Abstract
Predictive processing has revolutionized cognitive neuroscience, offering a comprehensive computational framework for understanding normative behavior and psychiatric illness. This narrative Review evaluates the role of predictive processing in understanding psychosis, revisiting the seminal work of Sterzer and colleagues. It consolidates recent experimental evidence on the alteration of priors and sensory likelihoods across different stages of psychosis in an attempt to reconcile top-down (that is, overly precise priors/noisy sensations) and bottom-up (that is, noisy priors/overly precise sensations) accounts. It evaluates predictive processing alterations across the continuum of psychosis, from non-clinical psychotic experiences to high-risk and first-episode psychosis to schizophrenia, exploring the explanatory potential of predictive processing as a transdiagnostic framework. We discuss the translational potential of predictive processing, including its use as a biomarker and in therapeutic interventions. We emphasize the need for standardized paradigms and longitudinal studies to advance predictive processing theories in clinical practice. By offering a unified theoretical perspective, this Review aims to inspire further research into the neuro-computational mechanisms underlying psychosis and enhance our understanding of psychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Velligan, D. I. & Rao, S. The epidemiology and global burden of schizophrenia. J. Clin. Psychiatry 84, MS21078COM5 (2023).
Millan, M. J. et al. Altering the course of schizophrenia: progress and perspectives. Nat. Rev. Drug Discov. 15, 485–515 (2016).
Charlson, F. J. et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr. Bull. 44, 1195 (2018).
McCutcheon, R. A., Reis Marques, T. & Howes, O. D. Schizophrenia—an overview. JAMA Psychiatry 77, 201–210 (2020).
Friston, K. A theory of cortical responses. Philos. Trans. R. Soc. B 360, 815–836 (2005).
Rao, R. P. N. & Ballard, D. H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87 (1999).
Corlett, P. R. et al. Hallucinations and strong priors. Trends Cogn. Sci. 23, 114–127 (2019).
Fletcher, P. C. & Frith, C. D. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat. Rev. Neurosci. 10, 48–58 (2009).
Powers, A. et al. A computational account of the development and evolution of psychotic symptoms. Biol. Psychiatry 97, 117–127 (2024).
Sterzer, P. et al. The predictive coding account of psychosis. Biol. Psychiatry 84, 634–643 (2018).
Adams, R., Stephan, K., Brown, H., Frith, C. & Friston, K. The computational anatomy of psychosis. Front. Psychiatry 4, 47 (2013).
Harding, J. N. et al. A new predictive coding model for a more comprehensive account of delusions. Lancet Psychiatry 11, 295–302 (2024).
Marr, D. Vision: A Computational Investigation into the Human Representation and Processing of Visual Information (MIT Press, 1982).
Schmack, K. et al. Delusions and the role of beliefs in perceptual inference. J. Neurosci. 33, 13701–13712 (2013).
Schmack, K., Schnack, A., Priller, J. & Sterzer, P. Perceptual instability in schizophrenia: probing predictive coding accounts of delusions with ambiguous stimuli. Schizophr. Res. Cogn. 2, 72–77 (2015).
Stuke, H., Weilnhammer, V. A., Sterzer, P. & Schmack, K. Delusion proneness is linked to a reduced usage of prior beliefs in perceptual decisions. Schizophr. Bull. 45, 80–86 (2019).
Teufel, C., Kingdon, A., Ingram, J. N., Wolpert, D. M. & Fletcher, P. C. Deficits in sensory prediction are related to delusional ideation in healthy individuals. Neuropsychologia 48, 4169–4172 (2010).
Weilnhammer, V. et al. Psychotic experiences in schizophrenia and sensitivity to sensory evidence. Schizophr. Bull. 46, 927–936 (2020).
Cassidy, C. M. et al. A perceptual inference mechanism for hallucinations linked to striatal dopamine. Curr. Biol. 28, 503–514 (2018).
Haarsma, J. et al. Influence of prior beliefs on perception in early psychosis: effects of illness stage and hierarchical level of belief. J. Abnorm. Psychol. 129, 581–598 (2020).
Powers, A., Mathys, C. & Corlett, P. R. Pavlovian conditioning—induced hallucinations result from overweighting of perceptual priors. Science 357, 596–600 (2017).
Stuke, H., Kress, E., Weilnhammer, V. A., Sterzer, P. & Schmack, K. Overly strong priors for socially meaningful visual signals are linked to psychosis proneness in healthy individuals. Front. Psychol. 12, 583637 (2021).
Teufel, C. et al. Shift toward prior knowledge confers a perceptual advantage in early psychosis and psychosis-prone healthy individuals. Proc. Natl. Acad. Sci. USA 112, 13401–13406 (2015).
Corlett, P. R., Frith, C. D. & Fletcher, P. C. From drugs to deprivation: a Bayesian framework for understanding models of psychosis. Psychopharmacology 206, 515–530 (2009).
Notredame, C.-E., Pins, D., Deneve, S. & Jardri, R. What visual illusions teach us about schizophrenia. Front. Integr. Neurosci. 8, 63 (2014).
Sterzer, P., Mishara, A. L., Voss, M. & Heinz, A. Thought insertion as a self-disturbance: an integration of predictive coding and phenomenological approaches. Front. Hum. Neurosci. 10, 502 (2016).
Eckert, A.-L., Gounitski, Y., Guggenmos, M. & Sterzer, P. Cross-modality evidence for reduced choice history biases in psychosis-prone individuals. Schizophr. Bull. 49, 397–406 (2023).
Jardri, R. et al. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain? Schizophr. Bull. 42, 1124–1134 (2016).
Schmack, K., Bosc, M., Sturgill, J., Ott, T. & Kepecs, A. Hallucination-like perception in mice provides neural-circuit insights into dopamine hypothesis of psychosis. Biol. Psychiatry 89, S44 (2021).
Heinz, A. & Schlagenhauf, F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr. Bull. 36, 472–485 (2010).
Corlett, P. R. & Fletcher, P. C. Delusions and prediction error: clarifying the roles of behavioural and brain responses. Cogn. Neuropsychiatry 20, 95–105 (2015).
Guloksuz, S. & van Os, J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol. Med. 48, 229–244 (2018).
Kapur, S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 160, 13–23 (2003).
Deserno, L. et al. Volatility estimates increase choice switching and relate to prefrontal activity in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 173–183 (2020).
Howes, O. D. et al. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr. Bull. 39, 807–814 (2013).
Davies, D. J., Teufel, C. & Fletcher, P. C. Anomalous perceptions and beliefs are associated with shifts toward different types of prior knowledge in perceptual inference. Schizophr. Bull. 44, 1245–1253 (2018).
Mourgues-Codern, C. et al. Emergence and dynamics of delusions and hallucinations across stages in early psychosis. Biol. Psychiatry 98, 679–688 (2025).
Sterzer, P., Voss, M., Schlagenhauf, F. & Heinz, A. Decision-making in schizophrenia: a predictive-coding perspective. NeuroImage 190, 133–143 (2019).
Javitt, D. C. Glutamatergic theories of schizophrenia. Isr. J. Psychiatry Relat. Sci. 47, 4–16 (2010).
Javitt, D. C. & Freedman, R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am. J. Psychiatry 172, 17–31 (2015).
Demler, V. F., Sterner, E. F., Wilson, M., Zimmer, C. & Knolle, F. Association between increased anterior cingulate glutamate and psychotic-like experiences, but not autistic traits in healthy volunteers. Sci. Rep. 13, 12792 (2023).
Merritt, K., Egerton, A., Kempton, M. J., Taylor, M. J. & McGuire, P. K. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry 73, 665–674 (2016).
Knolle, F., Sterner, E. F., Demler, V. F., MacGregor, L. J. & Mathys, C. Guided by expectations: overweighted semantic priors in schizotypy and their links to glutamate. Biol. Psychiatry https://doi.org/10.1016/j.biopsych.2025.06.025 (2025).
Rushworth, M. F. S. & Behrens, T. E. J. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 11, 389–397 (2008).
Adams, R., Brown, H. R. & Friston, K. J. Bayesian inference, predictive coding and delusions. AVANT V, 51–88 (2014).
Jardri, R. & Denève, S. Circular inferences in schizophrenia. Brain 136, 3227–3241 (2013).
Javitt, D. C. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol. 5, 249–275 (2009).
Stephan, K. E., Friston, K. J. & Frith, C. D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 35, 509–527 (2009).
Ranson, A. et al. Top-down suppression of sensory cortex in an NMDAR hypofunction model of psychosis. Schizophr. Bull. 45, 1349–1357 (2019).
Schmack, K., Bosc, M., Ott, T., Sturgill, J. F. & Kepecs, A. Striatal dopamine mediates hallucination-like perception in mice. Science 372, eabf4740 (2021).
Schmidt, A. et al. Modeling ketamine effects on synaptic plasticity during the mismatch negativity. Cereb. Cortex 23, 2394–2406 (2013).
Weber, L. A. et al. Ketamine affects prediction errors about statistical regularities: a computational single-trial analysis of the mismatch negativity. J. Neurosci. 40, 5658–5668 (2020).
Glausier, J. R. & Lewis, D. A. Dendritic spine pathology in schizophrenia. Neuroscience 251, 90–107 (2013).
Corlett, P. R., Honey, G. D. & Fletcher, P. C. Prediction error, ketamine and psychosis: an updated model. J. Psychopharmacol. 30, 1145–1155 (2016).
Kafadar, E. et al. Modeling perception and behavior in individuals at clinical high risk for psychosis: support for the predictive processing framework. Schizophr. Res. 226, 167–175 (2020).
Leptourgos, P. et al. Relating glutamate, conditioned, and clinical hallucinations via 1H-MR spectroscopy. Schizophr. Bull. 48, 912–920 (2022).
Benrimoh, D. et al. Evidence for reduced sensory precision and increased reliance on priors in hallucination-prone individuals in a general population sample. Schizophr. Bull. 50, 349–362 (2024).
Kafadar, E. et al. Conditioned hallucinations and prior over-weighting are state sensitive markers of hallucination susceptibility. Biol. Psychiatry 92, 772–780 (2022).
Altamura, M. et al. Are all forms of feature binding disturbed in schizophrenia? Evidence from a central vs. peripheral distinction in working memory. Psychiatry Res. 209, 9–14 (2013).
Chhabra, S., Badcock, J. C., Maybery, M. T. & Leung, D. Context binding and hallucination predisposition: evidence of intact intentional and automatic integration of external features. Pers. Individ. Differ. 50, 834–839 (2011).
Kang, S. S., MacDonald, A. W. & Sponheim, S. R. Dysfunctional neural processes underlying context processing deficits in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 644–654 (2019).
Waters, F. A. V., Maybery, M. T., Badcock, J. C. & Michie, P. T. Context memory and binding in schizophrenia. Schizophr. Res. 68, 119–125 (2004).
Alderson-Day, B. et al. Distinct processing of ambiguous speech in people with non-clinical auditory verbal hallucinations. Brain 140, 2475–2489 (2017).
Schmack, K., Rothkirch, M., Priller, J. & Sterzer, P. Enhanced predictive signalling in schizophrenia. Hum. Brain Mapp. 38, 1767–1779 (2017).
Javitt, D. C. Sensory processing in schizophrenia: neither simple nor intact. Schizophr. Bull. 35, 1059–1064 (2009).
Karvelis, P., Seitz, A. R., Lawrie, S. M. & Seriès, P. Autistic traits, but not schizotypy, predict increased weighting of sensory information in Bayesian visual integration. eLife 7, e34115 (2018).
Bévalot, C. & Meyniel, F. A dissociation between the use of implicit and explicit priors in perceptual inference. Commun. Psychol. 2, 111 (2024).
Dzafic, I. et al. Stronger top-down and weaker bottom-up frontotemporal connections during sensory learning are associated with severity of psychotic phenomena. Schizophr. Bull. 47, 1039–1047 (2021).
Daalman, K. & Diederen, K. M. A final common pathway to hearing voices: examining differences and similarities in clinical and non-clinical individuals. Psychosis 5, 236–246 (2013).
Gold, J. M. et al. Phenomenological and cognitive features associated with auditory hallucinations in clinical and nonclinical voice hearers. Schizophr. Bull. 49, 1591–1601 (2023).
Goodwin, I., Kugel, J., Hester, R. & Garrido, M. I. Bayesian accounts of perceptual decisions in the nonclinical continuum of psychosis: greater imprecision in both top-down and bottom-up processes. PLOS Comput. Biol. 19, e1011670 (2023).
Foussias, G. & Remington, G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr. Bull. 36, 359–369 (2010).
Blanchard, J. J. & Cohen, A. S. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr. Bull. 32, 238–245 (2006).
Fervaha, G., Foussias, G., Agid, O. & Remington, G. Amotivation and functional outcomes in early schizophrenia. Psychiatry Res. 210, 665–668 (2013).
Krause, M. et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268, 625–639 (2018).
Foussias, G., Agid, O., Fervaha, G. & Remington, G. Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. Eur. Neuropsychopharmacol. 24, 693–709 (2014).
Messinger, J. W. et al. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin. Psychol. Rev. 31, 161–168 (2011).
Chan, R. C. K., Wang, L. & Lui, S. S. Y. Theories and models of negative symptoms in schizophrenia and clinical implications. Nat. Rev. Psychol. 1, 454–467 (2022).
Bliksted, V. et al. Hyper- and hypomentalizing in patients with first-episode schizophrenia: fMRI and behavioral studies. Schizophr. Bull. 45, 377–385 (2019).
Jeganathan, J. & Breakspear, M. An active inference perspective on the negative symptoms of schizophrenia. Lancet Psychiatry 8, 732–738 (2021).
Kahnt, T., Heinzle, J., Park, S. Q. & Haynes, J.-D. The neural code of reward anticipation in human orbitofrontal cortex. Proc. Natl. Acad. Sci. USA 107, 6010–6015 (2010).
Chan, R. C. K. et al. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: cross-cultural validation and extension. Psychiatry Res. 175, 181–183 (2010).
Edwards, C. J., Cella, M., Tarrier, N. & Wykes, T. Predicting the future in schizophrenia: the discrepancy between anticipatory and consummatory pleasure. Psychiatry Res. 229, 462–469 (2015).
Gard, D. E., Kring, A. M., Gard, M. G., Horan, W. P. & Green, M. F. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 93, 253–260 (2007).
Cassidy, C. M., Lepage, M., Harvey, P.-O. & Malla, A. Cannabis use and anticipatory pleasure as reported by subjects with early psychosis and community controls. Schizophr. Res. 137, 39–44 (2012).
Da Silva, S. et al. Investigating consummatory and anticipatory pleasure across motivation deficits in schizophrenia and healthy controls. Psychiatry Res. 254, 112–117 (2017).
Strauss, G. P., Wilbur, R. C., Warren, K. R., August, S. M. & Gold, J. M. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 187, 36–41 (2011).
Strauss, G. P. & Gold, J. M. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry 169, 364–373 (2012).
Juckel, G. et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology 187, 222–228 (2006).
Juckel, G. et al. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage 29, 409–416 (2006).
Nielsen, M. Ø. et al. Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol. Psychiatry 71, 898–905 (2012).
Radua, J. et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry 72, 1243–1251 (2015).
Schlagenhauf, F. et al. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology 196, 673–684 (2008).
Waltz, J. A. The neural underpinnings of cognitive flexibility and their disruption in psychotic illness. Neuroscience 345, 203–217 (2017).
Kesby, J. P., Murray, G. K. & Knolle, F. Neural circuitry of salience and reward processing in psychosis. Biol. Psychiatry Glob. Open Sci. 3, 33–46 (2023).
Gradin, V. B. et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain J. Neurol. 134, 1751–1764 (2011).
Murray, G. K. et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol. Psychiatry 13, 267–276 (2008).
Waltz, J. A. et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology 34, 1567–1577 (2008).
Ermakova, A. O. et al. Abnormal reward prediction-error signalling in antipsychotic naive individuals with first-episode psychosis or clinical risk for psychosis. Neuropsychopharmacology 43, 1691–1699 (2018).
Montagnese, M. et al. Reinforcement learning as an intermediate phenotype in psychosis? Deficits sensitive to illness stage but not associated with polygenic risk of schizophrenia in the general population. Schizophr. Res. 222, 389–396 (2020).
Pelizza, L. & Ferrari, A. Anhedonia in schizophrenia and major depression: state or trait? Ann. Gen. Psychiatry 8, 22 (2009).
Dunlop, B. W. & Nemeroff, C. B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 64, 327–337 (2007).
McCabe, C., Mishor, Z., Cowen, P. J. & Harmer, C. J. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol. Psychiatry 67, 439–445 (2010).
Ubl, B. et al. Altered neural reward and loss processing and prediction error signalling in depression. Soc. Cogn. Affect. Neurosci. 10, 1102–1112 (2015).
Halahakoon, D. C. et al. Reward-processing behavior in depressed participants relative to healthy volunteers: a systematic review and meta-analysis. JAMA Psychiatry 77, 1286–1295 (2020).
Treadway, M. T. & Zald, D. H. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 35, 537–555 (2011).
Vrieze, E. et al. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry 73, 639–645 (2013).
Dev, A. S., Arditte Hall, K. A. & Timpano, K. R. The relationship between psychiatric symptoms and affective forecasting bias. J. Behav. Ther. Exp. Psychiatry 79, 101825 (2023).
Thompson, R. et al. Positive and negative affective forecasting in remitted individuals with bipolar I disorder, and major depressive disorder, and healthy controls. Cogn. Ther. Res. 41, 673–685 (2017).
Wenze, S. J., Gunthert, K. C. & German, R. E. Biases in affective forecasting and recall in individuals with depression and anxiety symptoms. Pers. Soc. Psychol. Bull. 38, 895–906 (2012).
Liang, S., Wu, Y., Hanxiaoran, L., Greenshaw, A. J. & Li, T. Anhedonia in depression and schizophrenia: brain reward and aversion circuits. Neuropsychiatr. Dis. Treat. 18, 1385–1396 (2022).
Keren, H. et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am. J. Psychiatry 175, 1111–1120 (2018).
Ng, T. H., Alloy, L. B. & Smith, D. V. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl. Psychiatry 9, 293 (2019).
Zhang, B. et al. Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav. 10, 920–939 (2016).
Leroy, A. et al. Reward anticipation in schizophrenia: a coordinate-based meta-analysis. Schizophr. Res. 218, 2–6 (2020).
McCabe, C. Neural signals of ‘intensity’ but not ‘wanting’ or ‘liking’ of rewards may be trait markers for depression. J. Psychopharmacol. 30, 1020–1027 (2016).
Knolle, F. et al. Investigating disorder-specific and transdiagnostic alterations in model-based and model-free decision-making. J. Psychiatry Neurosci. 49, E389–E401 (2024).
Banaraki, A. K., Toghi, A. & Mohammadzadeh, A. RDoC framework through the lens of predictive processing: focusing on cognitive systems domain. Comput. Psychiatry 8, 178–201 (2024).
Seth, A. K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17, 565–573 (2013).
Yao, B. & Thakkar, K. Interoception abnormalities in schizophrenia: a review of preliminary evidence and an integration with Bayesian accounts of psychosis. Neurosci. Biobehav. Rev. 132, 757–773 (2022).
Ainley, V., Apps, M. A. J., Fotopoulou, A. & Tsakiris, M. ‘Bodily precision’: a predictive coding account of individual differences in interoceptive accuracy. Philos. Trans. R. Soc. B 371, 20160003 (2016).
Poletti, M., Tortorella, A. & Raballo, A. Impaired corollary discharge in psychosis and at-risk states: integrating neurodevelopmental, phenomenological, and clinical perspectives. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 832–841 (2019).
Shergill, S. S., Samson, G., Bays, P. M., Frith, C. D. & Wolpert, D. M. Evidence for sensory prediction deficits in schizophrenia. Am. J. Psychiatry 162, 2384–2386 (2005).
Martinelli, C., Rigoli, F. & Shergill, S. S. Aberrant force processing in schizophrenia. Schizophr. Bull. 43, 417–424 (2017).
Wilquin, H. & Delevoye-Turrell, Y. Motor agency: a new and highly sensitive measure to reveal agency disturbances in early psychosis. PLoS ONE 7, e30449 (2012).
Thakkar, K. N., Schall, J. D., Logan, G. D. & Park, S. Response inhibition and response monitoring in a saccadic double-step task in schizophrenia. Brain Cogn. 95, 90–98 (2015).
Ford, J. M., Roach, B. J., Faustman, W. O. & Mathalon, D. H. Synch before you speak: auditory hallucinations in schizophrenia. Am. J. Psychiatry 164, 458–466 (2007).
Ford, J. M. Studying auditory verbal hallucinations using the RDoC framework. Psychophysiology 53, 298–304 (2016).
Sheffield, J. M., Brinen, A. P., Feola, B., Heckers, S. & Corlett, P. R. Understanding cognitive behavioral therapy for psychosis through the predictive coding framework. Biol. Psychiatry Glob. Open Sci. 4, 100333 (2024).
Smith, R., Badcock, P. & Friston, K. J. Recent advances in the application of predictive coding and active inference models within clinical neuroscience. Psychiatry Clin. Neurosci. 75, 3–13 (2021).
Miyake, N., Thompson, J., Skinbjerg, M. & Abi-Dargham, A. Presynaptic dopamine in schizophrenia. CNS Neurosci. Ther. 17, 104–109 (2011).
Morrison, A. P. & Barratt, S. What are the components of CBT for psychosis? A Delphi study. Schizophr. Bull. 36, 136–142 (2010).
Moritz, S. & Woodward, T. S. Metacognitive training in schizophrenia: from basic research to knowledge translation and intervention. Curr. Opin. Psychiatry 20, 619 (2007).
Pell, G. S., Roth, Y. & Zangen, A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog. Neurobiol. 93, 59–98 (2011).
Fiszdon, J. M., Choi, K. H., Bell, M. D., Choi, J. & Silverstein, S. M. Cognitive remediation for individuals with psychosis: efficacy and mechanisms of treatment effects. Psychol. Med. 46, 3275–3289 (2016).
Laukkonen, R. E. & Slagter, H. A. From many to (n)one: meditation and the plasticity of the predictive mind. Neurosci. Biobehav. Rev. 128, 199–217 (2021).
Fong, C. Y., Law, W. H. C., Uka, T. & Koike, S. Auditory mismatch negativity under predictive coding framework and its role in psychotic disorders. Front. Psychiatry 11, 557932 (2020).
Kirihara, K. et al. A predictive coding perspective on mismatch negativity impairment in schizophrenia. Front. Psychiatry 11, 660 (2020).
Donaldson, K. R. et al. Mismatch negativity and clinical trajectories in psychotic disorders: five-year stability and predictive utility. Psychol. Med. 53, 5818–5828 (2023).
Kafadar, E. et al. Conditioned hallucinations and prior overweighting are state-sensitive markers of hallucination susceptibility. Biol. Psychiatry 15, 772–780 (2022).
Fryer, S. L. et al. Deficits in auditory predictive coding in individuals with the psychosis risk syndrome: prediction of conversion to psychosis. J. Abnorm. Psychol. 129, 599–611 (2020).
Hauke, D. J. Aberrant hierarchical prediction errors are associated with transition to psychosis: a computational single-trial analysis of the mismatch negativity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 8, 1176–1185 (2023).
Knolle, F. et al. Action selection in early stages of psychosis: an active inference approach. J. Psychiatry Neurosci. 48, E78–E89 (2023).
Vilares, I. & Kording, K. P. Dopaminergic medication increases reliance on current information in Parkinson’s disease. Nat. Hum. Behav. 1, 0129 (2017).
Fusar-Poli, P. et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry 77, 755–765 (2020).
Suvisaari, J. et al. Is it possible to predict the future in first-episode psychosis? Front. Psychiatry 9, 580 (2018).
Fišar, Z. Biological hypotheses, risk factors, and biomarkers of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 120, 110626 (2023).
Kraguljac, N. V. et al. Neuroimaging biomarkers in schizophrenia. Am. J. Psychiatry 178, 509–521 (2021).
Lin, P. et al. Consensus on potential biomarkers developed for use in clinical tests for schizophrenia. Gen. Psychiatry 35, e100685 (2022).
Gillan, C. M. & Daw, N. D. Taking psychiatry research online. Neuron 91, 19–23 (2016).
Casaletto, K. B. & Heaton, R. K. Neuropsychological assessment: past and future. J. Int. Neuropsychol. Soc. 23, 778–790 (2017).
Goodwin, I., Hester, R. & Garrido, M. I. Temporal stability of Bayesian belief updating in perceptual decision-making. Behav. Res. Methods 56, 6349–6362 (2024).
Pálffy, Z., Farkas, K., Csukly, G., Kéri, S. & Polner, B. Cross-modal auditory priors drive the perception of bistable visual stimuli with reliable differences between individuals. Sci. Rep. 11, 16943 (2021).
McGorry, P. D. et al. Spurious precision: procedural validity of diagnostic assessment in psychotic disorders. Am. J. Psychiatry 152, 220–223 (1995).
Matuszak, J. & Piasecki, M. Inter-rater reliability in psychiatric diagnosis. Psychiatr. Times 29, 12–13 (2012).
Gibbs-Dean, T. et al. Belief updating in psychosis, depression and anxiety disorders: a systematic review across computational modelling approaches. Neurosci. Biobehav. Rev. 147, 105087 (2023).
Wilson, R. C. & Collins, A. G. Ten simple rules for the computational modeling of behavioral data. eLife 8, e49547 (2019).
Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013).
Haarsma, J. et al. Precision weighting of cortical unsigned prediction error signals benefits learning, is mediated by dopamine, and is impaired in psychosis. Mol. Psychiatry 26, 5320–5333 (2021).
Hauke, D. J. et al. Altered perception of environmental volatility during social learning in emerging psychosis. Comput. Psychiatry 8, 1–22 (2024).
Lalousis, P. A. et al. Heterogeneity and classification of recent onset psychosis and depression: a multimodal machine learning approach. Schizophr. Bull. 47, 1130–1140 (2021).
Taylor, J. A., Larsen, K. M. & Garrido, M. I. Multi-dimensional predictions of psychotic symptoms via machine learning. Hum. Brain Mapp. 41, 5151–5163 (2020).
Kahneman, D. Experiences of collaborative research. Am. Psychol. 58, 723–730 (2003).
Olcese, U. et al. Accelerating research on consciousness: an adversarial collaboration to test contrasting predictions of the integrated information theory and predictive processing accounts of consciousness—version 2. OSF https://doi.org/10.17605/OSF.IO/4RN85 (2024).
Cleeremans, A. Theory as adversarial collaboration. Nat. Hum. Behav. 6, 485–486 (2022).
Melloni, L. et al. An adversarial collaboration protocol for testing contrasting predictions of global neuronal workspace and integrated information theory. PLoS ONE 18, e0268577 (2023).
Yaron, I., Melloni, L., Pitts, M. & Mudrik, L. The ConTraSt database for analysing and comparing empirical studies of consciousness theories. Nat. Hum. Behav. 6, 593–604 (2022).
Mathys, C. D. et al. Uncertainty in perception and the Hierarchical Gaussian Filter. Front. Hum. Neurosci. 8, 825 (2014).
Katthagen, T., Fromm, S., Wieland, L. & Schlagenhauf, F. Models of dynamic belief updating in psychosis—a review across different computational approaches. Front. Psychiatry 13, 814111 (2022).
Weber, L. A. et al. The generalized Hierarchical Gaussian Filter. Preprint at https://doi.org/10.48550/arXiv.2305.10937 (2024).
Marek, S. et al. Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660 (2022).
Markiewicz, C. J. et al. The OpenNeuro resource for sharing of neuroscience data. eLife 10, e71774 (2021).
Rahnev, D. et al. The Confidence Database. Nat. Hum. Behav. 4, 317–325 (2020).
The International Brain Laboratory et al. Standardized and reproducible measurement of decision-making in mice. eLife 10, e63711 (2021).
Nassar, M. R., Waltz, J. A., Albrecht, M. A., Gold, J. M. & Frank, M. J. All or nothing belief updating in patients with schizophrenia reduces precision and flexibility of beliefs. Brain 144, 1013–1029 (2021).
Baker, S. C., Konova, A. B., Daw, N. D. & Horga, G. A distinct inferential mechanism for delusions in schizophrenia. Brain 142, 1797–1812 (2019).
Reed, E. J. et al. Paranoia as a deficit in non-social belief updating. eLife 9, e56345 (2020).
Limongi, R., Bohaterewicz, B., Nowicka, M., Plewka, A. & Friston, K. J. Knowing when to stop: aberrant precision and evidence accumulation in schizophrenia. Schizophr. Res. 197, 386–391 (2018).
Kaliuzhna, M. et al. No evidence for abnormal priors in early vision in schizophrenia. Schizophr. Res. 210, 245–254 (2019).
van Leeuwen, T. M. et al. Perceptual gains and losses in synesthesia and schizophrenia. Schizophr. Bull. 47, 722–730 (2021).
Bansal, S. et al. Association between failures in perceptual updating and the severity of psychosis in schizophrenia. JAMA Psychiatry 79, 169–177 (2022).
Scheliga, S., Schwank, R., Scholle, R., Habel, U. & Kellermann, T. A neural mechanism underlying predictive visual motion processing in patients with schizophrenia. Psychiatry Res. 318, 114934 (2022).
Larsen, K. M. et al. Aberrant connectivity in auditory precision encoding in schizophrenia spectrum disorder and across the continuum of psychotic-like experiences. Schizophr. Res. 222, 185–194 (2020).
Simonsen, A. et al. Taking others into account: combining directly experienced and indirect information in schizophrenia. Brain J. Neurol. 144, 1603–1614 (2021).
Alamia, A. et al. Oscillatory traveling waves provide evidence for predictive coding abnormalities in schizophrenia. Biol. Psychiatry 98, 167–174 (2025).
Castiello, S. et al. Delusional unreality and predictive processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 10, 709–717 (2025).
Kowalski, J. et al. Associations of cognitive expectancies with auditory hallucinations and hallucinatory-like experiences in patients with schizophrenia. Schizophr. Bull. 51, 780–791 (2025).
Fromm, S. P. et al. Neural correlates of uncertainty processing in psychosis spectrum disorder. Brain Commun. 7, fcaf073 (2025).
Cole, D. M. et al. Atypical processing of uncertainty in individuals at risk for psychosis. NeuroImage Clin. 26, 102239 (2020).
Silverstein, S. M. et al. Increased face detection responses on the mooney faces test in people at clinical high risk for psychosis. npj Schizophr. 7, 26 (2021).
Charlton, C. E. et al. Atypical prediction error learning is associated with prodromal symptoms in individuals at clinical high risk for psychosis. Schizophrenia 8, 105 (2022).
Rossi-Goldthorpe, R. et al. Different learning aberrations relate to delusion-like beliefs with different contents. Brain 147, 2854–2866 (2024).
Tran, T. et al. Increased face perception in individuals at clinical high-risk for psychosis: mechanisms, sex differences, and clinical correlates. Schizophrenia 11, 74 (2025).
Dzafic, I., Randeniya, R., Harris, C. D., Bammel, M. & Garrido, M. I. Statistical learning and inference is impaired in the nonclinical continuum of psychosis. J. Neurosci. 40, 6759–6769 (2020).
Kreis, I. et al. Aberrant uncertainty processing is linked to psychotic-like experiences, autistic traits, and is reflected in pupil dilation during probabilistic learning. Cogn. Affect. Behav. Neurosci. 23, 905–919 (2023).
Suthaharan, P. et al. Paranoia and belief updating during the COVID-19 crisis. Nat. Hum. Behav. 5, 1190–1202 (2021).
Haarsma, J., Deveci, N., Corbin, N., Callaghan, M. F. & Kok, P. Expectation cues and false percepts generate stimulus-specific activity in distinct layers of the early visual cortex. J. Neurosci. 43, 7946–7957 (2023).
Seymour, K., Sterzer, P. & Soto, N. Believing is seeing: the link between paranormal beliefs and perceiving signal in noise. Conscious. Cogn. 106, 103418 (2022).
Alderson-Day, B. et al. Susceptibility to auditory hallucinations is associated with spontaneous but not directed modulation of top-down expectations for speech. Neurosci. Conscious. 2022, niac002 (2022).
Lhotka, M., Ischebeck, A., Helmlinger, B. & Zaretskaya, N. No common factor for illusory percepts, but a link between pareidolia and delusion tendency: a test of predictive coding theory. Front. Psychol. 13, 1067985 (2023).
Tarasi, L., Martelli, M. E., Bortoletto, M., di Pellegrino, G. & Romei, V. Neural signatures of predictive strategies track individuals along the autism–schizophrenia continuum. Schizophr. Bull. 49, 1294–1304 (2023).
Bott, A., Steer, H. C., Faße, J. L. & Lincoln, T. M. Visualizing threat and trustworthiness prior beliefs in face perception in high versus low paranoia. Schizophrenia 10, 40 (2024).
Mazer, P. et al. How distinct autism and schizotypal trait dimensions influence neural predictive processing: an event-related potential study. Brain Cogn. 188, 106329 (2025).
Bartels-Velthuis, A. A., Willige, G., van de, Jenner, J. A. & Wiersma, D. Consistency and reliability of the auditory vocal hallucination rating scale (AVHRS). Epidemiol. Psychiatr. Sci. 21, 305–310 (2012).
Faustman, W. O. & Overall, J. E. in The Use of Psychological Testing for Treatment Planning and Outcomes Assessment (ed. Maruish, M. E.) 2nd edn 791–830 (Lawrence Erlbaum, 1999).
Yung, A. R. et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust. N. Z. J. Psychiatry 39, 964–971 (2005).
Konings, M., Bak, M., Hanssen, M., Van Os, J. & Krabbendam, L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr. Scand. 114, 55–61 (2006).
Bell, V., Halligan, P. W. & Ellis, H. D. The Cardiff Anomalous Perceptions Scale (CAPS): a new validated measure of anomalous perceptual experience. Schizophr. Bull. 32, 366–377 (2006).
Kern, B., Axelrod, J., Gao, Y. & Keedy, S. Exchange the magnifying glass for a microscope: the Chicago Hallucination Assessment Tool (CHAT). Schizophr. Bull. 41, S110 (2015).
Launay, G. & Slade, P. Launay–Slade Hallucination Scale. APA PsycNet https://doi.org/10.1037/t13160-000 (1981).
McGlashan, T. H., Miller, T. J., Woods, S. W., Hoffman, R. E., Davidson, L. in Early Intervention in Psychotic Disorders. NATO Science Series (eds Miller, T. et al.) Vol. 91, 135–149 (Springer, 2001).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Peters, E. R., Joseph, S. A. & Garety, P. A. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. delusions inventory). Schizophr. Bull. 25, 553–576 (1999).
Loewy, R. L., Bearden, C. E., Johnson, J. K., Raine, A. & Cannon, T. D. The prodromal questionnaire (PQ): preliminary validation of a self-report screening measure for prodromal and psychotic syndromes. Schizophr. Res. 79, 117–125 (2005).
Kline, E. et al. Psychosis risk screening in youth: a validation study of three self-report measures of attenuated psychosis symptoms. Schizophr. Res. 141, 72–77 (2012).
Freeman, D. et al. The revised Green et al., paranoid thoughts scale (R-GPTS): psychometric properties, severity ranges, and clinical cut-offs. Psychol. Med. 51, 244–253 (2021).
Morrison, A. P., Wells, A. & Nothard, S. Cognitive and emotional predictors of predisposition to hallucinations in non-patients. Br. J. Clin. Psychol. 41, 259–270 (2002).
Rust, J. The Rust inventory of schizotypal cognitions (RISC). Schizophr. Bull. 14, 317–322 (1988).
Shankman, S. A. et al. Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID). Int. J. Methods Psychiatr. Res. 27, e1590 (2018).
McGlashan, T. H., Walsh, B. & Woods, S. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up (Oxford Univ. Press, 2010).
Raine, A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr. Bull. 17, 555–564 (1991).
Author information
Authors and Affiliations
Contributions
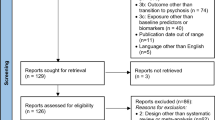
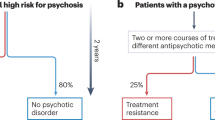
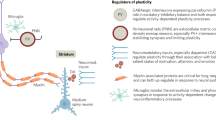
I.G., K.M.J.D., E.J.H., V.W., M.I.G. and F.K. contributed to the conceptualization of the structure and content of the manuscript, contributed written sections and reviewed all sections. M.I.G. created Fig. 1. F.K. created Fig. 3, and I.G. created Fig. 2 and Tables 1, 2.
Corresponding author
Ethics declarations
Competing interests
The authors report no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Tyrone Cannon, Andrea Raballo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goodwin, I., Diederen, K.M.J., Hird, E.J. et al. Predictive processing accounts of psychosis: bottom-up or top-down disruptions. Nat. Mental Health 4, 60–84 (2026). https://doi.org/10.1038/s44220-025-00558-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00558-5