Abstract
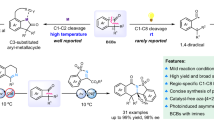
Multisubstituted azaarenes and conjugated polyazaarenes are important heterocycles in chemistry and materials science. Here we report the discovery of a carbon–carbon bond cleavage and alkyl transfer approach for the synthesis of azaarenes or conjugated polyazaarenes, which is promoted by potassium tert-butoxide. Neither precious-metal catalysts nor directing groups are required. This strategy is enabled by an alkyl transfer, releasing aryl methanes, such as toluene, as the only by-product. This general and versatile method enables the divergent synthesis of a variety of highly functionalized azaarenes and polyazaarenes. In addition, several azaarenes were found to have visible-light photocatalytic reactivities.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. The crystallographic data for the small molecules have been submitted to the Cambridge Structural Database (https://www.ccdc.cam.ac.uk): 5a (CCDC 2015538), 6a (CCDC 2015536), 8g (CCDC 2016533), 8o (CCDC 2016534), 10o (CCDC 2069031).
References
Maxwell, K. L. Cyclic pyrimidines jump on the anti-phage bandwagon. Cell 184, 5691–5693 (2021).
Becker, S. et al. Unified prebiotically plausible synthesis of pyrimidine and purine RNA ribonucleotides. Science 366, 76–82 (2019).
Hud, N. V. & Fialho, D. M. RNA nucleosides built in one prebiotic pot. Science 366, 32–33 (2019).
Matsumoto, S. et al. DNA damage detection in nucleosomes involves DNA register shifting. Nature 571, 79–84 (2019).
Parker, W. B. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem. Rev. 109, 2880–2893 (2009).
Hussain, H., Al-Harrasi, A., Al-Rawahi, A., Green, I. R. & Gibbons, S. Fruitful decade for antileishmanial compounds from 2002 to late 2011. Chem. Rev. 114, 10369–10428 (2014).
Wang, M. et al. Revealing the cooperative relationship between spin, energy, and polarization parameters toward developing high-efficiency exciplex light-emitting diodes. Adv. Mater. 31, 1904114 (2019).
Yang, L. et al. High-performance red quantum-dot light-emitting diodes based on organic electron transporting layer. Adv. Funct. Mater. 31, 2007686 (2021).
Chen, D., Su, S. J. & Cao, Y. Nitrogen heterocycle-containing materials for highly efficient phosphorescent OLEDs with low operating voltage. J. Mater. Chem. C 2, 9565–9578 (2014).
Wang, S. F. et al. Highly efficient near-infrared electroluminescence up to 800 nm using platinum(II) phosphors. Adv. Funct. Mater. 30, 2002173 (2020).
Lipunova, G. N., Nosova, E. V., Charushin, V. N. & Chupakhin, O. N. Functionalized quinazolines and pyrimidines for optoelectronic materials. Curr. Org. Synth. 15, 793–814 (2018).
Fresta, E. et al. Novel red-emitting copper(I) complexes with pyrazine and pyrimidinyl ancillary ligands for white light-emitting electrochemical cells. Adv. Opt. Mater. 10, 2101999 (2022).
Bailey, P. S. The reactions of ozone with organic compounds. Chem. Rev. 58, 925–1010 (1958).
Liu, J. et al. A metal-free synthesis of pyrimidines from amidines with α,β-unsaturated ketones via tandem [3 + 3] annulation and visible-light-enabled photo-oxidation. Org. Biomol. Chem. 21, 3411–3416 (2023).
Shi, S. H., Liang, Y. & Jiao, N. Electrochemical oxidation induced selective C–C bond cleavage. Chem. Rev. 121, 485–505 (2021).
Chen, F., Wang, T. & Jiao, N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev. 114, 8613–8661 (2014).
Chan, A. P. Y. & Sergeev, A. G. Metal-mediated cleavage of unsaturated C–C bonds. Coord. Chem. Rev. 413, 213213 (2020).
Gozin, M. et al. Activation of a carbon–carbon bond in solution by transition-metal insertion. Nature 364, 699–701 (1993).
Gozin, M. et al. Transfer of methylene groups promoted by metal complexation. Nature 370, 42–44 (1994).
Smaligo, A. J. et al. Hydrodealkenylative C(sp3)–C(sp2) bond fragmentation. Science 364, 681–685 (2019).
Dai, P. F., Wang, H., Cui, X. C., Qu, J. P. & Kang, Y. B. Recent progress in C(aryl)–C(alkyl) bond cleavage of alkylarenes. Org. Chem. Front. 7, 896–904 (2020).
Dai, P. F. et al. Cleavage of C(aryl)–CH3 bonds in the absence of directing groups under transition metal free conditions. Angew. Chem. Int. Ed. 58, 5392–5395 (2019).
Shan, X. H. et al. Copper-catalyzed oxidative benzylic C–H cyclization via iminyl radical from intermolecular anion-radical redox relay. Nat. Commun. 10, 908 (2019).
Li, Y. W. et al. tBuOK-promoted cyclization of imines with aryl halides. Org. Lett. 22, 4553–4556 (2020).
Shan, X. H., Wang, M. M., Tie, L., Qu, J. P. & Kang, Y. B. CuSO4‑catalyzed tandem C(sp3)–H insertion cyclization of toluenes with isonitriles to form indoles. Org. Lett. 22, 357–360 (2020).
Shan, X. H., Yang, B., Qu, J. P. & Kang, Y. B. CuSO4-catalyzed dual annulation to synthesize O, S or N-containing tetracyclic heteroacenes. Chem. Commun. 56, 4063–4066 (2020).
Zheng, H. X., Shan, X. H., Qu, J. P. & Kang, Y. B. Strategy for overcoming full reversibility of intermolecular radical addition to aldehydes: tandem C–H and C–O bonds cleaving cyclization of (phenoxymethyl)arenes with carbonyls to benzofurans. Org. Lett. 20, 3310–3313 (2018).
Zheng, H. X. et al. Transition-metal-free self-hydrogen-transferring allylic isomerization. Org. Lett. 17, 6102–6105 (2015).
Li, Q. Q., Xiao, Z. F., Yao, C. Z., Zheng, H. X. & Kang, Y. B. Direct alkylation of amines with alcohols catalyzed by base. Org. Lett. 17, 5328–5331 (2015).
Yao, C. Z., Li, Q. Q., Wang, M. M., Ning, X. S. & Kang, Y. B. (E)-Specific direct Julia-olefination of aryl alcohols without extra reducing agents promoted by bases. Chem. Commun. 51, 7729–7732 (2015).
Bao, W., Kossen, H. & Schneider, U. Formal allylic C(sp3)–H bond activation of alkenes triggered by a sodium amide. J. Am. Chem. Soc. 139, 4362–4365 (2017).
Toutov, A. et al. Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst. Nature 518, 80–84 (2015).
Müllen, K. & Scherf, U. Conjugated polymers: where we come from, where we stand, and where we might go. Macromol. Chem. Phys. 224, 2200337 (2023).
Allard, S., Forster, M., Souharce, B., Thiem, H. & Scherf, U. Organic semiconductors for solution-processable field-effect transistors (OFETs). Angew. Chem. Int. Ed. 47, 4070–4098 (2008).
Wang, C., Dong, H., Hu, W., Liu, Y. & Zhu, D. Semiconducting π-conjugated systems in field-effect transistors: a material odyssey of organic electronics. Chem. Rev. 112, 2208–2267 (2012).
Panwar, N. et al. Nanocarbons for biology and medicine: sensing, imaging, and drug delivery. Chem. Rev. 119, 9559–9656 (2019).
Tielens, A. G. G. M. Interstellar polycyclic aromatic hydrocarbon molecules. Annu. Rev. Astron. Astrophys. 46, 289–337 (2008).
Chen, X. Y. et al. KOt-Bu/DMF promoted intramolecular cyclization of 1,1′-biphenyl aldehydes and ketones: an efficient synthesis of phenanthrenes. RSC Adv. 5, 48046–48049 (2015).
Luan, Z. H., Qu, J. P. & Kang, Y. B. Discovery of oxygen α-nucleophilic addition to α,β-unsaturated amides catalyzed by redox-neutral organic photoreductant. J. Am. Chem. Soc. 142, 20942–20947 (2020).
Ito, S., Fujimoto, H. & Tobisu, M. Non-stabilized vinyl anion equivalents from styrenes by N-heterocyclic carbene catalysis and its use in catalytic nucleophilic aromatic substitution. J. Am. Chem. Soc. 144, 6714–6718 (2022).
Shan, X. H., Yang, B., Zheng, H. X., Qu, J. P. & Kang, Y. B. Phenanthroline‑tBuOK promoted intramolecular C–H arylation of indoles with ArI under transition-metal-free conditions. Org. Lett. 20, 7898–7901 (2018).
Bhuniaa, S. & Dasb, D. Carbon-based nucleophiles as leaving groups in organic synthesis via cleavage of C–C sigma bonds. Tetrahedron 112, 132738 (2022).
Fabry, D. C., Ronge, M. A. & Rueping, M. Immobilization and continuous recycling of photoredox catalysts in ionic liquids for applications in batch reactions and flow systems: catalytic alkene isomerization by using visible light. Chem. Eur. J. 21, 5350–5354 (2015).
Metternich, J. B. & Gilmour, A. R. Bio-inspired, catalytic E → Z isomerization of activated olefins. J. Am. Chem. Soc. 137, 11254–11257 (2015).
Tlahuext-Aca, A., Garza-Sanchez, R. A. & Glorius, F. Multicomponent oxyalkylation of styrenes enabled by hydrogen-bond-assisted photoinduced electron transfer. Angew. Chem. Int. Ed. 56, 3708–3711 (2017).
Jiang, M., Yang, H. J. & Fu, H. Visible-light photoredox borylation of aryl halides and subsequent aerobic oxidative hydroxylation. Org. Lett. 18, 5248–5251 (2016).
Wang, S. D., Yang, B., Zhang, H., Qu, J. P. & Kang, Y. B. Reductive cleavage of C–X or N–S bonds catalyzed by super organoreductant CBZ6. Org. Lett. 25, 816–820 (2023).
Wang, H., Qu, J. P. & Kang, Y. B. CBZ6 as a recyclable organic photoreductant for pinacol coupling. Org. Lett. 23, 2900–2903 (2021).
Okamoto, S., Kojiyama, K., Tsujioka, H. & Sudo, A. Metal-free reductive coupling of C=O and C=N bonds driven by visible light: use of perylene as a simple photoredox catalyst. Chem. Commun. 52, 11339–11342 (2016).
Acknowledgements
We thank the National Key R&D Program of China (2021YFA1500100, Y.-B.K.) and the National Natural Science Foundation of China (22271268, Y.-B.K.) for financial support. We thank S. Zhou for helping with X-ray molecular structure analysis.
Author information
Authors and Affiliations
Contributions
Y.-Z.C. and X.-H.S. performed the experiments for compounds 1–9 and PM and analysed the data, unless otherwise stated. B.Y. tested the applications of 6a, 6f, 6i and 7 in photocatalysis. L.T. performed experiments for the synthesis of 1C and 10 and analysed the data. Y.L. performed the characterization of 6a, 6f, 6i, 7 and 8a. J.-L.F. synthesized substrates 1a, 1b, 1l, 2m and 2n. Y.-B.K. and J.-P.Q. conceived the research, designed the experiments, supervised experiments and analyses, interpreted the data, generated figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary sections 1–20, Figs. 1–51 and Tables 1 and 2.
Supplementary Data 1
Supplementary NMR spectra.
Supplementary Data 2
X-ray crystallographic data for 5a, CCDC 2015538.
Supplementary Data 3
Structure factors for 5a, CCDC 2015538.
Supplementary Data 4
X-ray crystallographic data for 6a, CCDC 2015536.
Supplementary Data 5
Structure factors for 6a, CCDC 2015536.
Supplementary Data 6
X-ray crystallographic data for 8g, CCDC 2016533.
Supplementary Data 7
Structure factors for 8g, CCDC 2016533.
Supplementary Data 8
X-ray crystallographic data for 8o, CCDC 2016534.
Supplementary Data 9
Structure factors for 8o, CCDC 2016534.
Supplementary Data 10
X-ray crystallographic data for 10o, CCDC 2069031.
Supplementary Data 11
Structure factors for 10o, CCDC 2069031.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, YZ., Shan, XH., Yang, B. et al. Base-promoted azaarene and polyazaarene synthesis through C–C bond cleavage and alkyl transfer. Nat. Synth 4, 1288–1296 (2025). https://doi.org/10.1038/s44160-025-00833-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00833-4