Abstract
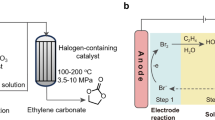
Electrochemical methods have emerged as crucial strategies in the pursuit of achieving net-zero emissions, addressing key objectives such as hydrogen production, CO2 capture and CO2 conversion. Here we propose an electrochemically initiated process that integrates these three tasks while producing valuable industrial chemicals, such as hydrogen, ethylene carbonate and urethane polymers. In this approach, CO2, ethylene and water are transformed into ethylene carbonate and hydrogen, and the key to enabling this transformation is the discovery that CO2, captured as sodium bicarbonate, can be utilized in organic carbonate synthesis in an aqueous solution. Importantly, this transformation is mediated by succinimide, an organic catalyst that interacts with protons, bromine and CO2 throughout this process. Technoeconomic analysis and life-cycle assessment of a commercial-scale process based on our proposed reaction pathway reveals its profitability and environmental viability. In the future, this approach could be generalized for the synthesis of various organic carbonates, with implications for producing lithium battery electrolytes, carbonate polymers and non-isocyanate urethane polymers.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Additional data related to this study are available from the corresponding author upon request.
References
UNFCCC, Glasgow Climate Pact, Decision -/CP.26, advance unedited version (2021); https://unfccc.int/documents/310475
Lee, M. et al. Current achievements and the future direction of electrochemical CO2 reduction: a short review. Crit. Rev. Environ. Sci. Technol. 50, 769–815 (2020).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Jin, S. et al. Low energy carbon capture via electrochemically induced pH swing with electrochemical rebalancing. Nat. Commun. 13, 2140 (2022).
Zhu, P. et al. Continuous carbon capture in an electrochemical solid-electrolyte reactor. Nature 618, 959–966 (2023).
Kim, C. et al. Highly efficient CO2 utilization via aqueous zinc– or aluminum–CO2 systems for hydrogen gas evolution and electricity production. Angew. Chem. Int. Ed. 131, 9606–9611 (2019).
Sullivan, I. et al. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 4, 952–958 (2021).
Gutiérrez-Sánchez, O. et al. A state‐of‐the‐art update on integrated CO2 capture and electrochemical conversion systems. ChemElectroChem 9, e202101540 (2022).
Lee, K. M. et al. Redox-neutral electrochemical conversion of CO2 to dimethyl carbonate. Nat. Energy 6, 733–741 (2021).
Gu, X. et al. Simulation and assessment of manufacturing ethylene carbonate from ethylene oxide in multiple process routes. Chin. J. Chem. Eng. 3, 135–144 (2021).
Fukuoka, S. et al. A novel non-phosgene process for polycarbonate production from CO2: green and sustainable chemistry in practice. Catal. Surv. Asia 14, 146–163 (2010).
Fernandes, D. et al. Investigations of primary and secondary amine carbamate stability by 1H NMR spectroscopy for post combustion capture of carbon dioxide. J. Chem. Thermodyn. 54, 183–191 (2012).
Mohite, A. R. et al. Thiourea-mediated halogenation of alcohols. J. Org. Chem. 85, 12901–12911 (2020).
Adimurthy, S. et al. Eco-friendly and versatile brominating reagent prepared from a liquid bromine precursor. Green Chem. 8, 916–922 (2006).
McClellan, P. P. Manufacture and uses of ethylene oxide and ethylene glycol. Ind. Eng. Chem. 42, 2402–2407 (1950).
Leow, W. R. et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368, 1228–1233 (2020).
Schalck, J. et al. The bromine mediated electrosynthesis of ethylene oxide from ethylene in continuous flow-through operation. Chem. Eng. J. 446, 136750 (2022).
Deno, N. C. & Fruit, R. E. Jr. The oxidative cleavage of amines by aqueous bromine at 25 deg. J. Am. Chem. Soc. 90, 3502–3506 (1968).
Horner, L. & Winkelmann, E. H. N-Bromosuccinimide, its properties and reactions the course of substitution. Newer Methods Prep. Org. Chem. 3, 151–198 (1964).
Khokarale, S. G. & Mikkola, J. P. Metal free synthesis of ethylene and propylene carbonate from alkylene halohydrin and CO2 at room temperature. RSC Adv. 9, 34023–34031 (2019).
Lyubimov, S. E. et al. A simple synthesis of ethylene carbonate from carbon dioxide and 2-chloroethanol using silica gel as a catalyst. Appl. Catal. A 592, 117433 (2020).
Zanatta, M. et al. Direct air capture and integrated conversion of carbon dioxide into cyclic carbonates with basic organic salts. ACS Sustain. Chem. Eng. 11, 9613–9619 (2023).
Maisonneuve, L. et al. Isocyanate-free routes to polyurethanes and poly(hydroxy urethane)s. Chem. Rev. 115, 12407–12439 (2015).
Dros, A. B. et al. Hexamethylenediamine (HMDA) from fossil- vs bio-based routes: an economic and life cycle assessment comparative study. Green Chem. 17, 4760–4772 (2015).
Saliu, F. & Rindone, B. Organocatalyzed synthesis of ureas from amines and ethylene carbonate. Tetrahedron Lett. 51, 6301–6304 (2010).
Kočí, V. & Loubal, T. LCA of liquid epoxy resin produced based on propylene and on glycerin. Acta Environ. Universitatis Comenianae 20, 62–67 (2012).
Scodeller, I. et al. Synthesis of renewable meta‐xylylenediamine from biomass‐derived furfural. Angew. Chem. Int. Ed. 57, 10510–10514 (2018).
Nwaoha, C. et al. Process simulation and parametric sensitivity study of CO2 capture from 115 MW coal–fired power plant using MEA–DEA blend. Int. J. Greenhouse Gas Control 76, 1–11 (2018).
Langie, K. M. G. et al. Toward economical application of carbon capture and utilization technology with near-zero carbon emission. Nat. Commun. 13, 1–10 (2022).
Cannavó, F. Sensitivity analysis for volcanic source modeling quality assessment and model selection. Comput. Geosci. 44, 52–59 (2012).
Environmental Management: Life Cycle Assessment; Principles and Framework (ISO, 2006).
Environmental Management: Life Cycle Assessment; Requirements and Guidelines, ISO 14044 (ISO, 2006).
Monie, F. et al. Chemo‐ and regioselective additions of nucleophiles to cyclic carbonates for the preparation of self‐blowing non‐isocyanate polyurethane foams. Angew. Chem. Int. Ed. 132, 17181–17189 (2020).
Korth, H. G. & Mulder, P. Phenolic hydrogen transfer by molecular oxygen and hydroperoxyl radicals. Insights into the mechanism of the anthraquinone process. J. Org. Chem. 85, 2560–2574 (2020).
Seider W. D., Seader J. D., & Lewin D. R., Product and Process Design Principles: Synthesis, Analysis and Evaluation (John Wiley, 2009).
Colella W. G., James B. D., & Moton J. M., Hydrogen Pathways Analysis for Polymer Electrolyte Membrane (PEM) Electrolysis (Strategic Analysis, 2014).
Huang, Z., Grim, R. G., Schaidle, J. A. & Tao, L. The economic outlook for converting CO2 and electrons to molecules. Energy Environ. Sci. 14, 3664–3678 (2021).
Assen, N. V. D., Voll, P., Peters, M. & Bardow, A. Life cycle assessment of CO2 capture and utilization: a tutorial review. Chem. Soc. Rev. 43, 7982–7994 (2014).
Wernet, G. et al. The Ecoinvent database version 3 (part I): overview and methodology. Int. J. Life Cycle Assess. 21, 1218–1230 (2016).
Delpierre, M. et al. Assessing the environmental impacts of wind-based hydrogen production in the Netherlands using ex-ante LCA and scenarios analysis. J. Clean. Prod. 299, 126866 (2021).
Acknowledgements
This research was supported by the Creative Materials Discovery Program (2017M3D1A1039377) and the DACU Program (RS-2023-00259920) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (MSIT), the Korea Environment Industry & Technology Institute (KEITI) funded by the Korea Ministry of Environment (2021002800009), the Ministry of Trade, Industry and Energy (MOTIE) (10076899), Development of Electro-chlorination Catalyst and System, and the National Research Foundation of Korea (NRF-2022M3C1A3092056). K.T.N. thanks the Institute of Engineering Research, the Research Institute of Advanced Materials (RIAM) and the Soft Foundry at Seoul National University for support.
Author information
Authors and Affiliations
Contributions
K.T.N. and J.H.J. conceived the idea and obtained the initial results. J.H.J., C.K., O.S.N., U.L., M.S.K. and K.T.N. designed the research and wrote the manuscript. J.H.J., J.B.Y. and Y.I.J. conducted experiments for steps RX1–RX4. O.S.N., G.R.K. and J.K. performed the synthesis, functionalization, characterization of BHUs and DFT calculations. C.K. simulated the model process and performed the technoeconomic analysis and the LCA. All authors discussed the experiments and contributed to the writing of the manuscript. U.L. guided the process simulation, the technoeconomic analysis and the LCA. M.S.K. guided the synthesis, functionalization and characterization of BHUs and the DFT calculation. K.T.N. guided all aspects of this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary Figs. 1–9 and Tables 1–7.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 5
Source data for Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jang, J.H., Kim, C., Nayal, O.S. et al. Electrochemically initiated synthesis of ethylene carbonate from CO2. Nat. Synth 3, 846–857 (2024). https://doi.org/10.1038/s44160-024-00543-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00543-3
This article is cited by
-
Bromide-mediated membraneless electrosynthesis of ethylene carbonate from CO2 and ethylene
Nature Communications (2025)
-
Ampere-level electroconversion of acetylene towards polymer grade ethylene via Pd-H mediated non-spillover hydrogenation
Nature Communications (2025)
-
Electrocatalytic N–C–N coupling over a hierarchically ordered open single-atom superstructure toward organonitrogen synthesis
Nature Communications (2025)
-
Electrochemical deprotonation of halohydrins enables cascading reactions for CO2 capture and conversion into ethylene carbonate
Nature Communications (2025)