted.
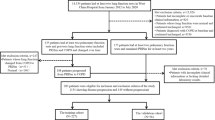
Since we are interested in the longitudinal transition of lung function, we only included individuals with repeated measurements of lung function (with 2 or more spirometry records). The criteria for eligible participant records in this study were: 1) had acceptable spirometry data; 2) had complete information for essential covariates (i.e., sex, age, BMI, smoking status, household income, education level); 3) had data on air pollutants investigated in this study (PM2.5, PM coarse, PM10, NO2, NOx).
The UK Biobank study was approved by the North West Multi-centre Research Ethics Committee (Ref: 19/NW/0644). Written informed consents were obtained from all participants.
Study outcomes
For each visit, participants were asked to complete a pre-bronchodilator lung function test, which recorded 2–3 blows using a spirometer (Pneumotrac 6800; Vitalograph, Buckingham, UK). The best measures of FEV1 and FVC from acceptable blows were used. The definition of “best measures” can be found in previous research13.
The outcomes of this study were the categorization of three developmental stages of COPD, e.g., normal spirometry (NS), PRISm and spirometry-defined COPD for the spirometry data of each participant visit. Normal spirometry was defined as having an FEV1 of 80% predicted or higher with a FEV1/FVC ratio of 0.70 or higher. PRISm was defined as a FEV1 of less than 80% predicted and a FEV1/FVC of 0.70 or higher. Predicted values of FEV1 were obtained using the Global Lung Function Initiative (GLI) 2012 lung function equations14. Spirometry-defined COPD was defined as a FEV1/FVC ratio of less than 0.7.
Covariates
In this study, demographic and behavioural style variables (i.e., sex, age, BMI, smoking status, history of doctor-diagnosed asthma, household income, education level, occupational exposure, and respiratory symptoms) were included as potential confounders4,7,13. Details and the recoding strategy of the adjusted variables were shown in Supplementary Table S1 and S2. For binary variables (doctor-diagnosed asthma, occupational exposure and respiratory symptoms) indicating the presence or absence of a condition, missing data were imputed as “absent”, while missing information on covariates with multiple levels was excluded from analysis without imputation.
Exposure assessment
Based on a land use regression (LUR) model developed by the ESCAPE study, annual-mean exposure of PM2.5, PM coarse, PM10, NO2, NOx in the year of 2010 were linked to the geocoded residential address of each participant in the UK Biobank study15,16. Briefly, Eeftens et al. developed the LUR model by using a supervised stepwise selection procedure and including GIS-derived predictor variables (e.g., traffic intensity, population, and land-use). The model has good performance for PM2.5, PM coarse, PM10, NO2 and NOx (median model R2 = 0.71, 0.68, 0.77, 0.82 and 0.78)16.
Statistics and reproducibility
Socio-demographic characteristics for the included participants were reported. Continuous and categorical variables were described as mean ± SD and counts. Analysis of variance (ANOVA) and chi-squared test were used to compare differences between groups for continuous and categorical variables, respectively. The correlation between air pollutant exposures was assessed by Spearman’s correlation coefficients.
To characterize the reversible transitions between the three developmental stages of COPD4,7,17,18, we used the multi-state Markov models to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the associations between per interquartile range (IQR) increase in air pollutants and the transitional probability among the stages. The three developmental statuses of COPD were corresponding to the states in the three-state Markov model, and we defined six transitional pathways among the states (three for disease progression and three for reversion), as shown in Fig. 1. As an extension to the commonly-used Cox proportional hazards model, the multi-state Markov model (MSMM) enables the characterization of more complex health state transitions with consideration of competing risks19. The MSMM has been applied in several epidemiological studies investigating the risk of air pollution exposure and transitions among disease stages19,20,21 and the methodology of MSMM has been elaborated in previous studies22,23.
For each pollutant, we constructed four MSMM models, in which we sequentially add more covariates to each model. This sequential adjustment method has been used in a number of epidemiological studies24,25,26. Due to the exploratory nature of our study, we included covariates with references to previous studies7,27 and with consideration of the data availability. In our study, we specified four models, and each model can be considered as a sensitivity analyses regarding covariate adjustment: Model 1 only included the air pollutant of interest; Model 2 additionally adjusted for sex, age, body mass index (BMI) and smoking status; Model 3 further included the disease history of asthma; Model 4 (the fully adjusted model) added family household income, education level, occupational exposure and respiratory symptoms. To assess potential multicollinearity among the adjusted covariates, we calculated the generalized variance inflation factor (GVIF) for each variable in the fully adjusted model. GVIF values were scaled to a comparable metric using the transformation \({{GVIF}}^{1/(2* {df})}\), where df denotes degrees of freedom28. The scaled values were interpreted using conventional VIF thresholds, with values > 10 indicating strong multicollinearity. All variables had scaled GVIF values below this threshold (Table S3), confirming minimal collinearity concerns.
To demonstrate the impact of air pollution on the longitudinal change among COPD developmental phases, transition probability was calculated in terms of Q using the Kolmogorov differential equations for a time-homogeneous process22. In this study, we predicted the 10-year cumulative probabilities of PRISm progression (from PRISm to COPD) and reversion (from PRISm to normal spirometry) for participants with PRISm using the fully adjusted model. We compared the predicted transitional probability under the real-world exposure levels and counterfactual scenarios, by setting the air pollution exposure level at the population average for the study sample (the real-world setting), and the World Health Organization Air Quality Guidelines (WHO-AQG) recommended levels for the included pollutants (PM2.5, PM10, and NO2) among the study participants (counterfactual setting with reduced exposure).
Stratification analyses were performed across different sexes (male and female) and BMI categories (obese: BMI ≥ 30 kg/m2; non-obese: BMI < 30 kg/m2). For sensitivity analysis, since smoking status can affect the transitional probabilities among different COPD phases and has possible interactive effects with air pollutants29,30, we repeated the analyses in a sample excluding current smokers. We also focused on the association between air pollutants and the dynamic transitions among pre-COPD and COPD stages in non-asthmatic observations, as asthma diagnosis is an important risk factor for COPD31. We excluded the current smokers in the sensitivity analysis to test the robustness of our results.
Analyses were conducted using R version 4.4.0, and the msm package was used for multi-state modelling. We used two-sided significance tests for all analyses, with statistical significance set at P < 0.05.
Results
Population characteristics
We included 31,625 participants (68,219 visits) for the PM dataset and 33,217 participants (71,287 visits) for the NOx dataset (Fig. 2). Males represented 48.84% of the PM dataset and 48.77% of the NOx dataset. The baseline characteristics of the study population were provided in Supplementary Table S4 and S5. For each participant, the record with the first valid best measure of lung function was considered the baseline investigation. At baseline, the average exposure concentration of PM2.5, PM coarse and PM10 were 9.92, 6.35, 15.97 μg/m3 for the PM dataset, the average exposure concentration of NO2, and NOx were 25.86 and 42.73 μg/m3 for NOx dataset. Correlation matrix between the included pollutants is shown in the Supplementary Table S6. The total person-years of observation were 244,740 and 261,117 years for the PM and NOx dataset,s respectively. The mean time intervals between two consecutive visits were 6.68 and 6.84 years for the PM and NOx dataset. During the study period, the observed transition patterns among the three COPD developmental stages were shown in Supplementary Table S7.
Association between air pollutant exposure and dynamic transitions of PRISm
The results of fully adjusted MSMMs were shown in Fig. 3. Regarding the progression from PRISm to COPD, we found that an IQR increment in PM10 exposure was associated with a significant risk increase [HR (95%CI): 1.16 (1.02,1.31)]. Such positive associations were not significant for other air pollutants. Interestingly, individuals with higher exposure of PM2.5, NO2 and NOx were less likely to revert back to normal spirometry from the PRISm status: Each IQR increase in exposure of PM2.5, NO2 and NOx associated with a lower probability of transiting from PRISm to normal spirometry [HR (95% CI): 0.88 (0.81, 0.96) for PM2.5; 0.86 (0.79, 0.93) for NO2 and 0.89 (0.83, 0.96) for NOx]. This finding was robust across different adjustment models (from Model 1 to Model 4) (Supplementary Table S8). No significant associations were found between exposure to five air pollutants and transitions from normal spirometry to PRISm, normal spirometry to COPD, COPD to normal spirometry, nor COPD to PRISm.
The IQR was 1.34 μg/m3 for PM2.5, 0.69 μg/m3 for PM2.5–10, 1.74 μg/m3 for PM10, 9.47 μg/m3 for NO2 and 16.61 μg/m3 for NOx). The number of observations included in the models was 68,219 for the analyses of PM2.5, PM2.5–10, and PM10, and 71,287 for NO2 and NOx. The adjusted variables included sex, age, BMI, smoking status, the disease history of asthma, family household income, education level, occupational exposure and respiratory symptoms. Dots represent the point estimate of hazard ratios and the lines represent the 95% confidence intervals. Red dots and lines indicate the progressive transitions (a); Blue dots and lines indicate the recovery transitions (b). The triangle points indicate statistically significant results.
Differences in cumulative transition probabilities by exposure levels in various age groups with PRISm
The predicted cumulative transition probabilities for participants with PRISm with 10-year follow-up in the real-world and counterfactual scenarios were presented in Fig. 4. When the air pollution exposure levels are dropping, the transition probabilities of PRISm progressing to COPD would be lower, while the probabilities of reversing from PRISm to normal spirometry would be higher. When we reduced PM2.5 exposure from the baseline population average exposure level to the WHO Air Quality Guidelines recommended levels, the study participants could have been 13.89% more likely to reverse from the PRISm condition to normal spirometry. Reducing the population level exposure of PM2.5 from 9.92 μg/m3 to 5 μg/m3 was associated with a 7.96% decrease in risk of progressing to COPD for 70-years-old PRISm participants.
A–C are for transition probabilities from PRISm to normal spirometry (the higher the better), while D–F are for transition probabilities from PRISm to COPD (the lower the better). The population average exposure at baseline for PM2.5, PM10 and NO2 were 9.92, 15.97, and 25.86 μg/m3; while the WHO Air quality guidelines recommend levels were 5 μg/m3 for PM2.5, 15 μg/m3 for PM10 and 10 μg/m3 for NO2.
Subgroup and sensitivity analyses
Results of subgroup analyses were displayed in Table S9 and S10. We found that each IQR increase in PM2.5 and PM10 exposure was associated with greater decreases [HR (95% CI): 0.85 (0.75, 0.95) for PM2.5; 0.80 (0.71, 0.91) for PM10] in the likelihood of recovering from PRISm to normal spirometry in females compared to males. Exposure to PM2.5, PM10, NO₂, and NOₓ were significantly associated with a reduced likelihood of transitioning from PRISm to normal spirometry in obese individuals, but not in non-obese individuals. After excluding current smokers, the associations between air pollutants and dynamic transitions of PRISm were mostly robust (Fig. 5 and Supplementary Table S11). Increase in PM2.5 exposure level was found to be significantly associated with an increased risk of the transition from PRISm to COPD [HR (95% CI): 1.22 (1.00, 1.47), P < 0.05]. Subjects with higher PM10 exposure had more difficulty recovering from PRISm to normal spirometry [HR (95% CI): 0.94 (0.88, 1.00), P < 0.05]. After focusing on non-asthmatic observations, we found that the results were mostly robust. There were no directional changes for the estimates compared to the main findings, while for PM10 and NOx, the associations between pollutants and 2 state transition pathways did not reach statistical significance (Table S12).
The number of observations included in the models was 63,724 for PM2.5, PM2.5-10 and PM10; and 66,586 for NO2 and NOx). The adjusted variables included sex, age, BMI, smoking status, the disease history of asthma, family household income, education level, occupational exposure and respiratory symptoms. Dots represent the point estimate of hazard ratios and the lines represent the 95% confidence intervals. Red dots and lines indicate the progressive transitions (a); Blue dots and lines indicate the recovery transitions (b). The triangle points indicate statistically significant results.
Discussion
This study assessed the association between the exposure of air pollution and the dynamic transition of PRISm, which is a pre-COPD phase that can either progress to COPD, revert back to normal spirometry, or persist as PRISm. To the best of our knowledge, this is the first epidemiological study that found individuals with higher exposure to PM2.5, NO2, and NOx were less likely to recover from PRISm to normal spirometry. We also discovered that exposure to PM2.5 and PM10 was associated higher risk of progression from PRISm to COPD after excluding current smokers. Reducing the exposure of PM2.5 from 9.92 μg/m3 to 5 μg/m3 may be associated with up to 4.42% of risk reduction for reversion from PRISm to COPD, and increased the probability by 13.89% for reversion from PRISm to normal spirometry.
Although exposure to air pollution is generally considered one of the risk factors for COPD32,33 or reductions in pulmonary function34,35, rarely had studies focused on the association between air pollution and the dynamic transition of different COPD states. Although changes in pre-COPD and COPD status are driven by underlying variations in spirometry measures such as FEV1 and FVC, we did not assess the direct associations between air pollution and these continuous lung function indicators. Instead, our analyses focused on the effects of air pollution on transitional probabilities among disease stages using multi-state Markov models. This approach was chosen to capture the transition across clinically interpretable and actionable pre-COPD and COPD stages. Doiron et al. 36, using cross-sectional baseline data from the UK Biobank cohort, have reported significant negative associations between long-term air pollutant exposures and lung function parameters, with differential effects observed for FEV1 versus FVC36. However, it remains unclear whether such impairment in pulmonary function can translate into changes across health states (normal spirometry, PRISm, COPD) in longitudinal settings. Moreover, for long-term outcomes, it is the direction of longitudinal transitions from and to the pre-COPD status that matters: it was found that individuals who recovered from PRISm showed similar risk level as their normal spirometry counterparts, while those who progressed from normal spirometry to PRISm and those who had persistent PRISm were associated with a much higher risk of morbidity and mortality37,38.
In this study, we found individuals with higher exposure of PM2.5, NO2 and NOx were significantly less likely to revert back to normal spirometry from the PRISm status, which represented one of the first population-level efforts to estimate the adverse effect of air pollutants in hampering the recovery of pulmonary function. Lung function recovery has been the subject and desired outcome in many studies39,40,41. It has been found among a small group of post-operative patients that high PM2.5 exposure was associated with reduced speed and degree of lung function recovery42; however, such findings have never been tested in general populations. In vitro and in vivo models were more concerned with the role of air pollutants in the development and progression of COPD43, instead of focusing on the potentially harmful effects of air pollutants in hampering lung function recovery. Based on our findings, further studies could focus on whether reducing exposure of air pollution may lead to higher likelihood of PRISm reversion, thus providing more evidences on the possible interventions for individuals in the pre-COPD phase.
In terms of disease progression, we found that exposure to PM2.5 and PM10 was associated higher risk of transiting from PRISm to COPD after excluding current smokers, but not from normal spirometry to COPD. Despite common understandings, evidence on the association between air pollution exposures and COPD incidence are still largely inconclusive44. This could be explained by the unstable and multifaceted nature of COPD development45, which highlighted the importance of considering the multiple transitional pathways of PRISm, as demonstrated in this study. Also, due to the differences in analytic strategies and the selection of study outcomes, the findings of some previous studies46 may not be directly comparable to this study. Future studies could also consider the effect of multi-pollutant mixture on the state transition probability of pre-COPD and COPD stages, since human beings are exposed to multiple ambient pollutants simultaneously47.
It should be noted that, by utilizing the multi-state model and estimating bi-directional transition probabilities for PRISm, we simultaneously considered the competing risk of all the possible transitional directions. This could reduce the bias of estimating the effect of air pollution on the dynamic transition of PRISm. Several longitudinal studies on PRISm have already found that PRISm, as an unstable phase, can have a dynamic transitional trajectory; nevertheless, the inherent limitation of the logistic or Cox regression models prevented them from characterizing multiple state transitions across the timeframe7,45. In this study, we opt for the multi-state regression model, which is an extension of the Cox model that enables us to estimate associations between air pollution and the dynamic transitions across different states (normal spirometry, PRISm and COPD)48. Although the clock-forward approach is often used in the multistate models to model the unidirectional changes of disease statuses (e.g., from pre-disease to disease onset, and then death), the incorporation of the bidirectional transitions for PRISm. This design provides a more comprehensive understanding on the dynamic nature of PRISm and COPD over time, while estimating the effect of air pollutants in the context of real-world transitive scenarios.
This study provided essential evidence on whether and how the dynamic transition of PRISm is influenced by air pollution exposure. Through the utilization of the multi-state model and data from a large, prospective cohort, this study quantified the impact of air pollution on the dynamic transition of pre-COPD status: exposure to higher levels of air pollutants is not only associated with increased risk of disease progression, but also relates to reduced likelihood of recovery to normal spirometry. This renewed the understanding on the potential pathway of air pollution and the development process of COPD could inform clinicians, basic researchers, and epidemiologists, while supporting the estimation of the potential health benefit brought by controlling air pollution.
This study had a few limitations. First, we used the annual air pollution exposure data in the year 2010 in all the analyses, which were publicly available. Although the exposure data had been employed in a number of studies investigating the health effect of air pollution using the UK Biobank dataset13,19,49, and it could be assumed that the air pollution exposure level did not vary drastically from baseline to the latest follow-up of the UK Biobank, this lack of temporal variation in exposure and the lack of data regarding possible participant relocation during the observational period could bring exposure misclassification50.
Second, due to the lack of data on computed tomography scan, or other lung physiology indicators, we used lung function indicators to define normal spirometry, PRISm and spirometry-defined COPD stages. This approximation could bring bias in the outcome measurement51. Future studies could use more detailed information to assess the dynamic transitions of respiratory functions, so that we can further the understanding of the mechanisms behind the adverse respiratory effect of air pollutants, while having a clearer pathological understanding of the PRISm state itself.
Third, cautions should be taken in generalizing the study results, since the UK Biobank participants are mostly of European ancestry7, and the air pollution exposure level was very low in the UK36. Nevertheless, the methodology used in this study is applicable in estimating the association between air pollution and the dynamic transition of lung function stages, as well as other diseases involving the reversible transition of physiological indicators. This study design is also urgently needed in developing countries with high air pollution levels and high burden of chronic respiratory diseases, since it can demonstrate the effect of air pollution on the whole process of disease development.
Lastly, we found that the associations between air pollutants and state transition probabilities became statistically insignificant in some subgroups or in sensitivity analyses. This could imply that although we have utilized all the eligible participants in the UK Biobank database, a larger sample size for each state transitional pathway could still bring more statistical power to the analyses. Therefore, future studies that focused on the risk factors for the dynamic transitions of PRISm or other pre-COPD conditions could consider more frequent measurements of spirometry data or longer observation time.
Conclusions
This study, using the multistate models, found that exposing to higher levels of air pollutants is not only associated with increased risk of progressing from PRISm to COPD, but also relate to reduced likelihood of recovery from pre-COPD condition to the normal spirometry. As this is one of the first efforts to unravel the role of air pollution and the dynamic transitions of PRISm, future studies could further the understanding on both the effect size and the underlying mechanisms behind the relation between air pollutants on the entire development phases of COPD, while examining the effectiveness of possible intervention in curtailing pre-COPD progression, and promoting reversion to normal spirometry.
Data availability
This research has been conducted using the UK Biobank Resource under Application Number 76248. The UK Biobank data are available upon application (www.ukbiobank.ac.uk). The numerical data (source data) for Figs. 3–5 are presented in Supplementary Data 1–3.
References
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD: 2023 report. Global Initiative for Chronic Obstructive Lung Disease; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
Venkatesan, P. GOLD COPD report: 2024 update. Lancet Resp. Med. 12, 16–18 (2024).
Wan, E. S. et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. Jama 326, 2287–2298 (2021).
Washio, Y. et al. Risks of mortality and airflow limitation in Japanese individuals with preserved ratio impaired spirometry. Am. J. Respir. Crit. Care Med. 206, 563–572 (2022).
Huang, J. et al. Preserved Ratio Impaired Spirometry (PRISm): A global epidemiological overview, radiographic characteristics, comorbid associations, and differentiation from chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 19, 753–764 (2024).
Zheng, J. et al. Preserved ratio impaired spirometry in relationship to cardiovascular outcomes: a large prospective cohort study. Chest 163, 610–623 (2023).
Higbee, D. H., Granell, R., Davey, S. G. & Dodd, J. W. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Resp. Med. 10, 149–157 (2022).
Luo, H., Zhang, Q., Niu, Y., Kan, H. & Chen, R. Fine particulate matter and cardiorespiratory health in China: A systematic review and meta-analysis of epidemiological studies. J. Environ. Sci. 123, 306–316 (2023).
Meng, X. et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: multilocation analysis in 398 cities. Bmj 372, n534 (2021).
Sin, D. D. et al. Air pollution and COPD: GOLD 2023 committee report. Eur. Respir. J. 61, 2202469 (2023).
To, T. et al. Progression from asthma to chronic obstructive pulmonary disease. is air pollution a risk factor?. Am. J. Respir. Crit. Care Med. 194, 429–438 (2016).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Guyatt, A. L., Cai, Y. S., Doiron, D., Tobin, M. D. & Hansell, A. L. Air pollution, lung function and mortality: survival and mediation analyses in UK Biobank. ERJ Open Res. 10, 93 (2024).
Quanjer, P. H. et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur. Respir. J. 40, 1324–1343 (2012).
Beelen, R. et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe – The ESCAPE project. Atmos. Environ. 72, 10–23 (2013).
Eeftens, M. et al. Development of land use regression models for PM2.5, PM2.5 absorbance, PM10 and PMcoarsein 20 European Study Areas; Results of the ESCAPE Project. Environ. Sci. Technol. 46, 11195–11205 (2012).
Wan, E. S. et al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am. J. Respir. Crit. Care Med. 198, 1397–1405 (2018).
Wan, E. S. et al. Significant spirometric transitions and preserved ratio impaired spirometry among ever smokers. Chest 161, 651–661 (2022).
Luo, H. et al. Long-term exposure to ambient air pollution is a risk factor for trajectory of cardiometabolic multimorbidity: A prospective study in the UK Biobank. EBioMedicine 84, 104282 (2022).
Wu, Y. et al. Ambient air pollution associated with incidence and dynamic progression of type 2 diabetes: a trajectory analysis of a population-based cohort. BMC Med. 20, 375 (2022).
Luo, H. et al. Impacts of physical activity and particulate air pollution on the onset, progression and mortality for the comorbidity of type 2 diabetes and mood disorders. Sci. Total Environ. 890, 164315 (2023).
Yuan, M., Xu, C. & Fang, Y. The transitions and predictors of cognitive frailty with multi-state Markov model: a cohort study. BMC Geriatr. 22, 550 (2022).
Jackson, C. Multi-State Models for Panel Data: The msm Package for R. J. Stat. Softw. 38, 1–28 (2011).
Li, J. et al. Associations between long-term night shift work and incidence of chronic obstructive pulmonary disease: a prospective cohort study of 277,059 UK Biobank participants. BMC Med. 22, 16 (2024).
Pan, Z. et al. Short-term exposure to ozone and ECG abnormalities in China: A nationwide longitudinal study. J. Hazard. Mater. 459, 132290 (2023).
Sun, W. et al. Middle-term nitrogen dioxide exposure and electrocardiogram abnormalities: A nationwide longitudinal study. Ecotoxicol. Environ. Saf. 266, 115562 (2023).
Gan, W. Q., FitzGerald, J. M., Carlsten, C., Sadatsafavi, M. & Brauer, M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am. J. Respir. Crit. Care Med. 187, 721–727 (2013).
Fox, J. & Weisberg, S. An R Companion to Applied Regression, 3rd edn. (SAGE Publications, Los Angeles, London, 2018).
Turner, M. C. et al. Interactions between cigarette smoking and fine particulate matter in the Risk of Lung Cancer Mortality in Cancer Prevention Study II. Am. J. Epidemiol. 180, 1145–1149 (2014).
Turner, M. C. et al. Interactions between cigarette smoking and ambient PM 2.5 for cardiovascular mortality. Environ. Res. 154, 304–310 (2017).
Asamoah-Boaheng, M. et al. Association between early history of asthma and COPD diagnosis in later life: a systematic review and meta-analysis. Int. J. Epidemiol. 47, 1865–1876 (2018).
Chen, L. et al. Risk/benefit tradeoff of habitual physical activity and air pollution on chronic pulmonary obstructive disease: findings from a large prospective cohort study. BMC Med 20, 70 (2022).
Stolz, D. et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet 400, 921–972 (2022).
Yang, T. et al. Association of fine particulate matter air pollution and its constituents with lung function: The China Pulmonary Health study. Environ. Int. 156, 106707 (2021).
Niu, Y. et al. Long-term ozone exposure and small airway dysfunction: The China Pulmonary Health (CPH) Study. Am. J. Respir. Crit. Care Med. 205, 450–458 (2022).
Doiron, D. et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur. Respir. J. 54, 1802140 (2019).
Marott, J. L., Ingebrigtsen, T. S., Çolak, Y., Vestbo, J. & Lange, P. Trajectory of preserved ratio impaired spirometry: natural history and long-term prognosis. Am. J. Respir. Crit. Care Med. 204, 910–920 (2021).
Melén, E. et al. Lung-function trajectories: relevance and implementation in clinical practice. The Lancet 403, 1494–1503 (2024).
Dai, J. et al. Pulmonary function recovery and displacement patterns after anatomic Segmentectomy vs Lobectomy. Ann. Thorac. Surg. 118, 365–374 (2024).
Saito, H. et al. Pulmonary function after lobectomy versus segmentectomy in patients with Stage I non-small cell lung cancer. World J. Surg. 38, 2025–2031 (2014).
Basil, M. C., Alysandratos, K. D., Kotton, D. N. & Morrisey, E. E. Lung repair and regeneration: Advanced models and insights into human disease. Cell Stem Cell 31, 439–454 (2024).
Liu, Y. et al. Association of high PM2.5 levels with short-term and medium-term lung function recovery in patients with pulmonary lobectomy. Front. Public Health 10, 1022199 (2022).
Zhao, J. et al. Role of PM2.5 in the development and progression of COPD and its mechanisms. Respir. Res. 20, 120 (2019).
Liu, S. et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: The ELAPSE project. Environ. Int. 146, 106267 (2021).
Agustí, A. et al. The many faces of COPD in real life: a longitudinal analysis of the NOVELTY cohort. ERJ Open Res. 10, 00895–02023 (2024).
Wang, L., Xie, J., Hu, Y. & Tian, Y. Air pollution and risk of chronic obstructed pulmonary disease: The modifying effect of genetic susceptibility and lifestyle. EBioMedicine 79, 103994 (2022).
Bobb, J. F. et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16, 493–508 (2015).
Hu, X. et al. Role of residential greenspace in the trajectory of major neurological disorders: A longitudinal study in UK Biobank. Sci. Total Environ. 912, 168967 (2024).
Zhang, S. et al. Associations between air pollution and the risk of first admission and multiple readmissions for cardiovascular diseases. Heart 110, 337–345 (2024).
Armstrong, B. G. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup. Environ. Med. 55, 651–656 (1998).
Han, X. et al. Association between long-term exposure to PM2.5 constituents and electrocardiographic abnormality: A nationwide longitudinal study in China. Environ. Int. 178, 108130 (2023).
Acknowledgements
This study is supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0506306) and the Science & Technology Fundamental Resources Investigation Programme (2023FY100604). We also acknowledged the help from our colleague Dr. Ruxianguli Aimuzi regarding the UK Biobank data application.
Author information
Authors and Affiliations
Contributions
X.H. and Z.P. designed the study and drafted the manuscript. X.H., Z.P. and T.G. designed and conducted the statistical analyses. Y.Q., J.G., H.Z., H.C., Y.W., and Q.W. edited the manuscript. K.H., Y.Q., T.G., T.Y. and C.W. supervised the study. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks David M. Mannino, Subhabrata Moitra and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, X., Pan, Z., Qu, Y. et al. Air pollution and dynamic transitions of preserved ratio impaired spirometry in UK Biobank. Commun Med 5, 520 (2025). https://doi.org/10.1038/s43856-025-01225-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-01225-7