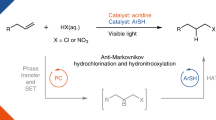
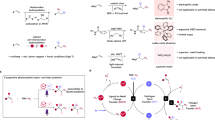
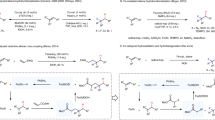
Abstract
The hydrochlorination of unsaturated hydrocarbons is a fundamental reaction in organic synthesis. Traditional acid-mediated approaches proceed with Markovnikov selectivity, but direct access to anti-Markovnikov hydrochlorination products is still a longstanding pursuit. Previous methods are restricted by the need for multiple synthetic steps, stoichiometric chlorine and hydride sources and/or highly oxidative photocatalysis, resulting in limited scope and, in some cases, low regioselectivity. So, the development of redox-neutral hydrochlorination with high anti-Markovnikov regioselectivity compatible with both alkenes and alkynes remains important. Here we report a photocatalytic anti-Markovnikov hydro- and deuterochlorination of unsaturated hydrocarbons enabling access to diverse alkyl and alkenyl chlorides regio- and stereoselectively. Broad scope (125 examples), mild conditions and regio- and isotopo-divergent syntheses are demonstrated. Key to this method is the combination use of ligand-to-metal charge transfer photoreactivity of earth-abundant iron and hydrogen atom transfer reactivity of redox-active thiol. This cooperative system offers a powerful strategy for anti-Markovnikov hydrofunctionalization of unsaturated hydrocarbons.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this research are available within the article and its Supplementary Information.
References
Gribble, G. W. in Naturally Occurring Organohalogen Compounds—A Comprehensive Update 349–365 (Springer, 2010).
Hernandes, Z. M., Cavalcanti, T. S. M., Moreira, M. D. R., de Azevedo Junior, F. W. & Leite, L. A. C. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr. Drug Targets 11, 303–314 (2010).
Tang, M. L. & Bao, Z. Halogenated materials as organic semiconductors. Chem. Mater. 23, 446–455 (2011).
Nunnery, J. K. et al. Biosynthetically intriguing chlorinated lipophilic metabolites from geographically distant tropical marine cyanobacteria. J. Org. Chem. 77, 4198–4208 (2012).
Togo, H. in Advanced Free Radical Reactions for Organic Synthesis (Elsevier, 2004).
Carey, F. A. & Sundberg, R. J. in Advanced Organic Chemistry: Part A: Structure and Mechanisms 1–117 (Springer, 2007).
Terao, J. & Kambe, N. Cross-coupling reaction of alkyl halides with grignard reagents catalyzed by Ni, Pd, or Cu Complexes with π-carbon ligand(s). Acc. Chem. Res. 41, 1545–1554 (2008).
Rudolph, A. & Lautens, M. Secondary alkyl halides in transition-metal-catalyzed cross-coupling reactions. Angew. Chem. Int. Ed. 48, 2656–2670 (2009).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Johansson Seechurn, C. C. C., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 51, 5062–5085 (2012).
Markownikoff, W. I. Ueber die Abhängigkeit der verschiedenen Vertretbarkeit des Radicalwasserstoffs in den isomeren Buttersäuren. Justus Liebigs Ann. Chem. 153, 228–259 (1870).
Kharasch, M. S. & Mayo, F. R. The peroxide effect in the addition of reagents to unsaturated compounds. I. The addition of hydrogen bromide to allyl bromide. JACS 55, 2468–2496 (1933).
Blanksby, S. J. & Ellison, G. B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 36, 255–263 (2003).
Mayo, F. R. Free radical addition and transfer reactions of hydrogen chloride with unsaturated compounds. JACS 76, 5392–5396 (1954).
Brown, H. C. & De Lue, N. R. Organoboranes for synthesis. 12. The reaction of organoboranes with nitrogen trichloride. A convenient procedure for the conversion of alkenes into alkyl chloridesvia hydroborationi. Tetrahedron 44, 2785–2792 (1988).
Zheng, J. et al. Synthesis of diverse well-defined functional polymers based on hydrozirconation and subsequent anti-markovnikov halogenation of 1,2-polybutadiene. Macromolecules 45, 1190–1197 (2012).
Appel, R. Tertiary phosphane/tetrachloromethane, a versatile reagent for chlorination, dehydration, and P–N linkage. Angew. Chem. Int. Ed. Engl. 14, 801–811 (1975).
Wilger, D. J., Grandjean, J.-M. M., Lammert, T. R. & Nicewicz, D. A. The direct anti-Markovnikov addition of mineral acids to styrenes. Nat. Chem. 6, 720–726 (2014).
Margrey, K. A. & Nicewicz, D. A. A general approach to catalytic alkene anti-markovnikov hydrofunctionalization reactions via acridinium photoredox catalysis. Acc. Chem. Res. 49, 1997–2006 (2016).
Li, X., Jin, J., Chen, P. & Liu, G. Catalytic remote hydrohalogenation of internal alkenes. Nat. Chem. 14, 425–432 (2022).
Kim, J., Sun, X., van der Worp, B. A. & Ritter, T. Anti-Markovnikov hydrochlorination and hydronitrooxylation of α-olefins via visible-light photocatalysis. Nat. Catal. 6, 196–203 (2023).
Petrone, D. A., Ye, J. & Lautens, M. Modern transition-metal-catalyzed carbon–halogen bond formation. Chem. Rev. 116, 8003–8104 (2016).
Gaspar, B. & Carreira, E. M. Catalytic hydrochlorination of unactivated olefins with para-toluenesulfonyl chloride. Angew. Chem. Int. Ed. 47, 5758–5760 (2008).
Dérien, S., Klein, H. & Bruneau, C. Selective ruthenium-catalyzed hydrochlorination of alkynes: one-step synthesis of vinylchlorides. Angew. Chem. Int. Ed. 54, 12112–12115 (2015).
Ebule, R., Liang, S., Hammond, G. B. & Xu, B. Chloride-tolerant gold(I)-catalyzed regioselective hydrochlorination of alkynes. ACS Catal. 7, 6798–6801 (2017).
Derosa, J. et al. Palladium(II)-catalyzed directed anti-hydrochlorination of unactivated alkynes with HCl. JACS 139, 5183–5193 (2017).
Yu, P., Bismuto, A. & Morandi, B. Iridium-catalyzed hydrochlorination and hydrobromination of alkynes by shuttle catalysis. Angew. Chem. Int. Ed. 59, 2904–2910 (2020).
Koh, M. J., Nguyen, T. T., Zhang, H., Schrock, R. R. & Hoveyda, A. H. Direct synthesis of Z-alkenyl halides through catalytic cross-metathesis. Nature 531, 459–465 (2016).
Abderrazak, Y., Bhattacharyya, A. & Reiser, O. Visible-light-induced homolysis of earth-abundant metal-substrate complexes: a complementary activation strategy in photoredox catalysis. Angew. Chem. Int. Ed. 60, 21100–21115 (2021).
Juliá, F. Ligand-to-metal charge transfer (LMCT) photochemistry at 3d-metal complexes: an emerging tool for sustainable organic synthesis. ChemCatChem 14, e202200916 (2022).
Kochi, J. K. Photolyses of metal compounds: cupric chloride in organic media. JACS 84, 2121–2127 (1962).
Shul’pin, G. B., Druzhinina, A. N. & Shul’pina, L. S. Photo-oxidation of cyclohexane by air in acetonitrile, catalysed by π-complexes of iron. Pet. Chem. 33, 321–325 (1993).
Giedyk, M., Goliszewska, K. & Gryko, D. Vitamin B12 catalysed reactions. Chem. Soc. Rev. 44, 3391–3404 (2015).
Shields, B. J. & Doyle, A. G. Direct C(sp3)–H cross coupling enabled by catalytic generation of chlorine radicals. JACS 138, 12719–12722 (2016).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
de Groot, L. H. M., Ilic, A., Schwarz, J. & Wärnmark, K. Iron photoredox catalysis—past, present, and future. JACS 145, 9369–9388 (2023).
Heitz, D. R., Tellis, J. C. & Molander, G. A. Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations. JACS 138, 12715–12718 (2016).
Lian, P., Long, W., Li, J., Zheng, Y. & Wan, X. Visible-light-induced vicinal dichlorination of alkenes through LMCT excitation of CuCl2. Angew. Chem. Int. Ed. 59, 23603–23608 (2020).
Chábera, P. et al. A low-spin Fe(III) complex with 100-ps ligand-to-metal charge transfer photoluminescence. Nature 543, 695–699 (2017).
Li, Z., Wang, X., Xia, S. & Jin, J. Ligand-accelerated iron photocatalysis enabling decarboxylative alkylation of heteroarenes. Org. Lett. 21, 4259–4265 (2019).
Gygi, D. et al. Capturing the complete reaction profile of a C–H bond activation. JACS 143, 6060–6064 (2021).
Jin, Y. et al. Photo-induced direct alkynylation of methane and other light alkanes by iron catalysis. Green Chem. 23, 9406–9411 (2021).
Kang, Y. C., Treacy, S. M. & Rovis, T. Iron-catalyzed photoinduced LMCT: a 1 °C–H abstraction enables skeletal rearrangements and C(sp3)–H alkylation. ACS Catal. 11, 7442–7449 (2021).
Wang, M., Wen, J., Huang, Y. & Hu, P. Selective degradation of styrene-related plastics catalyzed by iron under visible light. ChemSusChem 14, 5049–5056 (2021).
Zhang, G., Zhang, Z. & Zeng, R. Photoinduced FeCl3-catalyzed alkyl aromatics oxidation toward degradation of polystyrene at room temperature. Chin. J. Chem. 39, 3225–3230 (2021).
Dai, Z.-Y., Zhang, S.-Q., Hong, X., Wang, P.-S. & Gong, L.-Z. A practical FeCl3/HCl photocatalyst for versatile aliphatic C–H functionalization. Chem. Catal. 2, 1211–1222 (2022).
Tu, J.-L., Hu, A.-M., Guo, L. & Xia, W. Iron-catalyzed C(sp3)–H borylation, thiolation, and sulfinylation enabled by photoinduced ligand-to-metal charge transfer. JACS 145, 7600–7611 (2023).
Gonzalez, M. I. et al. Taming the chlorine radical: enforcing steric control over chlorine-radical-mediated C–H activation. JACS 144, 1464–1472 (2022).
Niu, B., Sachidanandan, K., Blackburn, B. G., Cooke, M. V. & Laulhé, S. Photoredox polyfluoroarylation of alkyl halides via halogen atom transfer. Org. Lett. 24, 916–920 (2022).
Oh, S. & Stache, E. E. Chemical upcycling of commercial polystyrene via catalyst-controlled photooxidation. JACS 144, 5745–5749 (2022).
Zhang, Q. et al. Iron-catalyzed photoredox functionalization of methane and heavier gaseous alkanes: scope, kinetics, and computational studies. Org. Lett. 24, 1901–1906 (2022).
Bian, K.-J., Kao, S.-C., Nemoto, D., Chen, X.-W. & West, J. G. Photochemical diazidation of alkenes enabled by ligand-to-metal charge transfer and radical ligand transfer. Nat. Commun. 13, 7881 (2022).
Bian, K.-J. et al. Photocatalytic, modular difunctionalization of alkenes enabled by ligand-to-metal charge transfer and radical ligand transfer. Chem. Sci. 15, 124–133 (2023).
Kao, S.-C. et al. Photochemical iron-catalyzed decarboxylative azidation via the merger of ligand-to-metal charge transfer and radical ligand transfer catalysis. Chem. Catal. 3, 100603 (2023).
Lu, Y.-C. & West, J. G. Chemoselective decarboxylative protonation enabled by cooperative earth-abundant element catalysis. Angew. Chem. Int. Ed. 62, e202213055 (2023).
Bian, K.-J. et al. Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids. Nat. Chem. 15, 1683–1692 (2023).
Isse, A. A., Lin, C. Y., Coote, M. L. & Gennaro, A. Estimation of standard reduction potentials of halogen atoms and alkyl halides. J. Phys. Chem. B 115, 678–684 (2011).
Börgel, J. & Ritter, T. Late-stage functionalization. Chem 6, 1877–1887 (2020).
Soulard, V., Villa, G., Vollmar, D. P. & Renaud, P. Radical deuteration with D2O: catalysis and mechanistic insights. JACS 140, 155–158 (2018).
Shi, Q. et al. Visible-light mediated catalytic asymmetric radical deuteration at non-benzylic positions. Nat. Commun. 13, 4453 (2022).
Zhu, Z., Qian, S. & Nicewicz, D. A. Divergent functionalization of alkynes enabled by organic photoredox catalysis. Synlett 34, 1023–1028 (2023).
Simmons, E. M. & Hartwig, J. F. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 51, 3066–3072 (2012).
Acknowledgements
We acknowledge financial support from CPRIT (RR190025), NIH (R35GM142738), the Welch Foundation (C-2085), RCSA (CS-CSA-2023-007), and ACS-PRF (62397-DNI1). J.G.W. is a CPRIT Scholar in Cancer Research. Y. H. Rezenom (TAMU/LBMS), I. M Riddington (UT Austin Mass Spectrometry Facility) and C. L. Pennington (Rice University Mass Spectrometry Facility) are acknowledged for assistance with mass spectrometry analysis.
Author information
Authors and Affiliations
Contributions
K.-J.B. and J.G.W. designed the project. K.-J.B., D.N.Jr., Y.C., Y.-C.L., S.-C.K. and X.-W.C. performed the experiments. K.-J.B., D.N.Jr. and J.G.W. wrote the paper. J.G.W. directed the project. All authors interpreted the results in the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, discussion, figures and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bian, KJ., Nemoto, D., Chen, Y. et al. Anti-Markovnikov hydro- and deuterochlorination of unsaturated hydrocarbons using iron photocatalysis. Nat. Synth 4, 314–326 (2025). https://doi.org/10.1038/s44160-024-00698-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00698-z
This article is cited by
-
Photocatalytic pathways for hydrochlorination
Nature Synthesis (2025)