Abstract
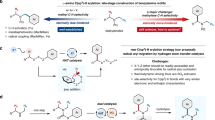
Regioselective arene C–H functionalization has widespread applications in synthetic chemistry, materials science and fine chemical industries. Catalytic methods for the ortho- and meta-C–H functionalization of arenes are well developed; however, para-C–H bond functionalization is much less developed and is more challenging due to the distal relationship of the para-C–H bond with the para-directing group. Here we report an umpolung method for the synthesis of para-functionalized anilides from arylhydroxylamines and O- and S-nucleophiles in the presence of fluorosulfuryl imidazolium triflates. Proceeding at low temperature under air, this process is tolerant of a diverse range of arylhydroxylamines and O- and S-nucleophiles giving para-functionalized anilide derivatives in good yields and excellent para-selectivity. Mechanistic studies reveal that the reaction process probably proceeds through a polarity inversion sequence, which comprises O-fluorosulfonation of the arylhydroxylamine substrate followed by N–O bond cleavage and nucleophilic addition. The synthetic utility of the reaction was demonstrated through reaction scale-up and onward synthetic transformations of the reaction products.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for the products reported in the present article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition nos. CCDC 2156664 (3p), CCDC 2160531 (5q) and CCDC 2165574 (7e). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. Experimental procedures and characterization of new compounds are available in the Supplementary Information.
References
Seebach, D. & Corey, E. J. Generation and synthetic applications of 2-lithio-1,3-dithianes. J. Org. Chem. 40, 231–237 (1975).
Dickstein, J. S., Fennie, M. W., Norman, A. L., Paulose, B. J. & Kozlowski, M. C. Three component coupling of α-iminoesters via umpolung addition of organometals: synthesis of α,α-disubstituted α-amino acids. J. Am. Chem. Soc. 130, 15794–15795 (2008).
Schedler, M., Wang, D.-S. & Glorius, F. NHC-catalyzed hydroacylation of styrenes. Angew. Chem. Int. Ed. 52, 2585–2589 (2013).
Wu, Y., Hu, L., Li, Z. & Deng, L. Catalytic asymmetric umpolung reactions of imines. Nature 523, 445–450 (2015).
Waser, M. & Novacek, J. An organocatalytic biomimetic strategy paves the way for the asymmetric umpolung of imines. Angew. Chem. Int. Ed. 54, 14228–14231 (2015).
Liu, J., Cao, C.-G., Sun, H.-B., Zhang, X. & Niu, D. Catalytic asymmetric umpolung allylation of imines. J. Am. Chem. Soc. 138, 13103–13106 (2016).
Patra, A. et al. N-Heterocyclic-carbene-catalyzed umpolung of imines. Angew. Chem. Int. Ed. 56, 2730–2734 (2017).
Seebach, D. & Enders, D. Umpolung of amine reactivity. Nucleophilic α-(secondary amino)-alkylation via metalated nitrosamines. Angew. Chem. Int. Ed. Engl. 14, 15–32 (1975).
Enders, D. & Balensiefer, T. Nucleophilic carbenes in asymmetric organocatalysis. Acc. Chem. Res. 37, 534–541 (2004).
Christmann, M. New developments in the asymmetric Stetter reaction. Angew. Chem. Int. Ed. 44, 2632–2634 (2005).
Nair, V., Vellalath, S. & Babu, B. P. Recent advances in carbon-carbon bond-forming reactions involving homoenolates generated by NHC catalysis. Chem. Soc. Rev. 37, 2691–2698 (2008).
Nair, V. et al. Employing homoenolates generated by NHC catalysis in carbon-carbon bond-forming reactions: state of the art. Chem. Soc. Rev. 40, 5336–5346 (2011).
Biju, A. T., Kuhl, N. & Glorius, F. Extending NHC-catalysis: coupling aldehydes with unconventional reaction partners. Acc. Chem. Res. 44, 1182–1195 (2011).
Berkessel, A. et al. Umpolung by N-heterocyclic carbenes: generation and reactivity of the elusive 2,2-diamino enols (Breslow intermediates). Angew. Chem. Int. Ed. 51, 12370–12374 (2012).
Berkessel, A., Yatham, V. R., Elfert, S. & Neudoerfl, J.-M. Characterization of the key intermediates of carbene-catalyzed umpolung by NMR spectroscopy and X-ray diffraction: Breslow intermediates, homoenolates, and azolium enolates. Angew. Chem. Int. Ed. 52, 11158–11162 (2013).
Menon, R. S., Biju, A. T. & Nair, V. Recent advances in employing homoenolates generated by N-heterocyclic carbene (NHC) catalysis in carbon-carbon bond-forming reactions. Chem. Soc. Rev. 44, 5040–5052 (2015).
Yoshimura, A. & Zhdankin, V. V. Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 116, 3328–3435 (2016).
Arava, S. et al. Enolonium species—umpoled enolates. Angew. Chem. Int. Ed. 56, 2599–2603 (2017).
Arava, S., Santra, S. K., Pathe, G. K., Kapanaiah, R. & Szpilman, A. M. Direct umpolung Morita–Baylis–Hillman like α-functionalization of enones via enolonium species. Angew. Chem. Int. Ed. 59, 15171–15175 (2020).
Kiefl, G. M. & Gulder, T. α-Functionalization of ketones via a nitrogen directed oxidative umpolung. J. Am. Chem. Soc. 142, 20577–20582 (2020).
Wang, T.-C. et al. Palladium-catalyzed enantioselective C(sp3)-H/C(sp3)-H umpolung coupling of N-allylimine and α-aryl ketones. J. Am. Chem. Soc. 143, 20454–20461 (2021).
Godula, K. & Sames, D. C-H bond functionalization in complex organic synthesis. Science 312, 67–72 (2006).
Alberico, D., Scott, M. E. & Lautens, M. Aryl-aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 107, 174–238 (2007).
McGlacken, G. P. & Bateman, L. M. Recent advances in aryl-aryl bond formation by direct arylation. Chem. Soc. Rev. 38, 2447–2464 (2009).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C-H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Cheng, C. & Hartwig, J. F. Rhodium-catalyzed intermolecular C-H silylation of arenes with high steric regiocontrol. Science 343, 853–857 (2014).
Li, B., Ali, A. I. M. & Ge, H. Recent advances in using transition-metal-catalyzed C-H functionalization to build fluorescent materials. Chem 6, 2591–2657 (2020).
Rej, S., Ano, Y. & Chatani, N. Bidentate directing groups: an efficient tool in C-H bond functionalization chemistry for the expedient construction of C-C bonds. Chem. Rev. 120, 1788–1887 (2020).
Zhao, Q., Meng, G., Szostak, M. & Nolan, S. P. N-Heterocyclic carbene (NHC) complexes in C-H activation reactions. Chem. Rev. 120, 1981–2048 (2020).
Shao, Q., Wu, K., Zhuang, Z., Qian, S. & Yu, J.-Q. From Pd(OAc)2 to chiral catalysts: the discovery and development of bifunctional mono-N-protected amino acid ligands for diverse C-H functionalization reactions. Acc. Chem. Res. 53, 833–851 (2020).
Phipps, R. J. & Gaunt, M. J. A meta-selective copper-catalyzed C-H bond arylation. Science 323, 1593–1597 (2009).
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C-H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Hofmann, N. & Ackermann, L. meta-Selective C-H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc. 135, 5877–5884 (2013).
Bag, S. et al. Remote para-C-H functionalization of arenes by a D-shaped biphenyl template-based assembly. J. Am. Chem. Soc. 137, 11888–11891 (2015).
Patra, T. et al. Palladium-catalyzed directed para C-H functionalization of phenols. Angew. Chem. Int. Ed. 55, 7751–7755 (2016).
Dey, A., Sinha, S. K., Achar, T. K. & Maiti, D. Accessing remote meta- and para-C(sp2)-H bonds with covalently attached directing groups. Angew. Chem. Int. Ed. 58, 10820–10843 (2019).
Shi, H. et al. Differentiation and functionalization of remote C-H bonds in adjacent positions. Nat. Chem. 12, 399–404 (2020).
Mao, J.-H. et al. Organocatalyst-controlled site-selective arene C-H functionalization. Nat. Chem. 13, 982–991 (2021).
Tabolin, A. A. & Ioffe, S. L. Rearrangement of N-oxyenamines and related reactions. Chem. Rev. 114, 5426–5476 (2014).
Kikugawa, Y. & Shimada, M. AlCl3-mediated regioselective migration of a methoxy group of N-methoxy-N-phenylamides to the ortho position of the phenyl ring. J. Chem. Soc. Chem. Commun. 19, 1450–1451 (1989).
Kikugawa, Y. & Mitsui, K. Migration of the hydroxyl group of N-hydroxy-N-phenylamides to the phenyl group with tertiary phosphines and tetrachloromethane. A novel transhydroxylation reaction. Chem. Lett. 22, 1369–1372 (1993).
Hojczyk, K. N., Feng, P., Zhan, C. & Ngai, M.-Y. Trifluoromethoxylation of arenes: synthesis of ortho-trifluoromethoxylated aniline derivatives by OCF3 migration. Angew. Chem. Int. Ed. 53, 14559–14563 (2014).
Porzelle, A., Woodrow, M. D. & Tomkinson, N. C. O. Rearrangement of differentially protected N-arylhydroxylamines. Eur. J. Org. Chem. 2008, 5135–5143 (2008).
Jones, K. L., Porzelle, A., Hall, A., Woodrow, M. D. & Tomkinson, N. C. O. Copper-catalyzed coupling of hydroxylamines with aryl iodides. Org. Lett. 10, 797–800 (2008).
Porzelle, A., Woodrow, M. D. & Tomkinson, N. C. O. Palladium-catalyzed coupling of hydroxylamines with aryl bromides, chlorides, and iodides. Org. Lett. 11, 233–236 (2009).
Porzelle, A., Woodrow, M. D. & Tomkinson, N. C. O. Synthesis of benzoxazolones from nitroarenes or aryl halides. Org. Lett. 12, 812–815 (2010).
Nakamura, I., Owada, M., Jo, T. & Terada, M. Concerted [1,3]-rearrangement in cationic cobalt-catalyzed reaction of O-(alkoxycarbonyl)-N-arylhydroxylamines. Org. Lett. 19, 2194–2196 (2017).
Yuan, H. et al. Copper-catalyzed tandem O-vinylation of arylhydroxylamines/[3,3]-rearrangement/cyclization: synthesis of highly substituted indoles and benzoindoles. ACS Catal. 9, 3906–3912 (2019).
Yuan, H. et al. Tandem approach to NOBIN analogues from arylhydroxylamines and diaryliodonium salts via [3,3]-sigmatropic rearrangement. Chem. Commun. 56, 8226–8229 (2020).
Porzelle, A., Woodrow, M. D. & Tomkinson, N. C. O. Rearrangement strategy for the synthesis of 2-aminoanilines. Org. Lett. 12, 1492–1495 (2010).
Shaaban, S., Tona, V., Peng, B. & Maulide, N. Hydroxamic acids as chemoselective (ortho-amino)arylation reagents via sigmatropic rearrangement. Angew. Chem. Int. Ed. 56, 10938–10941 (2017).
Bamberger, E. Phenylhydroxylamine. Ber. Dtsch. Chem. Ges. 27, 1548–1557 (1894).
Bamberger, E. Reduction of nitro-compounds. Chem. Ber. 27, 1347–1350 (1894).
Chuang, H.-Y., Schupp, M., Meyrelles, R., Maryasin, B. & Maulide, N. Redox-neutral selenium-catalysed isomerisation of para-hydroxamic acids into para-aminophenols. Angew. Chem. Int. Ed. 60, 13778–13782 (2021).
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014).
Dong, J., Sharpless, K. B., Kwisnek, L., Oakdale, J. S. & Fokin, V. V. SuFEx-based synthesis of polysulfates. Angew. Chem. Int. Ed. 53, 9466–9470 (2014).
Hanley, P. S., Ober, M. S., Krasovskiy, A. L., Whiteker, G. T. & Kruper, W. J. Nickel- and palladium-catalyzed coupling of aryl fluorosulfonates with aryl boronic acids enabled by sulfuryl fluoride. ACS Catal. 5, 5041–5046 (2015).
Gao, B. et al. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 9, 1083–1088 (2017).
Guo, T. et al. A new portal to SuFEx click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an ‘F-SO2+’ donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. 57, 2605–2610 (2018).
Yang, C., Flynn, J. P. & Niu, J. Facile synthesis of sequence-regulated synthetic polymers using orthogonal SuFEx and CuAAC click reactions. Angew. Chem. Int. Ed. 57, 16194–16199 (2018).
Liu, Z. et al. SuFEx click chemistry enabled late-stage drug functionalization. J. Am. Chem. Soc. 140, 2919–2925 (2018).
Barrow, A. S. et al. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 48, 4731–4758 (2019).
Zheng, Q. et al. Sulfur [18F]fluoride exchange click chemistry enabled ultrafast late-stage radiosynthesis. J. Am. Chem. Soc. 143, 3753–3763 (2021).
Zhou, H. et al. Introduction of a crystalline, shelf-stable reagent for the synthesis of sulfur(VI) fluorides. Org. Lett. 20, 812–815 (2018).
Liang, Q. et al. Palladium-catalyzed, ligand-free Suzuki reaction in water using aryl fluorosulfates. Org. Lett. 17, 1942–1945 (2015).
Schimler, S. D. et al. Nucleophilic deoxyfluorination of phenols via aryl fluorosulfonate intermediates. J. Am. Chem. Soc. 139, 1452–1455 (2017).
Ma, C. et al. Nickel-catalyzed carboxylation of aryl and heteroaryl fluorosulfates using carbon dioxide. Org. Lett. 21, 2464–2467 (2019).
Zhang, Y. et al. Palladium-catalyzed one-pot phosphorylation of phenols mediated by sulfuryl fluoride. Chem. Commun. 57, 4588–4591 (2021).
Epifanov, M. et al. One-pot 1,1-dihydrofluoroalkylation of amines using sulfuryl fluoride. J. Am. Chem. Soc. 140, 16464–16468 (2018).
Foth, P. J., Gu, F., Bolduc, T. G., Kanani, S. S. & Sammis, G. M. New sulfuryl fluoride-derived alkylating reagents for the 1,1-dihydrofluoroalkylation of thiols. Chem. Sci. 10, 10331–10335 (2019).
Fang, W.-Y., Zha, G.-F., Zhao, C. & Qin, H.-L. Regioselective installation of fluorosulfate (-OSO2F) functionality into aromatic C(sp2)-H bonds for the construction of para-amino-arylfluorosulfates. Chem. Commun. 55, 6273–6276 (2019).
Kikugawa, Y., Matsumoto, K., Mitsui, K. & Sakamoto, T. A facile regioselective aromatic fluorination of N-aryl-N-hydroxyamides with diethylaminosulfur trifluoride (DAST). J. Chem. Soc. Chem. Commun. 921–922 (1992).
Fu, L. et al. Ligand-enabled site-selectivity in a versatile rhodium(II)-catalysed aryl C-H carboxylation with CO2. Nat. Catal. 1, 469–478 (2018).
Cermak, J. K. & Cirkva, V. Copper-mediated synthesis of mono- and dichlorinated diaryl ethers. Tetrahedron Lett. 55, 4185–4188 (2014).
Tamaki, T. & Nagaki, A. Flash production of organophosphorus compounds in flow. Tetrahedron Lett. 81, 153364 (2021).
Bronner, S. M., Goetz, A. E. & Garg, N. K. Overturning indolyne regioselectivities and synthesis of indolactam V. J. Am. Chem. Soc. 133, 3832–3835 (2011).
Rodriguez-Otero, J., Hermida-Ramon, J. M. & Cabaleiro-Lago, E. M. DFT study of the nucleophilic addition of water to ketenes. Eur. J. Org. Chem. 2007, 2344–2351 (2007).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant nos. 22271176, 21933003 and 91856105), the Key Technology Research and Development Program of Shandong Province (grant no. 2019GSF108056) and Shandong University. This work was also supported by the High-Performance Computing Platform of Peking University. We thank D. Sun at Shandong University for the X-ray diffraction and data analysis.
Author information
Authors and Affiliations
Contributions
H.G. conceived and directed the project. Z.X. designed and performed experiments. X.-J.L. performed the DFT calculations and mechanism analysis. Z.Q.G. and Z.W.G. helped with the collection of some new compounds and data analysis. H.G., Z.-X.Y. and X.-J.L. wrote the paper with input from all other authors. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
Shandong University has filed a patent application (patent application no. 202210215790.X, China). The competing interest is related to H.G., Z.X., Z.Q.G. and Z.W.G. X.-J.L. and Z.-X.Y. declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Jesus Jover and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Discussion and Tables 1–8.
Supplementary Data 1
Crystallographic data for 3p, CCDC 2156664.
Supplementary Data 2
Crystallographic data for 5q, CCDC 2160531.
Supplementary Data 3
Crystallographic data for 7e, CCDC 2165574.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xi, Z., Liu, XJ., Guo, Z. et al. Regioselective umpolung para-C–H functionalization of arylhydroxylamines. Nat. Synth 2, 778–788 (2023). https://doi.org/10.1038/s44160-023-00293-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-023-00293-8
This article is cited by
-
Metal-free para-selective C-H amination and azidation of N-arylhydroxylamines
Nature Communications (2025)
-
Selective synthesis of phenylhydroxylamine under slug flow conditions using Bayesian optimization
Journal of Flow Chemistry (2025)
-
Tailoring photocatalysts to modulate oxidative potential of anilides enhances para-selective electrochemical hydroxylation
Nature Communications (2024)