Abstract
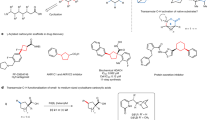
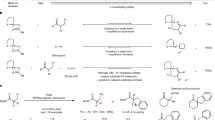
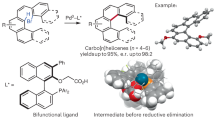
Methylene-selective C–H functionalization at distal positions is a challenge in the field of Pd(II) catalysis. We have previously reported a ligand-enabled β,γ-C–H coupling with dihaloarenes for the synthesis of benzocyclobutenes (BCBs) as a promising class of scaffolds in drug discovery. Here we report a Pd(II)-catalysed method for the γ,δ-methylene C–H activation of free aliphatic acids and subsequent coupling with dihaloarenes, which offers an efficient route for the synthesis of diversely functionalized BCBs. The development of a carboxyl-pyridone ligand is crucial for the remote C(sp3)–H activation. Notably, previous γ,δ-methylene C–H activation reactions of monoaliphatic acids are limited to carbocyclic substrates. The site-selective activation of γ,δ-C–H bonds installs the BCB pharmacophores that are one carbon atom further away from the carboxyl group than in previous studies. Given the carboxyl group can serve as hydrogen-bond donor or acceptor, such alternation of distance between two interactions can impact bioactivity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information. The crystallographic data for the structure reported in this study for compounds 4o have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under accession number 2357735. These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif.
References
Korth, H. G. & Sustman, R. in Carboxylic Acids and Carboxylic Acid Derivatives 4th edn (ed. Falbe, J.) 193–469 (Thieme, 1985).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalizations: mechanism and scope. Chem. Rev. 111, 1315–1345 (2011).
Daugulis, O., Roane, J. & Tran, L. D. Bidentate, monoanionic auxiliary-directed functionalization of carbon–hydrogen bonds. Acc. Chem. Res. 48, 1053–1064 (2015).
Giri, R. et al. Palladium-catalyzed methylation and arylation of sp2 and sp3 C−H bonds in simple carboxylic acids. J. Am. Chem. Soc. 129, 3510–3511 (2007).
Zhu, Y. et al. Pd-catalysed ligand-enabled carboxylate-directed highly regioselective arylation of aliphatic acids. Nat. Commun. 8, 14904 (2017).
Uttry, A., Mal, S. & van Gemmeren, M. Late-stage β-C(sp3)–H deuteration of carboxylic acids. J. Am. Chem. Soc. 143, 10895–10901 (2021).
Hu, L., Meng, G. & Yu, J.-Q. Ligand-enabled Pd(II)-catalyzed β-methylene C(sp3)–H arylation of free aliphatic acids. J. Am. Chem. Soc. 144, 20550–20553 (2022).
Yan, J.-L., Hu, L., Lu, Y. & Yu, J.-Q. Catalyst-controlled chemoselective γ‑C(sp3)–H lactonization of carboxylic acid: methyl versus methylene. J. Am. Chem. Soc. 146, 29311–29314 (2024).
Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2012).
Dolui, P., Das, J., Chandrashekar, H. B., Anjana, S. S. & Maiti, D. Ligand-enabled PdII-catalyzed iterative γ-C(sp3)–H arylation of free aliphatic acid. Angew. Chem. Int. Ed. 58, 13773–13777 (2019).
Park, H. S., Fan, Z., Zhu, R.-Y. & Yu, J.-Q. Distal γ-C(sp3)–H olefination of ketone derivatives and free carboxylic acids. Angew. Chem. Int. Ed. 59, 12853–12859 (2020).
Ghosh, K. K. et al. Ligand-enabled γ-C(sp3)–H olefination of free carboxylic acids. Angew. Chem. Int. Ed. 59, 12848–12852 (2020).
Meng, G., Hu, L., Tomanik, M. & Yu, J.-Q. β- and γ-C(sp3)–H heteroarylation of free carboxylic acids: a modular synthetic platform for diverse quaternary carbon centers. Angew. Chem. Int. Ed. 62, e202214459 (2023).
Das, J., Pal, T., Ali, W., Sahoo, S. R. & Maiti, D. Pd-catalyzed dual-γ-1,1-C(sp3)–H activation of free aliphatic acids with Allyl–O moieties. ACS Catal. 12, 11169–11176 (2022).
Kang, G., Strassfeld, D. A., Sheng, T., Chen, C.-Y. & Yu, J.-Q. Transannular C–H functionalization of cycloalkane carboxylic acids. Nature 618, 519–525 (2023).
Zhang, T. et al. Enantioselective remote methylene C–H (hetero)arylation of cycloalkane carboxylic acids. Science 384, 793–798 (2024).
Sadana, A. K., Saini, R. K. & Billups, W. E. Cyclobutarenes and related compounds. Chem. Rev. 103, 1539–1602 (2003).
Elnaggar, M. S. et al. Hydroquinone derivatives from the marine-derived fungus Gliomastix sp. RSC Adv. 7, 30640–30649 (2017).
Psotka, M. A. & Teerlink, J. R. Role in the chronic heart failure armamentarium. Circulation 133, 2066–2075 (2016).
Tsotinis, A., Afroudakis, P. A., Garratt, P. J., Bocianowska-Zbrog, A. & Sugden, D. Benzocyclobutane, benzocycloheptane and heptene derivatives as melatonin agonists and antagonists. ChemMedChem 9, 2238–2243 (2014).
Swedberg, K. et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376, 875–885 (2010).
Segura, J. L. & Martín, N. o-Quinodimethanes: efficient intermediates in organic synthesis. Chem. Rev. 99, 3199–3246 (1999).
Yang, B. & Gao, S. Recent advances in the application of Diels–Alder reactions involving o-quinodimethanes, aza-o-quinone methides and o-quinone methides in natural product total synthesis. Chem. Soc. Rev. 47, 7926–7953 (2018).
Kirchhoff, R. A. & Bruza, K. J. Benzocyclobutenes in polymer synthesis. Prog. Polym. Sci. 18, 85–185 (1993).
Harth, E. et al. A facile approach to architecturally defined nanoparticles via intramolecular chain collapse. J. Am. Chem. Soc. 124, 8653–8660 (2002).
Kotha, S., Lahiri, K. & Tangella, Y. Recent advances in benzocyclobutene chemistry. Asian J. Org. Chem. 10, 3166–3185 (2021).
Schiess, P., Heitzmann, M., Rutschmann, S. & Stäheli, R. Preparation of benzocyclobutenes by flash vacuum pyrolysis. Tetrahedron Lett. 19, 4569–4572 (1978).
Chaumontet, M. et al. Synthesis of benzocyclobutenes by palladium-catalyzed C–H activation of methyl groups: method and mechanistic study. J. Am. Chem. Soc. 130, 15157–15166 (2008).
Ye, J. et al. Remote C–H alkylation and C–C bond cleavage enabled by an in situ generated palladacycle. Nat. Chem. 9, 361–368 (2017).
Provencher, P. A. et al. Pd(II)-catalyzed synthesis of benzocyclobutenes by β-methylene-selective C(sp3)–H arylation with a transient directing group. J. Am. Chem. Soc. 143, 20035–20041 (2021).
Wei, W.-X. et al. Experimental and computational studies of palladium-catalyzed spirocyclization via a Narasaka–Heck/C(sp3 or sp2)–H activation cascade reaction. J. Am. Chem. Soc. 143, 7868–7875 (2021).
Liu, J., Hao, T., Qian, L., Shi, M. & Wei, Y. Construction of benzocyclobutenes enabled by visible-light-induced triplet biradical atom transfer of olefins. Angew. Chem. Int. Ed. 61, e202204515 (2022).
Talbot, F. J. T. et al. Modular synthesis of stereodefined benzocyclobutene derivatives via sequential Cu- and Pd-catalysis. ACS Catal. 11, 14448–14455 (2021).
Fujii, T., Gallarati, S., Corminboeuf, C., Wang, Q. & Zhu, J. Modular synthesis of benzocyclobutenes via Pd(II)-catalyzed oxidative [2 + 2] annulation of arylboronic acids with alkenes. J. Am. Chem. Soc. 144, 8920–8926 (2022).
Yang, J.-M. et al. Regio-controllable [2 + 2] benzannulation with two adjacent C(sp3)–H bonds. Science 380, 639–644 (2023).
Dubost, C. et al. Benzocyclobutane(thio) carboxamides. US patent 9878985 B2 (2018).
Acknowledgements
We thank Z. Li for help with ligand synthesis. We thank M. Gembicky and J. Bailey for X-ray crystallographic analysis. We thank L. Pasternack and G. Kroon for their assistance with NMR analysis. We gratefully acknowledge the National Institutes of Health (National Institute of General Medical Sciences, R01GM084019) and The Scripps Research Institute for financial support.
Author information
Authors and Affiliations
Contributions
J.-Q.Y. conceived the concept. L.H. discovered and developed the reaction. L.H., J.-L.Y. and Y.-K.L. developed the substrate scope. L.H., D.A.S. and J.-Q.Y. wrote the manuscript. J.-Q.Y. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Gong Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, experimental procedures, additional reaction optimization and characterization data.
Supplementary Data 1
Crystallographic data for compound 4o, CCDC 2357735.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, L., Yan, JL., Lin, YK. et al. Regiocontrollable [2 + 2] benzannulation of γ,δ-C(sp3)–H bonds with dihaloarenes using palladium catalysis. Nat. Synth 4, 1556–1564 (2025). https://doi.org/10.1038/s44160-025-00883-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00883-8