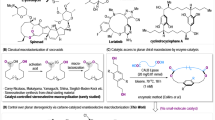
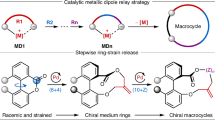
Abstract
Macrolactones are privileged motifs in materials science, aromachemicals and pharmaceuticals. The pivotal ester linkage is often formed from chiral secondary alcohols, with macrolactonization using stoichiometric reagents to ensure retention or inversion of stereochemistry without compromising enantiopurity. An ideal strategy for macrolactonization is via dynamic kinetic resolution (DKR), which involves the simultaneous formation of the ester bond and introduction of a chiral centre with high stereocontrol. Surprisingly, a DKR method within the context of macrocyclization is yet to be reported. Here, using a chemoenzymatic approach, the macrocyclic DKR of seco esters affords enantioenriched macrolactones. An optimized protocol (using Candida antarctica lipase B (~0.04 mol%) and Shvo’s catalyst) forms 14–19-membered macrocycles with excellent enantioselectivities (85–99% e.e.). A variety of macrolactones were synthesized including aliphatic macrocycles, meta- and paracyclophanes as well as a macrodiolide via a dimerization protocol that was converted to the natural product macrolide (−)-pyrenophorin.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data relating to the materials and methods, experimental procedures, mechanistic studies and NMR spectra are available in the Supplementary Information.
References
Driggers, E. M., Hale, S. P., Lee, J. & Terrett, N. K. The exploration of macrocycles for drug discovery — an underexploited structural class. Nature 7, 608–624 (2008).
Wessjohann, L. A., Ruijter, E., Garica-Rivera, D. & Brandt, W. What can a chemist learn from nature’s macrocycles? – a brief, conceptual view. Mol. Divers. 9, 171–186 (2005).
Henkel, T., Brunne, R. M., Mller, H. & Reichel, F. Statistical investigation into the structural complementarity of natural products and synthetic compounds. Angew. Chem. Int. Ed. 38, 643–647 (1999).
Kim, H. J., Pongdee, R., Wu, Q., Hong, L. & Liu, H.-W. The biosynthesis of spinosyn in Saccharopolyspora spinosa: synthesis of the cross-bridging precursor and identification of the function of SpnJ. J. Am. Chem. Soc. 129, 14582–14584 (2007).
Flynn, E. H., Sigal, M. V. Jr., Wiley, P. F. & Gerzon, K. Erythromycin. I. Properties and degradation studies. J. Am. Chem. Soc. 76, 3121–3131 (1954).
Nicolaou, K. C. et al. Design, synthesis, and biological properties of highly potent epothilone B analogues. Angew. Chem. Int. Ed. 42, 3515–3520 (2003).
Gaul, C. et al. The migrastatin family: discovery of potent cell migration inhibitors by chemical synthesis. J. Am. Chem. Soc. 126, 11326–11337 (2004).
Wender, P. A., DeChristopher, B. A. & Schrier, A. J. Formal synthesis of (−)-kendomycin featuring a Prins-cyclization to construct the macrocycle. J. Am. Chem. Soc. 130, 6658–6659 (2008).
Zin, P. P. K., Williams, G. J. & Ekins, S. Cheminformatics analysis and modeling with MacrolactoneDB. Sci. Rep. 10, 6284 (2020).
Seiple, I. B. et al. A platform for the discovery of new macrolide antibiotics. Nature 533, 338–345 (2016).
Saridakis, I., Kaiser, D. & Maulide, N. Unconventional macrocyclizations in natural product synthesis. ACS Cent. Sci. 6, 1869–1889 (2020).
Gradillas, A. & Perez-Castells, J. Macrocyclization by ring-closing metathesis in the total synthesis of natural products: reaction conditions and limitations. Angew. Chem. Int. Ed. 45, 6086–6101 (2006).
Garg, N. K., Hiebert, S. & Overman, L. E. Total synthesis of (−)-sarain A. Angew. Chem. Int. Ed. 45, 2912–2915 (2006).
Trost, B. M., Harrington, P. E., Chisholm, J. D. & Wrobleski, S. T. Total synthesis of (+)-amphidinolide A. Structure elucidation and completion of the synthesis. J. Am. Chem. Soc. 127, 13598–13610 (2005).
Hoye, T. R. & Wang, J. Alkyne haloallylation [with Pd(II)] as a core strategy for macrocycle synthesis: a total synthesis of (−)-haterumalide NA/(−)-oocydin A. J. Am. Chem. Soc. 127, 6950–6951 (2005).
Trost, B. M. Cyclizations via palladium-catalyzed allylic alkylations. Angew. Chem. Int. Ed. Engl. 28, 1173–1192 (1989).
Fraunhoffer, K. J., Prabagaran, N., Sirois, L. E. & White, M. C. Macrolactonization via hydrocarbon oxidation. J. Am. Chem. Soc. 128, 9032–9033 (2006).
Lumbroso, A., Koschker, P., Vautravers, N. R. & Breit, B. Enantioselective synthesis of branched allylic esters via rhodium-catalyzed coupling of allenes with carboxylic acids. J. Am. Chem. Soc. 133, 2386–2389 (2011).
Cooke, M. L., Xu, K. & Breit, B. Enantioselective rhodium-catalyzed synthesis of branched allylic amines by intermolecular hydroamination of terminal allene. Angew. Chem. Int. Ed. 51, 10876–10879 (2012).
Xu, K., Gilles, T. & Breit, B. Asymmetric synthesis of N-allylic indoles via regio- and enantioselective allylation of aryl hydrazines. Nat. Commun. 6, 7616 (2015).
de Léséleuc, M. & Collins, S. K. Direct macrolactonization of seco acids via hafnium(IV) catalysis. ACS Catal. 3, 1462–1467 (2015).
de Léséleuc, M. & Collins, S. K. Direct synthesis of macrodiolides via hafnium(IV) catalysis. Chem. Commun. 51, 10471–10474 (2015).
Parenty, A., Moreau, X. & Campagne, J.-M. Macrolactonizations in the total synthesis of natural products. Chem. Rev. 106, 911–939 (2006).
Parenty, A., Moreau, X., Niel, G. & Campagne, J.-M. Update 1 of: macrolactonizations in the total synthesis of natural products. Chem. Rev. 113, PR1–PR40 (2013).
Zhiwei, G., Ngooi, T. K., Scilimati, A., Fülling, G. & Sih, C. J. Macrocyclic lactones via biocatalysis in non-aqueous media. Tetrahedron Lett. 29, 5583–5586 (1998).
Gargouri, M., Drouet, P. & Legoy, M.-D. Synthesis of a novel macrolactone by lipase-catalyzed intra-esterification of hydroxy-fatty acid in organic media. J. Biotechnol. 92, 259–266 (2002).
Kiran, K. R. & Divakar, S. Lipase catalyzed synthesis of organic acid esters of lactic acid in non-aqueous media. J. Biotechnol. 87, 109–121 (2001).
Fortunati, T., D’Acunto, M., Caruso, T. & Spinella, A. Chemoenzymatic preparation of musky macrolactones. Tetrahedron 71, 2357–2362 (2015).
Bisht, K. S., Bhatt, S. & Muppalla, K. Synthesis of glycolipid analogs via highly regioselective macrolactonization catalyzed by lipase. Tetrahedron Lett. 47, 8645–8649 (2006).
Gagnon, C. et al. Biocatalytic synthesis of planar chiral macrocycles. Science 367, 917–921 (2020).
Sugai, T., Katoh, O. & Ohta, H. Chemo-enzymatic synthesis of (R,R)-(−)-pyrenophorin. Tetrahedron 51, 11987–11998 (1995).
Verho, O. & Bäckvall, J.-E. Chemoenzymatic dynamic kinetic resolution: a powerful tool for the preparation of enantiomerically pure alcohols and amines. J. Am. Chem. Soc. 137, 3996–4009 (2015).
Lia, J., Amatunia, A. & Renata, H. Recent advances in the chemoenzymatic synthesis of bioactive natural products. Curr. Opin. Chem. Biol. 55, 111–118 (2020).
Yang, L.-C., Deng, H. & Renata, H. Recent progress and developments in chemoenzymatic and biocatalytic dynamic kinetic resolution. Org. Process Res. Dev. 26, 1925–1943 (2022).
Li, X., Cao, X., Xiong, J. & Ge, J. Enzyme–metal hybrid catalysts for chemoenzymatic reactions. Small 16, 1902751 (2020).
Ferraccioli, R. Progress on the stereoselective synthesis of chiral molecules based on metal-catalyzed dynamic kinetic resolution of alcohols with lipases. Symmetry 13, 1744–1766 (2021).
Donald, J. R. & Unsworth, W. P. Ring-expansion reactions in the synthesis of macrocycles and medium-sized rings. Chem. Eur. J. 23, 8780–8799 (2017).
Stephens, T. C., Lawer, A., French, T. & Unsworth, W. P. Iterative assembly of macrocyclic lactones using successive ring expansion reactions. Chem. Eur. J. 24, 13947–13953 (2018).
Gustafson, K. P. J., Gumundsson, A., Lewis, K. & Bäckvall, J. E. Chemoenzymatic dynamic kinetic resolution of secondary alcohols using an air- and moisture-stable iron racemization catalyst. Chem. Eur. J. 23, 1048–1051 (2017).
Pamies, O. & Bäckvall, J.-E. Enzymatic kinetic resolution and chemoenzymatic dynamic kinetic resolution of δ-hydroxy esters. An efficient route to chiral δ-Lactones. J. Org. Chem. 67, 1261–1265 (2002).
Corbeil, C. R., Englebienne, P. & Moitessier, N. Docking ligands into flexible and solvated macromolecules. 1. Development and validation of FITTED 1.0. J. Chem. Inf. Model. 47, 435–449 (2007).
Moitessier, N. et al. Medicinal chemistry projects requiring imaginative structure-based drug design methods. Acc. Chem. Res. 49, 1646–1657 (2016).
Therrien, E. et al. Integrating medicinal chemistry, organic/combinatorial chemistry, and computational chemistry for the discovery of selective estrogen receptor modulators with FORECASTER, a novel platform for drug discovery. J. Chem. Inf. Model. 52, 210–224 (2012).
Kazlauskas, R. J., Weissfloch, A. N. E., Rappaport, A. T. & Cuccia, L. A. A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 56, 2656–2665 (1991).
Uppenberg, J. et al. Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry 34, 16838–16851 (1995).
Magnusson, A. O., Takwa, M., Hamberg, A. & Hult, K. An S-selective lipase was created by rational redesign and the enantioselectivity increased with temperature. Angew. Chem. Int. Ed. 44, 4582–4585 (2005).
Schopf, P. & Warshel, A. Validating computer simulations of enantioselective catalysis; reproducing the large steric and entropic contributions in Candida antarctica lipase B. Proteins 82, 1387–1399 (2014).
Ferrario, V., Ebert, C., Nitti, P., Pitacco, G. & Gardossi, L. Modelling and predicting enzyme enantioselectivity: the aid of computational methods for the rational use of lipase B from Candida antarctica. Curr. Biotechnol. 4, 87–99 (2015).
Park, A. et al. Structural and experimental evidence for the enantiomeric recognition toward a bulky sec-alcohol by Candida antarctica lipase B. ACS Catal. 6, 7458–7465 (2016).
Nozoe, S. et al. The structure of pyrenophorin. Tetrahedron Lett. 6, 4675–4677 (1995).
Rao, K. S., Reddy, S., Mukkanti, K., Pala, M. & Iqbala, J. A concise asymmetric route to the antibiotic macrolides patulolide A and pyrenophorin. Tetrahedron Lett. 47, 6623–6626 (2006).
Furstner, A., Thiel, O. R. & Ackermann, L. Exploiting the reversibility of olefin metathesis. Syntheses of macrocyclic trisubstituted alkenes and (R,R)-(−)-pyrenophorin. Org. Lett. 3, 449–451 (2001).
Benítez-Mateos, A. I., Padrosa, D. R., Contente, M. L. & Paradisi, F. Flow biocatalysis 101: design, development and applications. React. Chem. Eng. 6, 599–611 (2021).
Eijsink, V. G. H., Gaseidnes, S., Borchert, T. V. & van den Burg, B. Directed evolution of enzyme stability. Biomol. Eng. 22, 21–30 (2005).
Palomo, J. M. Nanobiohybrids: a new concept for metal nanoparticles synthesis. Chem. Commun. 55, 9583–9589 (2019).
González-Granda, S., Escot, L., Lavandera, I. & Gotor-Fernández, V. Chemoenzymatic cascades combining biocatalysis and transition metal catalysis for asymmetric synthesis. Angew. Chem. Int. Ed. 62, e202217713 (2023).
Acknowledgements
We acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery 1043344 and RGPIN-2020-05006), Université de Montréal and the Fonds de Recherche Nature et Technologies via the Centre in Green Chemistry and Catalysis (FRQNT-2020-RS4-265155-CCVC) for generous funding. We thank E. Godin for preliminary results. N. Moitessier, B. Weiser and S. Ma are thanked for computational help with the FORECASTER program.
Author information
Authors and Affiliations
Contributions
S.K.C. and J.G.-M. were involved in the discovery of the macrocyclization process. J.G.-M., M.S., E.F., G.L. and S.K.C. participated in the development of the methods and investigations. S.K.C. designed and directed the investigations. S.K.C. and J.G.-M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Jan-Erling Bäckvall, Jose M. Palomo, Xiang Sheng and William Unsworth for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, experimental details and characterization data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guerrero-Morales, J., Scaglia, M., Fauran, E. et al. Chemoenzymatic synthesis of macrocycles via dynamic kinetic resolution of secondary alcohols. Nat. Synth 3, 1275–1282 (2024). https://doi.org/10.1038/s44160-024-00591-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00591-9
This article is cited by
-
Enantio-, atrop-, and diastereoselective macrolactonization to access type III cyclophanes
Nature Communications (2025)