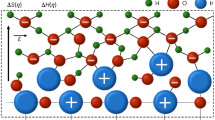
Abstract
Electrochemical O2 activation offers a green approach for efficient synthesis of singlet oxygen (1O2). However, it is commonly determined by adsorption-dependent O2 activation and transformation and can suffer from the sluggish desorption of surface-bound *OOH. Here we report an adsorption-independent O2 activation pathway for 1O2 electrosynthesis via an O2 mono-hydrogenation process on compressive-strained rutile TiO2 (CSR-TiO2). This CSR-TiO2 achieved an 1O2 generation rate of 148.26 μmol l−1 min−1 with near 100% Faradaic efficiency, outperforming the strain-free counterpart (35.97 μmol l−1 min−1) and other previously reported materials. Such superior performance of CSR-TiO2 stemmed from compressive strain, which can suppress the formation of reductive unsaturated sites for the O2 adsorption and enhance the reductive ability of atomic hydrogen (H*), favouring the O2 mono-hydrogenation pathway and bypassing the traditional surface-bound *OOH desorption pathway. The generated 1O2 could be utilized for the selective oxidation of thioanisole and its derivatives, offering a promising strategy for green organic synthesis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings of this study are present in the paper and the Supplementary Information. Source data are provided with this paper.
References
Borden, W. T., Hoffmann, R., Stuyver, T. & Chen, B. Dioxygen: what makes this triplet diradical kinetically persistent? J. Am. Chem. Soc. 139, 9010–9018 (2017).
Ghogare, A. A. & Greer, A. Using singlet oxygen to synthesize natural products and drugs. Chem. Rev. 116, 9994–10034 (2016).
Xie, L. et al. Pauling-type adsorption of O2 induced electrocatalytic singlet oxygen production on N–CuO for organic pollutants degradation. Nat. Commun. 13, 5560 (2022).
Lucky, S. S., Soo, K. C. & Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 115, 1990–2042 (2015).
Zhao, Y. et al. Janus electrocatalytic flow-through membrane enables highly selective singlet oxygen production. Nat. Commun. 11, 6228 (2020).
Feng, Z. et al. New insights into selective singlet oxygen production via the typical electroactivation of oxygen for water decontamination. Environ. Sci. Technol. 57, 17123–17131 (2023).
Holewinski, A., Idrobo, J.-C. & Linic, S. High-performance Ag–Co alloy catalysts for electrochemical oxygen reduction. Nat. Chem. 6, 828–834 (2014).
Aurbach, D., McCloskey, B. D., Nazar, L. F. & Bruce, P. G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 1, 1–11 (2016).
Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Yang, X. et al. Tuning two-electron oxygen-reduction pathways for H2O2 electrosynthesis via engineering atomically dispersed single metal site catalysts. Adv. Mater. 34, 2107954 (2022).
Shum, L. G. S. & Benson, S. W. Review of the heat of formation of the hydroperoxyl radical. J. Phys. Chem. 87, 3479–3482 (1983).
Dixon-Lewis, G. & Williams, A. Role of hydroperoxyl in hydrogen–oxygen flames. Nature 196, 1309–1310 (1962).
Foner, S. N. & Hudson, R. L. Mass spectrometry of the HO2 free radical. J. Chem. Phys. 36, 2681–2688 (1962).
Dai, J. et al. Hydrogen spillover in complex oxide multifunctional sites improves acidic hydrogen evolution electrocatalysis. Nat. Commun. 13, 1189 (2022).
Zheng, Q. et al. Cobalt single-atom reverse hydrogen spillover for efficient electrochemical water dissociation and dechlorination. Angew. Chem. Int. Ed. 63, e202401386 (2024).
Linh, N. H., Nguyen, T. Q., Diño, W. A. & Kasai, H. Effect of oxygen vacancy on the adsorption of O2 on anatase TiO2(001): a DFT-based study. Surf. Sci. 633, 38–45 (2015).
Macino, M. et al. Tuning of catalytic sites in Pt/TiO2 catalysts for the chemoselective hydrogenation of 3-nitrostyrene. Nat. Catal. 2, 873–881 (2019).
Lazzeri, M. & Selloni, A. Stress-driven reconstruction of an oxide surface: the anatase TiO2(001)−(1 × 4) surface. Phys. Rev. Lett. 87, 266105 (2001).
Yamamoto, Y., Kasamatsu, S. & Sugino, O. Scaling relation of oxygen reduction reaction intermediates at defective TiO2 surfaces. J. Phys. Chem. C 123, 19486–19492 (2019).
Mazza, T. et al. Raman spectroscopy characterization of TiO2 rutile nanocrystals. Phys. Rev. B 75, 045416 (2007).
Valeeva, A. A., Rempel’, A. A. & Gusev, A. I. Ordering of cubic titanium monoxide into monoclinic Ti5O5. Inorg. Mater. 37, 603–612 (2001).
Hÿtch, M. J., Snoeck, E. & Kilaas, R. Quantitative measurement of displacement and strain fields from HREM micrographs. Ultramicroscopy 74, 131–146 (1998).
Wu, G. et al. In-plane strain engineering in ultrathin noble metal nanosheets boosts the intrinsic electrocatalytic hydrogen evolution activity. Nat. Commun. 13, 4200 (2022).
Yi, X. & Zheng, A. Shine a light on the defect evolution over the TiO2 surface. Chem. Catal. 3, 100630 (2023).
Dai, J. et al. Spin polarized Fe1−Ti pairs for highly efficient electroreduction nitrate to ammonia. Nat. Commun. 15, 88 (2024).
Caramori, S., Cristino, V., Argazzi, R., Meda, L. & Bignozzi, C. A. Photoelectrochemical behavior of sensitized TiO2 photoanodes in an aqueous environment: application to hydrogen production. Inorg. Chem. 49, 3320–3328 (2010).
Miao, Y., Zhao, Y., Zhang, S., Shi, R. & Zhang, T. Strain engineering: a boosting strategy for photocatalysis. Adv. Mater. 34, 2200868 (2022).
Smith, J. W. & Saykally, R. J. Soft X-ray absorption spectroscopy of liquids and solutions. Chem. Rev. 117, 13909–13934 (2017).
Stöhr J. NEXAFS Spectroscopy (Springer, 2013).
de Groot, F. M. F., Fuggle, J. C., Thole, B. T. & Sawatzky, G. A. L2,3 x-ray-absorption edges of d0 compounds: K+, Ca2+, Sc3+, and Ti4+ in Oh (octahedral) symmetry. Phys. Rev. B 41, 928–937 (1990).
Haverkort, M. W. et al. Determination of the orbital moment and crystal-field splitting in LaTiO3. Phys. Rev. Lett. 94, 056401 (2005).
Chang, C. F. et al. C-axis dimer and its electronic breakup: the insulator-to-metal transition in Ti2O3. Phys. Rev. X 8, 021004 (2018).
Schlappa, J. et al. Resonant soft x-ray scattering from stepped surfaces of SrTiO3. J. Phys. Condens. Matter 24, 035501 (2012).
Dronskowski, R. & Bloechl, P. E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 97, 8617–8624 (1993).
Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Crystal orbital hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 115, 5461–5466 (2011).
Maintz, S., Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem. 34, 2557–2567 (2013).
Dickens, C. F., Montoya, J. H., Kulkarni, A. R., Bajdich, M. & Nørskov, J. K. An electronic structure descriptor for oxygen reactivity at metal and metal-oxide surfaces. Surf. Sci. 681, 122–129 (2019).
Shun, K. et al. Revealing hydrogen spillover pathways in reducible metal oxides. Chem. Sci. 13, 8137–8147 (2022).
Zhu, Z. et al. Highly sensitive and fast-response hydrogen sensing of WO3 nanoparticles via palladium reined spillover effect. Nanoscale 13, 12669–12675 (2021).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Chen, L. et al. Accurate identification of radicals by in-situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes. Water Res. 221, 118747 (2022).
Xu, M., Wang, R., Fu, H., Shi, Y. & Ling, L. Harmonizing the cyano-group and Na to enhance selective photocatalytic O2 activation on carbon nitride for refractory pollutant degradation. Proc. Natl Acad. Sci. USA 121, e2318787121 (2024).
Yang, J., Li, J., Ding, R., Liu, C. & Yin, X. Kinetic effects of temperature on Fe–N–C catalysts for 2e− and 4e− oxygen reduction reactions. J. Electrochem. Soc. 168, 096502 (2021).
Wen, X., Miao, J., Mandler, D. & Long, M. Rotating ring-disk electrode method to evaluate performance of electrocatalysts in hydrogen peroxide activation via rapid detection of hydroxyl radicals. Chem. Eng. J. 454, 140312 (2023).
Zhang, B. et al. A strongly coupled Ru–CrOx cluster–cluster heterostructure for efficient alkaline hydrogen electrocatalysis. Nat. Catal. 7, 441–451 (2024).
Cao, S. et al. Construction of an OCP-ATR-FTIR spectroscopy device to in situ monitor the interfacial reaction of contaminants: competitive adsorption of Cr(VI) and oxalate on hematite. Environ. Sci. Technol. 57, 16532–16540 (2023).
Ji, K. et al. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural promoted by a Ru1Cu single-atom alloy catalyst. Angew. Chem. Int. Ed. 61, e202209849 (2022).
Chen, C. et al. Adjacent Fe site boosts electrocatalytic oxygen evolution at Co site in single-atom-catalyst through a dual-metal-site design. Energy Environ. Sci. 16, 1685–1696 (2023).
Nakamura, R. & Nakato, Y. Primary intermediates of oxygen photoevolution reaction on TiO2 (rutile) particles, revealed by in situ FTIR absorption and photoluminescence measurements. J. Am. Chem. Soc. 126, 1290–1298 (2004).
Hammes-Schiffer, S. & Soudackov, A. V. Proton-coupled electron transfer in solution, proteins, and electrochemistry. J. Phys. Chem. B 112, 14108–14123 (2008).
Qian, S.-J. et al. Critical role of explicit lnclusion of solvent and electrode potential in the electrochemical description of nitrogen reduction. ACS Catal. 12, 11530–11540 (2022).
Wang, H. et al. Enhanced singlet oxygen generation in oxidized graphitic carbon nitride for organic synthesis. Adv. Mater. 28, 6940–6945 (2016).
Li, J. et al. Highly selective oxidation of organic sulfides by a conjugated polymer as the photosensitizer for singlet oxygen generation. ACS Appl. Mater. Interfaces 12, 35475–35481 (2020).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant nos. 2021YFA1201701 to Y.Y. and 2022YFA1205601 to Y.Y.), the National Natural Science Foundation of China (grant nos. U22A20402 to L.Z.Z., 22102100 to Y.Y., 22206121 to J.D., 22306119 to Y.S. and 22476126 to Y.Y.), Shenzhen Science and Technology Program (grant no. JCYJ20220818095601002 to L.Z.Z.) and the Natural Science Foundation of Shanghai (grant no. 22ZR1431700 to Y.Y.). The authors acknowledge the support from the Max Planck−POSTECH−Hsinchu Center for Complex Phase Materials, the Instrumental Analysis Center of Shanghai Jiao Tong University, Instrumental Analysis Center of School of Environmental Science and Engineering, State Key Laboratory for Pollution Control and Resource Reuse, and Shiyanjia Lab for the help in characterizations and experimental measurements. The computations in this paper were run on the π 2.0 cluster supported by the Center for High Performance Computing at Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Contributions
Y.Y. and L.Z.Z. conceived the idea. R.W. and J.D. carried out the sample synthesis, characterization and electrocatalytic measurements. R.W. performed the theoretical calculations. R.W., J.D., Y.Y. and L.Z.Z. wrote the paper. Z.H., C.C. and C.K. carried out the sXAS measurements. L.Z., Y.Z. and M.X. conducted TEM characterizations. G.Z. helped with the density functional theory (DFT) calculation. Y.S., J.W. and X.Z. offered help on the operando electrochemical ATR-IR. B.Z. and K.W. performed the EPR measurement. R.Z., Y.Z. and Y.S. took part in the operando Raman spectroscopy. All the authors discussed results and provided comments during the manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks W.-F. Lin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. The Primary Handling Editor: is Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details and Supplementary Figs. 1–63, Tables 1–3 and Notes 1–4.
Source data
Source Data Fig. 2
Source data for Fig. 2b,c,f,g–i.
Source Data Fig. 3
Source data for Fig. 3a,b,d,e.
Source Data Fig. 4
Source data for Fig. 4a–i.
Source Data Fig. 5
Source data for Fig. 5a–f,h.
Source Data Fig. 6
Source data for Fig. 6a,c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, R., Dai, J., Zhao, L. et al. Compressive-strained rutile TiO2 enables O2 mono-hydrogenation for singlet oxygen electrosynthesis. Nat. Synth 4, 754–764 (2025). https://doi.org/10.1038/s44160-025-00756-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00756-0
This article is cited by
-
Ultrafast energy-neutral molecular oxygen activation via atomically-adjacent bimetallic catalytic sites
Nature Communications (2025)
-
Role of metal oxides in direct syngas conversion via OXZEO catalysis: a review
Science China Chemistry (2025)