Abstract
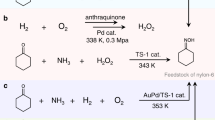
Cyclohexanone oxime (CHO) electrosynthesis from NO and cyclohexanone with high Faradaic efficiency at ampere-level current density is desirable but challenging. Here theoretical calculations reveal that NO coverage on silver catalysts plays a critical role in CHO electrosynthesis. We then experimentally adjust the NO coverage by tuning the bulk NO concentration and reaction rate. We find that low NO coverage benefits NH3 formation, whereas high coverage delivers CHO and N2. Mechanistic studies indicate that with increasing NO coverage, active sites transfer from bridge step to hollow terrace sites at which NH2OH* can stably exist, rather than its decomposition into NH3. However, N‒N coupling also readily occurs at high NO coverage. This understanding inspires us to develop a doping strategy to inhibit NO–NO coupling at high NO coverage. A ruthenium-doped silver catalyst is therefore developed, realizing 86% CHO Faradaic efficiency at 1.0 A cm−2, far exceeding previously reported performance.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The DFT-optimized atomic coordinates are available via Zenodo at https://zenodo.org/records/15590189 (ref. 45). The data that support the findings of this study are available in the paper and its Supplementary Information. Source data are provided with this paper.
References
Global nylon 6 production capacity reach to 8.86 million tons in 2024. HDIN Research www.hdinresearch.com/news/56 (2019).
Thomas, J. M. & Raja, R. Design of a ‘green’ one-step catalytic production of ε-caprolactam (precursor of nylon-6). Proc. Natl Acad. Sci. USA 102, 13732–13736 (2005).
Yuan, Y. et al. Electrocatalytic ORR–coupled ammoximation for efficient oxime synthesis. Sci. Adv. 10, eado1755 (2024).
Lewis, R. J. et al. Highly efficient catalytic production of oximes from ketones using in situ–generated H2O2. Science 376, 615–620 (2022).
Benson, R. E., Cairns, T. L. & Whitman, G. M. Synthesis of hydroxylamine. J. Am. Chem. Soc. 78, 4202–4205 (1956).
Mokaya, R. & Poliakoff, M. A cleaner way to nylon? Nature 437, 1243–1244 (2005).
Kong, X. et al. Synthesis of hydroxylamine from air and water via a plasma-electrochemical cascade pathway. Nat. Sustain. 7, 652–660 (2024).
Jia, S. et al. Synthesis of hydroxylamine via ketone-mediated nitrate electroreduction. J. Am. Chem. Soc. 146, 10934–10942 (2024).
Li, J., Zhang, Y., Kuruvinashetti, K. & Kornienko, N. Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis. Nat. Rev. Chem. 6, 303–319 (2022).
Li, J. et al. Heterogeneous electrosynthesis of C–N, C–S and C–P products using CO2 as a building block. Nat. Synth. 3, 809–824 (2024).
Liu, C., Gao, Y. & Zhang, B. Organonitrogen electrosynthesis from CO2 and nitrogenous sources in water. Nat. Synth. 3, 794–796 (2024).
Jouny, M. et al. Formation of carbon–nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 11, 846–851 (2019).
Tao, Z., Rooney, C. L., Liang, Y. & Wang, H. Accessing organonitrogen compounds via C–N coupling in electrocatalytic CO2 reduction. J. Am. Chem. Soc. 143, 19630–19642 (2021).
Jiao, Y., Li, H., Jiao, Y. & Qiao, S.-Z. Activity and selectivity roadmap for C–N electrocoupling on mxenes. J. Am. Chem. Soc. 145, 15572–15580 (2023).
Wu, Y., Jiang, Z., Lin, Z., Liang, Y. & Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 4, 725–730 (2021).
Liu, X., Jiao, Y., Zheng, Y., Jaroniec, M. & Qiao, S.-Z. Mechanism of C–N bonds formation in electrocatalytic urea production revealed by ab initio molecular dynamics simulation. Nat. Commun. 13, 5471 (2022).
Zhang, X. et al. Direct electrosynthesis of valuable C=N compound from NO. Chem. Catal. 2, 1807–1818 (2022).
Wu, Y. et al. Electrosynthesis of a nylon-6 precursor from cyclohexanone and nitrite under ambient conditions. Nat. Commun. 14, 3057 (2023).
Chen, F.-Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Han, S. et al. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat. Catal. 6, 402–414 (2023).
Shao, J. et al. Electrochemical synthesis of ammonia from nitric oxide using a copper–tin alloy catalyst. Nat. Energy 8, 1273–1283 (2023).
Jia, S. et al. Integration of plasma and electrocatalysis to synthesize cyclohexanone oxime under ambient conditions using air as a nitrogen source. Chem. Sci. 14, 13198–13204 (2023).
Liao, P., Kang, J., Xiang, R., Wang, S. & Li, G. Electrocatalytic systems for NOx valorization in organonitrogen synthesis. Angew. Chem. Int. Ed. 63, e202311752 (2024).
Wu, Y. et al. Electrocatalytic synthesis of nylon-6 precursor at almost 100 % yield. Angew. Chem. Int. Ed. 62, e202305491 (2023).
Sharp, J. et al. Sustainable electrosynthesis of cyclohexanone oxime through nitrate reduction on a Zn–Cu alloy catalyst. ACS Catal. 14, 3287–3297 (2024).
Luo, L. et al. Electrosynthesis of the nylon-6 precursor from nitrate and cyclohexanone over a rutile TiO2 catalyst. CCS Chem. 7, 266–278 (2025).
Ko, B. H., Hasa, B., Shin, H., Zhao, Y. & Jiao, F. Electrochemical reduction of gaseous nitrogen oxides on transition metals at ambient conditions. J. Am. Chem. Soc. 144, 1258–1266 (2022).
Yang, R. et al. Descriptor-based volcano relations predict single atoms for hydroxylamine electrosynthesis. Angew. Chem. Int. Ed. 63, e202317167 (2024).
Guo, P. et al. Computational insights on structural sensitivity of cobalt in NO electroreduction to ammonia and hydroxylamine. J. Am. Chem. Soc. 146, 13974–13982 (2024).
Wang, Z., Cao, X. M., Zhu, J. & Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: a deactivation scheme from density functional theory. J. Catal. 311, 469–480 (2014).
Li, J. et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat. Catal. 2, 1124–1131 (2019).
Hammer, B. & Nørskov, J. K. Adsorbate reorganization at steps: NO on Pd(211). Phys. Rev. Lett. 79, 4441–4444 (1997).
Beltramo, G. L. & Koper, M. T. M. Nitric oxide reduction and oxidation on stepped Pt [n(111) × (111)] electrodes. Langmuir 19, 8907–8915 (2003).
Li, T. et al. A spectroscopic study on nitrogen electrooxidation to nitrate. Angew. Chem. Int. Ed. 62, e202217411 (2023).
Guo, C. et al. Computational design of spinel oxides through coverage-dependent screening on the reaction phase diagram. ACS Catal. 12, 6781–6793 (2022).
Li, J. et al. Cascade dual sites modulate local CO coverage and hydrogen-binding strength to boost CO2 electroreduction to ethylene. J. Am. Chem. Soc. 146, 5693–5701 (2024).
Gao, D., Arán-Ais, R. M., Jeon, H. S. & Cuenya, B. R. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2, 198–210 (2019).
Karatok, M. Achieving ultrahigh selectivity to hydrogen production from formic acid on Pd–Ag alloys. J. Am. Chem. Soc. 145, 5114–5124 (2023).
Shi, J. et al. Promoting nitric oxide electroreduction to ammonia over electron-rich cu modulated by Ru doping. Sci. China Chem. 64, 1493–1497 (2021).
Xiang, R. et al. Electrocatalytic synthesis of pyridine oximes using in situ generated NH2OH from NO species on nanofiber membranes derived from NH2-MIL-53 (Al). Angew. Chem. Int. Ed. 62, e202312239 (2023).
Zhao, R. et al. Achieving over 90% Faradaic efficiency in cyclohexanone oxime electrosynthesis using the Cu–Mo dual-site catalyst. J. Am. Chem. Soc. 146, 27956–27963 (2024).
Zhang, F. et al. A Pickering-emulsion-droplet-integrated electrode for the continuous-flow electrosynthesis of oximes. Nat. Synth 4, 479–487 (2025).
Li, M. et al. Electrosynthesis of amino acids from NO and α-keto acids using two decoupled flow reactors. Nat. Catal. 6, 906–915 (2023).
Zhao, J. et al. NiFe nanoalloys derived from layered double hydroxides for photothermal synergistic reforming of CH4 with CO2. Adv. Funct. Mater. 32, 2204056 (2022).
Wu, Y. et al. [Dataset] Ampere-level electrosynthesis of a nylon-6 precursor by local NO coverage tuning. Zenodo https://zenodo.org/records/15590189 (2025).
Acknowledgements
We acknowledge the National Natural Science Foundation of China (22401212 to Y.W., 22271213 to B.Z. and 224B2306 to R.Y.).
Author information
Authors and Affiliations
Contributions
B.Z. conceived the idea and directed the project. Y.W. and B.Z. designed the experiments. X.L. and Y.W. carried out the experiments. R.Y. and C.C. performed the DFT calculations. Z.S. assisted with some experiments. X.L., Y.W. and B.Z. analysed the data. Y.W. wrote the paper. B.Z. revised the paper. All the authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Jeong Woo Han and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–55, Notes 1–19, Tables 1–8 and References 1–18.
Supplementary Data 1
Calculational models for Ag and AgRu.
Source data
Source Data Fig. 1
The source data underlying Fig. 1.
Source Data Fig. 2
The source data underlying Fig. 2.
Source Data Fig. 3
The source data underlying Fig. 3.
Source Data Fig. 4
The source data underlying Fig. 4.
Source Data Fig. 5
The source data underlying Fig. 5.
Source Data Fig. 6
The source data underlying Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Liu, X., Yang, R. et al. Ampere-level electrosynthesis of a nylon-6 precursor by local NO coverage tuning. Nat. Synth 4, 1504–1512 (2025). https://doi.org/10.1038/s44160-025-00851-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00851-2
This article is cited by
-
Electrocatalytic C‒N bond construction from inorganic nitrogen sources in water
Nature Protocols (2026)
-
Ampere-level electrosynthesis of a nylon-6 precursor by local NO coverage tuning
Nature Synthesis (2025)