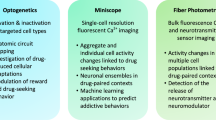
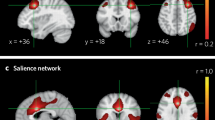
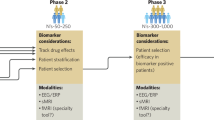
Abstract
As a neurobiological process, addiction involves pathological patterns of engagement with substances and a range of behaviors with a chronic and relapsing course. Neuroimaging technologies assess brain activity, structure, physiology, and metabolism at scales ranging from neurotransmitter receptors to large-scale brain networks, providing unique windows into the core neural processes implicated in substance use disorders. Identified aberrations in the neural substrates of reward and salience processing, response inhibition, interoception, and executive functions with neuroimaging can inform the development of pharmacological, neuromodulatory, and psychotherapeutic interventions to modulate the disordered neurobiology. Closed- or open-loop interventions can integrate these biomarkers with neuromodulation in real time or offline to personalize stimulation parameters and deliver precise intervention. This Analysis provides an overview of neuroimaging modalities in addiction medicine, potential neuroimaging biomarkers, and their physiologic and clinical relevance. Future directions and challenges in bringing these putative biomarkers from the bench to the bedside are also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The protocol and data for this systematic review are available on the open science framework (OSF) website (https://osf.io/79uc3/?view_only=1d92a6fd769f40119464b156f0c88912). The ClinicalTrials.gov search engine was used through the Study Fields query URL (https://ClinicalTrials.gov/api/gui/ref/api_urls) for searching the clinical trial protocols. For full-text screening, all available records were downloaded from the Aggregate Analysis of ClinicalTrials.gov (AACT) Database, Clinical Trials Transformation Initiative (CTTI) database203 (https://aact.ctti-clinicaltrials.org/) for the second stage. For searching the systematic reviews and meta-analyses, studies were identified using the Medline/PubMed database (https://pubmed.ncbi.nlm.nih.gov/).
Code availability
All codes are available on the study’s OSF project repository at the following link: https://osf.io/79uc3/?view_only=1d92a6fd769f40119464b156f0c88912. Data analyses and illustrations were conducted using R version 4.0.5204, with dplyr205 and ggplot2206 packages. The codes for data illustrations are freely available on the OSF repository of this project.
References
Degenhardt, L. et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 5, 987–1012 (2018).
Shield, K. D., Imtiaz, S., Probst, C. & Rehm, J. in Integrating Psychological and Pharmacological Treatments for Addictive Disorders: An Evidence-Based Guide 3–31 (Taylor & Francis, 2018).
Agency for Healthcare Research and Quality. 2022 National Healthcare Quality and Disparities Report https://www.ncbi.nlm.nih.gov/books/NBK587176/ (2022).
Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Association, 2013); https://doi.org/10.1176/appi.books.9780890425596
Volkow, N. D. & Boyle, M. Neuroscience of addiction: relevance to prevention and treatment. Am. J. Psychiatry 175, 729–740 (2018).
Kircher, J. & Pierson, C. Les atrophies cerebrales dans les toxicomanies: role de la pneumoencdphalographie. Essais therapeutiques. Maroc Med. 35, 668–670 (1956).
Koob, G. F. & Volkow, N. D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773 (2016).
Kwako, L. E., Bickel, W. K. & Goldman, D. Addiction biomarkers: dimensional approaches to understanding addiction. Trends Mol. Med. 24, 121–128 (2018).
Volkow, N. D., Koob, G. F. & McLellan, A. T. Neurobiologic advances from the brain disease model of addiction. New Engl. J. Med. 374, 363–371 (2016).
Ekhtiari, H., Zare-Bidoky, M. & Verdejo-Garcia, A. in Textbook of Addiction Treatment: International Perspectives (eds el-Guebaly, N. et al.) 1159–1176 (Springer, 2021); https://doi.org/10.1007/978-3-030-36391-8_81
Zilverstand, A., Huang, A. S., Alia-Klein, N. & Goldstein, R. Z. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron 98, 886–903 (2018).
Ekhtiari, H., Faghiri, A., Oghabian, M. A. & Paulus, M. P. in Neuroscience for Addiction Medicine: From Prevention to Rehabilitation—Methods and Interventions (eds Ekhtiari, H. & Paulus, M. P.) 129–153 (Elsevier, 2016); http://www.sciencedirect.com/science/article/pii/S0079612315001508
Moeller, S. J. & Paulus, M. P. Toward biomarkers of the addicted human brain: using neuroimaging to predict relapse and sustained abstinence in substance use disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 80, 143–154 (2018).
National Institutes of Health. National Institute on Drug Abuse. Resource Guide: Screening for Drug Use in General Medical Settings https://archives.drugabuse.gov/publications/resource-guide-screening-drug-use-in-general-medical-settings/biological-specimen-testing (2020).
Zakhari, S. & Li, T. K. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology 46, 2032–2039 (2007).
Bahji, A., Brietzke, E., Soares, C. & Stuart, H. Recent advances in biomarkers of addiction: a narrative review. Can. J. Addict. 12, 6–12 (2021).
Fernandes, B. S. et al. The new field of ‘precision psychiatry.’ BMC Med. 15, 80 (2017).
Mahmood, T. Biomarkers in psychiatry: a clinician’s viewpoint. Br. Med. Bull. 135, 23–27 (2020).
Carmichael, O. et al. The role of fMRI in drug development. Drug Discov. Today 23, 333–348 (2018).
Ekhtiari, H., Nasseri, P., Yavari, F., Mokri, A. & Monterosso, J. Neuroscience of drug craving for addiction medicine: from circuits to therapies. Prog. Brain Res. 223, 115–141 (2016).
Paulus, M. P. & Stewart, J. L. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry 77, 959–966 (2020).
O’Donnell, P. et al. Strategies to address challenges in neuroscience drug discovery and development. Int. J. Neuropsychopharmacol. 22, 445–448 (2019).
Heilig, M. et al. Addiction as a brain disease revised: why it still matters, and the need for consilience. Neuropsychopharmacology 46, 1715–1723 (2021).
Banks, M. L. & Negus, S. S. Insights from preclinical choice models on treating drug addiction. Trends Pharmacol. Sci. 38, 181–194 (2017).
Lewis, M. Addiction and the brain: development, not disease. Neuroethics 10, 7–18 (2017).
Kendler, K. S. Levels of explanation in psychiatric and substance use disorders: implications for the development of an etiologically based nosology. Mol. Psychiatry 17, 11–21 (2012).
Heather, N., Field, M., Moss, A. C. & Satel, S. Evaluating the Brain Disease Model of Addiction 1st edn (Routledge, 2022); https://doi.org/10.4324/9781003032762
Hart, C. L. Viewing addiction as a brain disease promotes social injustice. Nat. Hum. Behav. 1, 0055 (2017).
Heather, N. et al. Challenging the brain disease model of addiction: European launch of the addiction theory network. Addict. Res. Theory 26, 249–255 (2018).
MacKillop, J. et al. Hazardous drinking and alcohol use disorders. Nat. Rev. Dis. Primers 8, 80 (2022).
Pickard, H. Is addiction a brain disease? A plea for agnosticism and heterogeneity. Psychopharmacology 239, 993–1007 (2022).
FDA–NIH Biomarker Working Group BEST (Biomarkers, EndpointS, and other Tools) Resource (FDA and NIH, 2016).
Brook, R. D., Weder, A. B. & Rajagopalan, S. ‘Environmental hypertensionology’ the effects of environmental factors on blood pressure in clinical practice and research. J. Clin. Hypertens. 13, 836–842 (2011).
Kreatsoulas, C. & Anand, S. S. The impact of social determinants on cardiovascular disease. Can. J. Cardiol. 26, 8C–13C (2010).
Verdejo-Garcia, A. et al. A roadmap for integrating neuroscience into addiction treatment: a consensus of the Neuroscience Interest Group of the International Society of Addiction Medicine. Front. Psychiatry 10, 877 (2019).
Volkow, N. D., Wang, G. J., Fowler, J. S., Tomasi, D. & Baler, R. in Imaging of the Human Brain in Health and Disease (eds Seeman, P. & Madras, B.) 1–26 (Elsevier, 2014).
Strang, J. & Gurling, H. Computerized tomography and neuropsychological assessment in long-term high-dose heroin addicts. Br. J. Addict. 84, 1011–1019 (1989).
Whitwell, J. L. Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J. Neurosci. 29, 9661–9664 (2009).
Pando-Naude, V. et al. Gray and white matter morphology in substance use disorders: a neuroimaging systematic review and meta-analysis. Transl. Psychiatry 11, 29 (2021).
Sutherland, M. T. et al. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav. Brain Funct. 12, 16 (2016).
Hill-Bowen, L. D. et al. Convergent gray matter alterations across drugs of abuse and network-level implications: a meta-analysis of structural MRI studies. Drug Alcohol Depend. 240, 109625 (2022).
Sutherland, M. T. et al. Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies. Biol. Psychiatry 78, 711–720 (2015).
Zhang, M. et al. Shared gray matter alterations in subtypes of addiction: a voxel-wise meta-analysis. Psychopharmacology 238, 2365–2379 (2021).
Spindler, C. et al. Meta-analysis of grey matter changes and their behavioral characterization in patients with alcohol use disorder. Sci. Rep. 11, 5238 (2021).
Wollman, S. C. et al. Gray matter abnormalities in opioid-dependent patients: a neuroimaging meta-analysis. Am. J. Drug Alcohol Abuse 43, 505–517 (2017).
Mackey, S. et al. Mega-analysis of gray matter volume in substance dependence: general and substance-specific regional effects. Am. J. Psychiatry 176, 119–128 (2019).
Beard, C. L. et al. Regional differences in white matter integrity in stimulant use disorders: a meta-analysis of diffusion tensor imaging studies. Drug Alcohol Depend. 201, 29–37 (2019).
Monnig, M. A., Tonigan, J. S., Yeo, R. A., Thoma, R. J. & McCrady, B. S. White matter volume in alcohol use disorders: a meta-analysis. Addict. Biol. 18, 581–592 (2013).
Wollman, S. C. et al. White matter abnormalities in long-term heroin users: a preliminary neuroimaging meta-analysis. Am. J. Drug Alcohol Abuse 41, 133–138 (2015).
Suckling, J. & Nestor, L. J. The neurobiology of addiction: the perspective from magnetic resonance imaging present and future. Addiction 112, 360–369 (2017).
Duyn, J. in Slow Brain Oscillations of Sleep, Resting State and Vigilance (eds Van Someren, E. J. W. et al.) 295–305 (Progress in Brain Research, 2011).
Morgenstern, J., Naqvi, N. H., Debellis, R. & Breiter, H. C. The contributions of cognitive neuroscience and neuroimaging to understanding mechanisms of behavior change in addiction. Psychol. Addict. Behav. 27, 336–350 (2013).
Pariyadath, V., Gowin, J. L. & Stein, E. A. in Neuroscience for Addiction Medicine: From Prevention to Rehabilitation—Methods and Interventions (eds Ekhtiari, H. & Paulus, M. P.) 155–173 (2016); https://www.sciencedirect.com/science/article/pii/S0079612315001211
Ekhtiari, H. et al. A methodological checklist for fMRI drug cue reactivity studies: development and expert consensus. Nat. Protoc. 17, 567–595 (2022).
Borogovac, A. & Asllani, I. Arterial spin labeling (ASL) fMRI: advantages, theoretical constrains and experimental challenges in neurosciences. Int. J. Biomed. Imaging 2012, e818456 (2012).
Gu, X. et al. Prefrontal fNIRS-based clinical data analysis of brain functions in individuals abusing different types of drugs. J. Biomed. Semantics 12, 21 (2021).
Huettel, S. A., Song, A. W. & McCarthy, G. Functional Magnetic Resonance Imaging 3rd edn (Sinauer Associates, 2014).
Lu, H., Hua, J. & van Zijl, P. C. M. Noninvasive functional imaging of cerebral blood volume with vascular-space-occupancy (VASO) MRI. NMR Biomed. 26, 932–948 (2013).
Luijten, M., Schellekens, A. F., Kühn, S., Machielse, M. W. J. & Sescousse, G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry 74, 387–398 (2017).
Hill-Bowen, L. D. et al. The cue-reactivity paradigm: an ensemble of networks driving attention and cognition when viewing drug-related and natural-reward stimuli. Neurosci. Biobehav. Rev. 130, 201–213 (2021).
Wilcox, C. E., Abbott, C. C. & Calhoun, V. D. Alterations in resting-state functional connectivity in substance use disorders and treatment implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 91, 79–93 (2019).
Yang, L. Z. et al. Electrical stimulation reduces smokers’ craving by modulating the coupling between dorsal lateral prefrontal cortex and parahippocampal gyrus. Soc. Cogn. Affect. Neurosci. 12, 1296–1302 (2017).
Lopes da Silva, F. EEG and MEG: relevance to neuroscience. Neuron 80, 1112–1128 (2013).
Singh, S. P. Magnetoencephalography: basic principles. Ann. Indian Acad. Neurol. 17, S107–S112 (2014).
Houston, R. J. & Schlienz, N. J. Event-related potentials as biomarkers of behavior change mechanisms in substance use disorder treatment. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 30–40 (2018).
Parvaz, M. A., Moeller, S. J. & Goldstein, R. Z. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 73, 1127–1134 (2016).
Newson, J. J. & Thiagarajan, T. C. EEG frequency bands in psychiatric disorders: a review of resting state studies. Front. Hum. Neurosci. 12, 521 (2019).
Hu, B. et al. Effective brain network analysis with resting-state EEG data: a comparison between heroin abstinent and non-addicted subjects. J. Neural Eng. 14, 046002 (2017).
Naim-Feil, J. et al. Anomalies in global network connectivity associated with early recovery from alcohol dependence: a network transcranial magnetic stimulation and electroencephalography study. Addict. Biol. 27, e13146 (2022).
Ceccarini, J., Van Laere, K. & Koole, M. in PET and SPECT in Psychiatry (eds Dierckx, R. A. J. O. et al.) 17–44 (Springer, 2021).
Hellem, T., Shi, X., Latendresse, G. & Renshaw, P. F. The utility of magnetic resonance spectroscopy for understanding substance use disorders: a systematic review of the literature. J. Am. Psychiatr. Nurses Assoc. 21, 244–275 (2015).
Volkow, N. D., Koob, G. & Baler, R. Biomarkers in substance use disorders. ACS Chem. Neurosci. 6, 522–525 (2015).
Ashok, A. H., Mizuno, Y., Volkow, N. D. & Howes, O. D. Association of stimulants with dopaminergic alterations in users of cocaine, amphetamine, and methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 74, 511–519 (2017).
Proebstl, L. et al. Effects of stimulant drug use on the dopaminergic system: a systematic review and meta-analysis of in vivo neuroimaging studies. Eur. Psychiatry 59, 15–24 (2019).
Volkow, N. D. et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 24, 6583–6588 (2006).
Darcq, E. & Kieffer, B. L. Opioid receptors: drivers to addiction? Nat. Rev. Neurosci. 19, 499–514 (2018).
Jones, J. A., Russell, B. & Dalley, J. W. in PET and SPECT in Psychiatry (eds Dierckx, R. A. J. O. et al.) 713–739 (Springer, 2021).
Trick, L. et al. in PET and SPECT in Psychiatry (eds Dierckx, R. A. J. O. et al.) 653–712 (Springer, 2021).
Ae, K. et al. Brain metabolite alterations related to alcohol use: a meta-analysis of proton magnetic resonance spectroscopy studies. Mol. Psychiatry 27, 3223–3236 (2022).
Smucny, J. & Maddock, R. J. Spectroscopic meta-analyses reveal novel metabolite profiles across methamphetamine and cocaine substance use disorder. Drug Alcohol Depend. 248, 109900 (2023).
Chen, T., Tan, H., Lei, H., Su, H. & Zhao, M. Proton magnetic resonance spectroscopy in substance use disorder: recent advances and future clinical applications. Sci. China Inf. Sci. 63, 170101 (2020).
Chen, T. et al. Nature of glutamate alterations in substance dependence: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Psychiatry Res. Neuroimaging 315, 111329 (2021).
Califf, R. M. Biomarker definitions and their applications. Exp. Biol. Med. 243, 213–221 (2018).
Gromova, M., Vaggelas, A., Dallmann, G. & Seimetz, D. Biomarkers: opportunities and challenges for drug development in the current regulatory landscape. Biomark. Insights https://doi.org/10.1177/1177271920974652 (2020).
MacNiven, K. H. et al. Association of neural responses to drug cues with subsequent relapse to stimulant use. JAMA Netw. Open 1, e186466 (2018).
Bach, P. et al. FMRI-based prediction of naltrexone response in alcohol use disorder: a replication study. Eur. Arch. Psychiatry Clin. Neurosci. 271, 915–927 (2021).
Vollstädt-Klein, S. et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol. Psychiatry 69, 1060–1066 (2011).
Venkatasubramanian, G. & Keshavan, M. S. Biomarkers in psychiatry—a critique. Ann. Neurosci. 23, 3–5 (2016).
Smith, D. G. & Ersche, K. D. Using a drug-word Stroop task to differentiate recreational from dependent drug use. CNS Spectr. 19, 247–255 (2014).
Vollstädt-Klein, S. et al. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105, 1741–1749 (2010).
Chye, Y. et al. Orbitofrontal and caudate volumes in cannabis users: a multi-site mega-analysis comparing dependent versus non-dependent users. Psychopharmacology 234, 1985–1995 (2017).
McLellan, A. T., Koob, G. F. & Volkow, N. D. Preaddiction—a missing concept for treating substance use disorders. JAMA Psychiatry 79, 749–751 (2022).
Burnette, E. M., Grodin, E. N., Schacht, J. P. & Ray, L. A. Clinical and neural correlates of reward and relief drinking. Alcohol Clin. Exp. Res. 45, 194–203 (2021).
Gray, K. M. & Squeglia, L. M. Research review: what have we learned about adolescent substance use? J. Child Psychol. Psychiatry 59, 618–627 (2018).
Heitzeg, M. M., Cope, L. M., Martz, M. E. & Hardee, J. E. Neuroimaging risk markers for substance abuse: recent findings on inhibitory control and reward system functioning. Curr. Addict. Rep. 2, 91–103 (2015).
Tervo-Clemmens, B., Quach, A., Calabro, F. J., Foran, W. & Luna, B. Meta-analysis and review of functional neuroimaging differences underlying adolescent vulnerability to substance use. Neuroimage 209, 116476 (2020).
Moeller, S. J., Bederson, L., Alia-Klein, N. & Goldstein, R. Z. Neuroscience of inhibition for addiction medicine: from prediction of initiation to prediction of relapse. Prog. Brain Res. 223, 165–188 (2016).
Squeglia, L. M. & Cservenka, A. Adolescence and drug use vulnerability: findings from neuroimaging. Curr. Opin. Behav. Sci. 13, 164–170 (2017).
Camchong, J. et al. Changes in resting functional connectivity during abstinence in stimulant use disorder: a preliminary comparison of relapsers and abstainers. Drug Alcohol Depend. 139, 145–151 (2014).
Parvaz, M. A. et al. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict. Biol. 22, 1391–1401 (2017).
Wang, X. et al. Reversible brain white matter microstructure changes in heroin addicts: a longitudinal study. Addict. Biol. 18, 727–728 (2013).
Chou, Y. H. et al. Dopamine transporters and cognitive function in methamphetamine abuser after a short abstinence: a SPECT study. Eur. Neuropsychopharmacol. 17, 46–52 (2007).
Volkow, N. D. et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 21, 9414–9418 (2001).
Grabb, M. C., Hillefors, M. & Potter, W. Z. The NIMH ‘Fast-Fail Trials’ (FAST) initiative: rationale, promise, and progress. Pharmaceut. Med. 34, 233–245 (2020).
Young, K. A. et al. Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. J. Neurosci. 34, 5038–5043 (2014).
Beck, A. et al. Effects of high-dose baclofen on cue reactivity in alcohol dependence: a randomized, placebo-controlled pharmaco-fMRI study. Eur. Neuropsychopharmacol. 28, 1206–1216 (2018).
Wiers, C. E. et al. Effects of depressive symptoms and peripheral DAT methylation on neural reactivity to alcohol cues in alcoholism. Transl. Psychiatry 5, e648 (2015).
Medeiros, F. A. Biomarkers and surrogate endpoints: lessons learned from glaucoma. Invest. Ophthalmol. Vis. Sci. 58, BIO20–BIO26 (2017).
Schacht, J. P. et al. Predictors of naltrexone response in a randomized trial: reward-related brain activation, OPRM1 genotype, and smoking status. Neuropsychopharmacology 42, 2640–2653 (2017).
Nichols, T. T. et al. Cue-reactivity in experienced electronic cigarette users: novel stimulus videos and a pilot fMRI study. Brain Res. Bull. 123, 23–32 (2016).
Kroemer, N. B. et al. Sweet taste potentiates the reinforcing effects of e-cigarettes. Eur. Neuropsychopharmacol. 28, 1089–1102 (2018).
Coussens, N. P. et al. The opioid crisis and the future of addiction and pain therapeutics. J. Pharmacol. Exp. Ther. 371, 396–408 (2019).
Bach, P. et al. The effects of single nucleotide polymorphisms in glutamatergic neurotransmission genes on neural response to alcohol cues and craving. Addict. Biol. 20, 1022–1032 (2015).
Wang, W. et al. Cue-elicited craving, thalamic activity, and physiological arousal in adult non-dependent drinkers. J. Psychiatric Res. 116, 74–82 (2019).
Gouzoulis-Mayfrank, E. et al. Methamphetamine-related disorders. Dtsch. Ärztebl. Int. 114, 455 (2017).
Weigand, A. et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatry, 84, 28–37 (2018).
Siddiqi, S. H. et al. Repetitive transcranial magnetic stimulation with resting-state network targeting for treatment-resistant depression in traumatic brain injury: a randomized, controlled, double-blinded pilot study. J. Neurotrauma, 1361–1374 (2019).
Soleimani, G., Kupliki, R., Bodurka, J., Paulus, M. P. & Ekhtiari, H. How structural and functional MRI can inform dual-site tACS parameters: a case study in a clinical population and its pragmatic implications. Brain Stimul. 15, 337–351 (2022).
Joutsa, J. et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat. Med. 28, 1249–1255 (2022).
Ekhtiari, H. et al. A checklist for assessing the methodological quality of concurrent tES-fMRI studies (ContES checklist): a consensus study and statement. Nat. Protoc. 17, 596–617 (2022).
Esmaeilpour, Z. et al. Methodology for tDCS integration with fMRI. Hum. Brain Mapp. 41, 1950–1967 (2020).
Neuling, T. et al. Friends, not foes: magnetoencephalography as a tool to uncover brain dynamics during transcranial alternating current stimulation. Neuroimage 118, 406–413 (2015).
Parks, N. A. Concurrent application of TMS and near-infrared optical imaging: methodological considerations and potential artifacts. Front. Hum. Neurosci. 7, 592 (2013).
Siebner, H. R. et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2, 58–80 (2009).
Habelt, B., Arvaneh, M., Bernhardt, N. & Minev, I. Biomarkers and neuromodulation techniques in substance use disorders. Bioelectron. Med. 6, 4 (2020).
Karch, S. et al. Modulation of craving related brain responses using real-time fMRI in patients with alcohol use disorder. PLoS ONE 10, e0133034 (2015).
Karch, S. et al. Real-time fMRI neurofeedback in patients with tobacco use disorder during smoking cessation: functional differences and implications of the first training session in regard to future abstinence or relapse. Front. Hum. Neurosci. 13, 65 (2019).
Carroll, K. M. The profound heterogeneity of substance use disorders: implications for treatment development. Curr. Dir. Psychol. Sci. 30, 358–364 (2021).
Bach, P. et al. Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict. Biol. 25, e12717 (2020).
Regier, P. S. et al. Sustained brain response to repeated drug cues is associated with poor drug‐use outcomes. Addict. Biol. 26, e13028 (2021).
Steele, V. R. et al. Machine learning of functional magnetic resonance imaging network connectivity predicts substance abuse treatment completion. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 141–149 (2018).
Yan, C. et al. Treatment response prediction and individualized identification of short-term abstinence methamphetamine dependence using brain graph metrics. Front. Psychiatry 12, 583950 (2021).
Tisdall, L., MacNiven, K. H., Padula, C. B., Leong, J. K. & Knutson, B. Brain tract structure predicts relapse to stimulant drug use. Proc. Natl Acad. Sci. USA 119, e2116703119 (2022).
Garrison, K. A. & Potenza, M. N. Neuroimaging and biomarkers in addiction treatment. Curr. Psychiatry Rep. 16, 513 (2014).
Koban, L., Wager, T. D. & Kober, H. A neuromarker for drug and food craving distinguishes drug users from non-users. Nat. Neurosci. 26, 316–325 (2023).
Deacon, B. J. & McKay, D. The biomedical model of psychological problems: a call for critical dialogue. Behav. Ther. 38, 231–235 (2015).
Addiction Cue-Reactivity Initiative (ACRI) Network: Parameter space and potential for biomarker development in 25 years of fMRI drug cue reactivity: a systematic review. JAMA Psychiatry https://doi.org/10.1001/jamapsychiatry.2023.5483 (2024).
Bakker, E. et al. Biomarker qualification at the European Medicines Agency: a review of biomarker qualification procedures from 2008 to 2020. Clin. Pharmacol. Ther. 112, 69–80 (2022).
Kraus, V. B. Biomarkers as drug development tools: discovery, validation, qualification and use. Nat. Rev. Rheumatol. 14, 354–362 (2018).
Luking, K. R., Nelson, B. D., Infantolino, Z. P., Sauder, C. L. & Hajcak, G. Internal consistency of functional magnetic resonance imaging and electroencephalography measures of reward in late childhood and early adolescence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 289–297 (2017).
Elliott, M. L. et al. What is the test–retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychol. Sci. 31, 792–806 (2020).
Bach, P. et al. Test–retest reliability of neural alcohol cue-reactivity: is there light at the end of the magnetic resonance imaging tube? Addict. Biol. 27, e13069 (2022).
Amur, S., LaVange, L., Zineh, I., Buckman-Garner, S. & Woodcock, J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin. Pharmacol. Ther. 98, 34–46 (2015).
Concannon, T. W. et al. Practical guidance for involving stakeholders in health research. J. Gen. Intern. Med. 34, 458–463 (2019).
Harrison, J. D. et al. Patient stakeholder engagement in research: a narrative review to describe foundational principles and best practice activities. Health Expect. 22, 307–316 (2019).
Henderson, J., Sword, W., Niccols, A., Dobbins, M. & The Connections Research Team. Implementing stakeholder-informed research in the substance abuse treatment sector: strategies used by Connections, a Canadian knowledge translation and exchange project. Subst. Abuse Treat. Prev. Policy 9, 21 (2014).
Höller, Y. et al. Reliability of EEG measures of interaction: a paradigm shift is needed to fight the reproducibility crisis. Front. Hum. Neurosci. 11, 441 (2017).
Zuo, X. N., Biswal, B. B. & Poldrack, R. A. Editorial: reliability and reproducibility in functional connectomics. Front. Neurosci. 13, 117 (2019).
Hedges, E. P. et al. Reliability of structural MRI measurements: the effects of scan session, head tilt, inter-scan interval, acquisition sequence, FreeSurfer version and processing stream. Neuroimage 246, 118751 (2022).
Marek, S. et al. Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660 (2022).
Mackey, S. et al. in Neuroscience for Addiction Medicine: From Prevention to Rehabilitation—Methods and Interventions (eds Ekhtiari, H. & Paulus, M. P.) 203–223 (Elsevier, 2016).
Markiewicz, C. J. et al. The OpenNeuro resource for sharing of neuroscience data. eLife 10, e71774 (2021).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Garavan, H. et al. Recruiting the ABCD sample: design considerations and procedures. Dev. Cogn. Neurosci. 32, 16–22 (2018).
Van Essen, D. C. et al. The Human Connectome Project: a data acquisition perspective. Neuroimage 62, 2222–2231 (2012).
Botvinik-Nezer, R. et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88 (2020).
Schilling, K. G. et al. Fiber tractography bundle segmentation depends on scanner effects, vendor effects, acquisition resolution, diffusion sampling scheme, diffusion sensitization, and bundle segmentation workflow. Neuroimage 242, 118451 (2021).
Veronese, M. et al. Reproducibility of findings in modern PET neuroimaging: insight from the NRM2018 grand challenge. J. Cereb. Blood Flow Metab. 41, 2778–2796 (2021).
Poldrack, R. A. et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126 (2017).
Nichols, T. E. et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci. 20, 299–303 (2017).
Pernet, C. et al. Issues and recommendations from the OHBM COBIDAS MEEG committee for reproducible EEG and MEG research. Nat. Neurosci. 23, 1473–1483 (2020).
Neuner, I. et al. 7T ultra-high-field neuroimaging for mental health: an emerging tool for precision psychiatry? Transl. Psychiatry 12, 36 (2022).
Stanley, J. A. & Raz, N. Functional magnetic resonance spectroscopy: the ‘New’ MRS for cognitive neuroscience and psychiatry research. Front. Psychiatry 9, 76 (2018).
Hou, L. et al. Positron emission tomography imaging of the endocannabinoid system: opportunities and challenges in radiotracer development. J. Med. Chem. 64, 123–149 (2021).
Gordon, E. M. et al. Precision functional mapping of individual human brains. Neuron 95, 791–807.e7 (2017).
Demuru, M. & Fraschini, M. EEG fingerprinting: subject-specific signature based on the aperiodic component of power spectrum. Comput. Biol. Med. 120, 103748 (2020).
Ozdemir, R. A. et al. Cortical responses to noninvasive perturbations enable individual brain fingerprinting. Brain Stimul. 14, 391–403 (2021).
Fu, C. H. Y. & Costafreda, S. G. Neuroimaging-based biomarkers in psychiatry: clinical opportunities of a paradigm shift. Can. J. Psychiatry 58, 499–508 (2013).
McKenna, M. C., Murad, A., Huynh, W., Lope, J. & Bede, P. The changing landscape of neuroimaging in frontotemporal lobar degeneration: from group-level observations to single-subject data interpretation. Expert Rev. Neurother. 22, 179–207 (2022).
Hunter, M. A. et al. Baseline effects of transcranial direct current stimulation on glutamatergic neurotransmission and large-scale network connectivity. Brain Res. 1594, 92–107 (2015).
Giovannella, M. et al. Concurrent measurement of cerebral hemodynamics and electroencephalography during transcranial direct current stimulation. Neurophotonics 5, 015001–015001 (2018).
Lioi, G. et al. Simultaneous EEG-fMRI during a neurofeedback task, a brain imaging dataset for multimodal data integration. Sci. Data 7, 173 (2020).
Dipasquale, O. et al. Receptor-enriched analysis of functional connectivity by targets (REACT): a novel, multimodal analytical approach informed by PET to study the pharmacodynamic response of the brain under MDMA. Neuroimage 195, 252–260 (2019).
Li, X., Guo, N. & Li, Q. Functional neuroimaging in the new era of big data. Genomics Proteomics Bioinformatics 17, 393–401 (2019).
Poldrack, R. A., Gorgolewski, K. J. & Varoquaux, G. Computational and informatic advances for reproducible data analysis in neuroimaging. Annu. Rev. Biomed. Data Sci. 2, 119–138 (2019).
Carvalho, A. F. et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl. Psychiatry 10, 152 (2020).
Klugah-Brown, B. et al. Common abnormality of gray matter integrity in substance use disorder and obsessive–compulsive disorder: a comparative voxel-based meta-analysis. Hum. Brain Mapp. 42, 3871–3886 (2021).
Noori, H. R., Cosa Linan, A. & Spanagel, R. Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: a comprehensive meta-analysis. Eur. Neuropsychopharmacol. 26, 1419–1430 (2016).
Berridge, K. C. & Robinson, T. E. Liking, wanting and the incentive-sensitization theory of addiction. Am. Psychol. 71, 670–679 (2016).
Hogarth, L. Addiction is driven by excessive goal-directed drug choice under negative affect: translational critique of habit and compulsion theory. Neuropsychopharmacology 45, 720–735 (2020).
Littel, M., Euser, A. S., Munafò, M. R. & Franken, I. H. A. Electrophysiological indices of biased cognitive processing of substance-related cues: a meta-analysis. Neurosci. Biobehav. Rev. 36, 1803–1816 (2012).
Zhang, Y., Ou, H., Yuan, T. F. & Sun, J. Electrophysiological indexes for impaired response inhibition and salience attribution in substance (stimulants and depressants) use disorders: a meta-analysis. Int. J. Psychophysiol. 170, 133–155 (2021).
Zeng, J. et al. Neurobiological correlates of cue-reactivity in alcohol-use disorders: a voxel-wise meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 128, 294–310 (2021).
Devoto, F., Zapparoli, L., Spinelli, G., Scotti, G. & Paulesu, E. How the harm of drugs and their availability affect brain reactions to drug cues: a meta-analysis of 64 neuroimaging activation studies. Transl. Psychiatry 10, 429 (2020).
Everitt, B. J. & Robbins, T. W. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 37, 1946–1954 (2013).
Koob, G. F. & Le, MoalM. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129 (2001).
Park, S. Q. et al. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J. Neurosci. 30, 7749–7753 (2010).
Qiu, Z. & Wang, J. A voxel-wise meta-analysis of task-based functional MRI studies on impaired gain and loss processing in adults with addiction. J. Psychiatry Neurosci. 46, E128–E146 (2021).
Euser, A. S. et al. The P300 event-related brain potential as a neurobiological endophenotype for substance use disorders: a meta-analytic investigation. Neurosci. Biobehav. Rev. 36, 572–603 (2012).
Lim, T. V. & Ersche, K. D. Theory-driven computational models of drug addiction in humans: fruitful or futile? Addict. Neurosci. 5, 100066 (2023).
Fairbairn, C. E., Kang, D. & Federmeier, K. D. Alcohol and neural dynamics: a meta-analysis of acute alcohol effects on event-related brain potentials. Biol. Psychiatry 89, 990–1000 (2021).
Cao, Y. et al. The brain activity pattern in alcohol-use disorders under inhibition response Task. J. Psychiatr. Res. 163, 127–134 (2023).
Le, T. M., Potvin, S., Zhornitsky, S. & Li, C. S. R. Distinct patterns of prefrontal cortical disengagement during inhibitory control in addiction: a meta-analysis based on population characteristics. Neurosci. Biobehav. Rev. 127, 255–269 (2021).
Qiu, Z. & Wang, J. Altered neural activities during response inhibition in adults with addiction: a voxel-wise meta-analysis. Psychol. Med. 51, 387–399 (2021).
McClure, S. M. & Bickel, W. K. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Ann. N. Y. Acad. Sci. 1327, 62–78 (2014).
Everitt, B. J. et al. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil. Trans. R Soc. Lond. B 363, 3125–3135 (2008).
Rabin, R. A. et al. Common and gender-specific associations with cocaine use on gray matter volume: data from the ENIGMA addiction working group. Hum. Brain Mapp. 43, 543–554 (2022).
Litten, R. Z. et al. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin. Exp. Res. 39, 579–584 (2015).
Rezapour, T. et al. Neuroscience-informed classification of prevention interventions in substance use disorders: an RDoC-based approach. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2024.105578 (2024).
Mollick, J. A. & Kober, H. Computational models of drug use and addiction: a review. J. Abnorm. Psychol. 129, 544–555 (2020).
Stephan, K. E. et al. Computational neuroimaging strategies for single patient predictions. Neuroimage 145, 180–199 (2017).
Sangchooli, A., Zare-Bidoky, M. & Ekhtiari, H. Neuroimaging biomarkers of addiction: systematic review of the literature. Open Science Framework https://osf.io/79uc3 (2024).
Aggregate Analysis of ClinicalTrials.gov (AACT) Database (CTTI) https://aact.ctti-clinicaltrials.org
R Core Team. R: a language and environment for statistical computing, R Version 4.0.5 (R Foundation for Statistical Computing, 2013).
Wickham, H., François, R., Henry, L., Müller, K. & Vaughan, D. dplyr: a grammar of data manipulation, R package version 1.1.4 (2023).
Wickham H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Spindler, C., Mallien, L., Trautmann, S., Alexander, N. & Muehlhan, M. A coordinate-based meta-analysis of white matter alterations in patients with alcohol use disorder. Transl. Psychiatry 12, 40 (2022).
Suchting, R. et al. A meta-analysis of tract-based spatial statistics studies examining white matter integrity in cocaine use disorder. Addict. Biol. 26, e12902 (2021).
Xiao, P. et al. Regional gray matter deficits in alcohol dependence: a meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend. 153, 22–28 (2015).
Yan, H. et al. Functional and structural brain abnormalities in substance use disorder: a multimodal meta-analysis of neuroimaging studies. Acta Psychiatr. Scand. 147, 345–359 (2023).
Pan, P. et al. Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol. Sci. 34, 813–817 (2013).
Long, Y. et al. Distinct brain structural abnormalities in attention-deficit/hyperactivity disorder and substance use disorders: a comparative meta-analysis. Transl. Psychiatry 12, 368 (2022).
Hall, M. G. et al. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am. J. Drug Alcohol Abuse 41, 290–299 (2015).
Rocchetti, M. et al. Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in non-psychotic users. Psychiatry Clin. Neurosci. 67, 483–492 (2013).
Li, L. et al. Lower regional grey matter in alcohol use disorders: evidence from a voxel-based meta-analysis. BMC Psychiatry 21, 247 (2021).
Hahn, S. et al. Predicting alcohol dependence from multi-site brain structural measures. Hum. Brain Mapp. 43, 555–565 (2022).
Zhong, J. et al. Voxelwise meta-analysis of gray matter anomalies in chronic cigarette smokers. Behav. Brain Res. 311, 39–45 (2016).
Liu, Y., Masina, F., Ridderinkhof, K. R. & Pezzetta, R. Addiction as a brain disease? A meta-regression comparison of error-related brain potentials between addiction and neurological diseases. Neurosci. Biobehav. Rev. 148, 105127 (2023).
Hamidovic, A. & Wang, Y. The P300 in alcohol use disorder: a meta-analysis and meta-regression. Prog. Neuropsychopharmacol. Biol. Psychiatry 95, 109716 (2019).
Pollard, A. A. et al. Functional neuroanatomy of craving in heroin use disorder: voxel-based meta-analysis of functional magnetic resonance imaging (fMRI) drug cue reactivity studies. Am. J. Drug Alcohol Abuse 49, 418–430 (2023).
Schacht, J. P., Anton, R. F. & Myrick, H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict. Biol. 18, 121–133 (2013).
Kühn, S. & Gallinat, J. Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci. 33, 1318–1326 (2011).
Lin, X. et al. Neural substrates of smoking and reward cue reactivity in smokers: a meta-analysis of fMRI studies. Transl. Psychiatry 10, 97 (2020).
Engelmann, J. M. et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage 60, 252–262 (2012).
Chase, H. W., Eickhoff, S. B., Laird, A. R. & Hogarth, L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol. Psychiatry 70, 785–793 (2011).
Hanlon, C. A., Dowdle, L. T., Naselaris, T., Canterberry, M. & Cortese, B. M. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend. 143, 206–212 (2014).
Zeng, J. et al. A meta-analysis of the neural substrates of monetary reward anticipation and outcome in alcohol use disorder. Hum. Brain Mapp. 44, 2841–2861 (2023).
Zeng, J., You, L., Sheng, H., Luo, Y. & Yang, X. The differential neural substrates for reward choice under gain–loss contexts and risk in alcohol use disorder: evidence from a voxel-based meta-analysis. Drug Alcohol Depend. 248, 109912 (2023).
Taebi, A. et al. Shared network-level functional alterations across substance use disorders: a multi-level kernel density meta-analysis of resting-state functional connectivity studies. Addict. Biol. 27, e13200 (2022).
Dugré, J. R., Orban, P. & Potvin, S. Disrupted functional connectivity of the brain reward system in substance use problems: a meta-analysis of functional neuroimaging studies. Addict. Biol. 28, e13257 (2023).
Klugah-Brown, B. et al. Common and separable neural alterations in substance use disorders: a coordinate-based meta-analyses of functional neuroimaging studies in humans. Hum. Brain Mapp. 41, 4459–4477 (2020).
Dager, A. D. et al. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction 109, 585–595 (2014).
Goudriaan, A. E., Veltman, D. J., van den Brink, W., Dom, G. & Schmaal, L. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict. Behav. 38, 1509–1517 (2013).
Schacht, J. P. et al. Stability of fMRI striatal response to alcohol cues: a hierarchical linear modeling approach. Neuroimage 56, 61–68 (2011).
Egerton, A., Demjaha, A., McGuire, P., Mehta, M. A. & Howes, O. D. The test–retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. Neuroimage 50, 524–531 (2010).
Alakurtti, K. et al. Long-term test–retest reliability of striatal and extrastriatal dopamine D2/3 receptor binding: study with [11C]raclopride and high-resolution PET. J. Cereb. Blood Flow Metab. 35, 1199–1205 (2015).
Khan, A. R. et al. Biomarkers of Parkinson’s disease: striatal sub-regional structural morphometry and diffusion MRI. Neuroimage Clin. 21, 101597 (2019).
Albrecht, J. et al. potential impact of a 32-channel receiving head coil technology on the results of a functional MRI paradigm. Clin. Neuroradiol. 20, 223–229 (2010).
Colizoli, O., de Gee, J. W., van der Zwaag, W. & Donner, T. H. Functional magnetic resonance imaging responses during perceptual decision‐making at 3 and 7 T in human cortex, striatum, and brainstem. Hum. Brain Mapp. 43, 1265–1279 (2021).
Panman, J. L. et al. Bias introduced by multiple head coils in MRI research: an 8 channel and 32 channel coil comparison. Front. Neurosci. 13, 729 (2019).
Faria, D., Vale, J., Tavares, J. M. R. S., Oliveira, J. M. & Costa, D. Effect of reconstruction processing methods and analysis in the quantification of brain spect studies with DaTSCANTM. Phys. Med. 32, 311 (2016).
Zeng, H. et al. The action representation elicited by different types of drug-related cues in heroin-abstinent individuals. Front. Behav. Neurosci. 12, 123 (2018).
Hasler, B. P., Forbes, E. E. & Franzen, P. L. Time-of-day differences and short-term stability of the neural response to monetary reward: a pilot study. Psychiatry Res. Neuroimaging 224, 22–27 (2014).
Wetherill, R. R. et al. The impact of sex on brain responses to smoking cues: a perfusion fMRI study. Biol. Sex Differ. 4, 9 (2013).
Acknowledgments
The authors thank G. Soleimani, M. Farhadi and M. Ebrahimi for their contribution to designing some of the figures. H.E. is supported by funds from the Laureate Institute for Brain Research and Medical Discovery Team on Addiction and Brain Behavior Foundation (NARSAD Young Investigator Award 27305). O.C. is funded by NIH grants AG07425801, AG077497, AG077000, AG067765, AG041200, AG062309, AG062200, and AG069476, William K. Warren Foundation and the National Institute of General Medical Sciences Center Grant award number 1P20GM121312, and the National Institute on Drug Abuse (U01DA050989). A.R.C. is funded by NIH/NIDA mechanisms UG1DA050209, R01DA039215, T32-DA-028874, P30 DA046345, and U01DA048517. T.R. was substantially involved in UG1DA050209 and U01DA048517 consistent with her role as scientific officer. She has no substantial involvement in the other cited grants. The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy, or position of the US Department of Health and Human Services or any of its affiliated institutions or agencies.
Author information
Authors and Affiliations
Contributions
K.B., O.C., A.R.C., H.E., F.G.M., P.O., M.O., M.P.P., D.A.P., T.R., and J.S. conceived of the presented idea and designed the study, and H.E. coordinated the consensus process among all authors. A.S., M.Z.-B., and H.E. gathered data, designed the tables, and performed the initial analytic calculations. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
O.C. has received grant funding from Eli Lilly and Nestle. He has provided paid consulting to Novo Nordisk. Over the past 3 years, D.A.P. has received consulting fees from Boehringer Ingelheim, Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (formerly BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Sage Therapeutics, Sama Therapeutics and Takeda; he has received honoraria from the American Psychological Association, Psychonomic Society and Springer (for editorial work) and from Alkermes; he has received research funding from the BIRD Foundation, Brain and Behavior Research Foundation, Dana Foundation, DARPA, Millennium Pharmaceuticals, NIMH and Wellcome Leap MCPsych; and he has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics and Neuroscience Software. M.P.P. is an adviser to Spring Care, a behavioral health startup; he has received royalties for an article about methamphetamine in UpToDate and he has a consulting agreement with, and receives compensation from, F. Hoffmann–La Roche. P.O. is an employee and shareholder of Sage Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Yavin Shaham and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Tables 1–3 and Methods.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekhtiari, H., Sangchooli, A., Carmichael, O. et al. Neuroimaging biomarkers of addiction. Nat. Mental Health 2, 1498–1517 (2024). https://doi.org/10.1038/s44220-024-00334-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-024-00334-x