Abstract
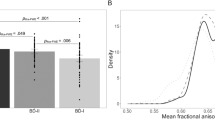
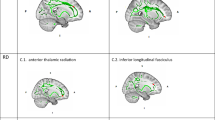
Childhood maltreatment has long-term effects on brain structure, inflammation and psychiatric symptoms, yet its impact on white matter (WM) integrity in bipolar disorder type II depression (BDII-D) remains understudied. Here, we investigate WM alterations in BDII-D and their associations with childhood maltreatment, inflammation and psychiatric symptoms. Using TractSeg, we analyzed WM integrity in 146 patients with BDII-D and 151 healthy controls, identifying significantly lower fractional anisotropy (FA) in the corpus callosum, left inferior longitudinal fasciculus and right striato-fronto-orbital tract, alongside higher FA in sensory-motor regions. WM alterations were correlated with inflammatory markers and psychiatric symptoms. Non-negative matrix factorization and clustering analysis revealed two BDII-D subgroups, with one subgroup showing lower corpus callosum FA, higher inflammatory markers, greater childhood emotional maltreatment and psychiatric symptoms. These findings suggest deficits in WM integrity in thalamo-subcortical-cortical and somatosensory areas in BDII-D. Childhood emotional maltreatment may contribute to long-term effects on inflammation and psychiatric symptoms in adulthood BDII-D.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Due to ethical concerns, patient confidentiality and our institutional restrictions, we are unable to publicly share the clinical and image data. However, we confirm that the data will be made available upon reasonable request to the corresponding authors, subject to appropriate ethical and institutional approvals.
Code availability
The code for white matter analysis used in this study was from TractSeg: https://github.com/MIC-DKFZ/TractSeg.
References
Benedetti, F. et al. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol. Psychiatry 69, 309–317 (2011).
Rosenblat, J. D. & McIntyre, R. S. Bipolar disorder and inflammation. Psychiatr. Clin. 39, 125–137 (2016).
Pereira, A. C. et al. Inflammation in bipolar disorder (BD): identification of new therapeutic targets. Pharmacol. Res. 163, 105325 (2021).
Kiecolt-Glaser, J. K. et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl Acad. Sci. USA 100, 9090–9095 (2003).
Saccaro, L., Schilliger, Z., Dayer, A., Perroud, N. & Piguet, C. Inflammation, anxiety, and stress in bipolar disorder and borderline personality disorder: a narrative review. Neurosci. Biobehav. Rev. 127, 184–192 (2021).
Poletti, S. et al. Long-term effect of childhood trauma: role of inflammation and white matter in mood disorders. Brain Behav. Immun. Health 26, 100529 (2022).
Dall’Aglio, L., Xu, B., Tiemeier, H. & Muetzel, R. L. Longitudinal associations between white matter microstructure and psychiatric symptoms in youth. J. Am. Acad. Child Adolesc. Psychiatry 62, 1326–1339 (2023).
Wasserthal, J., Neher, P. & Maier-Hein, K. H. TractSeg-Fast and accurate white matter tract segmentation. Neuroimage 183, 239–253 (2018).
Versace, A. et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol. Psychiatry 68, 560–567 (2010).
Manelis, A. et al. White matter abnormalities in adults with bipolar disorder type-II and unipolar depression. Sci. Rep. 11, 7541 (2021).
Wise, T. et al. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol. Psychiatry 79, 293–302 (2016).
Bracht, T. et al. Physical activity is associated with left corticospinal tract microstructure in bipolar depression. Neuroimage Clin. 20, 939–945 (2018).
Benedetti, F. et al. Inflammatory cytokines influence measures of white matter integrity in bipolar disorder. J. Affect. Disord. 202, 1–9 (2016).
Aronica, R., Enrico, P., Squarcina, L., Brambilla, P. & Delvecchio, G. Association between diffusion tensor imaging, inflammation and immunological alterations in unipolar and bipolar depression: a review. Neurosci. Biobehav. Rev. 143, 104922 (2022).
Zhang, W. et al. Neuroinflammation in the amygdala is associated with recent depressive symptoms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 8, 967–975 (2023).
Serpa, M. et al. Inflammatory cytokines and white matter microstructure in the acute phase of first-episode psychosis: a longitudinal study. Schizophr. Res. 257, 5–18 (2023).
Cao, Y. et al. Effects of inflammation, childhood adversity, and psychiatric symptoms on brain morphometrical phenotypes in bipolar II depression. Psychol. Med. 54, 775–784 (2024).
Cao, Y. et al. Brain-derived subgroups of bipolar II depression associate with inflammation and choroid plexus morphology. Psychiatry Clin. Neurosci. 77, 613–621 (2023).
Teicher, M. H. & Samson, J. A. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 57, 241–266 (2016).
Baumeister, D., Akhtar, R., Ciufolini, S., Pariante, C. M. & Mondelli, V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 21, 642–649 (2016).
Agnew-Blais, J. & Danese, A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry 3, 342–349 (2016).
Stevelink, R. et al. Childhood abuse and white matter integrity in bipolar disorder patients and healthy controls. Eur. Neuropsychopharmacol. 28, 807–817 (2018).
Congio, A. C., Rossaneis, A. C., Verri, W. A. Jr, Urbano, M. R. & Nunes, S. O. V. Childhood trauma, interleukin-17, C-reactive protein, metabolism, and psychosocial functioning in bipolar depression. J. Affect. Disord. Rep. 9, 100357 (2022).
Xiao, H. et al. Interleukin-1β moderates the relationships between middle frontal-mACC/insular connectivity and depressive symptoms in bipolar II depression. Brain Behav. Immun. 120, 44–53 (2024).
Leh, S. E., Ptito, A., Chakravarty, M. M. & Strafella, A. P. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci. Lett. 419, 113–118 (2007).
Perry, B. I. et al. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: a bi-directional two-sample mendelian randomization study. Brain Behav. Immun. 97, 176–185 (2021).
Sasaki, K. et al. Modulation of the neurotransmitter systems through the anti-inflammatory and antidepressant-like effects of squalene from Aurantiochytrium sp. PLoS ONE 14, e0218923 (2019).
Goldsmith, D., Rapaport, M. & Miller, B. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696–1709 (2016).
Lizano, P. et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am. J. Psychiatry 176, 564–572 (2019).
Klöppel, S. et al. Compensation in preclinical Huntington’s disease: evidence from the track-on HD study. EBioMedicine 2, 1420–1429 (2015).
Martino, M. et al. Abnormal functional relationship of sensorimotor network with neurotransmitter-related nuclei via subcortical-cortical loops in manic and depressive phases of bipolar disorder. Schizophr. Bull. 46, 163–174 (2020).
Judd, L. L. et al. Prevalence and clinical significance of subsyndromal manic symptoms, including irritability and psychomotor agitation, during bipolar major depressive episodes. J. Affect. Disord. 138, 440–448 (2012).
Wolf, R. C. & Sambataro, F. Neural compensation in Huntington’s disease: teaching mental disorders new tricks? EBioMedicine 2, 1288–1289 (2015).
Lizano, P. et al. Peripheral inflammatory subgroup differences in anterior Default Mode network and multiplex functional network topology are associated with cognition in psychosis. Brain Behav. Immun. 114, 3–15 (2023).
Quidé, Y., Tozzi, L., Corcoran, M., Cannon, D. M. & Dauvermann, M. R. The impact of childhood trauma on developing bipolar disorder: current understanding and ensuring continued progress. Neuropsychiatr. Dis. Treat. 16, 3095–3115 (2020).
Ascoli, B. M. et al. The role of macrophage polarization on bipolar disorder: identifying new therapeutic targets. Aust. N. Z. J. Psychiatry 50, 618–630 (2016).
Hett, D., Etain, B. & Marwaha, S. Childhood trauma in bipolar disorder: new targets for future interventions. BJPsych Open 8, e130 (2022).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (American Psychiatric Association, 2013).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56 (1960).
Hamilton, M. Hamilton anxiety rating scale. Br. J. Med. Psychol. 32, 50–55 (1959).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Posner, K. et al. Columbia-suicide severity rating scale (C-SSRS). New York, NY: Columbia University Medical Center 10, 2008 (2008).
Bernstein, D. P. et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Neg. 27, 169–190 (2003).
Jiang, W.-J. et al. Reliability and validity of the Chinese version of the Childhood Trauma Questionnaire-Short Form for inpatients with schizophrenia. PLoS ONE 13, e0208779 (2018).
Hassel, S. et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 10, 916–927 (2008).
Wasserthal, J., Neher, P. F. & Maier-Hein, K. H. Tract orientation mapping for bundle-specific tractography. In Medical Image Computing and Computer Assisted Intervention – MICCAI 2018: 21st International Conference, Granada, Spain, September 16-20, 2018, Proceedings, Part III 11 (eds Frangi, A. et al.) 36–44 (Springer, 2018).
Wasserthal, J., Neher, P. F., Hirjak, D. & Maier-Hein, K. H. Combined tract segmentation and orientation mapping for bundle-specific tractography. Med. Image Anal. 58, 101559 (2019).
Wasserthal, J. et al. Multiparametric mapping of white matter microstructure in catatonia. Neuropsychopharmacology 45, 1750–1757 (2020).
Han, S. et al. Parsing altered gray matter morphology of depression using a framework integrating the normative model and non-negative matrix factorization. Nat. Commun. 14, 4053 (2023).
Gaujoux, R. & Seoighe, C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics 11, 367 (2010).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant nos. 82471953 and 82271947 to Z.Y.J.), the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (grant no. 2020HXFH005 to Z.Y.J. and grant no. 2022HXFH029 to C.J.Q.), STI2030-Major Projects (grant no. 2021ZD0200600 to C.J.Q.), the Interdisciplinary Center of Clinical Research of the Medical Faculty Jena (L.C.) and the DZPG (German Center for Mental Health) (FKZ: grant no. 01EE2103 to M.W. and L.C.). We thank all participants for their kind contributions. P.L. was supported by a career development award from the National Institute of Mental Health (K23MH122701). The MRI machine in Supplementary Fig. 1 was drawn using FigDraw. We thank H. Xie, J. Mu and H. Sun for their help with the MRI scan; Y. Li, S. Liu and Y. Ma for their help in recruiting the BDII-D individuals; and S. Guo for laboratory testing of blood samples.
Author information
Authors and Affiliations
Contributions
Y.C. wrote the first draft of the paper; P.L., M.W. and L.C. edited the paper; Y.C., P. L. and M.W. designed the research; Y.C., M.L., N.J., X.Q.Z. and T.C. analyzed data; Y.C., H.S. and G.J.D. were involved in clinical data collection; Y.C. and X.P.L. participated in MRI scans; P.L., M.W., C.J.Q., Q.Y.G. and Z.Y.J. supervised the study. All authors edited the approved final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.W. is a member of the advisory boards and gave presentations for the following companies: Boehringer Ingelheim, Germany; Bayer AG, Germany; and Biologische Heilmittel Heel GmbH, Germany. M.W. has further conducted studies with institutional research support from HEEL and from Janssen Pharmaceutical Research for a clinical trial (IIT) on ketamine in patients with major depression unrelated to this investigation. M.W. has not received any financial compensation from the above-mentioned companies. The other authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Tania Silva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, results, Tables 1–6 and Figs. 1–4.
Supplementary Table 1
Partial correlation analysis between FA values that showed significant differences compared with HCs and inflammatory cytokines in BDII-D.
Supplementary Table 2
Partial correlation analysis between FA values that showed significant differences compared with HCs and indirect inflammatory indicators in BDII-D.
Supplementary Table 3
Partial correlation analysis between FA values that showed significant differences compared with HCs and psychiatric symptoms in BDII-D.
Supplementary Table 4
Partial correlation analysis between significant FA values that showed significant differences compared with HCs and clinical profiles in BDII-D.
Supplementary Table 5
Partial correlation analysis between FA values that showed significant differences compared with HCs and childhood maltreatment in BDII-D.
Supplementary Table 6
Partial correlation analysis between FA values that showed significant differences compared with HCs and childhood maltreatment in HCs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, Y., Lizano, P., Li, M. et al. Associations among bipolar II depression white matter subgroups, inflammation, symptoms and childhood maltreatment. Nat. Mental Health 3, 724–734 (2025). https://doi.org/10.1038/s44220-025-00432-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00432-4
This article is cited by
-
Social Determinants of Health, the developing brain, and risk and resilience for psychopathology
Neuropsychopharmacology (2026)