Abstract
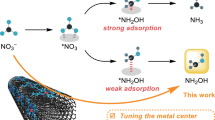
Hydroxylamine (NH2OH) is an important feedstock for oxime production. Coreduction of NOx and aldehydes or ketones enables sustainable one-step oximation by utilizing in situ *NH2OH intermediates but suffers from side reactions and reduced current density due to the presence of multiple reactants in one reactor. Here we decouple oximation into two steps, the electrochemical synthesis of free NH2OH via nitrite (NO2−) electroreduction and the aldehyde or ketone oximation chemical step, circumventing the negative effects (such as site blocking, aldehyde or ketone electroreduction, or crossover) encountered in one-step oximation. By using a Ketjen-black-supported iron phthalocyanine as the catalyst, we achieve an exceptionally high partial current density of free NH2OH (jNH2OH) of 262.9 mA cm−2 (corresponding to productivity of 2.452 mmol cm−2 h−1) in neutral conditions at an industrially relevant current density of 500 mA cm−2. By coupling NH2OH electrosynthesis with subsequent oximation in two steps, nearly stoichiometric oximes are produced with high efficiency and broad applicability. This work paves the way toward a sustainable oxime industry.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the finding of the study are available in the main text or Supplementary Information. Source data are provided with this paper, and at Zenodo via https://zenodo.org/records/16354033 (ref. 58).
References
Bolotin, D. S., Bokach, N. A., Demakova, M. Y. & Kukushkin, V. Y. Metal-involving synthesis and reactions of oximes. Chem. Rev. 117, 13039–13122 (2017).
Wu, Y. et al. Electrosynthesis of a nylon-6 precursor from cyclohexanone and nitrite under ambient conditions. Nat. Commun. 14, 3057 (2023).
Zhang, X. et al. Direct electro-synthesis of valuable C=N compound from NO. Chem Catal. 2, 1807–1818 (2022).
Kong, X. et al. Synthesis of hydroxylamine from air and water via a plasma-electrochemical cascade pathway. Nat. Sustain. 7, 652–660 (2024).
Lewis, R. J. et al. Highly efficient catalytic production of oximes from ketones using in situ-generated H2O2. Science 376, 615–620 (2022).
Wu, J. et al. Integrated tandem electrochemical–chemical–electrochemical coupling of biomass and nitrate to sustainable alanine. Angew. Chem. Int. Ed. 62, e202311196 (2023).
Xian, J. et al. Electrocatalytic synthesis of essential amino acids from nitric oxide using atomically dispersed Fe on N-doped carbon. Angew. Chem. Int. Ed. 62, e202304007 (2023).
Li, J. et al. Rechargeable biomass battery for electricity storage/generation and concurrent valuable chemicals production. Angew. Chem. Int. Ed. 62, e202304852 (2023).
Wu, Y. et al. Electrocatalytic synthesis of nylon-6 precursor at almost 100% yield. Angew. Chem. Int. Ed. 62, e202305491 (2023).
Lan, X. E. et al. Electrosynthesis of hydroxylamine from nitrate reduction in water. Sci. China Chem. 66, 1758–1762 (2023).
Chen, W. et al. Catalyst selection over an electrochemical reductive coupling reaction toward direct electrosynthesis of oxime from NOx and aldehyde. J. Am. Chem. Soc. 146, 6294–6306 (2024).
Sharp, J. et al. Sustainable electrosynthesis of cyclohexanone oxime through nitrate reduction on a Zn–Cu alloy catalyst. ACS Catal. 14, 3287–3297 (2024).
Sheng, Y. et al. Modulating hydrogen adsorption by unconventional p–d orbital hybridization over porous high-entropy alloy metallene for efficient electrosynthesis of nylon-6 precursor. Angew. Chem. Int. Ed. 63, e202410442 (2024).
Zhao, R. et al. Achieving over 90% Faradaic efficiency in cyclohexanone oxime electrosynthesis using the Cu–Mo dual-site catalyst. J. Am. Chem. Soc. 146, 27956–27963 (2024).
Tang, Y. et al. Selective electrosynthesis of hydroxylamine from aqueous nitrate/nitrite by suppressing further reduction. Nat. Commun. 15, 9800 (2024).
Xiang, R. et al. Electrocatalytic synthesis of pyridine oximes using in situ generated NH2OH from NO species on nanofiber membranes derived from NH2-MIL-53(Al). Angew. Chem. Int. Ed. 62, e202312239 (2023).
Dai, C. et al. Suppressing product crossover and C–C bond cleavage in a glycerol membrane electrode assembly reformer. Energy Environ. Sci. 17, 6350–6359 (2024).
Blanco, D. E., Prasad, P. A., Dunningan, K. & Modestino, M. A. Insights into membrane-separated organic electrosynthesis: the case of adiponitrile electrochemical production. React. Chem. Eng. 5, 136–144 (2020).
Li, M. et al. Electrosynthesis of amino acids from NO and α-keto acids using two decoupled flow reactors. Nat. Catal. 6, 906–915 (2023).
Han, S. et al. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat. Catal. 6, 402–414 (2023).
Wu, Z. Y. et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 12, 2870 (2021).
Xu, Z. et al. Continuous ammonia electrosynthesis using physically interlocked bipolar membrane at 1000 mA cm−2. Nat. Commun. 14, 1619 (2023).
Liang, J. et al. Amorphous boron carbide on titanium dioxide nanobelt arrays for high-efficiency electrocatalytic NO reduction to NH3. Angew. Chem. Int. Ed. 61, e202202087 (2022).
Fan, K. et al. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 13, 7958 (2022).
Fang, J. Y. et al. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 13, 7899 (2022).
He, W. et al. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. Nat. Commun. 13, 1129 (2022).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Li, P., Jin, Z., Fang, Z. & Yu, G. A single-site iron catalyst with preoccupied active centers that achieves selective ammonia electrosynthesis from nitrate. Energy Environ. Sci. 14, 3522–3531 (2021).
Ko, B. H., Hasa, B., Shin, H., Zhao, Y. & Jiao, F. Electrochemical reduction of gaseous nitrogen oxides on transition metals at ambient conditions. J. Am. Chem. Soc. 144, 1258–1266 (2022).
de Groot, M. T., Merkx, M., Wonders, A. H. & Koper, M. T. M. Electrochemical reduction of NO by hemin adsorbed at pyrolitic graphite. J. Am. Chem. Soc. 127, 7579–7586 (2005).
Sheng, X. et al. Carbon-supported iron complexes as electrocatalysts for the cogeneration of hydroxylamine and electricity in a NO–H2 fuel cell: a combined electrochemical and density functional theory study. J. Power Sources 390, 249–260 (2018).
Otsuka, K., Sawada, H. & Yamanaka, I. A hydrogen nitric oxide cell for the synthesis of hydroxylamine. J. Electrochem. Soc. 143, 3491–3497 (1996).
Kim, D. H. et al. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 12, 1856 (2021).
Zhao, S. et al. Selective nitric oxide electroreduction at monodispersed transition-metal sites with atomically precise coordination environment. Chem. Catal. 3, 100598 (2023).
Zhou, J. et al. Linear adsorption enables NO selective electroreduction to hydroxylamine on single Co sites. Angew. Chem. Int. Ed. 62, e202305184 (2023).
Long, L. A. The explosion at Concept Sciences: hazards of hydroxylamine. Process Saf. Prog. 23, 114–120 (2004).
Pio, G., Mocellin, P., Vianello, C. & Salzano, E. A detailed kinetic model for the thermal decomposition of hydroxylamine. J. Hazard. Mater. 416, 125641 (2021).
Xu, M.-Y. et al. Electrosynthesis of organonitrogen compounds via hydroxylamine-mediated cascade reactions. Angew. Chem. Int. Ed. 64, e202422637 (2025).
Zhou, H. et al. Scalable electrosynthesis of commodity chemicals from biomass by suppressing non-Faradaic transformations. Nat. Commun. 14, 5621 (2023).
Li, J. & Duan, H. Recent progress in energy-saving hydrogen production by coupling with value-added anodic reactions. Chem 10, 3008–3039 (2024).
Jia, S. et al. Synthesis of hydroxylamine via ketone-mediated nitrate electroreduction. J. Am. Chem. Soc. 146, 10934–10942 (2024).
Frear, D. S. & Burrell, R. C. Spectrophotometric method for determining hydroxylamine reductase activity in higher plants. Anal. Chem. 27, 1664–1665 (1955).
Zeng, Y. et al. Unraveling the electronic structure and dynamics of the atomically dispersed iron sites in electrochemical CO2 reduction. J. Am. Chem. Soc. 145, 15600–15610 (2023).
Ren, X. et al. In-situ spectroscopic probe of the intrinsic structure feature of single-atom center in electrochemical CO/CO2 reduction to methanol. Nat. Commun. 14, 3401 (2023).
Alsudairi, A. et al. Resolving the iron phthalocyanine redox transitions for ORR catalysis in aqueous media. J. Phys. Chem. Lett. 8, 2881–2886 (2017).
Melendres, C., Rios, C., Feng, X. & McMasters, R. In situ laser Raman spectra of iron phthalocyanine adsorbed on copper and gold electrodes. J. Phys. Chem. 87, 3526–3531 (1983).
Speelman, A. L. et al. Non-heme high-spin {FeNO}6–8 complexes: one ligand platform can do it all. J. Am. Chem. Soc. 140, 11341–11359 (2018).
Ding, Y., Cai, P. & Wen, Z. Electrochemical neutralization energy: from concept to devices. Chem. Soc. Rev. 50, 1495–1511 (2021).
Fan, L. et al. High entropy alloy electrocatalytic electrode toward alkaline glycerol valorization coupling with acidic hydrogen production. J. Am. Chem. Soc. 144, 7224–7235 (2022).
Jia, S. et al. Integration of plasma and electrocatalysis to synthesize cyclohexanone oxime under ambient conditions using air as a nitrogen source. Chem. Sci. 14, 13198–13204 (2023).
Luo, L. et al. Electrosynthesis of the nylon-6 precursor from nitrate and cyclohexanone over a rutile TiO2 catalyst. CCS Chem. 7, 266–278 (2025).
Zhang, F. et al. A Pickering-emulsion-droplet-integrated electrode for the continuous-flow electrosynthesis of oximes. Nat. Synth. 4, 479–487 (2025).
Wu, Y., Jiang, Z., Lu, X., Liang, Y. & Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 575, 639–642 (2019).
Lee, B.-H. et al. Supramolecular tuning of supported metal phthalocyanine catalysts for hydrogen peroxide electrosynthesis. Nat. Catal. 6, 234–243 (2023).
Li, Y. H. et al. Redox-mediated electrosynthesis of ethylene oxide from CO2 and water. Nat. Catal. 5, 185–192 (2022).
Luo, Y. T. et al. Selective electrochemical synthesis of urea from nitrate and CO via relay catalysis on hybrid catalysts. Nat. Catal. 6, 939–948 (2023).
Yu, L. et al. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 13, 3439–3446 (2020).
Li, J. Sustainable oxime production via the electrosynthesis of hydroxylamine in a free state. Zenodo https://doi.org/10.5281/zenodo.16354033 (2025).
Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFA1507400), the National Natural Science Foundation of China (grant numbers 22325805 and 22402107), the Beijing Natural Science Foundation (JQ22003), the Haihe Laboratory of Sustainable Chemical Transformations (24HHWCSS00007), and Tsinghua University Dushi Program and Center of High Performance Computing, Tsinghua University. J.L. was supported by the Postdoctoral Fellowship Program of CPSF (GZB20240475) and the Fundamental Research Funds for the Central Universities. The authors thank X. Gao, K. Kong, Q. Shi and K. Ji for useful discussions.
Author information
Authors and Affiliations
Contributions
J.L. and X.L. designed and carried out the synthesis, characterizations and catalytic reactions, analysed the data and wrote the paper. S.-M.X. performed DFT. M.X. and L.Z. performed XAS and analysis. Yunlong Wang and Y.P. performed flue gas analysis. Y.L. and X.W. carried out the LCA. A.-Z.L. and Ye Wang helped with in situ Fourier-transform infrared spectroscopy. X.L. performed Mӧssbauer spectroscopy. T.Z. assisted with catalyst synthesis. H.Z. provided help with electrolyser assembly. H.D. supervised the project, conceived the idea, helped design the experiments, analysed the data and wrote the paper. All the authors commented on the paper and have approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Feng Jiao, Hyungjun Kim, Tengfei Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–19, Figs. 1–68, Tables 1–8 and References.
Source data
Source Data Fig. 2
Free NH2OH production over a FePc-KB catalyst.
Source Data Fig. 3
Comparison between one-step oximation and two-step oximation.
Source Data Fig. 4
Understanding of selective production of free NH2OH over FePc-KB.
Source Data Fig. 5
MEA performance for oxime electrosynthesis at industrially relevant current density.
Source Data Fig. 6
Technoeconomic analysis and CO2 emission life-cycle assessment.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Liu, X., Xu, SM. et al. Sustainable oxime production via the electrosynthesis of hydroxylamine in a free state. Nat. Synth 4, 1598–1609 (2025). https://doi.org/10.1038/s44160-025-00879-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00879-4