Abstract
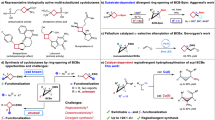
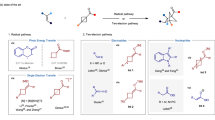
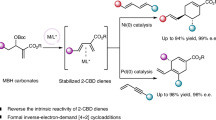
Cycloaddition reactions of bicyclo[1.1.0]butanes (BCBs) with 2π components are a powerful tool for preparing C(sp3)-rich arene bioisosteres. Despite enormous progress in this field, catalytic enantioselective cycloadditions of BCBs that produce enantioenriched three-dimensional bioisosteres are underdeveloped. Here we report a palladium-catalysed [3 + 2] cycloaddition reaction of vinyl-carbonyl-BCBs with carbonyl compounds, including formaldehyde, activated ketones, and aliphatic and aromatic aldehydes. This approach provides quick access to a wide variety of 2-oxabicyclo[2.1.1]hexanes. Density functional theory calculations indicate that the reaction occurs through a zwitterionic mechanism involving σ-bond cleavage, nucleophilic addition and allylic substitution. When (R,R)-ANDEN-phenyl Trost ligand is used, the stereoselectivity of the addition of palladium-zwitterionic enolates to carbonyl can be controlled to achieve enantioselective [3 + 2] cycloadditions. We further demonstrate the practicality of the method by carrying out several downstream transformations of cycloaddition products.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information files. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2358250 for (S)-4o and CCDC 2325465 for (R)-4ab. These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif.
References
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Ritchie, T. J. & Macdonald, S. J. F. The impact of aromatic ring count on compound developability—are too many aromatic rings a liability in drug design? Drug Discov. Today 14, 1011–1020 (2009).
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Auberson, Y. P. et al. Improving nonspecifc binding and solubility: bicycloalkyl groups and cubanes as para-phenyl bioisosteres. ChemMedChem 12, 590–598 (2017).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Tse, G. E. et al. Nonclassical phenyl bioisosteres as effective replacements in a series of novel open-source antimalarials. J. Med. Chem. 63, 11585–11601 (2020).
Brown, N. & Mannhold, R. Bioisosteres in Medicinal Chemistry (Wiley-VCH, 2012).
Blakemore, D. C. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Kanazawa, J. & Uchiyama, M. Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. Synlett 30, 1–11 (2019).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Kanazawa, J., Maeda, K. & Uchiyama, M. Radical multicomponent carboamination of [1.1.1]propellane. J. Am. Chem. Soc. 139, 17791–17794 (2017).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Stephenson, R. J. et al. Photochemical formal (4 + 2)-cycloaddition of imine-substituted bicyclo[1.1.1]pentanes and alkenes. J. Am. Chem. Soc. 143, 21223–21228 (2021).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Dong, W. et al. Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane. Nat. Chem. 14, 1068–1077 (2022).
Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022).
MacMillan, D. W. C. et al. General access to cubanes as benzene bioisosteres. Nature 618, 513–518 (2023).
Sarpong, R. et al. Skeletal editing approach to bridge-functionalized bicyclo[1.1.1]pentanes from azabicyclo[2.1.1]hexanes. J. Am. Chem. Soc. 145, 10960–10966 (2023).
Yu, I. F. et al. Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes. Nat. Chem. 15, 685–693 (2023).
Yang, Y. et al. Programmable late-stage functionalization of bridge-substituted bicyclo[1.1.1]pentane bis-boronates. Nat. Chem. 16, 285–293 (2024).
Alvarez, E. M. et al. O-, N- and C-bicyclopentylation using thianthrenium reagents. Nat. Synth. 2, 548–556 (2023).
Denisenko, A. et al. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Ed. 59, 20515–20521 (2020).
Kleinmans, R. et al. Intermolecular [2π + 2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Bychek, R. & Mykhailiuk, P. K. A. Practical and scalable approach to fluoro-substituted bicyclo[1.1.1]pentanes. Angew. Chem. Int. Ed. 61, e202205103 (2022).
Xu, M. et al. Diboron(4)-catalyzed remote [3 + 2] cycloaddition of cyclopropanes via dearomative/rearomative radical transmission through pyridine. Angew. Chem. Int. Ed. 61, e202214507 (2022).
Agasti, S. et al. A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. Nat. Chem. 15, 535–541 (2023).
Dhake, K. et al. Beyond bioisosteres: divergent synthesis of azabicyclohexanes and cyclobutenyl amines from bicyclobutanes. Angew. Chem. Int. Ed. 61, e202204719 (2022).
Liang, Y., Paulus, F., Daniliuc, C. G. & Glorius, F. Catalytic formal [2π + 2σ] cycloaddition of aldehydes with bicyclobutanes: expedient access to polysubstituted 2-oxabicyclo[2.1.1]hexanes. Angew. Chem. Int. Ed. 62, e20230504 (2023).
Tang, L. et al. Silver‐catalyzed dearomative [2π + 2σ] cycloadditions of indoles with bicyclobutanes: access to indoline fused bicyclo[2.1.1]hexanes. Angew. Chem. Int. Ed. 62, e202310066 (2023).
Ni, D. et al. Intermolecular formal cycloaddition of indoles with bicyclo[1.1.0]butanes by Lewis acid catalysis. Angew. Chem. Int. Ed. 62, e20230860 (2023).
Radhoff, N., Daniliuc, C. G. & Studer, A. Lewis acid catalyzed formal (3 + 2)-cycloaddition of bicyclo[1.1.0]butanes with ketenes. Angew. Chem. Int. Ed. 62, e202304771 (2023).
Zhang, J., Su, J.-Y. & Zheng, H. et al. Eu(OTf)3-catalyzed formal dipolar [4π + 2σ] cycloaddition of bicyclo-[1.1.0]butanes with nitrones: access to polysubstituted 2-oxa-3-azabicyclo[3.1.1]heptanes. Angew. Chem. Int. Ed. 63, e202318476 (2024).
Liang, Y. et al. Silver-enabled cycloaddition of bicyclobutanes with isocyanides for the synthesis of polysubstituted 3-azabicyclo[3.1.1]heptanes. Angew. Chem. Int. Ed. 63, e202402730 (2024).
Zheng, Y. et al. Photochemical intermolecular [3σ + 2σ]-cycloaddition for the construction of aminobicyclo[3.1.1]heptanes. J. Am. Chem. Soc. 144, 23685–23690 (2022).
Yu, T. et al. Selective [2σ + 2σ] cycloaddition enabled by boronyl radical catalysis: synthesis of highly substituted bicyclo[3.1.1] heptanes. J. Am. Chem. Soc. 145, 4304–4310 (2023).
Bellotti, P. & Glorius, F. Strain-release photocatalysis. J. Am. Chem. Soc. 145, 20716–20732 (2023).
Liu, Y. et al. Pyridine-boryl radical-catalyzed [2π + 2σ] cycloaddition of bicyclo[1.1.0]butanes with alkenes. ACS Catal. 13, 5096–5103 (2023).
Walczak, M. A., Krainz, T. & Wipf, P. Ring-strain-enabled reaction discovery: new heterocycles from bicyclo[1.1.0]butanes. Acc. Chem. Res. 48, 1149–1158 (2015).
Guo, R. et al. Strain-release [2π + 2σ] cycloadditions for the synthesis of bicyclo[2.1.1]hexanes initiated by energy transfer. J. Am. Chem. Soc. 144, 7988–7994 (2022).
Liang, Y. et al. Synthesis of polysubstituted 2-oxabicyclo[2.1.1]hexanes via visible-light-induced energy transfer. J. Am. Chem. Soc. 144, 20207–20213 (2022).
Kleinmans, R. et al. ortho-Selective dearomative [2π + 2σ] photocycloadditions of bicyclic aza-arenes. J. Am. Chem. Soc. 145, 12324–12332 (2023).
Robichon, M. et al. Enantioselective, intermolecular [π2 + σ2] photocycloaddition reactions of 2(1H)-quinolones and bicyclo[1.1.0]butanes. J. Am. Chem. Soc. 145, 24466–24470 (2023).
Dutta, S. et al. Photoredox-enabled dearomative [2π + 2σ] cycloaddition of phenols. J. Am. Chem. Soc. 146, 2789–2797 (2024).
Dutta, S. et al. Double strain-release [2π + 2σ]-photocycloaddition. J. Am. Chem. Soc. 146, 5232–5241 (2024).
Wang, H. et al. Dearomative ring expansion of thiophenes by bicyclobutane insertion. Science 381, 75–81 (2023).
Brooks, W. H., Guida, W. C. & Daniel, K. G. The significance and chirality in drug design and development. Curr. Top. Med. Chem. 11, 760–770 (2011).
Food and Drug Administration. FDA’s policy statement for the development of new stereoisomeric drugs. Chirality 4, 338–340 (1992).
Investigation of Chiral Active Substances (European Medicines Agency, 1994).
Fu, Q. et al. Enantioselective [2π + 2σ] cycloadditions of bicyclo[1.1.0]butanes with vinylazaarenes through asymmetric photoredox catalysis. J. Am. Chem. Soc. 146, 8372–8380 (2024).
Yang, C. et al. Development of dipolarophiles for catalytic asymmetric cycloadditions through Pd-π-allyl zwitterions. Chem. Rec. 21, 1442–1454 (2021).
Xu, B. et al. Recent advances in Pd-catalyzed asymmetric cyclization reactions. Chem. Soc. Rev. 53, 883–971 (2024).
Denisenko, A. et al. 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring. Nat. Chem. 15, 1155–1163 (2023).
Levterov, V. V. et al. 2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring. Nat. Commun. 14, 5608 (2023).
Levterov, V. V. et al. Water-soluble non-classical benzene mimetics. Angew. Chem. Int. Ed. 59, 7161–7167 (2020).
Fawcett, A., Murtaza, A., Gregson, C. H. U. & Aggarwal, V. K. Strain-release-driven homologation of boronicesters: application to the modular synthesis of azetidines. J. Am. Chem. Soc. 141, 4573–4578 (2019).
Gregson, C. H. U., Noble, A. & Aggarwal, V. K. Divergent, strain-release reactions of azabicyclo[1.1.0]butyl carbinols: semipinacol or spiroepoxy azetidine formation. Angew. Chem. Int. Ed. 60, 7360–7365 (2021).
Silvi, M. & Aggarwal, V. K. Radical addition to strained σ-bonds enables the stereocontrolled synthesis of cyclobutyl boronic esters. J. Am. Chem. Soc. 141, 9511–9515 (2019).
Fawcett, A., Biberger, T. & Aggarwal, V. K. Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem. 11, 117–122 (2019).
Pitzer, L., Schäfers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed. 58, 8572–8576 (2019).
Acknowledgements
We gratefully acknowledge funding support from the National Key R&D Program of China (numbers 2022YFA1503700, 2023YFA1506700), the National Natural Science Foundation of China (numbers 22071118, 22271162, 22188101) and the Natural Science Foundation of Tianjin (number 21JCZDJC00350). We thank the Haihe Laboratory of Sustainable Chemical Transformations and Frontiers Science Center for New Organic Matter for financial support. We thank F. Dean Toste for helpful discussions.
Author information
Authors and Affiliations
Contributions
W.Z. conceived and supervised this work. T.Q. and M.H. conducted the studies and prepared the Supplementary Information. All the authors co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, DFT calculations, X-ray crystallographic analysis and NMR spectra.
Supplementary Data 1
Crystallographic data for (S)-4o CCDC 2358250.
Supplementary Data 2
Crystallographic data for (R)-4ab CCDC 2325465.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, T., He, M. & Zi, W. Palladium-catalysed [2σ + 2π] cycloaddition reactions of bicyclo[1.1.0]butanes with aldehydes. Nat. Synth 4, 124–133 (2025). https://doi.org/10.1038/s44160-024-00659-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00659-6
This article is cited by
-
Thermal [2+2] cycloaddition as a route to gem-difluoro heterobicyclo[n.1.1]alkanes
Nature Chemistry (2026)
-
Catalytic asymmetric activation of bicyclobutanes
Nature Synthesis (2026)
-
Silver-catalyzed formal [2π+2σ] cycloaddition of bicyclobutanes with naphthols as surrogates for arynes
Science China Chemistry (2026)
-
Catalytic asymmetric tandem Heck/Sonogashira reaction enabling access to versatile chiral bridged ring scaffolds
Nature Communications (2025)
-
Regiodivergent hydrophosphination of Bicyclo[1.1.0]-Butanes under catalyst control
Nature Communications (2025)