Abstract
Cardiomyopathies, particularly, dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM) are significant and growing public health concerns in low resource settings, particularly Africa. This growing burden stems from undiagnosed genetic causes, rising hypertension and infectious diseases, and limited access to diagnostic and specialist cardiac care. However, the epidemiology of these conditions remains poorly defined. In Europe and North America, genetic testing has proven to be an important tool for early diagnosis for these conditions. Its use can be extended to risk assessment, personalized management, and increased understanding of disease mechanisms. However, the availability and implementation of genetic testing in Tanzania are limited, hindering its potential to improve patient outcomes. This Perspective evaluates the current epidemiology of cardiomyopathies in Tanzania, to provide a broader view on the underlying genetic factors, systemic barriers to implementing genetic testing and counseling, and proposes actionable solutions to overcome existing challenges. While similar needs have been recognized across Africa, this Perspective emphasizes how context-specific framing in Tanzania, grounded in real-world clinical and health system features, remains essential to catalyze local implementation and policy reform.
Similar content being viewed by others
Introduction
Cardiomyopathies denote a group of heart muscle diseases that can progressively lead to heart failure and sudden cardiac death1. These diseases are categorized into five subtypes based on their clinical pathophysiology and morphology: dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), non-dilated left ventricular cardiomyopathy (NDLVC), arrhythmogenic right ventricular cardiomyopathy (ARVC) and restrictive cardiomyopathy2,3. They arise due to a combination of genetic and non-genetic factors, or as secondary conditions associated with non-cardiac diseases. Despite multiple etiologies, genetic factors contribute to disease development in a significant number of patients with cardiomyopathies, even in cases where interaction with environmental factors cannot be excluded4,5.
Globally, the true burden of cardiomyopathy remains uncertain due to underdiagnosis and limited epidemiological data, particularly from low- and middle-income countries. Conservative estimates based on data from high-income countries suggest that more than 10 million individuals are affected worldwide, reflecting an increase of approximately 30% over the past decade6,7. Causes of cardiomyopathy include both non-genetic factors, such as myocarditis, toxins, endocrinopathies, nutritional deficiencies, and drugs, as well as genetic abnormalities that predispose individuals to the disease4. The prevalence of cardiomyopathy is generally estimated to be higher in low- and middle-income countries (LMICs) compared to high-income countries (HICs). However, the lack of population-based studies on the incidence and prevalence of cardiomyopathy means that available epidemiological data primarily come from local hospital-based studies that often vary in the application of diagnostic criteria. Studies conducted in Africa reveal significant regional variations in the prevalence of cardiomyopathy. Hospital-based studies in Southern Africa reported the highest prevalence of cardiomyopathy in sub-Saharan Africa, reaching 40.2%, compared to East Africa, where the prevalence was lowest at 18.2%8,9.
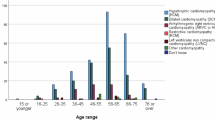
DCM is the most common form of cardiomyopathy in Africa, accounting for approximately 21.4% of all heart failure cases10,11,12. HCM, characterized by thickening of the heart muscle, affects approximately 0.2% of the population, a prevalence that is consistent across African and non-African populations. Once thought to be rare among Africans, HCM is now recognized to occur at similar rates globally and is well established as a genetic disorder9,13. These prevalence estimates underscore the substantial burden of cardiomyopathies in the region. In Tanzania, data from the nonischemic dilated cardioMyOpathY in a native Tanzanian cohort (MOYO) study demonstrated that nonischemic DCM was the dominant phenotype among patients presenting with heart failure, with many cases lacking clear etiological classification prior to referral14. Notwithstanding these statistics, specific data on the epidemiology and prevalence of cardiomyopathies in Africa in general and Tanzania in particular are limited. Limited epidemiological and genetic data constrain understanding of cardiomyopathies in Tanzania and impede the development of effective prevention and management strategies15. Both genetic testing and counseling are critical for identifying affected individuals, guiding family risk assessment, and supporting informed decision-making. However, access to these services remains extremely limited, leading to missed opportunities for early intervention, cascade screening, and improved patient outcomes.
While the availability and utilization of genetic testing in high income countries are increasingly commonplace, low resource settings such as Tanzania face numerous challenges in adopting and implementing these advancements. This Perspective discusses the challenges, opportunities, and future directions of implementing genetic counseling and testing for cardiomyopathies in Tanzania. While similar calls have been made across Africa, local implementation remains rare, and we aim to contextualize these gaps through a Tanzania-specific lens. In the sections that follow, we outline key systemic barriers to integrating genetic testing and counseling into cardiomyopathy care in Tanzania, examine opportunities such as telemedicine and regional collaboration, and propose actionable strategies to strengthen cardiogenetic services. Together, these insights highlight how a structured national approach could transform the diagnosis and management of inherited cardiac diseases in low-resource settings.
Addressing the unmet needs in cardiomyopathy care
Cardiomyopathies, present significant challenges to healthcare in Tanzania and research efforts to tackle this are currently limited. Genetic counseling and testing, with its potential for early diagnosis, risk assessment, cascade screening, and personalized management, can serve as a critical tool. However, its limited integration within Tanzanian healthcare settings currently hinders the optimization of the respective patient outcomes. In this respect, Tanzania faces unique challenges compared to other countries, including logistical issues in reaching rural areas, a limited number of specialized healthcare personnel, and reliance on centralized facilities, such as the Akaya Kikwete Cardiac Institute (JKCI), which constrains equitable access to advanced cardiac care.
JKCI is the national tertiary referral hospital for cardiac conditions in Tanzania, receiving patients from 26 regional and 4 zonal hospitals within the country. It also serves as the cardiac teaching hospital for the Muhimbili University of Health and Allied Sciences (MUHAS) and hosts the MSc Cardiology program. It is estimated that over 90% of the country’s cardiologists are based at JKCI (26 adult and 12 pediatric). The hospital primarily manages acute and advanced cardiac diseases, with a limited focus on preventive care. Echocardiography is the most commonly used diagnostic tool, while advanced imaging, such as cardiac MRI and CT, is available only at Muhimbili National Hospital and is not routinely used, limiting full characterization of cardiomyopathies. JKCI conducts 6–10 coronary angiograms (CAG) daily, mostly to confirm obstructive coronary artery disease (CAD). In DCM patients, CAG is not done routinely, mainly due to the added cost to the patient and the fact that there would be no added therapy for most patients. In the MOYO study14, for example, only 9% of the patients with DCM underwent CAG.
Tanzania’s step-up referral system often delays access to JKCI16, with patients being frequently misdiagnosed at primary care facilities, commonly with Tuberculosis due to the overlapping symptoms and stronger infectious disease emphasis at many healthcare providers. Outside major cities, patients often rely on general practitioners or clinical officers who lack specialized training. Also, regional and district hospitals often lack echocardiography, trained cardiologists, or capacity for follow-up. This results in late cardiac diagnoses, and many patients likely die before they get a correct diagnosis. There is therefore a strong need to train primary healthcare workers to diagnose cardiac disorders earlier and then refer cardiac patients to the facilities that can perform echocardiograms. Also, increasing the number of medical doctors who can perform and report on a basic echocardiogram will help in early diagnosis. To this end, MUHAS offers a 4-week short course to equip physicians with the basic knowledge and skills to perform and interpret echocardiograms. These doctors reliably make the right diagnosis, as having access to a cardiologist, with whom they can share echo clips via WhatsApp for diagnostic support, promotes earlier intervention. Formalizing this approach and integrating AI could enhance early referrals.
Understanding the infrastructure in Tanzania for detecting and managing cardiomyopathies is critical for developing better patient outcomes. Currently, referrals for advanced cardiac diagnostics and genetic counseling are routed through informal mechanisms such as WhatsApp messages to individual specialists. The absence of electronic health records or standardized care pathways often results in fragmented care and missed opportunities for important early intervention. This ad hoc model, while occasionally effective, is not sustainable for equitable and scalable care. The concentration of services at JKCI also limits access for rural and lower-income populations, reinforcing inequities within the care system. Furthermore, the prioritization of critical cardiac care has hampered attention to outpatient-level screening for inherited cardiomyopathies that could encourage earlier interventions and cascade testing16. A formal and national plan for structured referral and triage is therefore needed.
In addition, it is critical to recognize that improving the possibilities for early diagnosis and predicting its progress will be of limited use in the absence of the capacity to prevent and treat appropriately. JKCI’s services remain largely inaccessible due to financial barriers and geographical barriers, regional disparities, and limited health system capacity. Treatment remains challenging due to the low availability and use of advanced therapies such as ARNIs and SGLT2 inhibitors, largely due to high costs and limited health insurance coverage. These infrastructural limitations underscore the need for further investment in Tanzania’s cardiovascular care to support genetic testing and comprehensive management for DCM and HCM patients14.
Leveraging telemedicine to expand access to genetic counseling in Tanzania
In addition to the typical diagnostic review described above, genetic testing offers additional insight into disease presentation and risk. Genetic factors play a pivotal role in cardiomyopathy pathogenesis, with profound clinical implications, underscoring the need for genetic counseling and testing to reach optimized patient care. Identifying individuals at high genetic risk can enable clinicians to design individualized interventions that will improve patient outlook even before symptoms manifest17. Integrating genetic testing into cardiomyopathy care will also empower cascade screening, where phased implementation can focus initially on high-priority cases that can save both lives and money. Genetic counseling equips patients and families to make informed decisions about managing inherited risks, while knowledge on genetic markers can post presentation guided drug selection to improve patient outcomes with minimal side effects. In Tanzania, where most advanced cardiac and genetic services are concentrated in urban referral centers, telemedicine and mobile health (mHealth) can provide a critical bridge for integrating genetic testing into care. This approach offers a scalable model to extend specialized services into rural and underserved regions, compensating for workforce shortages and logistical constraints. Evidence from other African countries demonstrates that telemedicine can significantly reduce patient travel costs, minimize delays in care, and improve continuity by connecting patients to centralized expertise18,19. Recent studies from Tanzania, such as the REaCH trial18, show that community health workers using mobile data tools can deliver NCD care remotely without compromising patient trust or outcomes. These models demonstrate the feasibility of introducing remote follow-up in cardiogenetics. In practical terms, implementation outside of major cities could involve collecting buccal swabs or dried blood spots at district or regional hospitals, shipping them to a central reference laboratory such as JKCI or MUHAS for genetic analysis, and delivering results and counseling sessions remotely via secure telehealth platforms. Tanzania can apply similar innovations, such as SMS-based triage alerts, tele-echocardiography units for district hospitals, and remote genetic counseling sessions, to extend services beyond JKCI. Evidence from Muhimbili National Hospital supports the feasibility of structured telephone follow-up for patients with heart failure, showing improved appointment adherence and patient satisfaction20. These findings indicate that similar mobile-based models could be adapted for post-diagnostic follow-up and cascade screening.
Infrastructure costs for establishing telemedicine services in Africa vary depending on the level of technological sophistication and geographic coverage. A basic yet functional telemedicine configuration, including tablets or smartphones, a stable internet connection, and secure software platforms, can be implemented for under USD $5,000 per site, especially when leveraging existing public health infrastructure or primary care centers21. On the patient side, virtual genetic counseling sessions could cost USD $10–20 per session, markedly lower than the cumulative expenses of in-person visits requiring travel and accommodation. While out-of-pocket payments may still be necessary initially, there is a growing opportunity for health insurance schemes in Africa to include telehealth coverage, especially as pilot programs in Ghana, Kenya, and South Africa have shown promising results21.
This strategy not only alleviates logistical and financial burdens but also facilitates early diagnosis, cascade testing, and family counseling across geographically dispersed populations. With mobile penetration exceeding 85% in many parts of Tanzania and East Africa, the telemedicine model is increasingly feasible and sustainable18. Institutional anchoring through facilities like JKCI can provide centralized coordination and quality assurance, while decentralized outreach through community health workers can expand reach and uptake. These tools could be integrated into existing platforms such as maternal-child health and chronic disease programs, providing a backbone for sustainability. Such an approach not only decentralizes specimen collection but also centralizes specialized interpretation and counseling, ensuring quality and consistency while maintaining accessibility for geographically dispersed patients.
The clinical need in Tanzania differs from other countries due to its largely rural population and limited clinical and genetic expertise. Tailoring genetic testing initiatives to Tanzania’s context would first involve the identification of alleles that confer risk, particularly in this region, including those specific to local ancestral or ethnic groups. A phased approach, starting with probands/families at JKCI, and then expanding to other health centers, could demonstrate feasibility and support scalable, culturally sensitive solutions. Collaborative efforts among healthcare institutions, clinicians, researchers, and policymakers are essential for establishing genetic testing facilities, training genetic professionals, and ensuring equitable access. Ethical considerations, such as data privacy and informed consent, must guide these efforts in order to maximize impact and feasibility.
In addition, attention to religious and cultural dynamics is essential. In many Tanzanian communities, women are blamed for the transmission of hereditary disorders, leading to stigma and reluctance in family disclosure. Gender-sensitive counseling and engagement with religious leaders can play a crucial role in increasing acceptance of genetic services.
Genetic factors associated with cardiomyopathies
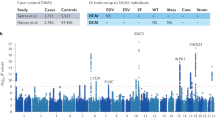
In addition to providing the infrastructure for remote screening that includes genetic analyses, it is important to better understand the genetic bases of cardiomyopathies in Tanzania, as the genetic architecture of DCM may differ substantially between African and European populations22,23. This will affect the ability to predict clinical outcomes and, importantly, the effectiveness of genetic testing. Research indicates that individuals of African ancestry are less likely to have clinically actionable variants (pathogenic and likely pathogenic; P/LP) in key DCM genes (Table 1). This population-specific distribution is evident in the TTN gene, where truncating variants show notable allelic heterogeneity. In African cohorts, several distinct variants have been identified that differ from those seen in European populations, highlighting the importance of ancestry-specific databases for accurate interpretation24. Historically, limited population frequency data have led to disproportionate misclassification of benign variants as pathogenic in African populations25. This disparity is driven largely by the limited representation of African populations in reference databases, resulting in a higher proportion of variants being classified as variants of uncertain significance and fewer variants confidently designated as LP23. In Africans, limited data indicate that the primary causative factors for DCM include unique variants in the TTN and lamin A/C (LMNA) genes, while HCM is linked to variants in genes encoding sarcomeric proteins such as MYH7, MYBPC3, and TNNT29. However, the complete genetic architecture and contribution of inherited factors to cardiomyopathies in African, and in particular Tanzanian populations, remain largely unknown. Variants in cardiac sarcomeric genes such as MYH7, MYBPC3, and TNNT2 are frequently associated with familial HCM, though their prevalence and spectrum differ across populations, including African cohorts26,27. Such population-specific differences underscore the importance of studying context-specific pathogenicity28, as genetic variants associated with cardiomyopathies in African populations may have different clinical implications compared to those found in European populations. African ancestry reported in US cohorts primarily represents West Africans whose genetic etiologies may differ from those in Tanzania. In contrast, European populations have more established pathogenic variant data, which supports better-informed clinical decision-making22. This disparity underscores the need for genetic studies that include diverse populations to enhance diagnostic accuracy and reduce potential health disparities in genetic testing29. The unique genetic characteristics in African populations and their diversity of environments highlight the importance of Africa-specific genomic programs30. Therefore, there is a pressing need to expand genomic data from African populations, validate findings within African cohorts, and develop guidelines tailored to the genetic landscape across Africa. Such programs could improve genetic counseling, enable targeted interventions, and support the establishment of region-specific standards that consider the sociocultural and logistical challenges of African healthcare systems.
Targeted gene sequencing and genome-wide association studies (GWAS) are valuable approaches for identifying genetic variants associated with cardiomyopathies2. Mendelian or monogenic cardiomyopathies with high penetrance offer clearer clinical relevance for early diagnosis, risk assessment, and targeted intervention. This indicates that investing in targeted gene sequencing of Tanzanian patients (and their families, particularly for single-gene cases) would be the preferred first approach, as that will have more immediate health care consequences. Identifying single-gene variants in genes such as TTN, MYH7, and LMNA has proven valuable for diagnosing dilated and hypertrophic cardiomyopathies and for enabling family cascade screening that is critical in managing inherited cardiomyopathies (for an overview of genes implicated in CM in African populations: see also Table 1). Comparing the genomes of individuals with cardiomyopathies to healthy controls allows researchers to identify specific genetic markers associated with increased risk31,32. This approach not only aids in identifying disease-specific variants but also opens the possibility of discovering novel genes and variants unique to the Tanzanian context. It is worth noting that even variants unique to Tanzania may allow for the development of treatments for all populations, as was shown for PCSK9 inhibitors33. In Tanzanian populations, genetic studies can reveal both allelic heterogeneity, different variants within the same genes, and locus heterogeneity, where variants in different genes may contribute to similar disease outcomes.
Understanding population-specific genetic variations, including modifiers that influence disease severity and progression, is essential for tailoring screening, clinical care, and developing targeted therapeutic interventions for cardiomyopathy patients. Notably, peripartum cardiomyopathy has a higher prevalence in African populations compared to European populations34, highlighting a distinct clinical need that may benefit from focused genetic and environmental studies to better address the unique risk factors and outcomes in such patients.
Environmental factors, including viral infections, nutritional deficiencies, and exposure to toxins, can interact with genetic predispositions to impact the development and progression of cardiomyopathies. For example, certain viral infections, such as enteroviruses and human immunodeficiency virus, have been associated with the development of DCM3,10,12. Investigating the interplay between these environmental risk factors and genetic variation in Tanzanian populations can help elucidate the complex etiology of cardiomyopathies and identify targeted prevention and management strategies.
Collaboration with international genetic research networks and initiatives focused on cardiomyopathies can facilitate knowledge sharing, resource allocation, and capacity building in genetic research in Tanzania. Moreover, establishing genetic databases and biobanks specific to Sub-Saharan Africa could serve as a valuable resource for future studies and contribute to a global understanding of cardiomyopathies. Studying genetic factors in these populations may enhance our understanding of the genetic architecture of cardiomyopathies in general, but also identify population-specific variants and modifiers, and enable exploration of interactions with environmental factors.
In studying cardiomyopathies, it is essential to distinguish between rare, family-based cases and broader population-based genetic variations. As noted above, family-based studies often focus on highly penetrant variants that are simply inherited and will lead to better prediction of risk for cardiomyopathy within specific families. Moreover, having (affected) family members available for co-segregation analyses will support and improve variant interpretation and classification. In contrast, population-based studies often examine more complex patterns of genetic variation, involving common variants with lower penetrance that interact with environmental factors to increase disease susceptibility at a population level. Both approaches are important for understanding cardiomyopathies: family-based studies aid in identifying highly impactful variants and enable targeted family counseling, while population-based research provides insights into genetic modifiers and broader trends that inform population-level risk assessments and preventative strategies in sporadic cases.
Availability of genetic counseling and testing and cost benefits in Tanzania
The availability of genetic counseling and testing for cardiomyopathies in Tanzania is currently limited, with few genetic testing services available and limited expertise to interpret the outcome of genetic tests. This scarcity leads to underdiagnoses and missed opportunities for early or pre-symptomatic intervention. While efforts such as those led by the Tanzania Human Genetics Organization (THGO)35 have begun to address these gaps, but they remain in early stages. In contrast, more established initiatives like the Cardiovascular Genetics Group in Cape Town, South Africa (Cardiovascular Genetics (CVG) | Cape Heart Institute), offer valuable models for integrating clinical care, research, and training from which Tanzania can draw practical lessons.
The availability of genetic counseling and testing in Tanzania is influenced by various factors, including healthcare infrastructure, technological resources, logistical barriers in rural areas, and financial considerations. Financial constraints limit accessibility, as genetic testing can be costly, often exceeding USD $1000 per sample if it involves advanced technologies such as next-generation sequencing. It is not currently covered under national health insurance plans, making it inaccessible to many individuals and families in Tanzania. However, once an array of risk variants is identified in Tanzania, more economic chip- or PCR-based technologies may become available, for example, through adaptation of existing platforms such as GeneXpert for targeted variant detection.
Our proposed application of genetic testing will not only contribute to better population health but may also offer a cost-saving36. Evidence from Europe and the United States indicates that early genetic diagnosis and intervention in cardiomyopathies can reduce the need for high-cost emergency and advanced-stage interventions. For example, studies from the United States and Europe demonstrated that family screening and early diagnosis can prevent expensive emergency treatments and hospitalizations, leading to substantial cost savings in the long term37,38. While such cost-benefit data are available from high-income countries, the long-term cost implications of genetic testing in Tanzania have not yet been evaluated, highlighting a critical evidence gap, underscoring the need for locally conducted studies to establish similar evidence.
To address these challenges, several strategies can be implemented. Building partnerships and collaborations between Tanzanian healthcare institutions and international organizations or research institutions specializing in genetic testing is crucial39. These partnerships provide technical expertise, training, and support for establishing and maintaining genetic testing services. Investing in training programs for healthcare professionals, including laboratory technicians, specialists, bioinformaticians and genetic counselors, can enhance skills and knowledge necessary for the implementation and accurate interpretation of genetic tests. Integration of genetic testing into national healthcare policies and guidelines, along with the establishment of referral pathways and protocols, facilitates accessibility and appropriate identification of patients who can benefit from testing40.
To implement the abovementioned proposals, it is essential to increase public awareness and education about genetic testing. Community engagement initiatives, public health campaigns, workshops, and involvement of local leaders and influencers can reduce stigma, address misconceptions, and encourage individuals and families to seek testing services. It can also provide the political motivation for the redirection of healthcare resources. Investments in genetic testing, coupled with targeted screening programs, could not only mitigate the clinical burden of untreated cardiomyopathies but also align with cost-effective healthcare delivery by pre-emptively identifying at-risk individuals early in the disease course and implementing timely, low-cost interventions. Political support for genetic testing initiatives could significantly transform Tanzania’s healthcare landscape. The combination of political support and the near-to-medium-term improvements in technology will reduce costs and be critical to policy changes.
Ethical implementation of genetic counseling and testing in Tanzania requires urgent attention. Currently, most genetic testing occurs in research settings, and there is no formal diagnostic framework or national recognition of genetic counseling as a clinical discipline. Universities do not offer dedicated training, and genetic services are often ad hoc or externally supported. The THGO is working to bridge this gap by establishing infrastructure. With support from African, American and European partners, THGO has co-developed informed consent forms in Kiswahili and English, piloted healthcare worker sensitization programs, and created culturally relevant educational materials for patients and the public. These efforts aim to lay the groundwork for national integration of ethical and context-specific genetic counseling. However, to ensure patient autonomy, confidentiality, and protection against discrimination, government policies and professional guidelines must be developed in parallel with service expansion.
Potential solutions to challenges
To effectively address the challenges of implementing genetic counseling and testing for cardiomyopathies in Tanzania, a balanced approach is essential. Unlike Europe, where populations may have relatively similar genetic backgrounds, Africa’s vast diversity, encompassing over 3000 ethnic groups and more than 2100 languages across the continent, presents unique genetic variations that demand population-specific approaches41. In Tanzania alone, with over 120 ethnolinguistic groups, it is crucial to conduct targeted studies to understand cardiomyopathy prevalence and identify common genetic variants specific to these groups42. Such research will provide the insights necessary to develop testing strategies that address the distinct genetic landscape and healthcare needs of Tanzanian communities41,42. Establishing both international North–South partnerships (between high- and low-income countries) and South–South collaborations (among African and other low- and middle-income nations) is essential to maximize resource sharing, knowledge transfer, and capacity building. These collaborations can support sustainable infrastructure development, comprehensive training programs, and adherence to quality standards across diverse contexts.
Raising awareness among healthcare providers and the public is equally important. Education campaigns can foster support for genetic testing, while establishing ethical guidelines will help protect privacy and address concerns around stigma. Affordability can be improved by leveraging economies of scale, securing public-private partnerships, and exploring innovative funding models, especially as global testing costs continue to decrease.
Building local and regional capacity is essential to equip Tanzanian healthcare professionals with training in genetics, appropriate counseling, lab techniques, data analysis and variant interpretation and classification. Investing in sustainable laboratory infrastructure and focusing on context-appropriate technology will also ensure consistency in genetic testing services. Integrating genetic counseling, especially in rural areas, through mobile units or telemedicine, will improve accessibility to genetic services.
Finally, integrating genetic testing into the national healthcare system will allow for a streamlined approach for patient care. Establishing guidelines and referral pathways, along with fostering partnerships among genetic specialists, cardiologists, and other healthcare providers, will help deliver comprehensive, patient-centered care. Achieving this will require strong collaboration among government bodies, healthcare institutions, and international partners, alongside adequate funding and policy support to make genetic testing a sustainable part of healthcare in Tanzania.
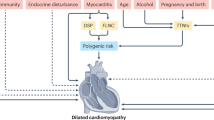
While this Perspective has primarily focused on Tanzania, the challenges and proposed solutions are broadly applicable to other low-resource settings across Africa, where cardiomyopathies and other genetic diseases are prevalent, but genetic testing capacity remains limited or unavailable (Table 1 and Fig. 1). Through sustained collaboration, investment, and commitment, it is possible to make genetic testing for cardiomyopathies a realistic and sustainable component of healthcare across the continent. A vision for addressing systemic gaps and building a sustainable cardiogenetics infrastructure in Tanzania is presented in Fig. 2.
Green areas represent countries where only clinical studies on cardiomyopathies have been conducted, indicating efforts to understand disease prevalence, types, and associated risk factors within the local population. Purple areas denote countries where genetic testing has been performed, reflecting an interest in identifying genetic variants and understanding hereditary aspects of cardiomyopathies. Green with stripes marks countries where both clinical studies and genetic testing have been undertaken, showcasing a more comprehensive approach that integrates epidemiological data with genetic insights. This map underscores the regional variability in research focus and resources, emphasizing areas where further genetic testing may enhance the understanding and management of cardiomyopathies in Africa.
This figure contrasts the current fragmented cardiogenetics landscape with a future, structured vision. The current system is characterized by informal referrals, lack of a national registry, limited diagnostic access, absence of trained professionals, and inadequate infrastructure. The future model proposes enabling pillars such as national policy support, family history-based triage, SMS-based appointment and follow-up systems, telemedicine integration, culturally and gender-sensitive counseling, bioinformatics training, AI-assisted variant classification, and sustainable partnerships. Central to the vision is a multidisciplinary and collaborative framework supported by local training, north–south and south–south collaboration, and digitized infrastructure for genetic testing and counseling.
Conclusions
In conclusion, while this paper does not introduce new genomic discoveries, it offers a practical and context-specific perspective to inform system-level planning for genetic counseling and testing of cardiomyopathies in Tanzania. Genetic testing can provide a pre-clinical diagnosis, allowing for precise classification that enables enhanced surveillance and personalized treatment approaches tailored to individual patients, optimizing treatment response, and reducing adverse effects. Investing in genetic testing would also contribute to building a comprehensive genetic database, advancing research, and potentially discovering novel therapeutic targets that can address not only CM but also other genetic conditions prevalent in the region.
References
Braunwald, E. Cardiomyopathies. Circ. Res. 121, 711–721 (2017).
Alimohamed, M. Z. et al. Diagnostic yield of targeted next generation sequencing in 2002 Dutch cardiomyopathy patients. Int. J. Cardiol. 332, 99–104 (2021).
Ntusi, N. A. B. & Sliwa, K. Impact of racial and ethnic disparities on patients with dilated cardiomyopathy: JACC focus seminar 7/9. J. Am. Coll. Cardiol. 78, 2580–2588 (2021).
Wexler, R. K., Elton, T., Pleister, A. & Feldman, D. Cardiomyopathy: an overview. Am. Fam. Physician 79, 778–784 (2009).
Moharem-Elgamal, S., Sammut, E. & Stuart, G. Genetic counseling in inherited cardiomyopathies. JACC Case Rep. 2, 392–395 (2020).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
McKenna, W. J., Maron, B. J. & Thiene, G. Classification, epidemiology, and global burden of cardiomyopathies. Circ. Res. 121, 722–730 (2017).
Minja, N. W. et al. Cardiovascular diseases in Africa in the twenty-first century: gaps and priorities going forward. Front. Cardiovasc. Med. 9, 1008335 (2022).
Shaboodien, G. et al. Genetics of inherited cardiomyopathies in Africa. Cardiovasc. Diagn. Ther. 10, 262–278 (2020).
Reichart, D., Magnussen, C., Zeller, T. & Blankenberg, S. Dilated cardiomyopathy: from epidemiologic to genetic phenotypes. J. Intern. Med. 286, 362–372 (2019).
Tsabedze, N. et al. The genetic basis for adult-onset idiopathic dilated cardiomyopathy in people of African descent. Heart Fail. Rev. 28, 879–892 (2023).
Fundikira, L. S. et al. Risk factors and prevalence of dilated cardiomyopathy in Sub-Saharan Africa: a systematic review. Glob. Heart 17, 76 (2022).
Sliwa, K., Damasceno, A. & Mayosi, B. M. Epidemiology and etiology of cardiomyopathy in Africa. Circulation 112, 3577–3583 (2005).
Fundikira, L. S. et al. Characterization of non-ischemic dilated cardiomyopathy in a native Tanzanian cohort: MOYO study. Glob. Heart 19, 26 (2024).
Musunuru, K. et al. Genetic testing for inherited cardiovascular diseases: a scientific statement from the American Heart Association. Circ. Genom. Precis. Med. 13, e000067 (2020).
Chillo, P., Humphrey, S. H., Meda, J. & Kerry, V. B. Cardiac critical care in resource-limited environments: lessons from Tanzania. Glob. Heart 9, 311–318 (2014).
Slunecka, J. L. et al. Implementation and implications for polygenic risk scores in healthcare. Hum. Genom. 15, 46 (2021).
Sturt, J. et al. Safety and upscaling of remote consulting for long-term conditions in primary health care in Nigeria and Tanzania (REaCH trials): stepped-wedge trials of training, mobile data allowance, and implementation. Lancet Glob. Health 11, e1753–e1764 (2023).
Onsongo, S. & Kagotho, E. Telemedicine in Africa: applications, opportunities, and challenges. in (eds Heston, T. F. & Doarn, C. R) A Comprehensive Overview of Telemedicine, https://doi.org/10.5772/intechopen.1005094 (IntechOpen, 2024).
Mmbali, S. & Chillo, P. Applicability of structured telephone monitoring to follow up heart failure patients discharged from Muhimbili National Hospital, Tanzania. Tanzan. J. Health Res. 19, 1–9 (2017).
Percept Actuaries & Consultants; Cenfri. Insurance and Telemedicine in Africa: A Moonshot in Response to COVID-19. United Nations Development Programme (UNDP) Insurance & Risk Finance Facility (IRFF); 2022. Available at: https://irff.undp.org/publications/insurance-and-telemedicine-africa-moonshot-response-covid-19
Jordan, E. et al. Genetic architecture of dilated cardiomyopathy in individuals of African and European ancestry. JAMA 330, 432–441 (2023).
Sirugo, G., Williams, S. M. & Tishkoff, S. A. The missing diversity in human genetic studies. Cell 177, 26–31 (2019).
Herman, D. S. et al. Truncations of titin causing dilated cardiomyopathy. N. Engl. J. Med. 366, 619–628 (2012).
Manrai, A. K. et al. Genetic misdiagnoses and the potential for health disparities. N. Engl. J. Med. 375, 655–665 (2016).
Marian, A. J. & Braunwald, E. Hypertrophic cardiomyopathy. Circ. Res. 121, 749–770 (2017).
Mukhopadhyay, S., Dixit, P., Khanom, N., Sanghera, G. & McGurk, K. A. The genetic factors influencing cardiomyopathies and heart failure across the allele frequency spectrum. J. Cardiovasc. Transl. Res. 17, 1119–1139 (2024).
Ciesielski, T. H., Sirugo, G., Iyengar, S. K. & Williams, S. M. Characterizing the pathogenicity of genetic variants: the consequences of context. npj Genom. Med. 9, 3 (2024).
Clarke, S. L., Assimes, T. L. & Tcheandjieu, C. The propagation of racial disparities in cardiovascular genomics research. Circ. Genom. Precis. Med. 14, e003178 (2021).
Kraus, S. M. et al. Rationale and design of the African Cardiomyopathy and Myocarditis Registry Program: the IMHOTEP study. Int. J. Cardiol. 333, 119–126 (2021).
Alimohamed, M. Diagnostic applications of next-generation sequencing. https://doi.org/10.33612/diss.215887939 (University of Groningen, 2022).
Alimohamed, M. Z. et al. Validation of new gene variant classification methods: a field-test in diagnostic cardiogenetics. Front. Genet. 13, 824510 (2022).
Cohen, J., Boerwinkle, E., Mosley, T. & Hobbs, H. Sequence variations in PCSK9, Low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Hilfiker-Kleiner, D., Haghikia, A., Nonhoff, J. & Bauersachs, J. Peripartum cardiomyopathy: current management and future perspectives. Eur. Heart J. 36, 1090–1097 (2015).
Alimohamed, M. Z. et al. Inauguration of the Tanzania Society of Human Genetics: biomedical research in Tanzania with emphasis on human genetics and genomics. Am. J. Trop. Med. Hyg. 104, 474–477 (2021).
Xi, Q., Jin, S. & Morris, S. Economic evaluations of predictive genetic testing: a scoping review. PLoS ONE 18, e0276572 (2023).
Ackerman, M. J. et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 8, 1308–1339 (2011).
Hershberger, R. E. et al. Genetic evaluation of cardiomyopathy—a heart failure society of america practice guideline. J. Card. Fail. 24, 281–302 (2018).
Martin, A. R., Teferra, S., Möller, M., Hoal, E. G. & Daly, M. J. The critical needs and challenges for genetic architecture studies in Africa. Curr. Opin. Genet. Dev. 53, 113–120 (2018).
Adebamowo, S. N. et al. Implementation of genomics research in Africa: challenges and recommendations. Glob. Health Action 11, 1419033 (2018).
Pfennig, A., Petersen, L. N., Kachambwa, P. & Lachance, J. Evolutionary genetics and admixture in African populations. Genome Biol. Evol. 15, evad054 (2023).
Tetikol, H. S. et al. Pan-African genome demonstrates how population-specific genome graphs improve high-throughput sequencing data analysis. Nat. Commun. 13, 4384 (2022).
Adadi, N. et al. Inherited dilated cardiomyopathy in a large Moroccan family caused by LMNA mutation. Anatol. J. Cardiol. 20, 65–68 (2018).
Woodiwiss, A. J. et al. Beta1- and alpha2c-adrenoreceptor variants as predictors of clinical aspects of dilated cardiomyopathy in people of African ancestry. Cardiovasc. J. Afr. 19, 188–193 (2008).
Fish, M. et al. Correction: corrigendum: mutation analysis of the phospholamban gene in 315 South Africans with dilated, hypertrophic, peripartum and arrhythmogenic right ventricular cardiomyopathies. Sci. Rep. 6, 25863 (2016).
Matolweni, L. O. et al. Arrhythmogenic right ventricular cardiomyopathy type 6 (ARVC6): support for the locus assignment, narrowing of the critical region and mutation screening of three candidate genes. BMC Med. Genet. 7, 29 (2006).
Watkins, D. A. et al. Clinical features, survival experience, and profile of plakophylin-2 gene mutations in participants of the Arrhythmogenic Right Ventricular Cardiomyopathy Registry of South Africa. Hear. Rhythm 6, S10–S17 (2009).
Mouton, J. M. et al. Diagnostic disparity and identification of two TNNI3 gene mutations, one novel and one arising de novo, in South African patients with restrictive cardiomyopathy and focal ventricular hypertrophy. Cardiovasc. J. Afr. 26, 63–69 (2015).
Author information
Authors and Affiliations
Contributions
M.Z.A. conceptualized the paper with S.M.W. M.Z.A. wrote the first draft of the paper. All authors, A.K., L.F., N.L., H.M., R.S., J.D.H.J., R.E.H., S.M.W., F.W.A., and P.C. provided critical input, contributed to manuscript revisions, and approved the final submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Gasnat Shaboodien and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alimohamed, M.Z., Kiula, A., Fundikira, L. et al. Systemic barriers and opportunities for equity in early implementation of genetic testing and counseling for cardiomyopathies in Tanzania. Commun Med 6, 3 (2026). https://doi.org/10.1038/s43856-025-01244-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-01244-4