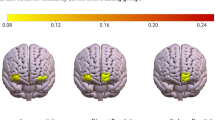
Abstract
Political identity shapes neural responses to political content, but how these responses change within individuals over time remains unexplored. Here, we tested this by leveraging a unique political crisis. We conducted two fMRI scans separated by two and a half years, during which 21 participants viewed identical political videos. This period coincided with political instability that potentially caused participants to shift their attitudes towards the videos. Analysis revealed a neural plasticity hierarchical pattern: primary sensory regions showed minimal changes, while limbic, reward, and memory networks exhibited the most substantial differences between sessions. Specifically, the amygdala, hippocampus, and caudate demonstrated activity patterns that tracked changes in interpretation. Notably, neural changes in these regions correlated with shifts in political in-group affiliations, but not statistically significantly with changes in ideological positions. These findings provide empirical support for the hypothesis that social and psychological processes shape neural responses to political content, rather than vice versa.
Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available at https://doi.org/10.5281/zenodo.1778768080. Additionally, neural maps of Figs. 3 and 4 are hosted on Neurovault at https://neurovault.org/collections/19578/81
Code availability
The code that supports the findings of this study is openly available at https://github.com/YeYaLab/Political-Identity-Change.git82.
References
Huddy, L. From social to political identity: a critical examination of social identity theory. Political Psychol. 22, 127–156 (2001).
Unsworth, K. L. & Fielding, K. S. It’s political: How the salience of one’s political identity changes climate change beliefs and policy support. Glob. Environ. Change 27, 131–137 (2014).
Bassan-Nygate, L. & Weiss, C. M. Party competition and cooperation shape affec-tive polarization: evidence from natural and survey experiments in Israel. Comparative Political Stud. 55 https://doi.org/10.1177/00104140211024283 (2022).
Freire, A. Left–right ideology as a dimension of identification and of competition. J. Political Ideol. 20, 43–68 (2015).
Schulze, H. Who uses right-wing alternative online media? An exploration of audience characteristics. Politics Gov. 8, 6–18 (2020).
Graham, J., Nosek, B. A. & Haidt, J. The moral stereotypes of liberals and conservatives: exaggeration of differences across the political spectrum. PLoS ONE 7, e50092 (2012).
Wojcik, S. P., Hovasapian, A., Graham, J., Motyl, M. & Ditto, P. H. Conservatives report, but liberals display, greater happiness. Science 347, 1243–1246 (2015).
Stewart, B. D. & Morris, D. S. Moving morality beyond the in-group: Liberals and conservatives show differences on group-framed moral foundations and these differences mediate the relationships to perceived bias and threat. Front. Psychol. 12, 579908 (2021).
Inbar, Y., Pizarro, D., Iyer, R. & Haidt, J. Disgust sensitivity, political conservatism, and voting. Soc. Psychol. Personal. Sci. 3, 537–544 (2012).
Frenda, S. J., Knowles, E. D., Saletan, W. & Loftus, E. F. False memories of fabricated political events. J. Exp. Soc. Psychol. 49, 280–286 (2013).
Vallone, R. P., Ross, L. & Lepper, M. R. The hostile media phenomenon: Biased perception and perceptions of media bias in coverage of the Beirut massacre. J. Personal. Soc. Psychol. 49, 577–585 (1985).
Ahn, W. Y. et al. Nonpolitical images evoke neural predictors of political ideology. Curr. Biol. 24, 2693–2699 (2014).
Galvan, A. et al. The role of ventral frontostriatal circuitry in reward-based learning in humans. J. Neurosci. 25, 8650–8656 (2005).
Gozzi, M., Zamboni, G., Krueger, F. & Grafman, J. Interest in politics modulates neural activity in the amygdala and ventral striatum. Hum. Brain Mapp. 31, 1763–1771 (2010).
Jost, J. T., Nam, H. H., Amodio, D. M. & Van Bavel, J. J. Political neuroscience: the beginning of a beautiful friendship. Political Psychol. 35, 3–42 (2014).
Rule, N. O. et al. Voting behavior is reflected in amygdala response across cultures. Soc. Cogn. Affect. Neurosci. 5, 349–355 (2010).
Schreiber, D. et al. Red Brain, Blue Brain: Evaluative Processes Differ in Democrats and Republicans. PLOS ONE 8, e52970 (2013).
Leong, Y. C., Chen, J., Willer, R. & Zaki, J. Conservative and liberal attitudes drive polarized neural responses to political content. Proc. Natl. Acad. Sci. 117, 27731–27739 (2020).
Dieffenbach, M. C. et al. Neural reference groups: a synchrony-based classification approach for predicting attitudes using fNIRS. Soc. Cogn. Affect. Neurosci. 16, 117–128 (2021).
van Baar, J. M., Halpern, D. J. & FeldmanHall, O. Intolerance of uncertainty modulates brain-to-brain synchrony during politically polarized perception. Proc. Natl. Acad. Sci. 118, e2022491118 (2021).
Broom, T. W., Stahl, J. L., Ping, E. E. & Wagner, D. D. They saw a debate: Political polarization is associated with greater multivariate neural synchrony when viewing the opposing candidate speak. J. Cogn. Neurosci. 35, 60–73 (2022).
Jacoby, N. et al. Partisans process policy-based and identity-based messages using dissociable neural systems. Cereb. Cortex 34, bhae368 (2024).
Katabi, N. et al. Deeper than you think: partisanship-dependent brain responses in early sensory and motor brain regions. J. Neurosci. 43, 1027–1037 (2023).
Rahat, G. & Kenig, O. From Party Politics to Personalized Politics?: Party Change And Political Personalization in Democracies. Oxford University Press.(2018).
Shahbari, I. Israel takes stock: the legacy of Benjamin Netanyahu. Political Insight 12, 34–36 (2021).
Gabay, N. The effect of news media political bias on pre-elections poll results: evidence from the 2019–20 Israeli elections. Isr. Aff. 28, 917–939 (2022).
Poll: Most Yamina voters displeased with Bennett over alliance with Lapid. The Times of Israel. May 31 (2021). Available from https://www.timesofisrael.com/liveblog_entry/poll-most-yamina-voters-displeased-with-bennett-over-alliance-with-lapid/.
Lu, X., Gao, J. & Szymanski, B. K. The evolution of polarization in the legislative branch of government. J. R. Soc. Interface 16, 20190010 (2019).
Finkel, E. J. et al. Political sectarianism in America. Science 370, 533–536 (2020).
Nelson, M. H. Resentment is like drinking poison? the heterogeneous health effects of affective polarization. J. Health Soc. Behav. 63, 508–524 (2022).
Iyengar, S., Lelkes, Y., Levendusky, M., Malhotra, N. & Westwood, S. J. The origins and consequences of affective polarization in the United States. Annu. Rev. Political Sci. 22, 129–146 (2019).
Yeshurun, Y. et al. Same story, different story: the neural representation of interpretive frameworks. Psychol. Sci. 28, 307–319 (2017).
Ames, D. L., Honey, C. J., Chow, M. A., Todorov, A. & Hasson, U. Contextual alignment of cognitive and neural dynamics. J. Cogn. Neurosci. 27, 655–664 (2015).
Pajula, J. & Tohka, J. How many is enough? Effect of sample size in inter-subject correlation analysis of fMRI. Comput. Intell. Neurosci. 2016, 2–2 (2016).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.: Ser. B (Methodol.) 57, 289–300 (1995).
Simony, E. et al. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat. Commun. 7, 12141 (2016).
Nichols, T. E. Multiple testing corrections, nonparametric methods, and random field theory. Neuroimage 62, 811–815 (2012).
Fan, L. et al. The human brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526 (2016).
BBC News. Israel’s Netanyahu and Gantz sign unity government deal. BBC News [Internet]. Aug 16 [cited 2025 Nov 30] (2019). Available from https://www.bbc.com/news/world-middle-east-52358479.
Akehurst L. Has Merav Michaeli rescued the Israeli Labor Party? We’ll find out in March. LabourList. Feb 04 (2021). Available from: https://labourlist.org/2021/02/has-merav-michaeli-rescued-the-israeli-labor-party-well-find-out-in-march/.
Hasson, U. et al. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Res. 2, 220–231 (2009).
Schmälzle, R., Imhof, M. A., Grall, C., Flaisch, T. & Schupp, H. T. Reliability of fMRI time series: similarity of neural processing during movie viewing. Biorxiv https://doi.org/10.1101/158188 (2017).
Knutson, B., Adams, C. M., Fong, G. W. & Hommer, D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 21, RC159 (2001).
Andrews, T. J., Smith, R. K., Hoggart, R. L., Ulrich, P. I. & Gouws, A. D. Neural correlates of group bias during natural viewing. Cereb. Cortex 29, 3380–3389 (2019).
Kobo, O., Yeshurun, Y. & Schonberg, T. Reward-related regions play a role in natural story comprehension. Iscience 27 (2024).
Phelps, E. A. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 57, 27–53 (2006).
Tusche, A., Kahnt, T., Wisniewski, D. & Haynes, J. D. Automatic processing of political preferences in the human brain. Neuroimage 72, 174–182 (2013).
Knutson, K. M., Wood, J. N., Spampinato, M. V. & Grafman, J. Politics on the brain: an FMRI investigation. Soc. Neurosci. 1, 25–40 (2006).
de Bruin, D., van Baar, J. M., Rodríguez, P. L. & FeldmanHall, O. Shared neural representations and temporal segmentation of political content predict ideological similarity. Sci. Adv. 9, eabq5920 (2023).
Moscovitch, M., Cabeza, R., Winocur, G. & Nadel, L. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67, 105–134 (2016).
Voss, J. L., Bridge, D. J., Cohen, N. J. & Walker, J. A. A closer look at the hippocampus and memory. Trends Cogn. Sci. 21, 577–588 (2017).
Chen, J. et al. Accessing real-life episodic information from minutes versus hours earlier modulates hippocampal and high-order cortical dynamics. Cereb. Cortex 26, 3428–3441 (2016).
Sheldon, S. & Levine, B. The role of the hippocampus in memory and mental construction. Ann. N. Y. Acad. Sci. 1369, 76–92 (2016).
Schafer, M. & Schiller, D. Navigating social space. Neuron 100, 476–489 (2018).
Welborn, B. L., Dieffenbach, M. C. & Lieberman, M. D. Default egocentrism: an MVPA approach to overlap in own and others’ socio-political attitudes. Soc. Cogn. Affect. Neurosci. 18, nsad028 (2023).
Yeshurun, Y., Nguyen, M. & Hasson, U. The default mode network: where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 22, 181–192 (2021).
Honey, C. J., Thompson, C. R., Lerner, Y. & Hasson, U. Not lost in translation: neural responses shared across languages. J. Neurosci. 32, 15277–15283 (2012).
Regev, M., Honey, C. J., Simony, E. & Hasson, U. Selective and invariant neural responses to spoken and written narratives. J. Neurosci. 33, 15978–15988 (2013).
Chen, J. et al. Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci. 20, 115–125 (2017).
Zadbood, A., Chen, J., Leong, Y. C., Norman, K. A. & Hasson, U. How we transmit memories to other brains: constructing shared neural representations via communication. Cereb. Cortex 27, 4988–5000 (2017).
Baldassano, C., Hasson, U. & Norman, K. A. Representation of real-world event schemas during narrative perception. J. Neurosci. 38, 9689–9699 (2018).
Bruneau, E. G. & Saxe, R. Attitudes towards the outgroup are predicted by activity in the precuneus in Arabs and Israelis. NeuroImage 52, 1704–1711 (2010).
Falk, E. B., Spunt, R. P. & Lieberman, M. D. Ascribing beliefs to ingroup and outgroup political candidates: neural correlates of perspective-taking, issue importance and days until the election. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 367, 731–743 (2012).
Mitchell, J. P., Macrae, C. N. & Banaji, M. R. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50, 655–663 (2006).
Morrison, S., Decety, J. & Molenberghs, P. The neuroscience of group membership. Neuropsychologia 50, 2114–2120 (2012).
Rilling, J. K., Dagenais, J. E., Goldsmith, D. R., Glenn, A. L. & Pagnoni, G. Social cognitive neural networks during in-group and out-group interactions. Neuroimage 41, 1447–1461 (2008).
Amodio, D. M. The neuroscience of prejudice and stereotyping. Nat. Rev. Neurosci. 15, 670–682 (2014).
Kaplan, J. T., Freedman, J. & Iacoboni, M. Us versus them: Political attitudes and party affiliation influence neural response to faces of presidential candidates. Neuropsychologia 45, 55–64 (2007).
Kaplan, J. T., Gimbel, S. I. & Harris, S. Neural correlates of maintaining one’s political beliefs in the face of counterevidence. Sci. Rep. 6, 39589 (2016).
Cikara, M. & Van Bavel, J. J. The neuroscience of intergroup relations: An integrative review. Perspect. Psychol. Sci. 9, 245–274 (2014).
Saarinen, A. et al. Neural basis of in-group bias and prejudices: a systematic meta-analysis. Neurosci. Biobehav. Rev. 131, 1214–1227 (2021).
Molenberghs, P. & Louis, W. R. Insights from fMRI studies into ingroup bias. Front. Psychol. 9, 1–12 (2018).
Hart, A. J. et al. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport 11, 2351–2354 (2000).
Phelps, E. A. et al. Performance on indirect measures of race evaluation predicts amygdala activation. J. Cogn. Neurosci. 12, 729–738 (2000).
Cunningham, W. A. et al. Separable neural components in the processing of black and white faces. Psychol. Sci. 15, 806–813 (2004).
Van Bavel, J. J., Packer, D. J. & Cunningham, W. A. The neural substrates of in-group bias: A functional magnetic resonance imaging investigation. Psychol. Sci. 19, 1131–1139 (2008).
Beer, J. S. et al. The Quadruple Process model approach to examining the neural underpinnings of prejudice. NeuroImage 43, 775–783 (2008).
Hein, G., Silani, G., Preuschoff, K., Batson, C. D. & Singer, T. Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron 68, 149–160 (2010).
Jost, J. T., Noorbaloochi, S. & Van Bavel, J. J. The “chicken-and-egg” problem in political neuroscience. Behav. Brain Sci. 37 (2014).
YeYaLab. Political-Identity-Change. GitHub (2025). https://github.com/YeYaLab/Political-Identity-Change.
Boiman, G., Ohad, T., Zvi, Y., Katabi, N. & Yeshurun, Y. Neural correlates of political attitudes identity change: a longitudinal fMRI study. Zenodo https://github.com/YeYaLab/Political-Identity-Change.git (2025).
NeuroVault collection 19578. Neural correlates of political identity change: a longitudinal fMRI study (2025). https://identifiers.org/neurovault.collection:19578.
Acknowledgements
The authors would like to thank the Israel Science Foundation (388\24) for funding this study. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors thank Shahar Dishon for generating illustrations for Fig.1.
Author information
Authors and Affiliations
Contributions
G.B.—methodology, analysis, investigation, writing, project administration; T.O.—methodology, analysis, investigation, writing; Y.Z.—data curation, methodology; N.K.—data curation, methodology, Y.Y.—conceptualization, methodology, writing, funding, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Psychology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Hannah Nam and Troby Ka-Yan Lui. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Boiman, G., Ohad, T., Zvi, Y. et al. Changes in political attitudes are associated with changes in neural responses to political content. Commun Psychol (2026). https://doi.org/10.1038/s44271-026-00395-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44271-026-00395-x