Abstract
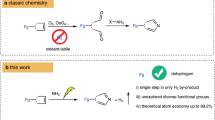
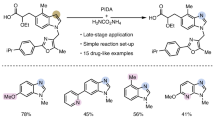
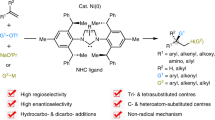
Skeletal editing via nitrogen atom insertion into cyclic frameworks is a non-conventional and powerful strategy for constructing functionalized N heterocycles—privileged scaffolds in both synthetic chemistry and pharmaceutical science. Despite their importance, general methods for the direct insertion of nitrogen into carbocycles, particularly saturated ones, remain limited due to the challenge of selectively activating of inert C–C bonds. Here we report an electrochemical platform that enables efficient nitrogen atom insertion into saturated carbocycles under mild conditions. Two distinct protocols have been developed, allowing access to either functionalized quinolines or N-alkylated saturated N heterocycles, both with excellent selectivity and broad functional group tolerance. Mechanistic studies reveal the involvement of benzylic carbocation and cyclic imine intermediates, which undergo divergent pathways to furnish structurally diverse products. This methodology for N heterocycle synthesis provides a robust route to bioactive scaffolds. The synthetic utility of the approach is highlighted by the synthesis of two ion-channel antagonists.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All other experimental and characterization data are available in Supplementary Information. Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC 2405528 (35) and CCDC 2406850 (54). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. Source data are provided with this paper.
References
Muthukrishnan, I., Sridharan, V. & Menendez, J. C. Progress in the chemistry of tetrahydroquinolines. Chem. Rev. 119, 5057–5191 (2019).
Elebiju, O. F., Ajani, O. O., Oduselu, G. O., Ogunnupebi, T. A. & Adebiyi, E. Recent advances in functionalized quinoline scaffolds and hybrids—exceptional pharmacophore in therapeutic medicine. Front. Chem. 10, 1074331 (2022).
Khadem, S. & Marles, R. J. Tetrahydroquinoline-containing natural products discovered within the last decade: occurrence and bioactivity. Nat. Prod. Res. 39, 182–194 (2025).
Ahmad, G. et al. N-heterocycles as promising antiviral agents: a comprehensive overview. Molecules 29, 2232 (2024).
Kumar, D. & Jain, S. K. A comprehensive review of N-heterocycles as cytotoxic agents. Curr. Med. Chem. 23, 4338–4394 (2016).
Atukuri, D. et al. Contribution of N-heterocycles towards anti-tubercular drug discovery (2014-2019); predicted and reengineered molecular frameworks. Drug Dev. Res. 82, 767–783 (2021).
El-Sherief, H. A. M., Youssif, B. G. M., Bukhari, S. N. A., Abdel-Aziz, M. & Abdel-Rahman, H. M. Novel 1,2,4-triazole derivatives as potential anticancer agents: design, synthesis, molecular docking and mechanistic studies. Bioorg. Chem. 76, 314–325 (2018).
Mermer, A. et al. Piperazine–azole–fluoroquinolone hybrids: conventional and microwave irradiated synthesis, biological activity screening and molecular docking studies. Bioorg. Chem. 85, 308–318 (2019).
Oniciuc, L. et al. Benzoquinoline derivatives: an attractive approach to newly small molecules with anticancer activity. Int. J. Mol. Sci. 24, 8124 (2023).
Joynson, B. W. & Ball, L. T. Skeletal editing: interconversion of arenes and heteroarenes. Helv. Chim. Acta 106, e202200182 (2023).
Li, X., Xu, J. & Xu, Z. G. Precision single-atom editing: new frontiers in nitrogen insertion and substitution for the generation of N-heterocycles. Org. Chem. Front. 11, 4041–4053 (2024).
Patel, C. K. et al. Skeletal editing through single-atom insertion and transmutation: an insight into a new era of synthetic organic chemistry. Synthesis 56, 3793–3814 (2024).
Lu, H., Chang, J. & Wei, H. Transition metal-catalyzed nitrogen atom insertion into carbocycles. Acc. Chem. Res. 58, 933–946 (2025).
Cheng, Z., Hu, Z. & Jiao, N. Molecular ring remodeling through C–C bond cleavage. Acc. Chem. Res. 58, 1003–1022 (2025).
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
Zhang, B.-S. et al. Electrochemical skeletal indole editing via nitrogen atom insertion by sustainable oxygen reduction reaction. Angew. Chem. Int. Ed. 63, e202407384 (2024).
Reisenbauer, J. C. et al. Direct access to quinazolines and pyrimidines from unprotected indoles and pyrroles through nitrogen atom insertion. Org. Lett. 25, 8419–8423 (2023).
He, Y., Wang, J., Zhu, T., Zheng, Z. & Wei, H. Nitrogen atom insertion into arenols to access benzazepines. Chem. Sci. 15, 2612–2617 (2024).
Kelly, P. Q., Filatov, A. S. & Levin, M. D. A synthetic cycle for heteroarene synthesis by nitride insertion. Angew. Chem. Int. Ed. 61, e202213041 (2022).
Liu, S. & Cheng, X. Insertion of ammonia into alkenes to build aromatic N-heterocycles. Nat. Commun. 13, 425 (2022).
Wang, J., Lu, H., He, Y., Jing, C. & Wei, H. Cobalt-catalyzed nitrogen atom insertion in arylcycloalkenes. J. Am. Chem. Soc. 144, 22433–22439 (2022).
Wang, Z., Xu, H., Han, X., Fan, S. & Zhu, J. Manganese-catalyzed cycloalkene ring expansion synthesis of azaheterocycles. Org. Lett. 26, 8559–8564 (2024).
Jun, C. H. Transition metal-catalyzed carbon–carbon bond activation. Chem. Soc. Rev. 33, 610–618 (2004).
Liu, H., Feng, M. H. & Jiang, X. F. Unstrained carbon–carbon bond cleavage. Chem. Asian J. 9, 3360–3389 (2014).
Song, F. J., Gou, T., Wang, B. Q. & Shi, Z. J. Catalytic activations of unstrained C‒C bond involving organometallic intermediates. Chem. Soc. Rev. 47, 7078–7115 (2018).
Xia, Y. & Dong, G. B. Temporary or removable directing groups enable activation of unstrained C‒C bonds. Nat. Rev. Chem. 4, 600–614 (2020).
Liang, Y. F. et al. Carbon–carbon bond cleavage for late-stage functionalization. Chem. Rev. 123, 12313–12370 (2023).
Zhang, Z., Li, Q., Cheng, Z., Jiao, N. & Zhang, C. Selective nitrogen insertion into aryl alkanes. Nat. Commun. 15, 6016 (2024).
Amber, C., Göttemann, L. T., Steele, R. T., Petitjean, T. M. & Sarpong, R. Reductive amination of carbonyl C–C bonds enables formal nitrogen insertion. J. Org. Chem. 89, 17655–17663 (2024).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Siu, J. C., Fu, N. & Lin, S. Catalyzing electrosynthesis: a homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 53, 547–560 (2020).
Meyer, T. H., Choi, I., Tian, C. & Ackermann, L. Powering the future: how can electrochemistry make a difference in organic synthesis?. Chem 6, 2484–2496 (2020).
Yuan, Y., Yang, J. & Lei, A. Recent advances in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals. Chem. Soc. Rev. 50, 10058–10086 (2021).
Xiong, P. & Xu, H.-C. Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 52, 3339–3350 (2019).
Shi, S. H., Liang, Y. J. & Jiao, N. Electrochemical oxidation induced selective C‒C bond cleavage. Chem. Rev. 121, 485–505 (2021).
Huang, J. S., Jian, Y. M., Zhou, M. & Wu, H. G. Oxidative C‒C bond cleavage of lignin via electrocatalysis. Front. Chem. 10, 1007707 (2022).
Hoffman, R. V. & Kumar, A. Cationic carbon-to-nitrogen rearrangements in N-(arylsulfonoxy)amines. J. Org. Chem. 50, 1859–1863 (1985).
Wang, T. et al. Hydroxylamine-mediated C‒C amination via an aza-Hock rearrangement. Nat. Commun. 12, 7029 (2021).
Anugu, R. R. & Falck, J. R. Site-selective amination and/or nitrilation via metal-free C(sp2)‒C(sp3) cleavage of benzylic and allylic alcohols. Chem. Sci. 13, 4821–4827 (2022).
Niu, C. et al. Selective ring-opening amination of isochromans and tetrahydroisoquinolines. Angew. Chem. Int. Ed. 63, e202401318 (2024).
Kumar, P., Divedi, A., Chandra, D., Ranjan De, S., & Jat, J. L. Hydroxylamine-O-sulfonic acid (HOSA): a recent synthetic overview. ChemistrySelect 9, e202401805 (2024).
Kirste, A., Elsler, B., Schnakenburg, G. & Waldvogel, S. R. Efficient anodic and direct phenol-arene C,C cross-coupling: the benign role of water or methanol. J. Am. Chem. Soc. 134, 3571–3576 (2012).
Westaway, S. M. et al. N-Tetrahydroquinolinyl, N-quinolinyl and N-isoquinolinyl biaryl carboxamides as antagonists of TRPV1. Bioorg. Med. Chem. Lett. 16, 4533–4536 (2006).
Rami, H. K., Thompson, M., Macdonald, G. J., Westaway, S. M. & Mitchell, D. J. Vanillord receptor modulators. International patent WO 03/068749A1 (2003).
Utley, J. H. P. & Rozenberg, G. G. Electroorganic reactions. Part 56: Anodic oxidation of 2-methyl- and 2-benzylnaphthalenes: factors influencing competing pathways. Tetrahedron 58, 5251–5265 (2002).
Utley, J. H. P. & Rozenberg, G. G. Electroorganic reactions. Part 57. DDQ mediated anodic oxidation of 2-methyl- and 2-benzylnaphthalenes. J. Appl. Electrochem. 33, 525–532 (2003).
Natori, I., Natori, S., Sekikawa, H. & Ogino, K. Effect of solvent on the dehydrogenation of poly(1,3-cyclohexadiene): formation and characteristics of benzoquinone–aromatic hydrocarbon charge-transfer complexes. J. Polym. Sci. Pol. Chem. 48, 342–350 (2010).
Bosnidou, A. E. et al. Tandem InCl3-promoted hydroperoxide rearrangements and nucleophilic additions: a straightforward entry to benzoxacycles. J. Org. Chem. 88, 9277–9282 (2023).
Nobusue, S., Fujita, K. & Tobe, Y. Skeletal rearrangement of twisted polycyclic aromatic hydrocarbons under scholl reaction conditions. Org. Lett. 19, 3227–3230 (2017).
Kurimoto, Y., Yamashita, J., Mitsudo, K., Sato, E. & Suga, S. Electrosynthesis of phosphacycles via dehydrogenative C‒P bond formation using DABCO as a mediator. Org. Lett. 23, 3120–3124 (2021).
Tao, S. K. et al. Electrochemical cross-dehydrogenative aromatization protocol for the synthesis of aromatic amines. Org. Lett. 24, 1011–1016 (2022).
Acknowledgements
This research was supported by the Ministry of Education of Singapore Academic Research Fund (tier 1: A-8001693-00-00 (M.J.K.) and A-8001040-00-00 (Y.Z.); tier 2: A-8001893-00-00 (Y.Z.)) and by National University of Singapore Foresight Grant: A-8002845-00-00, A-8002845-01-00 and A-8002845-02-00 (M.J.K.). I. I. Roslan assisted with X-ray crystallographic measurements.
Author information
Authors and Affiliations
Contributions
G.-Q.S., M.J.K. and Y.Z. conceived of the work. G.-Q.S., X.W., R.H. and W.R. conducted the optimization, reaction scope and mechanistic studies. M.J.K. and Y.Z. directed the research. All authors contributed to the writing of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Xu Cheng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Stephanie Greed, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary sections 1–13, Figs. 1–15, Tables 1–4 and Scheme 1.
Supplementary Data 1
Raw NMR data for verifying the charge-transfer complex.
Supplementary Data 2
Original data for Supplementary Figs. 4–7.
Supplementary Data 3
Single-crystal X-ray diffraction data for compound 35 (CCDC 2405528).
Supplementary Data 4
Single-crystal X-ray diffraction data for compound 54 (CCDC 2406850).
Source data
Source Data Fig. 6
The raw data for cyclic voltammograms in Fig. 6a,b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, GQ., Wang, X., Hu, R. et al. Divergent synthesis of N heterocycles from carbocycles enabled by electrochemical nitrogen atom insertion. Nat. Synth (2025). https://doi.org/10.1038/s44160-025-00945-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44160-025-00945-x