Abstract
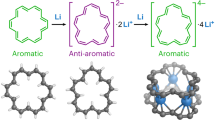
π-Conjugated molecules with non-trivial topologies, such as catenanes and molecular knots, offer aromaticity and through-space electronic and magnetic interactions absent in traditional planar π systems. However, their synthesis remains challenging, with previous examples showing only localized aromaticity in individual benzenoid rings. Here we report the synthesis of a [2]catenane comprising 2 intertwined octaphyrinoid rings, each with 34 globally delocalized π electrons, achieved using a passive metal-template strategy with 2,2′-dipyrromethene as the directing ligand. X-ray crystallographic analysis reveals a nearly orthogonal spatial arrangement of the rings in neutral catenane, stabilized by multiple [NH···N] and [S···N] close contacts. These rings exhibit global aromaticity with entangled magnetic shielding interactions. Upon four-electron oxidation, the system converts to a tetracation with two globally antiaromatic (32π) rings, in which through-space bonding interactions diminish the antiaromatic destabilization. Notably, counterions also affect the (anti)aromaticity of the tetracations in the single-crystal state, highlighting a dynamic interplay between molecular topology, electronic structure and external interactions.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2407480 (3), 2407481 (1), 2407482 (1-Zn), 2407483 (8), 2407485 (1-Zn4+·(SbF6−)4), 2407486 (1-Zn4+·(SbCl6−)4), 2407487 (OCT-1), 2407488 (14+·(SbCl6−)4), 2407489 ([OCT-2 + H]+·(SbF6−)), 2407490 (OCT-1+··(SbF6−)) and 2407491 (OCT-12+·(BF4−)(Cl−)). Copies of the data can be obtained free of charge via the Cambridge Crystallographic Data Center at https://www.ccdc.cam.ac.uk/structures/.
References
Herges, R. Topology in chemistry: designing Möbius molecules. Chem. Rev. 106, 4820–4842 (2006).
Segawa, Y., Levine, D. R. & Itami, K. Topologically unique molecular nanocarbons. Acc. Chem. Res. 52, 2760–2767 (2019).
Leonhardt, E. J. & Jasti, R. Emerging applications of carbon nanohoops. Nat. Chem. Rev. 3, 672–686 (2019).
Guo, Q.-H., Qiu, Y., Wang, M.-X. & Stoddart, J. F. Aromatic hydrocarbon belts. Nat. Chem. 13, 402–419 (2021).
Imoto, D., Yagi, A. & Itami, K. Carbon nanobelts: brief history and perspective. Precis. Chem. 1, 516–523 (2023).
Ajami, D., Oeckler, O., Simon, A. & Herges, R. Synthesis of a Möbius aromatic hydrocarbon. Nature 426, 819–821 (2003).
Naulet, G. et al. Cyclic tris-[5]helicenes with single and triple twisted Möbius topologies and Möbius aromaticity. Chem. Sci. 9, 8930–8936 (2018).
Jiang, X. et al. Kinetic control in the synthesis of a Möbius tris((ethynyl)[5]helicene) macrocycle using alkyne metathesis. J. Am. Chem. Soc. 142, 6493–6498 (2020).
Terabayashi, T. et al. Synthesis of twisted [n]cycloparaphenylene by alkene insertion. Angew. Chem. Int. Ed. 62, e202214960 (2023).
Zhou, Q. et al. [5]Helicene based π-conjugated macrocycles with persistent figure-eight and Möbius shapes: efficient synthesis, chiral resolution and bright circularly polarized luminescence. Angew. Chem. Int. Ed. 63, e202417749 (2024).
Segawa, Y. et al. Synthesis of a Möbius carbon nanobelt. Nat. Synth. 1, 535–541 (2022).
Fan, W. et al. Synthesis and chiral resolution of a triply twisted Möbius carbon nanobelt. Nat. Synth. 2, 880–887 (2023).
Nogami, J. et al. Catalytic stereoselective synthesis of doubly, triply and quadruply twisted aromatic belts. Nat. Synth. 2, 888–897 (2023).
Stoddart, J. F. The chemistry of the mechanical bond. Chem. Soc. Rev. 38, 1802–1820 (2009).
Stoddart, J. F. Mechanically interlocked molecules (MIMs)—molecular shuttles, switches, and machines. Angew. Chem. Inter. Ed. 56, 11094–11125 (2017).
Albrecht-Gary, A. M. et al. Interlocked macrocyclic ligands: a kinetic catenand effect in copper(I) complexes. J. Am. Chem. Soc. 107, 3205–3209 (1985).
Mena-Hernando, S. & Pérez, E. M. Mechanically interlocked materials. Rotaxanes and catenanes beyond the small molecule. Chem. Soc. Rev. 48, 5016–5032 (2019).
Bäuerle, P. et al. Oligothiophene-based catenanes: synthesis and electronic properties of a novel conjugated topological structure. Angew. Chem. Int. Ed. 46, 363–368 (2006).
Ammann, M., Rang, A., Schalley, C. A. & Bäuerle, P. A Synthetic approach towards interlocked π-conjugated macrocycles. Eur. J. Org. Chem. 9, 1940–1948 (2006).
Götz, G. et al. π-Conjugated [2]catenanes based on oligothiophenes and phenanthrolines: efficient synthesis and electronic properties. Chem. Eur. J. 21, 7193–7210 (2015).
Fan, Y.-Y. et al. An isolable catenane consisting of two Möbius conjugated nanohoops. Nat. Commun. 9, 3037 (2018).
Bu, A. et al. A conjugated covalent template strategy for all-benzene catenane synthesis. Angew. Chem. Int. Ed. 61, 363–368 (2022).
Segawa, Y. et al. Topological molecular nanocarbons: all-benzene catenane and trefoil knot. Science 365, 272–276 (2019).
May, J. H., Van Raden, J. M., Maust, R. L., Zakharov, L. N. & Jasti, R. Active template strategy for the preparation of π-conjugated interlocked nanocarbons. Nat. Chem. 15, 170–176 (2023).
Oka, Y., Masai, H. & Terao, J. Multiple structural switching of [3]catenanes with cyclic porphyrin dimers by complexation with amine ligands. Angew. Chem. Int. Ed. 62, e202217002 (2023).
Lash, T. D. Origin of aromatic character in porphyrinoid systems. J. Porphyr. Phthalocyanines 15, 1093–1115 (2011).
Ivanov, A. S. & Boldyrev, A. I. Deciphering aromaticity in porphyrinoids via adaptive natural density partitioning. Org. Biomol. Chem. 12, 6145–6150 (2014).
Peeks, M. D., Claridge, T. D. W. & Anderson, H. L. Aromatic and antiaromatic ring currents in a molecular nanoring. Nature 541, 200–203 (2017).
Tanaka, T. & Osuka, K. Chemistry of meso-aryl-substituted expanded porphyrins: aromaticity and molecular twist. Chem. Rev. 117, 2584–2640 (2017).
Shin, J.-Y. et al. Aromaticity and photophysical properties of various topology-controlled expanded porphyrins. Chem. Soc. Rev. 39, 2751–2767 (2010).
Heilbronner, E. Hückel molecular orbitals of Möbius-type conformations of annulenes. Tetrahedron Lett. 29, 1923–1928 (1964).
Rzepa, H. S. Möbius aromaticity and delocalization. Chem. Rev. 105, 3697–3715 (2005).
Stępień, M., Latos-Grażyński, L., Sprutta, N., Chwalisz, P. & Szterenberg, L. Expanded porphyrin with a split personality: a Hückel–Möbius aromaticity switch. Angew. Chem. Int. Ed. 46, 7869–7873 (2007).
Yoon, Z. S., Osuka, A. & Kim, D. Möbius aromaticity and antiaromaticity in expanded porphyrins. Nat. Chem. 1, 113–122 (2009).
Stępień, M., Sprutta, N. & Latos-Grażyński, L. Figure eights, Möbius bands, and more: conformation and aromaticity of porphyrinoids. Angew. Chem. Int. Ed. 50, 4288–4340 (2011).
Ni, Y. et al. 3D global aromaticity in a fully conjugated diradicaloid cage at different oxidation states. Nat. Chem. 12, 242–248 (2020).
Ren, L., Han, Y., Hou, X., Ni, Y. & Wu, J. All are aromatic: a 3D globally aromatic cage containing five types of 2D aromatic macrocycles. Chem 7, 3442–3453 (2021).
Nozawa, R. et al. Stacked antiaromatic porphyrins. Nat. Commun. 7, 13620 (2016).
Nozawa, R. et al. Three-dimensional aromaticity in an antiaromatic cyclophane. Nat. Commun. 10, 3576 (2019).
Dietrich-Buchecker, C. O., Sauvage, J.-P. & Kintzinger, J. P. Une nouvelle famille de molecules: les metallo-catenanes. Tetrahedron Lett. 24, 5095–5098 (1983).
Fujita, M., Ibukuro, F., Hagihara, H. & Ogura, K. Quantitative self-assembly of a [2]catenane from two preformed molecular rings. Nature 367, 720–723 (1994).
Hunter, C. A. Synthesis and structure elucidation of a new [2]-catenane. J. Am. Chem. Soc. 114, 5303–5311 (1992).
Ashton, P. R. et al. A [2] catenane made to order. Angew. Chem. Int. Ed. 28, 1396–1399 (1989).
Aucagne, V., Hänni, K. D., Leigh, D. A., Lusby, P. J. & Walker, D. B. Catalytic ‘click’ rotaxanes: a substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 128, 2186–2187 (2006).
Berná, J. et al. Cadiot–Chodkiewicz active template synthesis of rotaxanes and switchable molecular shuttles with weak intercomponent interactions. Angew. Chem. Int. Ed. 47, 4392–4396 (2008).
Singh, R. S., Paitandi, R. P., Gupta, R. K. & Pandey, D. S. Recent developments in metal dipyrrin complexes: design, synthesis, and applications. Coord. Chem. Rev. 414, 213269 (2020).
Schenk, R. & Müllen, K. Multiply charged anions from molecules with extended π-systems. Tetrahedron Lett. 31, 7367–7370 (1990).
Sprutta, N. & Latos-Grazyński, L. Figure-eight tetrathiaoctaphyrin and dihydrotetrathiaoctaphyrin. Chem. Eur. J. 23, 5099–5112 (2001).
Tanaka, Y. et al. Metalation of expanded porphyrins: a chemical trigger used to produce molecular twisting and Möbius aromaticity. Angew. Chem. Int. Ed. 47, 681–684 (2008).
Lu, T. & Chen, Q. A simple method of identifying π orbitals for non-planar systems and a protocol of studying π electronic structure. Theor. Chem. Acc. 139, 25 (2020).
Geuenich, D., Hess, K., Köhler, F. & Herges, R. Anisotropy of the induced current density (ACID), a general method to quantify and visualize electronic delocalization. Chem. Rev. 105, 3758–3772 (2005).
Sessler, J. L., Weghorn, S. J., Lynch, V. & Johnson, M. R. Turcasarin, the largest expanded porphyrin to date. Angew. Chem. Int. Ed. 33, 1509–1512 (1994).
Lash, T. D. Giant prohyrinoids: from figure eights to nanomolecular cavities. Angew. Chem. Int. Ed. 39, 1763–1767 (2000).
Choi, C. H. & Kertesz, M. Bond length alternation and aromaticity in large annulenes. J. Chem. Phy. 108, 6681–6688 (1998).
Sundholm, D., Fliegl, H. & Berger, R. J. F. Calculations of magnetically induced current densities: theory and applications. WIREs Comput. Mol. Sci. 6, 639–678 (2016).
Humphrey, W., Dalke, A. & Schulten, K. VMD-visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Bleaney, B. & Bowers, K. D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. Lond. A 214, 451–465 (1952).
Zeng, Z. et al. Pro-aromatic and antiaromatic π-conjugated molecules: an irresistible wish to be diradicals. Chem. Soc. Rev. 44, 6578–6596 (2015).
Wiberg, K. B. Antiaromaticity in monocyclic conjugated carbon rings. Chem. Rev. 101, 1317–1331 (2001).
Kaufman, H. S., Fankuchen, I. & Mark, H. Structure of cyclo-octatetraene. Nature 161, 165 (1948).
Baird, N. C. Quantum organic photochemistry. II. Resonance and aromaticity in the lowest 3ππ* state of cyclic hydrocarbons. J. Am. Chem. Soc. 94, 4941–4948 (1972).
Rosenberg, M., Dahlstrand, C., Kilså, K. & Ottosson, H. Excited state aromaticity and antiaromaticity: opportunities for photophysical and photochemical rationalizations. Chem. Rev. 114, 5379–5425 (2014).
Liu, C. et al. Macrocyclic polyradicaloids with unusual super-ring structure and global aromaticity. Chem 4, 1586–1595 (2018).
Kimball, J. C. & Frisch, H. L. Aharonov–Bohm effects in entangled molecules. Phys. Rev. Lett. 93, 093001 (2004).
Acknowledgements
Y.N. acknowledges financial support from the National Natural Science Foundation of China (grant number 22375085), the Guangdong Provincial Key Laboratory of Catalysis (grant number 22201124), the Guangdong Provincial Key Laboratory of Sustainable Biomimetic Materials and Green Energy (grant number 2024B121201003) and the Innovation Commission of Shenzhen Municipality (grant number 20231116124346001). J.W. acknowledges financial support from a Singapore MOE Tier 2 grant (MOE-T2EP10222-0003), the MOE Tier 3 program (MOE-000755-00) and the A*STAR MTC IRG project (M22K2c0083). Z.Z. acknowledges financial support from the National Natural Science Foundation of China (grant numbers 52350058, 22375059 and 52525306). We thank X. Chang for his support with X-ray diffraction data collection. We also thank H. Xia and Z. Xie for helpful discussions.
Author information
Authors and Affiliations
Contributions
Z.Z., J.W. and Y.N. conceived of the idea and supervised the project. Y.N. and Y.S. synthesized the compounds and collected the spectral data. Y.N., Y.S., Z.W., L.R. and S.W. performed theoretical calculations and X-ray analysis. Y.N., Y.S. and J.H. performed the magnetic measurements and analysis. All authors participated in writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Mercedes Alonso, Milan Gembicky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Representations of the conformation and π-electronic configuration of 8.
a, Crystallographic structures depicted as oak ridge thermal ellipsoid plots (ORTEP) at 30% probability, which clearly clarify the double-twisted figure-eight conformation of 8. b, Calculated LOL-π isosurface map (isovalue: 0.3), the pink and blue surfaces are interlocked in space, revealing a double-sided lemniscular topology for the π system of 8. c, Calculated AICD plots with the magnetic field aligned along the Z-axis (perpendicular to the paper, isovalue 0.02). d, Calculated EDDB isosurface (isovalue 0.02). The AICD and EDDB plots showed discontiguous electron circuit along the conjugated backbone, manifesting the nonaromatic character of 8.
Supplementary information
Supplementary Information
Experimental methods and Supplementary Figs. 1–92 and Tables 1–15.
Supplementary Video 1
GIMIC of 1-Zn.
Supplementary Video 2
GIMIC of 1.
Supplementary Video 3
GIMIC of 1-Zn⁴⁺.
Supplementary Video 4
GIMIC of 1⁴⁺.
Supplementary Data 1
Crystal data for 3, CCDC 2407480.
Supplementary Data 2
Crystal data for 8, CCDC 2407483.
Supplementary Data 3
Crystal data for 1-Zn, CCDC 2407482.
Supplementary Data 4
Crystal data for 1, CCDC 2407481.
Supplementary Data 5
Crystal data for 1-Zn4+·(SbF6−)4, CCDC 2407485.
Supplementary Data 6
Crystal data for 1-Zn4+·(SbCl6−)4, CCDC 2407486.
Supplementary Data 7
Crystal data for OCT-1, CCDC 2407487.
Supplementary Data 8
Crystal data for 14+·(SbCl6−)4, CCDC 2407488.
Supplementary Data 9
Crystal data for [OCT-2 + H]+·(SbF6−), CCDC 2407489.
Supplementary Data 10
Crystal data for OCT-1+··(SbF6−), CCDC 2407490.
Supplementary Data 11
Crystal data for OCT-12+·(BF4−)(Cl−), CCDC 2407491.
Source data
Source Data Fig. 1
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sui, Y., Wang, Z., Hao, J. et al. Aromaticity and through-space electronic coupling in [2]catenanes comprising two intertwined globally electron-delocalized octaphyrinoid rings. Nat. Synth (2025). https://doi.org/10.1038/s44160-025-00918-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44160-025-00918-0