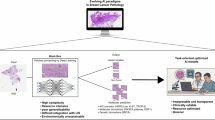
Abstract
Artificial intelligence is transforming breast cancer management through various machine learning applications. Artificial intelligence supports precision medicine by enhancing detection, diagnosis, prognosis, and treatment response prediction. It achieves this by analysing data from medical imaging, histopathology, genomics and multi-omics sources to improve patient recovery. This review summarises AI-driven advancements across the entire continuum of breast cancer management, spanning detection, diagnosis, prognosis, treatment and recovery. It evaluates their efficacy and limitations, explores their impact on healthcare costs and clinical practice, and addresses key challenges including generalisability, reproducibility and regulatory barriers. Evidence from recent studies highlights AI’s role in improving breast cancer detection, molecular subtyping and prognostic accuracy. It also facilitates more patient-tailored therapeutic strategies and supports quality of life interventions. Nonetheless, the translation of these benefits into clinical practice requires rigorous validation, transparent model development, and equitable implementation.

Similar content being viewed by others
References
Quaglino, E., Conti, L. & Cavallo, F. Breast cancer stem cell antigens as targets for immunotherapy. Semin. Immunol. 47, 101386 (2020).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Chen, Z. et al. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun. 41, 1100–1115 (2021).
Kumar, K. & Gandhi, H. Artificial intelligence for the management of breast cancer: an overview. Curr. Drug Discov. Technol. 12, e031123223115 (2024).
Yousif, M. et al. Artificial intelligence applied to breast pathology. Virchows Arch. 480, 191–209 (2022).
Darbandi, M. R. et al. Artificial intelligence breakthroughs in pioneering early diagnosis and precision treatment of breast cancer: a multimethod study. Eur. J. Cancer 209, 114227 (2024).
Tan, X. J., Cheor, W. L., Cheng, E. M., Lim, C. C. & Rahman, K. S. A. Generative Artificial intelligence (GAI) in breast cancer diagnosis and treatment: a systematic review. Comput. Mater. Contin. 84, 2015–2060 (2025).
Kumar, A., Sharma, A., Singh, A. K., Singh, S. K. & Saxena, S. Data augmentation for medical image classification based on Gaussian Laplacian pyramid blending with a similarity measure. IEEE J. Biomed. Health Inform. 29, 3886–3893 (2025).
Inan, M. S. K., Hossain, S. & Uddin, M. N. Data augmentation guided breast cancer diagnosis and prognosis using an integrated deep-generative framework based on breast tumor’s morphological information. Inform. Med. Unlocked 37, 101171 (2023).
Latha, V., Gomathi, V., Rajeshkanna, A. & Hari Ram, S. Generating a potent inhibitor against MCF7 breast cancer cell through artificial intelligence based virtual screening and molecular docking studies. Indian J. Biochem. Biophys. 60, 844–856 (2023).
Moghbel, M., Ooi, C. Y., Ismail, N., Hau, Y. W. & Memari, N. A review of breast boundary and pectoral muscle segmentation methods in computer-aided detection/diagnosis of breast mammography. Artif. Intell. Rev. 53, 1873–1918 (2020).
Sechopoulos, I., Teuwen, J. & Mann, R. Artificial intelligence for breast cancer detection in mammography and digital breast tomosynthesis: state of the art. Semin. Cancer Biol. 72, 214–225 (2021).
Murtaza, G. et al. Deep learning-based breast cancer classification through medical imaging modalities: state of the art and research challenges. Artif. Intell. Rev. 53, 1655–1720 (2020).
Kozegar, E., Soryani, M., Behnam, H., Salamati, M. & Tan, T. Computer aided detection in automated 3-D breast ultrasound images: a survey. Artif. Intell. Rev. 53, 1919–1941 (2020).
Ibrahim, A. et al. Artificial intelligence in digital breast pathology: techniques and applications. Breast 49, 267–273 (2020).
Chen, J.-M. et al. Computer-aided prognosis on breast cancer with hematoxylin and eosin histopathology images: a review. Tumor Biol. 39, 101042831769455 (2017).
Corti, C. et al. Artificial intelligence for prediction of treatment outcomes in breast cancer: systematic review of design, reporting standards, and bias. Cancer Treat. Rev. 108, 102410 (2022).
Scimeca, M., Urbano, N., Toschi, N., Bonanno, E. & Schillaci, O. Precision medicine in breast cancer: from biological imaging to artificial intelligence. Semin. Cancer Biol. 72, 1–3 (2021).
Phan, P., Mitragotri, S. & Zhao, Z. Digital therapeutics in the clinic. Bioeng. Transl. Med. 8, (2023).
Sahni, N. R., Stein, G., Zemmel, R. & Cutler, D. The potential impact of artificial intelligence on health care spending. In The Economics of Artificial Intelligence: Health Care Challenges (National Bureau of Economic Research, 2023). This study estimates that AI has the potential to significantly reduce US healthcare spending by improving administrative efficiency, enhancing clinical decision-making, and reducing diagnostic and treatment errors, highlighting AI’s role as a major driver of future cost savings across the healthcare system.
Lee, D. & Yoon, S. N. Application of artificial intelligence-based technologies in the healthcare industry: opportunities and challenges. Int. J. Environ. Res. Public. Health 18, 271 (2021).
Sezgin, E. Artificial intelligence in healthcare: complementing, not replacing, doctors and healthcare providers. Digit. Health 9, 20552076231186520 (2023).
Sandeep, F., Kiran, N., Rahaman, Z., Devi, P. & Bendari, A. Pathology in the age of artificial intelligence (AI): redefining roles and responsibilities for tomorrow’s practitioners. Cureus 16, e56040 (2024).
Jaglan, P., Dass, R. & Duhan, M. Breast cancer detection techniques: issues and challenges. J. Inst. Eng. India Ser. B 100, 379–386 (2019).
Suh, Y. J., Jung, J. & Cho, B.-J. Automated breast cancer detection in digital mammograms of various densities via deep learning. J. Pers. Med. 10, 211 (2020).
Lotter, W. et al. Robust breast cancer detection in mammography and digital breast tomosynthesis using an annotation-efficient deep learning approach. Nat. Med. 27, 244–249 (2021).
Miglioretti, D. L. et al. Radiologist characteristics associated with interpretive performance of diagnostic mammography. J. Natl. Cancer Inst. 99, 1854–1863 (2007).
Shah, S. M., Khan, R. A., Arif, S. & Sajid, U. Artificial intelligence for breast cancer analysis: trends & directions. Comput. Biol. Med. 142, 105221 (2022).
Ganesan, K. et al. Computer-aided breast cancer detection using mammograms: a review. IEEE Rev. Biomed. Eng. 6, 77–98 (2013).
Chan, H.-P. et al. Image feature analysis and computer-aided diagnosis in digital radiography. I. Automated detection of microcalcifications in mammography: Image feature analysis. I. Microcalcification detection. Med. Phys. 14, 538–548 (1987).
Chan, H. P., Doi, K., Vyborny, C. J., Lam, K. L. & Schmidt, R. A. Computer-aided detection of microcalcifications in mammograms. Methodology and preliminary clinical study. Invest. Radiol. 23, 664–671 (1988).
Zheng, B., Chang, Y.-H. & Gur, D. Computerized detection of masses in digitized mammograms using single-image segmentation and a multilayer topographic feature analysis. Acad. Radiol. 2, 959–966 (1995).
Yoshida, H., Doi, K., Nishikawa, R. M., Giger, M. L. & Schmidt, R. A. An improved computer-assisted diagnostic scheme using wavelet transform for detecting clustered microcalcifications in digital mammograms. Acad. Radiol. 3, 621–627 (1996).
Karssemeijer, N. & Te Brake, G. M. Detection of stellate distortions in mammograms. IEEE Trans. Med. Imaging 15, 611–619 (1996).
Rangayyan, R. M., El-Faramawy, N. M., Desautels, J. E. L. & Alim, O. A. Measures of acutance and shape for classification of breast tumors. IEEE Trans. Med. Imaging 16, 799–810 (1997).
Ke, L., Mu, N. & Kang, Y. Mass computer-aided diagnosis method in mammogram based on texture features. In 2010 3rd International Conference on Biomedical Engineering and Informatics 354–357 (IEEE, Yantai, China, 2010).
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. 42, 60–88 (2017).
Frazer, H. M. L. et al. Comparison of AI-integrated pathways with human-AI interaction in population mammographic screening for breast cancer. Nat. Commun. 15, 7525 (2024). This study demonstrates that AI-integrated mammography screening pathways can significantly reduce radiologist workload while maintaining or improving cancer detection performance, underscoring the potential of AI to enhance the efficiency of population screening programmes.
Yoon, J. H. et al. Standalone AI for breast cancer detection at screening digital mammography and digital breast tomosynthesis: a systematic review and meta-analysis. Radiology 307, e222639 (2023).
McKinney, S. M. et al. International evaluation of an AI system for breast cancer screening. Nature 577, 89–94 (2020).
Rodriguez-Ruiz, A. et al. Stand-alone artificial intelligence for breast cancer detection in mammography: Comparison with 101 radiologists. J. Natl. Cancer Inst. 111, 916–922 (2019).
Rodríguez-Ruiz, A. et al. Detection of breast cancer with mammography: effect of an artificial intelligence support system. Radiology 290, 305–314 (2019).
Kim, H.-E. et al. Changes in cancer detection and false-positive recall in mammography using artificial intelligence: a retrospective, multireader study. Lancet Digit. Health 2, e138–e148 (2020).
Wu, N. et al. Deep neural networks improve radiologists’ performance in breast cancer screening. IEEE Trans. Med. Imaging 39, 1184–1194 (2020).
Frazer, H. M., Qin, A. K., Pan, H. & Brotchie, P. Evaluation of deep learning-based artificial intelligence techniques for breast cancer detection on mammograms: results from a retrospective study using a BreastScreen Victoria dataset. J. Med. Imaging Radiat. Oncol. 65, 529–537 (2021).
Leibig, C. et al. Combining the strengths of radiologists and AI for breast cancer screening: a retrospective analysis. Lancet Digit. Health 4, e507–e519 (2022).
Byng, D. et al. AI-based prevention of interval cancers in a national mammography screening program. Eur. J. Radiol. 152, 110321 (2022).
Larsen, M. et al. Artificial intelligence evaluation of 122 969 mammography examinations from a population-based screening program. Radiology 303, 502–511 (2022).
Marinovich, M. L. et al. Artificial intelligence (AI) for breast cancer screening: BreastScreen population-based cohort study of cancer detection. eBioMedicine 90, 104498 (2023).
AlSamhori, J. F. et al. Artificial intelligence for breast cancer: implications for diagnosis and management. J. Med. Surg. Public Health 3, 100120 (2024).
Lång, K. et al. Artificial intelligence-supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence trial (MASAI): a clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol. 24, 936–944 (2023).
Ng, A. Y. et al. Prospective implementation of AI-assisted screen reading to improve early detection of breast cancer. Nat. Med. 29, 3044–3049 (2023).
Dembrower, K., Crippa, A., Colón, E., Eklund, M. & Strand, F. Artificial intelligence for breast cancer detection in screening mammography in Sweden: a prospective, population-based, paired-reader, non-inferiority study. Lancet Digit. Health 5, e703–e711 (2023).
Eisemann, N. et al. Nationwide real-world implementation of AI for cancer detection in population-based mammography screening. Nat. Med. 31, 917–924 (2025).
Díaz, O., Rodríguez-Ruíz, A. & Sechopoulos, I. Artificial intelligence for breast cancer detection: technology, challenges, and prospects. Eur. J. Radiol. 175, 111457 (2024).
Abrantes, J. External validation of a deep learning model for breast density classification. In European Congress of Radiology 2023 C–16014 (European Society of Radiology, 2023).
Pattacini, P. et al. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the Reggio Emilia tomosynthesis randomized trial. Radiology 288, 375–385 (2018).
Caumo, F. et al. Digital breast tomosynthesis with synthesized two-dimensional images versus full-field digital mammography for population screening: Outcomes from the Verona screening program. Radiology 287, 37–46 (2018).
Bernardi, D. et al. Application of breast tomosynthesis in screening: incremental effect on mammography acquisition and reading time. Br. J. Radiol. 85, e1174–e1178 (2012).
Aase, H. S. et al. A randomized controlled trial of digital breast tomosynthesis versus digital mammography in population-based screening in Bergen: interim analysis of performance indicators from the To-Be trial. Eur. Radiol. 29, 1175–1186 (2019).
Balleyguier, C. et al. Improving digital breast tomosynthesis reading time: a pilot multi-reader, multi-case study using concurrent computer-aided detection (CAD). Eur. J. Radiol. 97, 83–89 (2017).
Benedikt, R. A., Boatsman, J. E., Swann, C. A., Kirkpatrick, A. D. & Toledano, A. Y. Concurrent computer-aided detection improves reading time of digital breast tomosynthesis and maintains interpretation performance in a multireader multicase study. Am. J. Roentgenol. 210, 685–694 (2018).
Chae, E. Y. et al. Decrease in interpretation time for both novice and experienced readers using a concurrent computer-aided detection system for digital breast tomosynthesis. Eur. Radiol. 29, 2518–2525 (2019).
Conant, E. F. et al. Improving accuracy and efficiency with concurrent use of artificial intelligence for digital breast tomosynthesis. Radiol. Artif. Intell. 1, e180096 (2019).
Van Winkel, S. L. et al. Impact of artificial intelligence support on accuracy and reading time in breast tomosynthesis image interpretation: a multi-reader multi-case study. Eur. Radiol. 31, 8682–8691 (2021).
Goldberg, J. E. et al. New horizons: artificial intelligence for digital breast tomosynthesis. RadioGraphics 43, e220060 (2023).
Magni, V., Cozzi, A., Schiaffino, S., Colarieti, A. & Sardanelli, F. Artificial intelligence for digital breast tomosynthesis: impact on diagnostic performance, reading times, and workload in the era of personalized screening. Eur. J. Radiol. 158, 110631 (2023).
Mann, R. M., Kuhl, C. K., Kinkel, K. & Boetes, C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur. Radiol. 18, 1307–1318 (2008).
Feig, S. Cost-Effectiveness of mammography, MRI, and ultrasonography for breast cancer screening. Radiol. Clin. North Am. 48, 879–891 (2010).
Freer, P. E. Mammographic breast density: impact on breast cancer risk and implications for screening. RadioGraphics 35, 302–315 (2015).
Sheth, D. & Giger, M. L. Artificial intelligence in the interpretation of breast cancer on MRI. J. Magn. Reson. Imaging 51, 1310–1324 (2020).
Jiang, Y., Edwards, A. V. & Newstead, G. M. Artificial intelligence applied to breast MRI for improved diagnosis. Radiology 298, 38–46 (2021).
Morais, M., Calisto, F. M., Santiago, C., Aleluia, C. & Nascimento, J. C. Classification of breast cancer in MRI with multimodal fusion. In 2023 IEEE 20th International Symposium on Biomedical Imaging (ISBI) 1–4 (IEEE, Cartagena, Colombia, 2023).
Witowski, J. et al. Improving breast cancer diagnostics with deep learning for MRI. Sci. Transl. Med. 14, eabo4802 (2022).
Dalmış, M. U. et al. Fully automated detection of breast cancer in screening MRI using convolutional neural networks. J. Med. Imaging 5, 1 (2018).
Chung, M. et al. Deep learning to simulate contrast-enhanced breast MRI of invasive breast cancer. Radiology 306, e213199 (2023).
Diogo, P. et al. Weakly-supervised diagnosis and detection of breast cancer using deep multiple instance learning. In 2023 IEEE 20th International Symposium on Biomedical Imaging (ISBI) 1–4 (IEEE, Cartagena, Colombia, 2023).
Liu, H. et al. Artificial intelligence-based breast cancer diagnosis using ultrasound images and grid-based deep feature generator. Int. J. Gen. Med. 15, 2271–2282 (2022).
Yan, L. et al. A domain knowledge-based interpretable deep learning system for improving clinical breast ultrasound diagnosis. Commun. Med. 4, 90 (2024).
Liao, J. et al. Artificial intelligence-assisted ultrasound image analysis to discriminate early breast cancer in Chinese population: a retrospective, multicentre, cohort study. eClinicalMedicine 60, 102001 (2023).
Barinov, L. et al. Decision quality support in diagnostic breast ultrasound through artificial Intelligence. In 2016 IEEE Signal Processing in Medicine and Biology Symposium (SPMB) 1–4 (IEEE, 2016).
Nielsen, T. O. et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor–positive breast cancer. Clin. Cancer Res. 16, 5222–5232 (2010).
Wu, J. & Hicks, C. Breast cancer type classification using machine learning. J. Pers. Med. 11, 61 (2021).
Gomes, D. S., Porto, S. S., Balabram, D. & Gobbi, H. Inter-observer variability between general pathologists and a specialist in breast pathology in the diagnosis of lobular neoplasia, columnar cell lesions, atypical ductal hyperplasia and ductal carcinoma in situ of the breast. Diagn. Pathol. 9, 121 (2014).
Jaber, M. I. et al. A deep learning image-based intrinsic molecular subtype classifier of breast tumors reveals tumor heterogeneity that may affect survival. Breast Cancer Res. 22, 12 (2020). This study shows that a deep learning classifier can accurately predict intrinsic molecular subtypes of breast tumours from histopathology images, potentially improving personalised prognostic assessment.
Cruz-Roa, A. et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci. Rep. 7, 46450 (2017).
Couture, H. D. et al. Image analysis with deep learning to predict breast cancer grade, ER status, histologic subtype, and intrinsic subtype. Npj Breast Cancer 4, 30 (2018).
Bae, K. et al. Data-efficient computational pathology platform for faster and cheaper breast cancer subtype identifications: development of a deep learning model. JMIR Cancer 9, e45547 (2023).
Hossain, M. S. et al. Automatic quantification of HER2 gene amplification in invasive breast cancer from chromogenic in situ hybridization whole slide images. J. Med. Imaging 6, 1 (2019).
Adir, O. et al. Integrating artificial intelligence and nanotechnology for precision cancer medicine. Adv. Mater. 32, e1901989 (2020).
Yang, H.-Y., Wang, Y.-C., Peng, H.-Y. & Huang, C.-H. Breath biopsy of breast cancer using sensor array signals and machine learning analysis. Sci. Rep. 11, 103 (2021).
Alafeef, M., Srivastava, I. & Pan, D. Machine learning for precision breast cancer diagnosis and prediction of the nanoparticle cellular internalization. ACS Sens. 5, 1689–1698 (2020).
Wang, Y. et al. Improved breast cancer histological grading using deep learning. Ann. Oncol. 33, 89–98 (2002). This study demonstrates that deep learning–based histological grading of whole slide images provides accurate and prognostically informative breast cancer grades, offering an objective and reproducible approach to tumour evaluation.
Whitney, J. et al. Quantitative nuclear histomorphometry predicts oncotype DX risk categories for early stage ER+ breast cancer. BMC Cancer 18, 610 (2018).
Li, H. et al. Quantitative nuclear histomorphometric features are predictive of Oncotype DX risk categories in ductal carcinoma in situ: preliminary findings. Breast Cancer Res. 21, 114 (2019).
Romo-Bucheli, D., Janowczyk, A., Gilmore, H., Romero, E. & Madabhushi, A. A deep learning based strategy for identifying and associating mitotic activity with gene expression derived risk categories in estrogen receptor positive breast cancers. Cytom. A 91, 566–573 (2017).
Zhang, X. et al. Unsupervised representation learning of chromatin images identifies changes in cell state and tissue organization in DCIS. Nat. Commun. 15, 6112 (2024).
Thakur, S. S. et al. The use of automated Ki67 analysis to predict Oncotype DX risk-of-recurrence categories in early-stage breast cancer. PLoS ONE 13, e0188983 (2018).
Basavanhally, A. N. et al. Computerized image-based detection and grading of lymphocytic infiltration in HER2+ breast cancer histopathology. IEEE Trans. Biomed. Eng. 57, 642–653 (2010).
Hu, H. et al. A breast cancer classification and immune landscape analysis based on cancer stem-cell-related risk panel. Npj Precis. Oncol. 7, 130 (2023).
Xiao, Y. et al. Comprehensive metabolomics expands precision medicine for triple-negative breast cancer. Cell Res 32, 477–490 (2022).
Braman, N. et al. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. 19, 57 (2017). This study shows that radiomic features extracted from both intratumoral and peritumoral regions on pre-treatment DCE-MRI can accurately predict pathological complete response to neoadjuvant chemotherapy, demonstrating the value of radiomics for early personalised treatment stratification.
Shi, Z. et al. MRI-based quantification of intratumoral heterogeneity for predicting treatment response to neoadjuvant chemotherapy in breast cancer. Radiology 308, e222830 (2023).
Sammut, S.-J. et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature 601, 623–629 (2022).
Gu, J. et al. Deep learning radiomics of ultrasonography for comprehensively predicting tumor and axillary lymph node status after neoadjuvant chemotherapy in breast cancer patients: a multicenter study. Cancer 129, 356–366 (2023).
Peterson, J. R. et al. Novel computational biology modeling system can accurately forecast response to neoadjuvant therapy in early breast cancer. Breast Cancer Res. 25, 54 (2023).
Malik, V., Kalakoti, Y. & Sundar, D. Deep learning assisted multi-omics integration for survival and drug-response prediction in breast cancer. BMC Genomics 22, 214 (2021).
Park, S. et al. A deep learning model of tumor cell architecture elucidates response and resistance to CDK4/6 inhibitors. Nat. Cancer 5, 996–1009 (2024).
Chang, W.-T. et al. An artificial intelligence approach for predicting cardiotoxicity in breast cancer patients receiving anthracycline. Arch. Toxicol. 96, 2731–2737 (2022).
Jiang, Y.-Z. et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the FUTURE trial. Cell Res 31, 178–186 (2021).
Sarhangi, N. et al. Breast cancer in the era of precision medicine. Mol. Biol. Rep. 49, 10023–10037 (2022).
Fan, K., Cheng, L. & Li, L. Artificial intelligence and machine learning methods in predicting anti-cancer drug combination effects. Brief. Bioinform. 22, bbab271 (2021).
O’Neil, J. et al. An unbiased oncology compound screen to identify novel combination strategies. Mol. Cancer Ther. 15, 1155–1162 (2016).
Li, X. et al. Prediction of synergistic anti-cancer drug combinations based on drug target network and drug induced gene expression profiles. Artif. Intell. Med. 83, 35–43 (2017).
Celebi, R., Bear Don’t Walk, O., Movva, R., Alpsoy, S. & Dumontier, M. In-silico prediction of synergistic anti-cancer drug combinations using multi-omics data. Sci. Rep. 9, 8949 (2019).
Sidorov, P., Naulaerts, S., Ariey-Bonnet, J., Pasquier, E. & Ballester, P. J. Predicting synergism of cancer drug combinations using NCI-ALMANAC data. Front. Chem. 7, 509 (2019).
Jeon, M., Kim, S., Park, S., Lee, H. & Kang, J. In silico drug combination discovery for personalized cancer therapy. BMC Syst. Biol. 12, 16 (2018).
Julkunen, H. et al. Leveraging multi-way interactions for systematic prediction of pre-clinical drug combination effects. Nat. Commun. 11, 6136 (2020).
Preuer, K. et al. DeepSynergy: predicting anti-cancer drug synergy with deep learning. Bioinformatics 34, 1538–1546 (2018).
Sun, Z., Huang, S., Jiang, P. & Hu, P. DTF: deep tensor factorization for predicting anticancer drug synergy. Bioinformatics 36, 4483–4489 (2020).
Xia, F. et al. Predicting tumor cell line response to drug pairs with deep learning. BMC Bioinform. 19, 486 (2018).
Zhang, H., Feng, J., Zeng, A., Payne, P. & Li, F. Predicting tumor cell response to synergistic drug combinations using a novel simplified deep learning model. AMIA Annu. Symp. Proc. 2020, 1364–1372 (2020).
Zhang, T., Zhang, L., Payne, P. R. O. & Li, F. Synergistic drug combination prediction by integrating multiomics data in deep learning models. In Methods in Molecular Biology 223–238 (Humana Press Inc., 2021).
Chen, G., Tsoi, A., Xu, H. & Zheng, W. J. Predict effective drug combination by deep belief network and ontology fingerprints. J. Biomed. Inform. 85, 149–154 (2018).
Jiang, P. et al. Deep graph embedding for prioritizing synergistic anticancer drug combinations. Comput. Struct. Biotechnol. J. 18, 427–438 (2020).
Srithanyarat, T. et al. Interpreting drug synergy in breast cancer with deep learning using target-protein inhibition profiles. BioData Min. 17, 8 (2024).
He, L. et al. Patient-customized drug combination prediction and testing for t-cell prolymphocytic leukemia patients. Cancer Res. 78, 2407–2418 (2018).
Lim, J. J. et al. Rational drug combination design in patient-derived avatars reveals effective inhibition of hepatocellular carcinoma with proteasome and CDK inhibitors. J. Exp. Clin. Cancer Res. 41, 249 (2022).
Rashid, M. B. M. A. et al. Optimizing drug combinations against multiple myeloma using a quadratic phenotypic optimization platform (QPOP). Sci. Transl. Med. 10, eaan0941 (2018).
de Mel, S. et al. Application of an ex-vivo drug sensitivity platform towards achieving complete remission in a refractory T-cell lymphoma. Blood Cancer J. 10, 9 (2020).
Goh, J. et al. An ex vivo platform to guide drug combination treatment in relapsed/refractory lymphoma. Sci. Transl. Med. 14, eabn7824 (2022).
Tan, B. K. J. et al. Personalised, rational, efficacy-driven cancer drug dosing via an artificial intelligence system (PRECISE): a protocol for the PRECISE CURATE.AI pilot clinical trial. Front. Digit. Health 3, 635524 (2021).
Pantuck, A. J. et al. Modulating BET bromodomain inhibitor ZEN-3694 and enzalutamide combination dosing in a metastatic prostate cancer patient using CURATE.AI, an artificial intelligence platform. Adv. Ther. 1, 1800104 (2018).
Visvanathan, K. et al. The ENGAGE study: evaluation of a conversational virtual agent that provides tailored pre-test genetic education to cancer patients. J. Cancer Surviv. 19, 623–632 (2023). This study found that a conversational virtual agent delivering personalised pre-test genetic education was well-received by cancer patients and achieved comprehension levels comparable to traditional counselling, demonstrating its potential to expand access to genetic education services.
Al-Hilli, Z. et al. A randomized trial comparing the effectiveness of pre-test genetic counseling using an artificial intelligence automated chatbot and traditional in-person genetic counseling in women newly diagnosed with breast cancer. Ann. Surg. Oncol. 30, 5990–5996 (2023).
Nardin, S. et al. Breast cancer survivorship, quality of life, and late toxicities. Front. Oncol. 10, 864 (2020).
Delrieu, L. et al. Feasibility and health benefits of an individualized physical activity intervention in women with metastatic breast cancer: intervention study. JMIR MHealth UHealth 8, e12306 (2020).
Schaffer, K. et al. Systematic review of randomized controlled trials of exercise interventions using digital activity trackers in patients with cancer. J. Natl. Compr. Canc. Netw. 17, 57–63 (2019).
Signorelli, G. R., Monteiro-Guerra, F., Rivera-Romero, O., Núñez-Benjumea, F. J. & Fernández-Luque, L. Breast cancer physical activity mobile intervention: early findings from a user experience and acceptability mixed methods study. JMIR Form. Res. 6, e32354 (2022).
Smith, A. et al. Feasibility and preliminary efficacy of iConquerFear: a self-guided digital intervention for fear of cancer recurrence. J. Cancer Surviv. 18, 425–438 (2022).
Zhang, A. et al. Evaluating an engaging and coach-assisted online cognitive behavioral therapy for depression among adolescent and young adult cancer survivors: a pilot feasibility trial. J. Psychosoc. Oncol. 41, 20–42 (2023).
Sella, T. et al. Young, empowered and strong: a web-based education and supportive care intervention for young women with breast cancer across the care continuum. JCO Clin. Cancer Inf. 5, 933–943 (2021).
Gudmundsson, G. H. et al. Evaluating the feasibility of a digital therapeutic program for patients with cancer during active treatment: pre-post interventional study. JMIR Form. Res. 6, e39764 (2022).
Thorvardardottir, G. E. et al. A digital therapeutic intervention for breast cancer patients during active treatment: a feasibility study. Ann. Oncol. 33, S230 (2022).
Tzelves, L. et al. Artificial intelligence supporting cancer patients across Europe—The ASCAPE project. PLoS ONE 17, e0265127 (2022). This study presents the ASCAPE AI platform, demonstrating its ability to predict quality of life issues and support personalised interventions for cancer patients across Europe, highlighting its potential to enhance long-term survivorship care.
Soto-Ruiz, N., Escalada-Hernández, P., Martín-Rodríguez, L. S., Ferraz-Torres, M. & García-Vivar, C. Web-based personalized intervention to improve quality of life and self-efficacy of long-term breast cancer survivors: study protocol for a randomized controlled trial. Int. J. Environ. Res. Public. Health 19, 12240 (2022).
Pukancsik, D. et al. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: an aesthetic and functional prospective cohort study. Eur. J. Surg. Oncol. 43, 303–310 (2017).
Macmillan, R. D. & McCulley, S. J. Oncoplastic breast surgery: what, when and for whom?. Curr. Breast Cancer Rep. 8, 112–117 (2016).
Kaidar-Person, O. et al. Evaluating the ability of an artificial-intelligence cloud-based platform designed to provide information prior to locoregional therapy for breast cancer in improving patient’s satisfaction with therapy: The CINDERELLA trial. PLoS ONE 18, e0289365 (2023). This study shows that the CINDERELLA AI platform, which provides personalised pre-treatment information and predicted aesthetic outcomes for breast cancer patients, significantly improves patient satisfaction and supports more informed decision-making before locoregional therapy.
Cevik, J., Seth, I. & Rozen, W. M. Transforming breast reconstruction: the pioneering role of artificial intelligence in preoperative planning. Gland Surg. 12, 1271–1275 (2023).
Mavioso, C. et al. Automatic detection of perforators for microsurgical reconstruction. Breast 50, 19–24 (2020).
Mital, S. & Nguyen, H. V. Cost-effectiveness of using artificial intelligence versus polygenic risk score to guide breast cancer screening. BMC Cancer 22, 501 (2022). This study demonstrates that using AI to guide breast cancer screening is more cost-effective than relying on polygenic risk scores, highlighting AI’s potential to optimize personalized screening strategies and reduce healthcare costs.
Vargas-Palacios, A., Sharma, N. & Sagoo, G. S. Cost-effectiveness requirements for implementing artificial intelligence technology in the women’s UK breast cancer screening service. Nat. Commun. 14, 6110 (2023).
Hill, H., Roadevin, C., Duffy, S., Mandrik, O. & Brentnall, A. Cost-effectiveness of AI for risk-stratified breast cancer screening. JAMA Netw. Open 7, e2431715 (2024).
Brix, M. A. K. et al. Financial impact of incorporating deep learning reconstruction into magnetic resonance imaging routine. Eur. J. Radiol. 175, 111434 (2024).
Ezeana, C. F. et al. A deep learning decision support tool to improve risk stratification and reduce unnecessary biopsies in BI-RADS 4 mammograms. Radiol. Artif. Intell. 5, e220259 (2023).
Khanna, N. N. et al. Economics of artificial intelligence in healthcare: diagnosis vs. treatment. Healthcare 10, 2493 (2022).
Chubak, J., Boudreau, D. M., Fishman, P. A. & Elmore, J. G. Cost of breast-related care in the year following false positive screening mammograms. Med. Care 48, 815–820 (2010).
Ong, M.-S. & Mandl, K. D. National expenditure for false-positive mammograms and breast cancer overdiagnoses estimated at $4 billion a year. Health Aff. 34, 576–583 (2015).
Wolff, J., Pauling, J., Keck, A. & Baumbach, J. The economic impact of artificial intelligence in health care: systematic review. J. Med. Internet Res. 22, e16866 (2020).
Ahmed, M. I. et al. A systematic review of the barriers to the implementation of artificial intelligence in healthcare. Cureus 15, e46454 (2023). This systematic review identifies key technological, organisational, and ethical barriers to implementing AI in healthcare, emphasizing the need to address these challenges to enable effective and safe AI integration in clinical practice.
Rahman, M.dA. et al. Impact of artificial intelligence (AI) technology in healthcare sector: a critical evaluation of both sides of the coin. Clin. Pathol. 17, 2632010X241226887 (2024).
Cai, C. J., Winter, S., Steiner, D., Wilcox, L. & Terry, M. ‘Hello AI’: Uncovering the onboarding needs of medical practitioners for human-ai collaborative decision-making. Proc. ACM Hum. Comput. Interact. 3, 1–24 (2019). This study reveals that medical practitioners require targeted training to effectively engage in human-AI collaborative decision-making, underscoring the importance of designing AI systems that support clinician understanding and trust.
Alowais, S. A. et al. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med. Educ. 23, 689 (2023).
Calisto, F. M., Abrantes, J. M., Santiago, C., Nunes, N. J. & Nascimento, J. C. Personalized explanations for clinician-AI interaction in breast imaging diagnosis by adapting communication to expertise levels. Int. J. Hum. Comput. Stud. 197, 103444 (2025).
Cabitza, F., Rasoini, R. & Gensini, G. F. Unintended consequences of machine learning in medicine. JAMA 318, 517 (2017).
Montemayor, C., Halpern, J. & Fairweather, A. In principle obstacles for empathic AI: why we can’t replace human empathy in healthcare. AI Soc. 37, 1353–1359 (2022).
Vo, V. et al. Multi-stakeholder preferences for the use of artificial intelligence in healthcare: a systematic review and thematic analysis. Soc. Sci. Med. 338, 116357 (2023).
Wahl, B., Cossy-Gantner, A., Germann, S. & Schwalbe, N. R. Artificial intelligence (AI) and global health: how can AI contribute to health in resource-poor settings? BMJ Glob. Health 3, e000798 (2018).
Hickman, S. E., Baxter, G. C. & Gilbert, F. J. Adoption of artificial intelligence in breast imaging: evaluation, ethical constraints and limitations. Br. J. Cancer 125, 15–22 (2021).
Obermeyer, Z. & Emanuel, E. J. Predicting the future — big data, machine learning, and clinical medicine. N. Engl. J. Med. 375, 1216–1219 (2016).
Chockley, K. & Emanuel, E. The end of radiology? Three threats to the future practice of radiology. J. Am. Coll. Radiol. 13, 1415–1420 (2016).
Krittanawong, C. The rise of artificial intelligence and the uncertain future for physicians. Eur. J. Intern. Med. 48, e13–e14 (2018).
Chiwome, L., Okojie, O. M., Rahman, A. K. M. J., Javed, F. & Hamid, P. Artificial intelligence: is it armageddon for breast radiologists? Cureus 12, e8923 (2020). This article argues that while AI is transforming breast imaging, it is unlikely to replace radiologists, instead serving as a complementary tool that can enhance diagnostic accuracy and efficiency.
Langlotz, C. P. Will artificial intelligence replace radiologists?. Radiol. Artif. Intell. 1, e190058 (2019).
Pesapane, F. et al. Myths and facts about artificial intelligence: why machine- and deep-learning will not replace interventional radiologists. Med. Oncol. 37, 40 (2020).
Gampala, S., Vankeshwaram, V. & Gadula, S. S. P. Is artificial intelligence the new friend for radiologists? A review article. Cureus 12, e11137 (2020).
Pesapane, F., Codari, M. & Sardanelli, F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur. Radiol. Exp. 2, 35 (2018).
Schaffter, T. et al. Evaluation of combined artificial intelligence and radiologist assessment to interpret screening mammograms. JAMA Netw. Open 3, e200265 (2020).
Din, N. M. U., Dar, R. A., Rasool, M. & Assad, A. Breast cancer detection using deep learning: datasets, methods, and challenges ahead. Comput. Biol. Med. 149, 106073 (2022).
Goh, S. et al. Challenges in implementing artificial intelligence in breast cancer screening programs: systematic review and framework for safe adoption. J. Med. Internet Res 27, e62941 (2025). This systematic review identifies key technical, ethical, and operational challenges in implementing AI for breast cancer screening and proposes a framework to guide its safe and effective adoption in clinical programs.
Hanna, M. G. et al. Ethical and bias considerations in artificial intelligence/machine learning. Mod. Pathol. 38, 100686 (2025).
Koçak, B. et al. Bias in artificial intelligence for medical imaging: fundamentals, detection, avoidance, mitigation, challenges, ethics, and prospects. Diagn. Interv. Radiol. 31, 75–88 (2024).
Calisto, F. M. Human-centered design of personalized intelligent agents in medical imaging diagnosis. Instituto Superior Técnico https://doi.org/10.13140/RG.2.2.28353.33126 (2024).
Petersson, L. et al. Challenges to implementing artificial intelligence in healthcare: a qualitative interview study with healthcare leaders in Sweden. BMC Health Serv. Res 22, 850 (2022).
Dangi, R. R., Sharma, A. & Vageriya, V. Transforming healthcare in low-resource settings with artificial intelligence: recent developments and outcomes. Public Health Nurs. 42, 1017–1030 (2025).
Marcu, L. G., Boyd, C. & Bezak, E. Current issues regarding artificial intelligence in cancer and health care. Implications for medical physicists and biomedical engineers. Health Technol. 9, 375–381 (2019).
Acknowledgements
All figures were created on https://BioRender.com. This work was supported by funding from the WisDM Seed Fund (WisDM/Seed/002/2021, NUS).
Author information
Authors and Affiliations
Contributions
B.N.C., D.H., and T.B.T. conceptualised the review. D.H. and T.B.T. were responsible for the acquisition of funding for the study. B.N.C. wrote and edited the manuscript. B.N.C., D.K.H.T., and T.B.T. reviewed the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Francisco Maria Calisto and Asim Waqas for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chua, B.N., Thng, D.K.H., Toh, T.B. et al. Artificial intelligence for breast cancer management. Commun Med (2026). https://doi.org/10.1038/s43856-025-01342-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-025-01342-3