Abstract
Accurate diagnosis of frontotemporal dementia (FTD) with right anterior temporal lobe (RATL) predominance remains challenging due to lack of clinical characterization, and standardized terminology. The recent research of the International Working Group (IWG) identified common symptoms but also unveiled broad terminologies lacking precision and operationalization, with risk of misdiagnoses, inappropriate referrals and poor clinical management. Based on the published evidence (91267 articles screened) and expert opinion (105 FTD specialists across 52 centers) by using the nominal group technique, the IWG delineates three primary domains of impairment causing behavioral, memory and language problems: (i) multimodal knowledge of non-verbal information including people, living beings, landmarks, flavors/odors, sounds, bodily sensations, emotions and social cues; (ii) socioemotional behavior encompassing emotion expression, social response and motivation; and (iii) prioritization for focus on specific interests, hedonic valuation and personal preferences. This study establishes a consensus on clinical profile, phenotypic nomenclature, and future directions to enhance diagnostic precision and therapeutic interventions.
Similar content being viewed by others
Introduction
Frontotemporal dementia (FTD) is one of the leading causes of dementia before age 65, and selectively impacts circuits in the frontal and anterior temporal lobes (ATL) that support behavior and language1,2,3. Despite the extensive study of semantic aphasia (left temporal dominant) and behavioral variant (frontal dominant) FTD syndromes, the syndrome of FTD related to pathology predominant in the right ATL (RATL) lacks standardized nomenclature and consensus diagnostic criteria4. Although the diagnostic criteria for svPPA and earlier criteria for FTD and semantic dementia (SD) allude to RATL involvement, the syndrome has been relatively neglected as a separate entity in the literature. The problem goes beyond the lack of a categorical term for the syndrome, with confusion or inconsistency in the very phenomenological characterization.
Diverse and sometimes conflicting descriptions have been applied to the symptoms of FTD with RATL predominance. Hyper-religiosity, once regarded as virtually pathognomonic for this subtype, has been widely observed in numerous case reports, with influences from the “Geschwind Syndrome,” a concept derived from epilepsy literature that includes hyper-religiosity, hypergraphia, hyposexuality, and irritability5. Early publications with larger sample sizes highlighted prosopagnosia, parsimony, preoccupations, lack of empathy and compulsive behavior, while some emphasized loss of object knowledge and visual semantic deficits as core features3,6,7,8,9. Other studies pointed to memory deficits, mental rigidity, somatization, topographagnosia, atypical depression, slowness, hallucinations, and delusions10,11,12. Furthermore, a wide spectrum of singular case studies reported dysprosody, altered emotional expression, parosmia, gustatory agnosia, phonagnosia, musicophilia or amusia, visceral agnosia, and an array of emergent obsessions ranging from strong political or religious beliefs to artistic skills13,14,15,16,17,18,19,20,21. Most recently, some groups have proposed that socioemotional semantic deficits and altered hedonic valuation are the primary mechanisms underpinning the behavioral changes commonly seen in the RATL syndrome22,23,24.
We established an international working group (IWG) in 2020 to elucidate the clinical characteristics of the syndrome, resolve contradictions in the field, and promote consensus on terminology for FTD presenting with RATL predominance. Our initial multi-cultural publication, which included 360 patients, showed that common symptoms included mental rigidity/preoccupations (78%), disinhibition/socially inappropriate behavior (74%), naming/word-finding difficulties (70%), memory deficits (67%), apathy (65%), loss of empathy (65%), and face-recognition deficits (60%), as well as impairments regarding landmarks, smells, sounds, tastes, and bodily sensations (74%). Some of these were not specifically inquired about in many centers and others were interpreted heterogeneously using diverse terminologies4. Many symptoms in this study were described using broad terminologies such as “disinhibition” and “word-finding difficulties”, which either lacked or misattributed underlying mechanisms. These descriptions were typically based on clinicians’ observations or informant-based surveys. The limited use of objective, face-to-face assessments for common and specific RATL symptoms led to heterogeneous interpretations. While available test results indicated deficits in the semantics of emotion, people, social interactions, and visual stimuli, we lacked objective assessments and operationalization for mental rigidity and preoccupations, despite the high prevalence4.
Lastly, our most recent multicultural cohort revealed that 80% of patients have no genetic variant or family history, which will be detailed in our forthcoming publication. Nevertheless, given the sporadic nature of the disease, early and accurate diagnosis is particularly challenging worldwide, often leading to undiagnosed or misdiagnosed cases as psychiatric disorders. Therefore, early and precise diagnoses, which are essential for improving patient care, advancing research, and facilitating clinical trial inclusion, primarily rely on thorough clinical assessments.
These results underscore the urgent need for consensus on terminologies that elucidate primary cortical dysfunctions and can be readily applied in everyday clinical practice globally. This is the first international initiative to address existing gaps and offer consensus recommendations by conducting a thorough systematic review and gathering expert opinions.
Methods
Establishment of the IWG
HU and YP reviewed the international literature to identify the authors/centers that might have an interest in FTD with RATL, based on their scientific reports and/or clinical cohort characteristics. Clinician and researcher partners including neurologists, psychiatrists, psychologist specializing in FTD were invited via email and/or zoom meetings to collaborate in the project. Initial invitations were extended to multiple centers, and subsequent Zoom meetings were held to outline the objectives of the IWG and the specific project. This resulted in a positive reaction for collaboration from 18 centers including the United States, United Kingdom, Italy, France, Belgium, Germany, Spain, Sweden, Canada, Turkey, Brazil, and the Netherlands. The invitation remained open to allow additional centers to join subsequent round table meetings, which aimed to achieve consensus on various aspects including aims, methodology, study designs, generation of the symptom checklist for data collection, data analyses, interpretation of the collected data, terminology, recommendations, and future directions. Over time, new collaborators have joined the collaboration that contributed to the round table discussions, although they could not provide patient data for the retrospective study. Currently, the IWG contains 52 centers across the world with more than 100 early-career and senior FTD experts. The full list of participating investigators is provided below (see Investigators of the International Working Group).
Systematic review
A comprehensive review in MEDLINE (PubMed) and Embase until February 2024 was performed. Thirteen separate systematic reviews were conducted to tackle each RATL-relevant clinical symptom identified in our previous study and literature. Included symptoms were “person specific knowledge deficit” (Supplementary Fig. 1), “lack of empathy“ (Supplementary Fig. 2), “disinhibition” (Supplementary Fig. 3), deficits regarding “taste” (Supplementary Fig. 4), “sound” (Supplementary Fig. 5), “smell” (Supplementary Fig. 6), “landmarks” (Supplementary Fig. 7), “bodily sensations” (Supplementary Fig. 8), “visual information” (Supplementary Fig. 9), as well as “memory deficits” (Supplementary Fig. 10), “apathy” (Supplementary Fig. 11), “mental rigidity” (Supplementary Fig. 12), and “psychiatric symptoms” (Supplementary Fig. 13). Search terms for each symptom were discussed and identified by the IWG consensus to increase the sensitivity of the search. (i.e., for person specific knowledge, search terms; “prosopagnosia” OR “associative prosopagnosia” OR “face recognition deficit” OR “person knowledge” OR “semantics for people” OR “face agnosia” OR “face blindness” OR “person identification” OR “person-specific” OR “person recognition” OR “face recognition”, [see the Supplementary Table 1 in Supplementary Data File 1 for the search-terms of other symptoms]). Subsequently each symptom was attached with the following terms “frontotemporal dementia” OR “semantic dementia” OR “semantic variant primary progressive aphasia” OR “behavioral variant frontotemporal dementia” OR “temporal variant frontotemporal dementia” OR “frontotemporal lobar degeneration” OR “Pick’s Disease” OR “right temporal” OR “right anterior*” OR “temporal pole”, “frontotemporal dementia AND right temporal”, “semantic dementia”, “semantic variant primary progressive aphasia AND right”, “behavioral variant frontotemporal dementia AND right”, “temporal variant frontotemporal dementia”, and “frontotemporal lobar degeneration AND right temporal”.
Search strategy
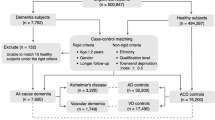
The systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Relevant studies were retrieved using keywords in Supplementary Table 1 in Supplementary Data File 1 from MEDLINE (PubMed) and Embase databases as well as other sources, covering the literature until February 2024. Search results are summarized in Supplementary Table 2 in Supplementary Data File 2, with PRISMA flow diagrams provided in Supplementary Figs. 1–13. The titles and abstracts of the citations were screened by 3 independent authors (HU, MM, and KY) to determine their relevance for inclusion. Full-text articles of the relevant citations were then assessed to determine whether the study meets the predefined criteria for RATL case identification (Fig. 1).
Individuals were included if they met at least one established clinical diagnostic criterion for frontotemporal dementia (FTD) (Neary 1994; Neary 1998; McKhann 2001; Rascovsky 2011), semantic dementia (SD; Neary 1998), primary progressive aphasia (PPA; Gorno-Tempini 2011), or frontotemporal lobar degeneration (FTLD; MacKenzie & Neumann 2016). Patients were included when clinical, imaging, or pathological findings indicated right-temporal variant FTD, right-predominant semantic-variant PPA (svPPA), right SD, semantic behavioral variant FTD (sbvFTD, or right anterior temporal lobe atrophy. Cases were excluded if they demonstrated left-predominant svPPA/SD, non-fluent variant PPA (nfvPPA), logopenic variant PPA (lvPPA), or frontal-predominant FTD without right temporal predominance.
Studies were excluded when no original data were reported (letters to the editor, meta-analyses, or review studies). Mendeley reference manager (v2.114.0) was used to register all citations. Duplicated studies were removed based on overlapping authorship, study description, year of publication, and journal. Unlike other published systematic reviews on this topic, even if the prevalent RATL atrophy was not mentioned in the titles or abstracts, if there was a bvFTD, svPPA, or SD cohort, full texts were screened to identify potential cases with predominant RATL atrophy. The identification of FTD cases with predominant RATL atrophy is illustrated in Fig. 1, and guidelines for visual atrophy rating are provided below. Initial discordance between the three primary assessors was resolved through consensus or through the decision of a fourth author (YP). Consequently, all included articles were shared with the IWG for their review, and additional articles were included by the IWG if they were not in the initial search. Full-text articles of the relevant citations were then assessed to determine whether the study met the predefined inclusion criteria. The study quality of all included articles was assessed using the IWG’s guidelines including a 6-point checklist assesses the rigor of inclusion criteria and subject selection, sample size, diversity (ethnicity/ country), biomarker, genetic and pathological confirmation availability, and measurement of symptoms (Supplementary Table 3 in Supplementary Data File 3).
Atrophy rating guidelines
If not explicitly reported in the original articles, RATL atrophy was determined through visual inspection of structural neuroimaging, following established visual atrophy rating guidelines25. All included patients demonstrated greater atrophy severity (at least one grade higher) in the RATL compared with other brain regions. Patients with marked frontal or left temporal atrophy (≥3, on a 0–4 scale), even if they had predominant RATL atrophy, were excluded to minimize gross effects of general neurodegeneration.
Consensus approach
A series of round table meetings was organized to reach consensus on aims, methodology, generation of the terminologies, and future directions. The nominal group technique (NGT) which combines qualitative and quantitative data and encourages and enhances the participation of group members was used to deliver rapid and reliable results to have a consensus and generate ideas/solutions to be used in the project26. Given the large number of experts involved, their varying levels of expertise, the complexity of the subject matter, and the fact that several IWG members are renowned specialists who have devoted their careers to specific symptoms in our study, we required a method that would allow for focused qualitative depth, real-time dialog, direct face-to-face interaction (via Zoom), and rapid turnaround without relying on multiple iterations or participant anonymization. Because of these needs, the NGT was deemed more suitable than other methods such as the Delphi technique27. After completing the systematic review, preliminary results were documented, and surveys were prepared to systematically collect the opinions of the collaborators. The surveys addressed the following topics: (1) interpreting and categorizing the listed symptoms; (2) rating previously used terminologies; (3) proposing new terminologies if existing ones did not adequately represent the clinical presentation; (4) rating previously used formal names for the syndrome; (5) proposing new formal name if previously used names did not adequately represent the clinical syndrome. All survey results were shared and discussed in the structured round table meetings to establish consensus on recommended terminology for symptoms and a formal name for the syndrome. All round table meetings were recorded to be further coded to capture the interpretations shared by the experts. In cases where members were unable to participate in the discussion or had additional comments, they were encouraged to watch the recorded videos and share their opinions via email. Subsequently, to determine the most appropriate terminology, another survey was conducted wherein each proposed term for each symptom was rated by the experts. A 70% agreement threshold was set for consensus. Finally, prior to manuscript submission, a final round table meeting and online discussion forum was held to determine whether the findings of this retrospective study were sufficient to establish operational diagnostic criteria (for further details regarding the design of the round table meetings, see the IWG’s previous publication28).
Results
Terminology
Supplementary Table 4 in Supplementary Data File 4 presents real-life examples from the IWG’s dataset and illustrates how these examples are interpreted by caregivers in terms that belie the underlying cognitive deficit. For instance, knowledge loss for people may be perceived as a memory (e.g., “She doesn’t remember people…”), language (e.g., “He cannot name actors on TV…”), or a behavioral issue (e.g., “She behaves as if she is talking to strangers on the phone when she is actually talking to close friends and family”) (see examples for each symptom in Supplementary Table 4 in Supplementary Data File 4). In the following section, the IWG meticulously reviewed each identified symptom from our previous work and the existing literature. The group conceptualized these symptoms by considering underlying neural mechanisms and provided recommended terminology and clinical guidelines to better capture these symptoms in daily clinical practice (Supplementary Table 4 in Supplementary Data File 4, Box 1). All terminologies identified in this process met or exceeded the consensus threshold.
Multimodal knowledge loss for non-verbal information
The IWG clustered the following symptoms under this broad category, pointing out that the main deficit is “knowledge loss” for many categories (each discussed in detail below) via multimodal (more than one modality, i.e., not only visual) “non-verbal” stimuli (without using language, i.e., visual, auditory, gustatory, or olfactory).
RATL and knowledge loss for people
Face recognition deficits are prominently observed in patients with predominant RATL atrophy, and extensively studied across various research groups (Supplementary Table 3 in Supplementary Data File 3). While commonly labeled as “prosopagnosia,” recent literature employing rigorous face-to-face assessments indicates that RATL-related impairments differ significantly from those related to posterior cortical areas primarily involved in basic face perception. Instead, these deficits are associated with a multimodal loss of person-specific knowledge, encompassing recognition difficulties with voices, biographical details, and faces, collectively termed as “person-specific knowledge” or “person-based semantics”8,17,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44. These studies have shown that patients with RATL atrophy often demonstrate intact basic face perception and discrimination skills, but struggle to identify people from photographs or recognize them by voice. They therefore also exhibit deficits in assessing familiarity, semantic associations and providing semantic information. Some studies have reported that knowledge of infrequent acquaintances or famous public figures deteriorates earlier than that of close family members, analogous to the early loss of less familiar/frequent words in the left hemisphere counterpart, svPPA38. Several authors have highlighted that semantic knowledge about people can be spared when individuals are provided with names or verbal definitions rather than pictures33,36,38,39,40,45. Moreover, comparative studies of RATL versus LATL atrophy in semantic dementia reveal distinct patterns of impairment. A strong anatomical correlation has been found between face-to-name and voice-to-name matching performance and the right temporal lobe but not the left46, and patients with predominant LATL atrophy identify faces from pictures (visual task) better than their names (verbal task), whereas those with RATL atrophy show the opposite pattern32,34,35, suggesting the crucial role of LATL in verbal semantics, and RATL in non-verbal processing. These laterality effects are likely to be a matter of degree, rather than binary distinctions47 and may dissipate as disease progression leads to bilateral ATL damage. In a detailed case study, the patient was also unable to describe her feelings about the ‘recognized’ person, as an aspect of semantic loss, beyond difficulties in providing biographical information. In addition, she used general semantic knowledge to identify people. For example, she would promptly recognize her grandson in his mechanic’s overall but not in ordinary clothes. Similarly, she would identify the local priest only when he wore his black cassock during church services39. These examples highlight the complexity of patients’ adaptive mechanisms, underscoring the difficulties in capturing such symptoms unless they are systematically inquired about and objectively tested. Existing face-to-face tests are mainly available for Western populations’ references images and delivered in English. Cultural adaptation of those tests is essential, both in language and in the cultural familiarity of test materials. Additionally, current validated tools assess person identity via static pictures. Novel ecologically valid, and culturally sensitive tools that combine visual and auditory information are warranted (Supplementary Table 4 in Supplementary Data File 4).
RATL and knowledge loss for other living beings
Object recognition deficits, naming, and word-finding difficulties are other common symptoms reported by several groups6,10,22,48. However, studies using standardized tests indicate that these deficits are category-specific. Beyond person-based knowledge, research has shown that patients with RATL atrophy experience knowledge loss for living beings, leading to recognition, naming, and word-finding difficulties39,49,50,51,52. Comparative studies have demonstrated that while left-predominant SD affects both animate and inanimate words equally due to language involvement, right-predominant SD, with greater language sparing, continues to impair other semantic aspects related to animals49. Other studies using multi-modal stimuli reported poor knowledge of sensory attributes and consistently greater impairment for living things52,53. A more specialized study documented difficulties in recognizing birds by their calls in a bird expert with RATL atrophy51, while another reported a deficit in mushroom identification in an experienced mushroom gatherer with RATL atrophy39. This evidence highlights the need for more detailed cognitive assessments to identify such deficits in patients with RATL atrophy. Better test designs and prospective multicultural studies are needed to elucidate whether the impairment is confined to socioemotionally relevant living beings or extends to all living entities, and to assess if this symptom is an early marker of the syndrome. Additionally, given the reported similar deficits in patients with predominant LATL atrophy54, prospective comparative studies are warranted to elucidate whether these deficits are part of a broader conceptual system underpinned by a bilaterally-implemented, functionally-unitary semantic hub in the ATLs. Currently, a few experimental tasks are available, but there is no validated tool for clinical use. During tool development and/or validation, cultural sensitivities should be carefully considered (Supplementary Table 4 in Supplementary Data File 4).
RATL and knowledge loss for landmarks, monuments and places
Deficits regarding landmarks and monuments have been reported by several groups, despite the use of heterogeneous terminologies for the ensuing phenomena, including “getting lost,” “topographagnosia,” “wayfinding difficulties,” “knowledge loss for places” or “semantic deficit for landmarks”10,11,12,22,29,30,38,42. Although most studies rely on clinical observations, some groups have assessed this deficit using quantitative tests, reflecting knowledge loss for landmarks and monuments29,30,38,42. One detailed case study provided deeper insights into the nature of the deficit in RATL degeneration30. In this study, the patient’s wayfinding abilities in a familiar environment (i.e., his hometown) were preserved despite an inability to recognize familiar and famous buildings, monuments, and landmarks. Wayfinding was achieved through heavy reliance on written indications (e.g., names of restaurants and streets), preservation of a pre-existing cognitive map of the familiar environment, normal executive functions necessary to plan the execution of a given trajectory, and an over-reliance on processing local features. This distinguishes the deficit from navigational impairment following damage to the medial temporal lobe. Naming (4/20) and identifying (6/20) famous monuments, as well as familiarity, were significantly impaired when presented with photographs. In contrast, upon verbal presentation of the names of these famous monuments, he correctly identified 17/20 of them. Although not systematically tested initially, the patient was also unable to recognize pictures of famous places he had visited and pictures of famous monuments in his hometown30. Considering the high prevalence of place orientation deficits and getting lost in Alzheimer’s disease (AD), improved terminologies and standardized assessments are crucial for differentiation and accurate diagnosis of FTD patients with RATL predominance. It is crucial to investigate in multicultural cohorts whether these modalities manifest at early stages. Although some experimental tasks are currently available, there is a lack of validated clinical tools. It is crucial to consider cultural sensitivities during the development and validation processes (Supplementary Table 4 in Supplementary Data File 4).
RATL and knowledge loss for flavors and odors
Clinical studies based on retrospective analyses of medical records frequently identify this deficit4,32,39,51,55,56. Although this domain has not been as extensively studied by employing objective tests, as ‘person knowledge,’ substantial evidence indicates that patients with predominant RATL atrophy experience semantic knowledge loss for flavors and odors, tested with gustatory and olfactory stimuli, despite retaining intact taste and smell perception abilities14,17,57. One study reported impaired identification of food elements and the inability to associate them with semantically related content (e.g., edible versus inedible items), despite recognizing them when provided with their names39. Another study described a patient who noted his favorite food smelled strange, and the slightest food odor became intolerable, described as “foul,” “rotten,” or “like sewage,” leading to significant weight loss due to reduced food intake14. Several authors suggest that “the strong food preference” observed in RATL neurodegeneration may be due to semantic degradation for foodstuffs, narrowing their preferences14,57. However, it remains debated whether chemosensory alterations (taste, smell) represent a pure semantic deficit or a broader deficit in hedonic valuation24. It is necessary to objectively test whether deficits related to taste and smell stimuli constitute a multimodal non-verbal semantic deficit and if so, whether these deficits are category-specific (e.g., living beings, social context-related items) in larger samples. Furthermore, although such deficits have been reported as early symptoms of the syndrome, prospective multicultural comparative studies are needed to elucidate the contribution of LATL atrophy and to determine the impact of the culture in food appreciation, as well as the onset and distinctiveness of these symptoms. At present, a few experimental tasks exist, but no tools have been validated for clinical application. Attention to cultural nuances is essential throughout development and validation (Supplementary Table 4 in Supplementary Data File 4).
RATL and knowledge loss for sounds
Studies focusing on RATL degeneration and sounds have primarily examined human voices, as discussed above (see Section, RATL and knowledge loss for people). However, data using detailed assessments that include otorhinolaryngological examinations and tests for perception, identification, discrimination, familiarity, semantic association, and naming have revealed knowledge loss for accents, songs, vocal tones, and melodies as well as prosody, in patients with RATL neurodegeneration43,58,59,60,61,62. Neuroimaging studies have found significant correlations between famous music recognition deficits and right temporal pole atrophy60, and between atrophy in the right supra-marginal and superior temporal gyri and deficits in detecting violated sounds and melodies. Additionally, atrophy in the bilateral anterior temporal poles and left medial temporal structures was related to deficits in environmental sound recognition59. Furthermore, post-hoc analysis in this study showed that the right predominant SD group had significant impairments in both melody and environmental sound tasks, scoring lower for living superordinate categories (animals, humans), although this was not statistically significant59. Another case study reported a patient with bilateral superior temporal lobe atrophy who lost expertise as a telephone operator, demonstrating deficits in human voice identification, naming, and familiarity63. Despite these observations, the limited amount of evidence highlights that this domain still awaits better-designed studies to determine its category specificity, clinical prevalence in the early stages of the syndrome, and distinctiveness from LATL neurodegeneration. The field has some experimental tasks but lacks clinically validated tools. Cultural considerations should be integral to the development and validation phases (Supplementary Table 4 in Supplementary Data File 4).
RATL and knowledge loss for bodily sensations
Another ubiquitous symptom in the RATL literature is altered responses of patients to sensations, mostly termed as “somatization”, “hypochondria”, or “alexisomia”10,64. The lack of knowledge related to interoceptive stimuli and their interpretation, leading to misidentification of normal bodily sensations, has been suggested as the underlying mechanism18,64. Patients exhibit a variety of complaints of behaviors related to this impairment, including unidentified pains, aches, numbness, itching, tinnitus, discomforts; feelings of warmth or cold; abnormal sensations in the bladder, bowel, thorax, abdomen, head and stomach; inappropriate reactions to own bodily odors, hunger, regurgitation, borborygmi, running nose, sweating, and fatigue. Normal interceptive signals may be considered as indicators of disease, which may be reported as hypochondriasis, illness anxiety disorder, or Cotard syndrome10,18,64. Recent studies using cardiac monitoring during emotional stimuli have shown significantly impaired interoception in predominant RATL neurodegeneration compared to those with predominant LATL atrophy65. The authors suggested that impaired emotion recognition in the RATL syndrome is driven by inaccurate internal monitoring65. Conversely, another group found preserved cardiac reactivity during emotional stimuli in ATL syndromes, whereas cardiac reactivity was attenuated in groups with predominant fronto-insular atrophy (bvFTD and nonfluent PPA)66. Another group suggested that a decline in the parasympathetic nervous system may contribute to reductions in interpersonal engagement and gregariousness/extraversion, personality changes that are especially common in the RATL syndrome67. Nevertheless, these hypotheses need to be tested in larger cohorts to elucidate the neural mechanisms and determine whether they are early characteristic symptoms of the syndrome and whether the bodily sensation of emotion dissipates before the cognitive recognition of the emotion, or vice versa68. As discussed in the previous sections, a consistent theme emerges: while there are a few experimental tasks available, the absence of validated tools for clinical use remains a significant gap. Moving forward, it is imperative that cultural sensitivities are incorporated into every stage of tool development and validation (Supplementary Table 4 in Supplementary Data File 4).
RATL and knowledge loss for emotions, social information and paralinguistic cues
Emotion recognition deficit is one of the most frequently reported symptoms in patients with RATL atrophy. Various emotion recognition tasks (see Supplementary Table 3 in Supplementary Data File 3) have been used globally, confirming these deficits across different studies22,65,69,70,71,72. Comparative studies have shown that patients with right temporal predominance at early stages perform worse on facial emotion selection tasks compared to other FTD subtypes, including bvFTD and svPPA22,72. Recent work has shown that comprehending facial cues is not limited to emotions. A study using video-based dynamic stimuli (including facial expressions, body gestures, and vocal cues) demonstrated a sarcasm detection deficit in patients with predominant RATL atrophy, who considered the actors’ statements literally without reading paralinguistic cues. This deficit was worse in RATL patients than in their left temporal and frontal FTD counterparts22. A PET study indicated that FTLD patients with right superior ATL hypometabolism were significantly more impaired on social concepts (e.g., “polite,” “stingy”) than on animal function concepts (e.g., “trainable,” “nutritious”)73. The same group, administering a similar word task in a healthy population with fMRI, confirmed their results, finding that the bilateral superior ATLs, particularly the right side, are selectively activated when participants judge the meaning relatedness of social concepts (e.g., honor–brave) compared with concepts describing general animal functions (e.g., nutritious–useful)74. A case report assessing knowledge of social information using a standardized test of general knowledge of public events and figures, found that the patient’s scores were significantly impaired in the visual identification part of the semantic test (a picture of a famous public event, such as the explosion of the atomic bomb, the destruction of the Berlin Wall, etc.,) while performance was within the normal range for the verbal part of the test38. Another study using a social interaction vocabulary test found a correlation between bilateral ATL atrophy and lower performances on this test. Notably, in this test, the definition of the social interaction was provided verbally, and participants were asked to match the word with a picture75. These studies support the argument that while the RATL is crucial for non-verbal comprehension of socioemotional concepts (paralinguistic, visual, vocal), the LATL supports verbal comprehension76,77. However, more meticulous work with larger sample sizes is needed to disentangle the contributions of each ATL to knowledge for socioemotional concepts. Existing face-to-face tests are mainly available for Western, English-speaking populations. During tool development and/or validation, cultural sensitivities and ecological validity should be considered (Supplementary Table 4 in Supplementary Data File 4).
Altered socioemotional behavior
A second domain affected in FTD with RATL predominance according to the IWG was altered socioemotional behaviors, characterized by either exaggerated responses or a lack of reaction, or altered motivation for social interactions.
RATL and altered emotional expression
Beyond emotion recognition deficits, several groups have reported other social cognition deficits such as inappropriate emotional expression (facial and prosodic) or theory of mind (ToM) (mentalizing) impairments in patients with RATL atrophy. This is often described as a ‘lack of empathy’ by caregivers and clinicians,13,16,20,22,78,79,80,81. A recent study found a restricted prosodic range in this group compared to svPPA and healthy controls, which was associated with a reduction in empathy, as observed by caregivers13. Another study showed that the subgroup with RATL atrophy exhibited a unique phenotype, characterized by globally reduced facial reactivity and aberrant coupling of muscle reactivity to facial expression identification. This group showed impaired emotion recognition, but their facial mimics were not correlated with emotion identification performances, unlike other FTD subgroups and healthy controls, and were described as “poker-faced (no reaction)” or “caricatures” of normal emotional reactions (over/inappropriate reaction)16,79. Similarly, another group reported that compared with healthy older adults, bvFTD patients showed an overall dampening of physiological responses, whereas SD patients exhibited abnormal facial expressiveness discordant with the emotional content of the stimuli78. The right fusiform gyrus has been implicated in the processes of facial and emotional recognition of laughter, explaining inconsistent shared laughter experiences in patients with RATL80. Additionally, ToM deficits on ToM tasks have been associated with RATL regions, a recent study assessing a large number of patients with focal RATL atrophy at early stages using dynamic face-to-face tests showed that patients with predominant RATL atrophy displayed lower scores in the emotional ToM task, unlike patients with predominant frontal atrophy who exhibited worse performance in the cognitive ToM task22. It has been suggested that if an individual can no longer understand the semantic meaning of emotional information, the associated response appears compromised76,78. However, it remains to be tested whether these altered emotional reactions are purely due to knowledge loss for emotions or whether alterations in other brain networks following neurodegeneration in RATL lead to the abnormal behavioral response. Given the lack of evidence elucidating the neural mechanisms, the IWG recommends the term “altered emotional expression”. Although it is still relatively broad and does not fully reflect impaired neural domains, it refers to the objective observation of the external expression of emotion, rather than attempting to infer the patient’s internal emotional state. Additionally, it is more specific than current terms such as “lack of empathy”, and clearly describes the clinical phenomenon. Next to neural mechanisms, it is crucial to investigate whether these modalities manifest at early stages. The same gap persists regarding the lack of culturally sensitive validated tests for clinical use (Supplementary Table 4 in Supplementary Data File 4).
RATL and altered social reaction
Previous work has shown that knowledge loss for socioemotional information as well as people, living beings, landmarks, flavors, odors, sounds and bodily sensations was mainly reported as ‘disinhibition’ by many clinicians, as the clinical outcome was socially inappropriate behavior4,76 (i.e., drinking a bottle of soap, confusing it with food, see more real life examples in Supplementary Table 4 in Supplementary Data File 4). This has been widely discussed in several theoretical models, suggesting that conceptual knowledge of social constructs and socially relevant cues are represented in the ATLs70,76,77,82, and an anatomical model for the umbrella term “disinhibition” was offered suggesting that ATL related disinhibition is associated with loss of knowledge of social norms and expectations rather than a problem with control or inhibition per se83. However, no large sample size study has yet unveiled the neural components of inappropriate social behavior in RATL using objective measurements. It remains unknown whether there are other components causing such reactions beyond semantic deficits. Given the lack of evidence elucidating the neural mechanisms, the IWG recommends the term “altered social reaction,” which is still relatively broad and does not fully reflect impaired neural domains. Yet it is more specific and accurate than current terms such as “disinhibition”, and clearly describes the clinical phenomenon without implying the unknown mechanism. Currently, no tasks are available for clinical use.
RATL and altered motivation for social interactions
Apathy is another highly reported symptom in RATL cohorts, mainly measured with the neuropsychiatric inventory apathy subscale10,84,85,86,87,88. However, the characteristics of “lack of motivation” in the RATL syndrome differ from classic cognitive apathy, which typically involves losing motivation for almost all daily tasks and novelty seeking. Instead, patients exhibit a strong shift from socially motivated activities to narrowed solitary activities for which they show heightened motivation4,23 (see Section 3). Moreover, in the early stages, patients maintain their non-social daily life activities such as showering, cooking, driving etc., but they may express inertia towards social activities4. Available studies using quantitative assessments are limited, however, one study using the Snaith-Hamilton Pleasure Scale and the motivation subscale of the Cambridge Behavior Inventory indicated a unique role for right temporal lobe structures in modulating anhedonia in SD. The study suggested that degeneration of predominantly right-hemisphere structures deleteriously impacts the capacity to experience pleasure in SD, leading to a lack of motivation89. However, no distinction regarding social vs solitary activities has been made in this study. Moreover, patients with semantic and behavioral deficits violate the assumptions implicit in self-report questionnaires and Likert scales90. Although there are no objective measurements to assess such behaviors, clinical studies indicate that the motivation for social interactions is often misplaced rather than completely absent. Given the lack of evidence elucidating the neural mechanisms, the IWG recommends the term “Altered motivation for social interactions,” which is still relatively broad and does not fully reflect impaired neural domains. Yet it is more specific than current complex terms such as ‘apathy’, and clearly describes the clinical phenomenon without implying a mechanism. Investigating whether these modalities manifest at early stages and identifying the underlying neural mechanisms is crucial. Beyond informant-based surveys for broad symptoms such as pleasure, apathy, motivation scales, more specific, objective, culturally sensitive face-to-face tests targeting neural mechanisms are warranted (Supplementary Table 4 in Supplementary Data File 4).
Altered prioritization
Another symptom group that is very prominent and even characteristic of patients with RATL is altered prioritization. This category includes symptoms such as hyper-religiosity (excessive preoccupation with religious activities, e.g., spending considerable time at church reading the Bible), developing strong appreciation for certain topics (e.g., musicophilia), rigidity around food (e.g., only eating spaghetti), color (e.g., only wearing blue), or scheduling (e.g., eating breakfast only at 8 a.m.). Instead of describing this symptom group based on individual examples, as has been done previously in the literature, the IWG conceptualized it under the title ‘altered prioritization’, categorizing it into two subgroups: hyperfocus on specific interests and altered hedonic valuation and personal preferences.
RATL and hyperfocus on specific interests
Perhaps the least understood symptom group of the syndrome is mental rigidity, preoccupations, ritualistic and obsessive-compulsive behavior, despite their high prevalence (78% in early stages) in the IWG dataset4. A total of 505 specific examples were reported, including time and schedule (21%), food (17%), puzzles/sudoku/computer games (12%), global warming/recycling/saving gas, water, electricity (8%), sports (6%), walking/cycling/driving (6%), hoarding/collecting (5%), health-related (4%), shopping/ordering (3%), colors (3%), clothes (3%), religion (3%), writing (2%), art (music/drawing/painting/sculpture) (2%), saving money/parsimony (2%), cleaning (1%), clock-watching (1%), checking/controlling (1%), gardening (1%), and other (4%). Similar examples have been described by several authors displayed in Supplementary Table 3 in Supplementary Data File 3. These activities are part of patients’ daily lives, and they exhibit heightened motivation and attention spans toward such actions. There is no objective test available to assess such behavior; however, caregiver reports and clinical observations suggest a unique nature, indicating that patients spend considerable time and attention, exhibiting hyperfocus on certain activities and specific interests. Additionally, unlike individuals with psychiatrically diagnosed obsessive-compulsive disorder, patients with RATL atrophy exhibit less anxiety, self-criticism, or insight. Thus, the IWG advocates for improved terminologies instead of mental rigidity, preoccupations, ritualistic or obsessive-compulsive behavior to better phenotype the distinct characteristics of these symptoms. Future studies are warranted to identify the neural underpinnings of such deficits, to interrogate the thought process behind these typical behaviors, and objective tests are needed to examine these symptoms in daily clinical practice.
RATL and altered hedonic valuation and personal preferences
Alongside specific interests, alterations in personal preferences such as food choices, colors, clothes, and esthetic tastes have been noted in international data4. A group of authors has suggested that the clinical syndrome associated with RATL atrophy may partly involve disturbances in reward processing, shifting hedonic values away from people towards inanimate objects23,91. Similar arguments have highlighted that semantic knowledge of social interactions is influenced by the hedonic evaluation system, emphasizing the close connection between ATLs and the medial orbitofrontal regions75,82. Several clinical scientists claimed semantic loss as the reason of strong personal preference, particularly for food14,17,57. However, to what extent semantic deficits contribute to personal preferences and whether valence processing (i.e., recognizing the pleasant or unpleasant nature of emotions) also depends on semantic knowledge remains to be determined through objective evaluations (Supplementary Table 4 in Supplementary Data File 4).
Other symptoms
RATL and apparent memory deficits
To date, episodic, semantic and autobiographical memory deficits have been documented in RATL with discrepant frequencies by several groups10,11,92,93,94, and the occurrence of amnestic presentations (episodic memory deficits), remains a controversial topic in the field. Although episodic memory disturbances (i.e., forgetting appointments) have been reported in multiple studies4,10,93,95,96,97,98, these were objectified with episodic memory tests in relatively fewer studies4,10,81,99. The latter showed impairments in standard episodic memory tests, particularly in those using visual stimuli rather than verbal stimuli. When comparing dementia subtypes, worse semantic memory performances in FTD with RATL atrophy were found compared to AD and worse episodic memory performances in AD compared to FTD with RATL atrophy10,81,99. In IWG’s recent study, chart reviews showed that 67% of patients had reported memory problems, whereas objective abnormalities varied between 21% and 87% across eight different episodic memory tests4. Studies using detailed memory tests have identified category specific memory deficits29,100. In those studies, memory for famous people and social events were selectively disturbed. Current neuroscientific evidence suggests that due to the categorization problem, patients with semantic deficits demonstrate a “over-generalization” tendency and exhibit learning difficulties101, and semantic processing may underlie forms of episodic and autobiographical memory by providing schemas and meaning for remembering the past, even for imagining the future94. However, those publications include patients with either predominant LATL or bilateral temporal atrophy. Given the advent of disease-modifying therapies for AD, the IWG calls for more focused studies that aim to disentangle the neural and molecular underpinnings of memory impairment in RATL syndrome.
RATL and psychiatric symptoms
Besides those core symptoms, affective dysregulation, anxiety/panic, delusions/ hallucinations, have also been reported with lower frequencies in previous publications10,93. Although, former literature suggested depression as a distinctive symptom10,93, our joint data also showed cases with mania and fluctuating mood4. A case with RATL atrophy whose severe claustrophobia had disappeared 7 years after onset of her first symptoms has been reported102. Another case with mania responded very well to symptomatic treatment103. On the other hand, another group has drawn attention to increased potential risk for suicidal behavior in this disease group, as they found preoccupations around depressive thoughts and suicidal ideas104. All reported psychiatric symptoms cited above rely on clinical observations and caregiver declarations, lacking direct face-to-face psychometric assessments to better understand the neural mechanisms causing such problems. However, RATL neurodegeneration should be considered in cases with late-onset psychiatric problems, and other RATL-specific symptoms listed in this paper should be further assessed to detect potential underlying neurodegeneration.
RATL and language problems
As discussed in the previous sections and exemplified in Supplementary Table 4 in Supplementary Data File 4, the loss of knowledge across several categories were reported as language problems by many caregivers and clinicians. In the IWG dataset, 70% of patients were reported to have naming and word-finding difficulties, although available cognitive test scores revealed that nearly all patients who underwent cognitive assessment exhibited severe visual and person-specific semantic deficits while performances on general naming were relatively better4. Additionally, comparative studies have shown that patients with predominant RATL atrophy performed better on verbal semantics and fluency tests compared to svPPA, however worse than bvFTD and healthy controls10,22. However, more robust studies are warranted to elucidate these findings and to determine whether certain semantic categories (i.e., animate, inanimate, socioemotional, proper names) may be more susceptible to degradation. It also remains unclear whether anomia primarily arises from deficits in visual confrontation naming (based on visual presentation) or verbal confrontation naming (based on verbal description). Therefore, further work is required to understand the role of RATL in language functions, particularly in naming and elucidate the contributions of LATL atrophy which is commonly observed in patients with RATL predominant atrophy. Experimental paradigms that specifically differentiate between visual versus verbal anomia and test category specificity would be especially valuable in advancing our knowledge in this area.
RATL and motor symptoms
Previous clinical studies and case reports have predominantly focused on the initial and characteristic symptoms of RATL neurodegeneration, often omitting motor symptoms. However, a single-center study reported motor slowness as an initial symptom in 27% of the RATL patients10. Additionally, a post-mortem study revealed that 35% of patients with RATL developed parkinsonism over the course of the disease, which is linked to tau pathology11. Several studies have also noted associations between RATL and motor neuron disease (MND), as well as parkinsonism10,85,105,106,107,108,109,110,111,112. Some studies have emphasized the relationship between RATL neurodegeneration and co-existing MND or corticospinal tract degeneration (CTD) features observed in pathological examinations85,105,106,107,110,111,113, which has been accumulated in a systematic review showing that 28.6% of RATL patients exhibit co-existing CTD in brain autopsy85. While MND is predominantly associated with FTLD-TDP type B, the aforementioned studies have also identified CTD features in FTLD-TDP types A and C. Forthcoming research by the IWG aims to study the genetic and pathological features of the syndrome, which would shed light on the frequency of the relationship between genetic and pathological risk factors associated with MND and parkinsonism.
RATL and spared functions
It should be noted that almost all papers have reported no symptoms related to visuospatial and attention functions4,6,8,9,10,22,93,114. Patients have either performed within normal expectations or showed mild impairment on standardized tests. Visuospatial functions, in particular, have been highlighted as well-preserved, a finding confirmed by standardized test assessments4,6,8,9,10,11,22,93. Comparative studies showed although executive and attention functions may not be normal, they are less severely affected compared to AD, bvFTD, and svPPA10,22. And in RATL, there are better verbal semantic skills, less surface dyslexia in English; or fewer accent and tone regularization errors in other languages compared to svPPA10,22,84, and better episodic memory performances compared to AD10,81,99.
Conclusions
This consensus paper on FTD with RATL predominant neurodegeneration breaks new ground on four levels. First, it represents the first international initiative, employing a 4-year nominal group approach that includes neurologists, psychiatrists, psychologists, and dementia neuroimaging experts from around the world as equal partners to tackle discrepancies and resolve conflicts in the field. Second, it implements a meticulous systematic review aimed at identifying patients with RATL atrophy harmonized in SD, svPPA, and bvFTD cohorts, resulting in the largest collection of cases to disentangle the nature of the symptoms in a neuroscientifically informed manner. Third, it provides transparent, evidence-based nomenclature that moves beyond subjective caregiver and clinician observations, clearly highlighting limitations and avoiding the imposition of personal opinions. Lastly, it identifies the lack of or limited evidence in many domains, indicating areas where physicians need more information, thus shaping future direction goals. In particular, neuroimaging studies that integrate both structural and functional techniques offer a promising avenue for elucidating the syndrome’s poorly understood symptoms. By applying these advanced imaging methods, future investigations can clarify underlying pathophysiological mechanisms, ultimately enhancing both diagnostic precision and therapeutic strategies.
Although the IWG reached consensus on terminologies, no agreement was reached on finalizing a formal name or publishing the symptom checklist in Box 1 as international diagnostic criteria. Our future goal is to perform cross-cultural validation of the clinician-faced symptom checklist identified in this study (Box 1) by utilizing culturally sensitive objective tests described in Supplementary Table 4 in Supplementary Data File 4. The IWG is formulating targeted interview questions that capture the identified symptoms, assembling a reliable and adaptable test battery to supplement these interviews. Our multicenter prospective study will thoroughly validate these tools across various cultural and linguistic settings. This study will include direct comparisons with other diagnostic groups to differentiate the syndrome from AD and psychiatric disorders, and delineate the ambiguous boundaries with bvFTD, and particularly with left predominant SD/svPPA. Furthermore, we will work towards achieving consensus on a formal nomenclature for this syndrome to facilitate precise communication and diagnosis within the international medical community.
References
Neary, D. et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554 (1998).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Gorno-Tempini, M. L. et al. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014 (2011).
Ulugut, H. et al. Clinical recognition of frontotemporal dementia with right anterior temporal predominance: a multicenter retrospective cohort study. Alzheimers Dement. https://doi.org/10.1002/alz.14076 (2024).
Veronelli, L., Makaretz, S. J., Quimby, M., Dickerson, B. C. & Collins, J. A. Geschwind Syndrome in frontotemporal lobar degeneration: neuroanatomical and neuropsychological features over 9 years. Cortex J. Devoted Study Nerv. Syst. Behav. 94, 27–38 (2017).
Snowden, J. S. et al. Semantic dementia and the left and right temporal lobes. Cortex J. Devoted Study Nerv. Syst. Behav. 107, 188–203 (2018).
Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J. Neurol. Neurosurg. Psychiatry 57, 416–418 (1994).
Joubert, S. et al. The right temporal lobe variant of frontotemporal dementia. J. Neurol. 253, 1447–1458 (2006).
Thompson, S. A., Patterson, K. & Hodges, J. R. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 61, 1196–1203 (2003).
Ulugut Erkoyun, H. et al. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain J. Neurol. 143, 2831–2843 (2020).
Josephs, K. A. et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 73, 1443–1450 (2009).
Chan, D. et al. The clinical profile of right temporal lobe atrophy. Brain J. Neurol. 132, 1287–1298 (2009).
Geraudie, A. et al. Expressive prosody in patients with focal anterior temporal neurodegeneration. Neurology 101, e825–e835 (2023).
Joo, J. Y. et al. Parosmia in right-lateralized semantic variant primary progressive aphasia a case report. Alzheimer Dis. Assoc. Disord. 35, 160–163 (2021).
Shinagawa, S. & Nakayama, K. A case of musicophilia with right predominant temporal lobe atrophy]. Brain Nerve Shinkei Kenkyu No Shinpo 67, 1443–1448 (2015).
Marshall, C. R. et al. Motor signatures of emotional reactivity in frontotemporal dementia. Sci. Rep. 8, 1030 (2018).
Mendez, M. F. & Ghajarnia, M. Agnosia for familiar faces and odors in a patient with right temporal lobe dysfunction. Neurology 57, 519–521 (2001).
Gan, J. J., Lin, A., Samimi, M. S. & Mendez, M. F. Somatic symptom disorder in semantic dementia: the role of alexisomia. Psychosomatics 57, 598–604 (2016).
Evans, J. J., Heggs, A. J., Antoun, N. & Hodges, J. R. Progressive prosopagnosia associated with selective right temporal lobe atrophy: a new syndrome? Brain 118, 1–13 (1995).
Edwards-Lee, T. et al. The temporal variant of frontotemporal dementia. Brain J. Neurol 120, 1027–1040 (1997).
Hailstone, J. C., Crutch, S. J., Vestergaard, M. D., Patterson, R. D. & Warren, J. D. Progressive associative phonagnosia: a neuropsychological analysis. Neuropsychologia 48, 1104–1114 (2010).
Younes, K. et al. Right temporal degeneration and socioemotional semantics: semantic behavioural variant frontotemporal dementia. Brain 145, 4080–4096 (2022).
Belder, C. R. S. et al. The problematic syndrome of right temporal lobe atrophy: unweaving the phenotypic rainbow. Front. Neurol. 13, 1082828 (2023).
Chokesuwattanaskul, A. et al. The architecture of abnormal reward behaviour in dementia: multimodal hedonic phenotypes and brain substrate. Brain Commun. 5, fcad027 (2023).
Kipps, C. M. et al. Clinical significance of lobar atrophy in frontotemporal dementia: application of an MRI visual rating scale. Dement. Geriatr. Cogn. Disord. 23, 334–342 (2007).
GALLAGHER, M., HARES, T., SPENCER, J., BRADSHAW, C. & WEBB, I. The nominal group technique: a research tool for general practice? Fam. Pract. 10, 76–81 (1993).
McMillan, S. S., King, M. & Tully, M. P. How to use the nominal group and Delphi techniques. Int. J. Clin. Pharm. 38, 655–662 (2016).
Ulugut, H. et al. Clinical recognition of frontotemporal dementia with right anterior temporal predominance: a multicenter retrospective cohort study. Alzheimers Dement. J. Alzheimers Assoc. 20, 5647–5661 (2024).
Kazui, H., Tanabe, H., Ikeda, M., Hashimoto, M. & Yamada, N. A case of predominantly right-temporal lobe atrophy with disturbance of identifying familiar faces. Brain Nerve 47, 77–85 (1995).
Rainville, C. et al. Wayfinding in familiar and unfamiliar environments in a case of progressive topographical agnosia. Neurocase 11, 297–309 (2005).
Borghesani, V. et al. ‘Looks familiar, but I do not know who she is’: The role of the anterior right temporal lobe in famous face recognition. Cortex J. Devoted Study Nerv. Syst. Behav. 115, 72–85 (2019).
Cosseddu, M., Gazzina, S., Borroni, B., Padovani, A. & Gainotti, G. Multimodal face and voice recognition disorders in a case with unilateral right anterior temporal lobe atrophy. Neuropsychology 32, 920–930 (2018).
Gainotti, G., Barbier, A. & Marra, C. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain J. Neurol. 126, 792–803 (2003).
Snowden, J. S., Thompson, J. C. & Neary, D. Knowledge of famous faces and names in semantic dementia. Brain J. Neurol. 127, 860–872 (2004).
Snowden, J. S., Thompson, J. C. & Neary, D. Famous people knowledge and the right and left temporal lobes. Behav. Neurol. 25, 35–44 (2012).
Nakachi, R. et al. Progressive prosopagnosia at a very early stage of frontotemporal lobar degeneration. Psychogeriatrics 7, 155–162 (2007).
Thompson, S. A. et al. Dissociating person-specific from general semantic knowledge: roles of the left and right temporal lobes. Neuropsychologia 42, 359–370 (2004).
Joubert, S. et al. Impaired configurational processing in a case of progressive prosopagnosia associated with predominant right temporal lobe atrophy. Brain 126, 2537–2550 (2003).
Gentileschi, V., Sperber, S. & Spinnler, H. Progressive defective recognition of familiar people. Neurocase 5, 407–424 (1999).
Grossi, D. et al. Structural connectivity in a single case of progressive prosopagnosia: the role of the right inferior longitudinal fasciculus. Cortex 56, 111–120 (2014).
Mendez, M. F., Ringman, J. M. & Shapira, J. S. Impairments in the face-processing network in developmental prosopagnosia and semantic dementia. Cogn. Behav. Neurol. 28, 188–197 (2015).
Gainotti, G., Ferraccioli, M., Quaranta, D. & Marra, C. Cross-modal recognition disorders for persons and other unique entities in a patient with right fronto-temporal degeneration. Cortex 44, 238–248 (2008).
Hailstone, J. C. et al. Voice processing in dementia: a neuropsychological and neuroanatomical analysis. Brain 134, 2535–2547 (2011).
Piccininni, C. et al. Which components of famous people recognition are lateralized? A study of face, voice and name recognition disorders in patients with neoplastic or degenerative damage of the right or left anterior temporal lobes. Neuropsychologia 181, 108490 (2023).
Péron, J. A. et al. Preservation of person-specific semantic knowledge in semantic dementia: does direct personal experience have a specific role? Front. Hum. Neurosci. 9, 625 (2015).
Luzzi, S. et al. Famous faces and voices: differential profiles in early right and left semantic dementia and in Alzheimer’s disease. Neuropsychologia 94, 118–128 (2017).
Rouse, M. A. et al. Social-semantic knowledge in frontotemporal dementia and after anterior temporal lobe resection. Brain Commun. 6, fcae378 (2024).
Ghirelli, A. et al. Clinical and neuroanatomical characterization of the semantic behavioral variant of frontotemporal dementia in a multicenter Italian cohort. J. Neurol. https://doi.org/10.1007/s00415-024-12338-9 (2024).
Mendez, M. F., Kremen, S. A., Tsai, P.-H. & Shapira, J. S. Interhemispheric differences in knowledge of animals among patients with semantic dementia. Cogn. Behav. Neurol. 23, 240–246 (2010).
Lambon Ralph, M. A., Cipolotti, L., Manes, F. & Patterson, K. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain 133, 3243–3255 (2010).
Mole, J. A. et al. Avian agnosia: a window into auditory semantics. Neuropsychologia 134, 107219 (2019).
Barbarotto, R., Capitani, E., Spinnler, H. & Trivelli, C. Slowly progressive semantic impairment with category specificity. Neurocase 1, 107–119 (1995).
Lambon Ralph, M. A., Patterson, K., Garrard, P. & Hodges, J. R. Semantic dementia with category specificity:acomparative case-series study. Cogn. Neuropsychol. 20, 307–326 (2003).
Muhammed, L. et al. Agnosia for bird calls. Neuropsychologia 113, 61–67 (2018).
Funayama, M., Nakajima, A., Kurose, S. & Takata, T. Putative alcohol-related dementia as an early manifestation of right temporal variant of frontotemporal dementia. J. Alzheimers Dis. 83, 531–537 (2021).
Carnemolla, S. E. et al. Olfactory bulb integrity in frontotemporal dementia and Alzheimer’s disease. J. Alzheimers Dis. 89, 51–66 (2022).
Sakai, M. et al. Gustatory dysfunction as an early symptom of semantic dementia. Dement. Geriatr. Cogn. Disord. EXTRA 7, 395–405 (2017).
Fletcher, P. D. et al. Agnosia for accents in primary progressive aphasia. Neuropsychologia 51, 1709–1715 (2013).
Lin, P.-H. et al. Anatomical correlates of non-verbal perception in dementia patients. Front. Aging Neurosci. 8, 207 (2016).
Hsieh, S., Hornberger, M., Piguet, O. & Hodges, J. R. Neural basis of music knowledge: evidence from the dementias. Brain J. Neurol. 134, 2523–2534 (2011).
19th IPA International Congress, 31 August − 3 September 2019 Santiago de Compostela, Spain. Int. Psychogeriatr. 31, i–172 (2019).
González-Caballero, G., Abellán-Miralles, I. & Sáenz-Sanjuan, M. J. Right temporal lobe variant of frontotemporal dementia. J. Clin. Neurosci. 22, 1139–1143 (2015).
Didic, M. et al. Progressive phonagnosia in a telephone operator carrying a C9orf72 expansion. Cortex 132, 92–98 (2020).
Mendez, M. F. Frontotemporal dementia: a window to alexithymia. J. Neuropsychiatry Clin. Neurosci. 33, 157–160 (2021).
Hazelton, J. L. et al. Hemispheric contributions toward interoception and emotion recognition in left-vs right-semantic dementia. Neuropsychologia 188, 108628 (2023).
Marshall, C. R. et al. Cardiac responses to viewing facial emotion differentiate frontotemporal dementias. Ann. Clin. Transl. Neurol. 5, 687–696 (2018).
Hua, A. Y. et al. Diminished baseline autonomic outflow in semantic dementia relates to left-lateralized insula atrophy. NeuroImage Clin. 40, 103522 (2023).
Damasio, A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness (Harcourt Brace, 1999).
Kumfor, F. et al. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain J. Neurol. 139, 986–998 (2016).
Bertoux, M. et al. When affect overlaps with concept: emotion recognition in semantic variant of primary progressive aphasia. Brain 143, 3850–3864 (2020).
Binney, R. J. et al. Reading words and other people: a comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex J. Devoted Study Nerv. Syst. Behav. 82, 147–163 (2016).
Gressie, K. et al. Error profiles of facial emotion recognition in frontotemporal dementia and Alzheimer’s disease. Int. Psychogeriatr. 1–10 https://doi.org/10.1017/S1041610223000297 (2023).
Zahn, R. et al. The neural basis of human social values: evidence from functional MRI. Cereb. Cortex 19, 276–283 (2009).
Zahn, R. et al. Social concepts are represented in the superior anterior temporal cortex. Proc. Natl. Acad. Sci. USA 104, 6430–6435 (2007).
Rijpma, M. G. et al. Semantic knowledge of social interactions is mediated by the hedonic evaluation system in the brain. Cortex J. Devoted Study Nerv. Syst. Behav. 161, 26–37 (2023).
Rouse, M. A., Binney, R. J., Patterson, K., Rowe, J. B. & Lambon Ralph, M. A. A neuroanatomical and cognitive model of impaired social behaviour in frontotemporal dementia. Brain 147, 1953–1966 (2024).
Binney, R. J. & Ramsey, R. Social semantics: the role of conceptual knowledge and cognitive control in a neurobiological model of the social brain. Neurosci. Biobehav. Rev. 112, 28–38 (2020).
Kumfor, F., Hazelton, J. L., Rushby, J. A., Hodges, J. R. & Piguet, O. Facial expressiveness and physiological arousal in frontotemporal dementia: phenotypic clinical profiles and neural correlates. Cogn. Affect. Behav. Neurosci. 19, 197–210 (2019).
Clark, C. N. & Warren, J. D. Emotional caricatures in frontotemporal dementia. Cortex 76, 134–136 (2016).
Pressman, P. S. et al. Neuroanatomy of shared conversational laughter in neurodegenerative disease. Front. Neurol. 9, 464 (2018).
Perry, R. J. et al. Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase 7, 145–160 (2001).
Rankin, K. P. Brain networks supporting social cognition in dementia. Curr. Behav. Neurosci. Rep. 7, 203–211 (2020).
Magrath Guimet, N., Miller, B. L., Allegri, R. F. & Rankin, K. P. What do we mean by behavioral disinhibition in frontotemporal dementia? Front. Neurol. 12, 707799 (2021).
Ulugut Erkoyun, H. et al. The right temporal variant of frontotemporal dementia is not genetically sporadic: a case series. J. Alzheimers Dis. 79, 1195–1201 (2021).
Ulugut, H. et al. Right temporal variant frontotemporal dementia is pathologically heterogeneous: a case-series and a systematic review. Acta Neuropathol. Commun. 9, 131 (2021).
Koros, C. et al. Right temporal atrophy versus frontal atrophy in patients with frontotemporal lobar degeneration: cognitive and behavioural profiles. J. Neurol. 259, S62 (2012).
Sato, S. et al. Characteristics of behavioral symptoms in right-sided predominant semantic dementia and their impact on caregiver burden: a cross-sectional study. Alzheimers Res. Ther. 13, 166 (2021).
Kashibayashi, T. et al. Transition of distinctive symptoms of semantic dementia during longitudinal clinical observation. Neurosci. Res. 68, e191 (2010).
Shaw, S. R. et al. Anhedonia in semantic dementia-exploring right hemispheric contributions to the loss of pleasure. Brain Sci. 11, 998 (2021).
Williams, R. S. et al. Syndromes associated with frontotemporal lobar degeneration change response patterns on visual analogue scales. Sci. Rep. 13, 8939 (2023).
Horne, K. & Irish, M. Reconciling profiles of reward-seeking versus reward-restricted behaviours in frontotemporal dementia. Brain Commun. 5, fcad045 (2023).
Hornberger, M. & Piguet, O. Episodic memory in frontotemporal dementia: a critical review. Brain 135, 678–692 (2012).
Chan, D. et al. The clinical profile of right temporal lobe atrophy. Brain 132, 1287–1298 (2009).
Irish, M. & Piguet, O. The pivotal role of semantic memory in remembering the past and imagining the future. Front. Behav. Neurosci. 7, 27 (2013).
Clark, C. N. et al. Temporal variant frontotemporal dementia is associated with globular glial tauopathy. Cogn. Behav. Neurol. 28, 92–97 (2015).
Chen, K. et al. The neuropsychological profiles and semantic-critical regions of right semantic dementia. NeuroImage Clin. 19, 767–774 (2018).
Koros, C. et al. Prosopagnosia, other specific cognitive deficits, and behavioral symptoms: comparison between right temporal and behavioral variant of frontotemporal dementia. Vision 6, 75 (2022).
Scahill, V. L., Hodges, J. R. & Graham, K. S. Can episodic memory tasks differentiate semantic dementia from Alzheimer’s disease? Neurocase 11, 441–451 (2005).
Pleizier, C. M. et al. Episodic memory and the medial temporal lobe: not all it seems. Evidence from the temporal variants of frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 83, 1145–1148 (2012).
Simons, J. S., Graham, K. S., Galton, C. J., Patterson, K. & Hodges, J. R. Semantic knowledge and episodic memory for faces in semantic dementia. Neuropsychology 15, 101–114 (2001).
Patterson, K., Nestor, P. J. & Rogers, T. T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8, 976–987 (2007).
Clarke, C., Fletcher, P., Cifelli, A. & Warren, J. D. ‘The mind is its own place’: amelioration of claustrophobia in a patient with semantic dementia. J. Neurol. Neurosurg. Psychiatry 2014, 1–5 (2013).
Turan, Ç, Kesebir, S., Meteris, H. & Ülker, M. Aphasia, prosopagnosia and mania: a case diagnosed with right temporal variant semantic dementia. Turk. J. Psychiatry 24, 68–72 (2013).
Sabodash, V., Mendez, M. F., Fong, S. & Hsiao, J. J. Suicidal behavior in dementia: a special risk in semantic dementia. Am. J. Alzheimers Dis. Other Demen. 28, 592–599 (2013).
Coon, E. A., Whitwell, J. L., Parisi, J. E., Dickson, D. W. & Josephs, K. A. Right temporal variant frontotemporal dementia with motor neuron disease. J. Clin. Neurosci. 19, 85–91 (2012).
Josephs, K. A. et al. Corticospinal tract degeneration associated with TDP-43 type C pathology and semantic dementia. Brain 136, 455–470 (2013).
Miki, Y. et al. Corticospinal tract degeneration and temporal lobe atrophy in frontotemporal lobar degeneration TDP-43 type C pathology. Neuropathol. Appl. Neurobiol. 46, 296–299 (2020).
Kobayashi, Z. et al. Clinicopathological characteristics of FTLD-TDP showing corticospinal tract degeneration but lacking lower motor neuron loss. J. Neurol. Sci. 298, 70–77 (2010).
Lee, S. E. et al. Clinical characterization of bvFTD due to FUS neuropathology. Neurocase 18, 305–317 (2012).
Kelley, B. J. et al. Alzheimer disease-like phenotype associated with the c.154delA mutation in progranulin. Arch. Neurol. 67, 171–177 (2010).
Kelley, B. J. et al. Prominent phenotypic variability associated with mutations in Progranulin. Neurobiol. Aging 30, 739–751 (2009).
Davion, S. et al. Clinicopathologic correlation in PGRN mutations. Neurology 69, 1113–1121 (2007).
Koriath, C. A. M. et al. The clinical, neuroanatomical, and neuropathologic phenotype of TBK1-associated frontotemporal dementia: a longitudinal case report. Alzheimers Dement. Diagn. Assess. Dis. Monit. 6, 75–81 (2017).
Seeley, W. W. et al. The natural history of temporal variant frontotemporal dementia. Neurology 64, 1384–1390 (2005).
Acknowledgements
We would like to thank to Alexandra Paizee for her help in technical details of the systematic review. This project is supported by an Alzheimer’s Association Grant (AACSF-22-849085, PI: Ulugut). K.Y. is supported by the NIH/NIA (1K23AG09073301A1), the Alzheimer’s Association AACSF-24-1307411, the Iqbal Farrukh, Asad Jamal Fund, and the Erb Family Foundation. K.Y. also serves as a consultant for Taudia. Irish is supported by a National Health and Medical Research Council of Australia Investigator Grant (GNT2025228). K.A.J. and J.L.W. are supported by R01-AG37491, R01-DC010367, R21-NS94684. Van den Stock is supported by KU Leuven (IDN/21/010 and C24/18/095) and the Sequoia Fund for research on ageing and mental health. A.F.S. is supported by the Swedish federal government under the ALF agreement. M.B. is supported by the Fondation Planiol, the Fondation France Alzheimer, and Fondation Vaincre Alzheimer. Laforce; Chaire de recherche sur les aphasies primaires progressives—Fondation de la Famille Lemaire. Warren is supported by the Alzheimer’s Society, Alzheimer’s Research UK, the Royal National Institute for Deaf People, the NIHR UCLH Biomedical Research Centre and a Frontotemporal Dementia Research Fellowship in Memory of David Blechner (funded through The National Brain Appeal). K.P.R. is supported by RF1-AG029577. J.B.R. is funded by the Medical Research Council (MC_UU_00030/14; MR/T033371/1); the NIHR Cambridge Biomedical Research Centre (NIHR203312) and Wellcome Trust (220258). R.V. receives support from the Mady Browaeys Fonds voor Onderzoek naar Frontotemporale Degeneratie. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication. For the purpose of open access, the authors have applied a CC BY public copyright license to any author accepted manuscript version arising from this submission.
Author information
Authors and Affiliations
Consortia
Contributions
H.U. and Y.P. established the international working group (IWG) and the study was designed by them with the contributions of the IWG. K.Y., M.M. and H.U. conducted the systematic review. HU collected and integrated the qualitative data (expert recommendations) generated by IWG. HU wrote the manuscript. HU designed the tables and figures. All authors (H.U., K.Y., M.M., M.B., M.I., F.K., G.G.F., B.S., I.I.G., J.C.T., A.F.S., E.E., M.L.W., L.R., J.V.D.S., M.V., R.V., R.J.L., S.D., P.S.P., P.C., L.C.d.S., L.T.T., H.G., J.D.S., D.G., F.P., S.W., B.L.M., V.E.S., J.L.W., B.B., J.D.R., O.P., M.L.G.T., K.A.J., J.S., J.B.R., J.D.W., K.P.R., and Y.P.) contributed to the interpretation of the results and provided a critical review and approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Varvara Valotassiou, Shuangwu Liu, Charles Marshall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ulugut, H., Younes, K., Montembeault, M. et al. Clinical recognition of frontotemporal dementia with right temporal predominance: a consensus statement from the International Working Group. Commun Med 5, 523 (2025). https://doi.org/10.1038/s43856-025-01252-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-01252-4