Abstract
The increased cardiovascular risk associated with hypertensive disorders of pregnancy (HDP) necessitates methods to improve cardiometabolic health in this population. Postpartum hypertension clinics providing cardiovascular assessment, lifestyle counseling, and medication management have emerged as one such strategy. This review examines current recommendations and interventions for care of postpartum HDP patients and highlights the need for research into solutions merging clinical practice with digital health innovations, ultimately improving long-term maternal health.
Similar content being viewed by others
Introduction
Hypertensive disorders of pregnancy (HDP), including preeclampsia and gestational hypertension, are among the most common adverse pregnancy outcomes (APOs) and significantly elevate the risk of long-term cardiovascular disease (CVD)1,2,3,4,5,6,7. For example, estimates derived from a meta-analysis of 22 studies investigating risks associated with preeclampsia suggest an approximately doubled risk of stroke or coronary heart disease and a quadrupled risk of heart failure3. Given this increased risk, the American College of Obstetricians and Gynecologists (ACOG) and the American Heart Association (AHA) recognize the postpartum period as a critical time for blood pressure (BP) management, CVD risk factor screening, and additional optimization of cardiovascular health (CVH)2,5,6,7,8,9. As a result, structured postpartum follow-up for HDP is essential but remains underutilized, with data from one retrospective cohort study demonstrating low (around 20%) rates of glucose or lipid screening among patients with HDP in the first year postpartum10.
Multidisciplinary postpartum hypertension clinics have emerged as strategies to improve postpartum follow-up and CVH screening and management (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)7. Clinic visits may address medication management, cardiometabolic screening, lifestyle assessment, and patient education, as well as serve as a bridge to primary care (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)7. However, studies investigating these clinics, as well as additional digital health interventions to facilitate follow-up and lifestyle change, are limited by their relatively small size and heterogeneous protocols. In this review, we will discuss the importance of the postpartum timeframe in maternal CVH, the multidisciplinary structure of postpartum cardiovascular care clinics, recommended domains for assessment and counseling in the postpartum timeframe, and research surrounding interventions to improve postpartum follow-up and ultimately maternal health outcomes.
Postpartum follow-up and the fourth trimester
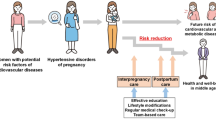
The “fourth trimester” (the first 12 weeks post-delivery) as well as the first year postpartum present a critical opportunity to identify, assess, and address cardiovascular risk factors that may have emerged during pregnancy4,6,7,8. For many reproductive-age individuals, the postpartum timeframe represents a period where they are more significantly engaged in the healthcare system and may be more likely to have adequate health insurance, facilitating CVH screening and risk factor management4,6,8,11. Several AHA publications, including a recent 2024 statement, have emphasized the importance of conducting a thorough CVH evaluation after an APO and specifically after HDP2,6,8,9,12. The AHA recommends regular screening for cardiovascular risk factors in the first year postpartum with visits at intervals of 6 weeks, 8–12 weeks, 6 months, and 12 months postpartum2,6. This screening should be conducted using the components of Life’s Essential 8: lipids, BP, blood glucose, weight, sleep, diet, physical activity, and avoidance of nicotine, as well as accounting for factors specific to the postpartum patient, including contraception and lactation counseling and planning for repeat pregnancies (Figs. 1 and 2, Table 1)2,6,13. An analysis of the UK Biobank cohort found that individuals with prior APO and high CVH using a Life’s Essential 8 framework had significantly decreased rates of incident CVD compared to those with a low CVH assessed via Life’s Essential 8, demonstrating the utility of this framework in assessing CVH in this population14.
The postpartum period is not merely a recovery phase after childbirth; it is a crucial window for identifying individuals at risk of developing CVD and for implementing effective preventive strategies. Unfortunately, this vital opportunity is frequently overlooked in current clinical practices2,6,10. Effective interventions during this crucial period—including lifestyle changes like a balanced diet, regular physical activity, diligent weight management, and appropriate pharmacologic treatments for persistent hypertension—can dramatically shape the long-term health outcomes of individuals. Embracing these strategies not only paves the way for improved individual health but also enhances overall well-being6.
It is important to consider the structural barriers and other social drivers of health (SDOH), such as access to healthcare, socioeconomic challenges, and racial disparities, that result in many postpartum individuals, especially those from underrepresented populations, lacking essential follow-up care after delivery6,8,15. For example, adequacy of health insurance coverage may significantly limit access to postpartum care. In the United States, 41% of births were covered by Medicaid in 2021, and postpartum Medicaid coverage varies from a federally mandated 60 days to 12 months (https://www.medicaid.gov/medicaid/benefits/downloads/2024-maternal-health-at-a-glance.pdf). Other factors that may impact ability to access postpartum care include lack of transportation or childcare and difficulty accommodating work schedules. Clinic design may consider these issues by locating clinics near public transportation options when possible and offering flexible appointment options, such as weekend hours or telehealth11. Home visiting programs for low-income postpartum populations have shown some benefit in reducing postpartum emergency department usage and increasing participation in government aid programs and may be considered as a complement to postpartum clinics16,17. Overall, integrating multidisciplinary postpartum CVH clinics can address gaps in care on a local level as well as work to improve structural barriers, ensuring vulnerable populations receive timely and effective interventions6.
Care models for postpartum maternal health or cardiovascular clinics
The following section focuses on the role of structured postpartum hypertension clinics in facilitating cardiovascular risk assessment and management of individuals with prior HDP. The importance and benefit of prompt postpartum screening in this population is highlighted by a retrospective cohort study of 4194 postpartum women with HDP, which demonstrated that those who received early BP screening (6–12 months postpartum) were more likely to have cardiovascular risk factors identified compared to those who received BP screening more than one year after delivery18. Increasing evidence highlights the effectiveness of postpartum clinics in improving postpartum follow-up, risk stratification, and long-term cardiovascular care (Table 2)19,20,21.
These postpartum clinics facilitate early postpartum follow-up, risk stratification, and engagement with primary care providers. Celi et al. report on the success of a postpartum clinic based at Brigham and Women’s Hospital staffed by an internist seeing patients with recent HDP21. A total of 412 patients were referred in five years, and a total of 48.3% required antihypertensive medication adjustments, underscoring the utility of structured postpartum follow-up in optimizing CVH21. Another cohort study in Ontario, Canada, demonstrated that the introduction of a maternal health clinic accurately identified postpartum patients with cardiovascular risk: individuals in the postpartum maternal health clinic were identified to have increased 30 year- and lifetime CVD risk, compared to controls19. A small retrospective cohort study in Canada involving 21 participants demonstrated noteworthy improvements in physical activity rates among those who attended a postpartum preeclampsia clinic20. This underscores the significant role these clinics can play in promoting healthier lifestyles in this population, suggesting that they are crucial in enhancing postnatal care and well-being20.
The Follow-Up PreEClampsia Outpatient Clinic (FUPEC) study is a large ongoing prospective cohort study in the Netherlands that collects CVH data of individuals with a history of severe preeclampsia seen by FUPEC22. The FUPEC model is a multidisciplinary clinic between obstetrics and internal medicine that provides detailed and long-term cardiovascular screening and follow-up for patients with prior severe preeclampsia22. The FUPEC model focuses on the very high-risk population of patients with prior severe preeclampsia, and this study’s ongoing data collection and biobank offers a wealth of important data that may inform further risk stratification and tailoring cardiovascular interventions in this population22. Depending on local resources and need, postpartum hypertension clinics may be limited to the highest-risk populations (such as patients with severe preeclampsia) or broadened to include all patients with HDP.
Developing an effective postpartum CVH clinic is crucial as a bridge between obstetric to cardiology care (Figs. 1 and 2). A guide to clinic development can be the American College of Cardiology’s Postpartum Hypertension Clinic Development Toolkit and the AHA guidelines on managing APO and psychological health, which provides a structured framework for postpartum care, beginning immediately after delivery, continuing through the fourth trimester and extending into long-term follow-up (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6,23. Using this framework and AHA guidelines, clinics should monitor individuals periodically as described earlier. The initial phase includes regular BP monitoring, lifestyle counseling, and cardiovascular risk assessments to identify emerging issues. After the first year, clinics may emphasize health behavior counseling and continuous assessment of cardiovascular risk factors to detect any lingering or emerging concerns or issues (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf). Free toolkits are available for download from the California Maternal Quality Care Collaborative (CMQCC) to enhance HDP care (https://www.cmqcc.org/quality-improvement-toolkits/hypertensive-disorders-pregnancy/hypertension-and-preeclampsia-data.; https://www.cmqcc.org/toolkits-quality-improvement/hypertensive-disorders-pregnancy). Additionally, member hospitals can utilize maternal data centers to monitor progress in quality improvement, which provides extra guidelines and models for improving care delivery (https://www.cmqcc.org/quality-improvement-toolkits/hypertensive-disorders-pregnancy/hypertension-and-preeclampsia-data.; https://www.cmqcc.org/toolkits-quality-improvement/hypertensive-disorders-pregnancy).
Postpartum hypertension clinics may follow two models: combined or single specialty7,21. The integrated care model offers a groundbreaking approach by potentially uniting cardiology, primary care, and nephrology with obstetrics and maternal-fetal medicine in one comprehensive visit, typically held within a specialized cardio-obstetrics clinic7. This innovative method stands in stark contrast to the traditional single specialty model, which operates separately across obstetrics, cardiology, primary care, or nephrology, ultimately enhancing patient convenience and outcomes7.
Multidisciplinary teams, including physicians, advanced practice providers, nurses, and pharmacists, enhance care (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6,7,11. Dedicated nurses and pharmacists support patient visits, medication adjustments, and education on BP measurement, antihypertensives, and risk reduction (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6,7. In addition, nutritionists, health coaches, clinical psychologists, and patient (peer) mentors/advocates all may play vital roles6. These professionals may foster a sustained and engaged support system that facilitates continued follow-up. Additionally, targeted patient education initiatives can empower women with knowledge about their cardiovascular risks, promoting adherence to lifestyle modifications and facilitating shared decision-making6. For example, as described previously, Janmohamed et al. (2015) report on the increased physical activity found in postpartum patients seen at their multidisciplinary postpartum clinic comprised of a physician, a nurse practitioner, a dietitian, and a pharmacist20.
Domains for comprehensive cardiovascular screening and management in postpartum clinics
Comprehensive cardiovascular screening and management in postpartum clinics should include BP, blood glucose, lipids, assessment of health behaviors, evaluation of lactation, contraception, and future pregnancy planning, as well as assessment of SDOH (Table 1) (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6,7. Several studies that have evaluated postpartum interventions aimed at reducing CVD risk among women with prior APOs (Table 2).
Blood pressure
HDPs, such as gestational hypertension and preeclampsia, are defined by elevated BP (>140/90 mmHg) after 20 weeks of gestation in pregnancy or while postpartum5,24. Preeclampsia is defined as gestational hypertension accompanied by proteinuria5,24. It may be classified as severe if one or more of the following features are present: BP that equals or exceeds 160/110 mmHg, thrombocytopenia, impaired renal or liver function, pulmonary edema, or neurological symptoms such as refractory headache or visual disturbances5,24. BP trajectories in the postpartum period can vary significantly5,6,7,24,25. New-onset hypertension or preeclampsia may also develop in this time frame5,7,24,25,26. Given the risks of severe hypertension and the potential for significant maternal morbidity immediately after delivery, ACOG recommends that postpartum individuals with HDP have their BP checked within 10 days of hospital discharge6,7,25. While BP typically normalizes within twelve weeks postpartum, a subset of individuals may still experience elevated BP at this time and should be closely monitored for the development of chronic hypertension4,6,7.
Postpartum hypertension care clinic visits within 6–12 weeks postpartum should include a BP check, education about lifestyle changes, and pharmacologic treatment as necessary to target goal BP of <120/80 mmHg (or <130/80 mmHg for patients with treated chronic hypertension), based on AHA guidance4,6,7. As will be discussed further in a subsequent section, remote BP monitoring programs may be considered based on clinic resources, given evidence from preliminary studies that demonstrate benefits with regards to BP control, hypertension diagnosis, and other outcomes27,28,29,30,31,32. Evaluation of serum creatinine and proteinuria may be considered in individuals with HDP and a history of kidney injury or proteinuria during or prior to pregnancy6. Duration of longitudinal follow-up for BP management in the postpartum clinic may vary depending on patient circumstances and clinic resources7. Multiple clinical models merit development as to serve the heterogenous postpartum patient population and care settings7.
A complete discussion of BP agents in postpartum hypertension is beyond the scope of this review and has been reviewed elsewhere7. However, commonly used agents for BP management in the postpartum population include labetalol, nifedipine, amlodipine, and enalapril, which are also considered safe in lactation7. Additional research is necessary to further investigate and specify the optimal treatment goals and strategies for postpartum hypertension, as there is not a clear consensus on the appropriate BP target for postpartum hypertension, which consequently leads to variation in clinical management5,6,24. Extrapolating from ACOG guidelines for antepartum HDP suggests a goal of <140/90 mmHg, which is higher than AHA suggestions and may miss a subset of patients5,6,24. Variations exist in the diagnosis and treatment guidelines for HDP established by international medical societies (Table 3) (https://www.ncbi.nlm.nih.gov/books/NBK546004/)5,6,7,24,33,34. Further investigation is needed to determine optimal treatment goals for chronic hypertension during pregnancy and to evaluate the safety of targeting lower BP thresholds (e.g., 130/80 mmHg), like those recommended for the nonpregnant population (https://www.ncbi.nlm.nih.gov/books/NBK546004/)5,6,7,24,33,34.
Blood glucose
Although BP management is often the primary focus of postpartum care for patients with HDP, evaluation of blood glucose is an important component of comprehensive CVH screening, especially given the association between HDPs and the risk of Type 2 DM1,6. In addition, HDPs can co-occur with GDM; retrospective cohort data suggests these individuals have increased cardiovascular risk compared to HDP or GDM alone10,35. Screening with hemoglobin A1c (HbA1c) or fasting glucose in the first year postpartum should be strongly considered in all individuals with a history of HDP (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6. Individuals with history of GDM should be screened with a 2-hour oral glucose tolerance test, ideally within 12 weeks postpartum, according to recommendations from the American Diabetes Association and ACOG2,6,25,36. A fasting plasma glucose test or HbA1c can be used to screen in the first year postpartum (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6,36.
Programs following Diabetes Prevention Program (DPP) protocols may be considered for individuals with evidence of glucose intolerance on screening6. The DPP was a large multicenter RCT that investigated the impact of intensive lifestyle modification and metformin therapy on individuals at risk of developing Type 2 DM37,38. In analyses of patients with a history of GDM over a follow-up period of three years, participants in the lifestyle modification arm and participants in the metformin arm both had reduced incidence of type 2 DM by ~50% compared to the placebo group, reinforcing the importance of postpartum glycemic control strategies in women at high metabolic risk38. The National DPP is a 12-month lifestyle intervention program established by the U.S. Center for Disease Control using similar protocols as the original DPP study and aimed to decrease rates of type 2 DM6,39. National DPP programs are conducted by coaches with a standard curriculum and include education on diet and exercise strategies and motivational strategies to sustain weight loss6,39. These programs have demonstrated success in postpartum individuals with prior GDM and can be considered depending on local resources and patient needs6,39.
Lipids
Lipid levels, including low density lipoprotein cholesterol (LDL-C) and triglycerides, physiologically rise during pregnancy and are expected to decrease to approximately baseline pre-pregnancy levels by around three months postpartum6,40,41. APOs, including HDPs such as preeclampsia, have been linked to the subsequent development of dyslipidemia1. Given this, lipid screening should be completed once in the first year postpartum, ideally after 12 weeks postpartum (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf; https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation)6. Lactation promotes lipolysis and mobilization of accumulated fat stores, which may impact lipid levels6,42. Lactation additionally may limit pharmacotherapy options (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation)6. Consequently, clinicians may prefer to postpone lipid screening until after completion of lactation, although both the AHA and National Lipid Association suggest that screening can be considered after the 6–12 week postpartum timeframe despite lactation status (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation)6,41.
Results of lipid screening may prompt counseling on lifestyle measures or consideration of pharmacologic treatments based on the assessment of atherosclerotic CVD risk and AHA guidelines6,41,43. Lifestyle modifications include weight management and dietary modifications, such as reducing saturated fat intake and refined carbohydrates (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation.)6,41. Lactation and plans for future pregnancies should be taken into account when considering pharmacologic treatment, and treatment should be individualized for each patient6,44.
Patients meeting the criteria for statins who are not pregnant or lactating should be placed on appropriate pharmacotherapy along with reliable contraception6,41,44. Analysis of the U.S. Patient and Provider Assessment of Lipid Management registry demonstrates women are less likely to receive appropriate statin treatment, underscoring the need to offer treatment to these patients when indicated41,45. Patients should additionally be counseled on the potential need to interrupt statin therapy during future pregnancies and during lactation, although this decision should be individualized based on patient risk6,41,44. For very high-risk individuals, such as those with familial hypercholesterolemia (FH), clinicians should have a risk vs. benefit discussion about continuing statin treatment during pregnancy6,41,46. Decisions on statin treatment in the postpartum timeframe should be individualized based on patient risk and plans for lactation6,41,46. Research suggests that patients with FH may lose up to 14 years of statin treatment associated with pregnancy and lactation-related interruptions, which may deleteriously impact atherosclerotic CVD risk management46. Regarding other lipid-lowering alternatives, bile-acid sequestrants are not systemically absorbed and thus may be used during pregnancy and lactation, although may require supplementation of fat-soluble vitamins and folic acid (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation.)41. Ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and bempedoic acid are not recommended in pregnancy or lactation (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation)41.
Severe hypertriglyceridemia in pregnancy (>500 mg/dL) is associated with increased pancreatitis risk (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation)41. Elevated triglycerides pre-pregnancy, along with other types of dyslipidemia, have been associated with increased APO risk (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation)41. Individuals with GDM are at increased risk of severe gestational hypertriglyceridemia (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation)41. The cornerstone of triglyceride management is lifestyle modification, especially a low-fat diet. Medications such as omega-3 fatty acids may be considered. In patients with symptoms of or history of pancreatitis or severe hypertriglyceridemia, plasma exchange may be considered (https://www.lipid.org/lipid-spin/spring-2024/specialty-corner-lipid-management-pregnancy-and-lactation)41. Bile acid sequestrants should be used with caution in patients with hypertriglyceridemia, as they may increase triglyceride levels41.
Health behaviors: diet, exercise, and tobacco use
As described previously, the postpartum timeframe offers an important opportunity for patient education and counseling on optimization of lifestyle factors for CVH4,6,8. Dietary recommendations are similar to the non-postpartum individual and emphasize the DASH (Dietary Approaches to Stop Hypertension) or Mediterranean diet as the most evidence-based diets to decrease cardiovascular risk6,9. Dietary adjustments in the postpartum period can improve outcomes in subsequent pregnancies – one prospective cohort study of 7798 individuals found that high adherence to a Mediterranean diet around the time of conception is associated with a 21% decreased risk of APO in subsequent pregnancy (adjusted OR 0.79; 95% CI 0.68–0.92)47. Individuals should strive for at least 150 min/week of moderate intensity exercise once they are cleared postpartum for physical activity6,48. Patients should be screened for use of tobacco or nicotine products and provided resources and interventions for lifelong cessation6,49.
Burgeoning evidence demonstrates the benefits of digital health interventions to promote lifestyle changes in postpartum individuals50,51. The Heart Health 4 Moms (HH4M) RCT evaluated a comprehensive digital health intervention involving 9 months of online educational modules, lifestyle coaching, and access to a community forum in 151 participants with a recent history of preeclampsia50. Among the intervention group, 89% completed at least three scheduled coaching calls50. At follow-up, participants in the intervention group demonstrated significantly greater awareness of CVD risk factors (p = 0.01), increased self-efficacy for healthy dietary habits (p = 0.03), and reduced physical inactivity compared to controls (p = 0.0006)50. An RCT of 127 postpartum patients with recent HDP assessed the attainment of activity goals with a wearable step tracker over 12 weeks51. The trial found that a digital health intervention incorporating gamification with virtual teams and scores significantly increased mean daily step count compared to the control group who solely received daily assessment of progress towards step goals51. However, it should be noted that SDOH, such as socioeconomic status, broadband access, and low health or digital literacy, all may impact access to and uptake of digital health technologies52,53. As has been reviewed elsewhere and highlighted in a recent AHA statement, equitable implementation of digital health interventions requires both thoughtful consideration of individual patient factors that may affect adoption of the technology and advocacy for research and policy changes, such as improved broadband access and increased recruitment of underserved populations in digital health clinical trials52,53.
Interventions to promote lifestyle modifications in the postpartum period should take into account the diversity of patient experiences as well as the specific context of the postpartum period and new parenthood. Community-based participatory research models that survey patients and the community to assess their priorities and may include community members in project leadership may be used to optimally tailor research studies and health interventions to patient needs52,53. For example, Kókai et al. report on a survey conducted among patients of the FUPEC study to assess patients’ desires regarding an app to promote lifestyle modifications for improved CVH54. Similar surveys could be used in clinics and in other research projects to facilitate designing targeted interventions for this population, although caution should be used when generalizing surveys with a small sample size.
Weight management
Given the high rates of pre-pregnancy obesity in reproductive-age women, which may be exacerbated by pregnancy weight gain, weight management in the postpartum timeframe is important to reduce cardiovascular and reproductive health risks associated with obesity6,55. Weight and potentially waist circumference are thus important metrics to evaluate at postpartum clinic visits (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf). Behavioral counseling on diet and physical activity as described above facilitates postpartum weight management (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6. Clinics based on the National DPP described earlier may be beneficial to promote lifestyle changes and weight loss6,39. Referrals to other resources, such as dietitians or other weight loss clinics, should additionally be considered as needed (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6.
Glucagon-like peptide 1 (GLP1) receptor agonists (semaglutide, liraglutide) and combined glucose-dependent insulinotropic peptide (GIP)/GLP1 agonists (tirzepatide) offer new strategies to medically treat obesity56,57,58. These medications are not approved for pregnancy or lactation due to lack of data but may be considered in non-pregnant, non-lactating individuals on reliable contraception41,59,60. Limited retrospective data available investigating GLP1 receptor agonist exposure in early pregnancy showed no increased risk of pregnancy loss61 or fetal malformation62, which is reassuring when considering possible inadvertent exposures, but significantly more research is needed on the subject59,60. Additional research is also necessary to investigate the impact of these medications on APO risk and long-term outcomes in reproductive-age women. One retrospective cohort study of over 8,000 individuals demonstrated an association between preconception GLP1 receptor agonist use and decreased risk of several APOs (GDM: OR 0.81, 95% CI 0.72–0.91; HDP: OR 0.84; 95% CI 0.76–0.94), but further data is needed on the subject63.
Reproductive health: lactation, contraception, and pregnancy planning
Postpartum clinic visits may be an opportunity for education about the benefits of breastfeeding, screening for issues with lactation, and referral to lactation consultants as needed(https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6. Lactation has been associated with decreased rates of cardiac risk factors and CVD, although confounding associated with the ability to breastfeed in these retrospective studies does limit the strength of these conclusions (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6,64. Breastfeeding assessment is important to determine appropriate pharmacologic treatment of hypertension, hyperlipidemia, and/or obesity (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)6.
Clinicians should assess contraceptive use for several purposes. Short interpregnancy intervals (<6 months) are associated with increased APO risk in subsequent pregnancy and may interfere with postpartum cardiovascular risk factor management that can decrease risks associated with subsequent pregnancies6,65. In addition, use of certain anti-hypertensives, such as angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, weight management medications, such as GLP-1 receptor agonists, or some lipid-lowering agents may additionally necessitate reliable contraceptive use due to their possible teratogenic effects (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)7.
Significant disparities exist in contraceptive access and use66. Utilization of a multidisciplinary team demonstrated higher rates of postpartum contraception uptake and planning, supporting the important role of postpartum hypertension clinics in facilitating contraceptive use66,67. When determining the type of contraceptive, long-acting reversible contraceptives, such as intrauterine devices and subdermal implants, are highly effective with a less than 1% rate of failure in one year and, for this reason, are often highly recommended66. Estrogen-containing oral contraceptives should be used with caution in those with difficult to control hypertension, as they may exacerbate hypertension6.
Given the risk of reoccurrence of APOs, patients should be counseled on the benefits of improved cardiometabolic health in decreasing APO risk and the importance of careful pregnancy planning to allow for appropriate management during pregnancy to reduce risk (e.g., aspirin use and strict BP control to minimize risk of recurrent preeclampsia) (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)2,6. Clinicians may additionally screen for the risk of severe maternal morbidity in pre-conception visits. The use of validated risk scores may be useful for this purpose68,69.
Social drivers of health
SDOH include socioeconomic, environmental, structural, and psychosocial factors that affect health status and outcomes70,71. SDOH should be assessed and considered when making recommendations regarding cardiovascular risk management, as these factors may affect an individual’s ability to follow recommendations and are associated with poor cardiovascular outcomes6,70,71. For example, considering HDP as a risk factor for CVD, findings from the Nulliparous Pregnancy Outcomes Study indicate that social environmental factors—particularly social demographics—had a greater impact on hypertension development during pregnancy than behavioral factors (i.e., diet, exercise, smoking)72. Additionally, one analysis of pregnant women in National Health Interview Survey data demonstrated that those with the highest quartile SDOH scores were twice as likely to have suboptimal CVH, defined as two or more cardiovascular risk factors, compared to individuals with the lowest quartile SDOH scores (risk ratio 2.05; 95% CI 1.46–2.88)73. There are multiple screening tools available to assess SDOH, and health systems may have a preferred tool incorporated into the electronic medical record (EMR)70,71,74,75. Clinics may cultivate a list of local resources, such as food banks, for SDOH-related needs70,71,75. Use of social workers, nurse navigators, or postpartum doulas may be beneficial for overall care coordination in all patients, regardless of SDOH status6,71.
It is also important to consider SDOH and systemic barriers to care that exist on the basis of race, ethnicity, socioeconomic status, and other marginalized identities when developing a postpartum clinic to optimize access for all individuals6,8,70,71,76. For example, clinics may strive to cultivate a diverse clinic staff that appropriately represents the clinic’s patient population76. Structural and systemic factors such as access to education and insurance, neighborhood socioeconomic status, structural racism, and others are key elements of SDOH and require policy interventions to adequately address70,71,76,77,78. As described earlier, insurance status is a key factor in healthcare access and may be limited depending on the length of Medicaid coverage in the postpartum period (https://www.medicaid.gov/medicaid/benefits/downloads/2024-maternal-health-at-a-glance.pdf). Clinicians in postpartum clinics can advocate for policy interventions related to these barriers to care71,76.
Evaluation of psychological health and sleep in postpartum clinics
As highlighted by a 2025 AHA statement on the topic, maternal psychological health in the perinatal period is intimately linked with APO risk and CVH outcomes23. Meta-analysis and prospective cohort study data both support an increased risk of postpartum depression after a pregnancy complicated by preeclampsia23,79,80. Given this risk of poor postpartum psychological health after APO and associations between perinatal depression and increased CVD risk, assessment of postpartum psychological health is strongly recommended by both the AHA and ACOG23,81. Postpartum clinic visits offer an important opportunity for screening (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)7,23. The Edinburgh Postnatal Depression Scale or Patient Health Questionnaire-9 may be used as validated screening tools for postpartum depression (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)23,82. Clinicians should consider screening for additional risk factors for postpartum depression, such as sleep disturbance, social support, and previous mental health diagnoses6,23,83,84. Clinicians may offer education on symptoms of postpartum depression as well as lifestyle interventions, such as stress management techniques and physical activity23. Referrals to behavioral health can be beneficial to address concerns in this domain, and dependent on local resources, direct integration of psychology into a postpartum hypertension clinic can improve access to care (https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/Sections-Councils/Reproductive-Health-and-Cardio-Obstetrics/ACC-Post-Partum-Hypertension-Clinical-Toolkit.pdf)23,85.
Strategies to improve retention in postpartum clinics
Telehealth and remote blood pressure monitoring
Recent studies have explored the role of telehealth and self BP monitoring in improving postpartum hypertension management (Table 2)11,27,28,30,31,32,86,87. These interventions have shown promising improvements in outcomes and follow-up rates, but best practices for their implementation are still unclear due to significant heterogeneity in protocols and study design11,27,28,30,31,32,86,87. Telehealth follow-up for postpartum hypertension management demonstrated improved visit completion rates at the Hospital of the University of Pennsylvania86 and served a patient population on average living at a greater distance from the clinic at the University of Pittsburgh Medical Center11, suggesting this strategy may increase postpartum care access.
Multiple studies have additionally investigated outcomes associated with remote or self-BP monitoring, with improved BP control noted27,28, decreased postpartum adverse events and emergency department visits31, and increased rates of 6-week postpartum follow-up and anti-hypertensive medication adjustment32. In addition to the improved BP control observed with self-BP monitoring, the Physician-Optimized Postpartum hypertension Treatment Trial (POP-HT) findings suggest improvement in adverse cardiac remodeling in response to BP control as well as improvement in cardiac function27,29. One retrospective cohort study of outcomes after ambulatory BP monitoring at one year postpartum in 200 women with severe preeclampsia demonstrated that 41.5% of women with prior severe preeclampsia had hypertension one year postpartum, underscoring the importance of ambulatory BP monitoring in diagnosing masked hypertension and white-coat hypertension30.
Ultimately, while the evolving data on remote BP monitoring, telehealth, and EMR integration is promising with regard to improving outcomes, increasing follow-up rates, and facilitating access to care, these studies are limited by their heterogeneity and relatively small sample size11,27,28,30,31,32,86,87. Large RCTs on comparative effectiveness and long-term outcomes are necessary to further investigate these strategies. In addition, as described earlier, the impact of digital health interventions such as remote BP monitoring and telehealth, may be limited by accessibility issues and SDOH52,53. Implementation in clinics should be done with careful consideration of the patient population served by this clinic and resources needed, such as broadband and digital devices, to access these strategies52,53. Clinics may work locally to increase resource accessibility as well as advocate for policy change on the state and federal level52,53.
Electronic medical record integration and referral types
An EMR-integrated clinic model for postpartum hypertension care can enhance follow-up, improve outcomes, and reduce long-term cardiovascular risks7,87. A remote BP monitoring protocol with EMR integration facilitating communication among providers and documentation of BP reading and management showed high rates of participant retention at three weeks (83% out of 409 participants) and high rates of attendance at the six-week postpartum visit (88%)87. As outlined by Countouris et al., there are multiple options for referral mechanisms and patient outreach7. Referral mechanisms may include automated/default referrals, clinician-initiated referrals, and patient self-referrals7. Automated referrals, with appointments scheduled before discharge, can streamline access and enrollment7. Systematic chart reviews can identify eligible patients post-discharge, particularly in BP monitoring programs7. Patient outreach methods include brochures, BP program messages, or educational videos, with institutional communications teams assisting in developing awareness materials7. Social media can be an important delivery method for patient education88. EMR-integrated referral pathways have shown benefits in facilitating postpartum screening - Cameron et al. found that a clinician-initiated EMR-integrated postpartum referral pathway to primary care increased the likelihood of primary care visits (48.1%), cholesterol screening (31.8%), and hemoglobin A1c testing (41.9%)89.
Conclusion
In conclusion, structured postpartum hypertension follow-up is vital for reducing maternal morbidity and long-term cardiovascular risks, particularly for individuals with hypertensive disorders of pregnancy such as preeclampsia and gestational hypertension. Research underscores the heightened risk these women face for chronic hypertension, stroke, and cardiovascular disease later in life. Additionally, these clinics offer healthcare providers the opportunity to screen for and initiate the management of other cardiovascular risk factors, such as diabetes, hyperlipidemia, and obesity. Advances like EMR-based tracking, remote BP monitoring, and multidisciplinary clinics have shown significant improvements in blood pressure control and medication adherence. Additionally, integrated community models enhance access to care, especially for underserved populations such as Black and Hispanic individuals, who are disproportionately impacted by these conditions.
However, challenges remain, including inconsistent clinical guidelines and the need for a standardized approach to postpartum hypertension management. While both clinic-based and telehealth interventions have shown promise, further research is necessary to evaluate their scalability and long-term effectiveness. Some of this research could be conducted through postpartum clinics, as these clinics can be a useful site for additional data collection and analysis that may further inform care practices, exemplified by research conducted through the FUPEC study in the Netherlands. Federal and state-level policy initiatives, such as extension of Medicaid coverage to 12 months postpartum or expansion of broadband access, also play an important role in improving postpartum health and facilitating implementation of postpartum CVH programs. The inclusivity and accessibility of any care strategy targeted towards improving CVH in this high-risk population should be evaluated to ensure that strategies address and reduce care disparities. Addressing these issues is crucial for improving maternal health outcomes and preventing future cardiovascular disease. This review calls for ongoing research into comprehensive and scalable care models that effectively bridge clinical practice with digital health innovations, thereby enhancing postpartum hypertension management and reducing health disparities.
Data availability
This manuscript is based on publicly available medical literature and references. All sources used are cited within the manuscript and can be accessed through respective publishers, databases, or repositories.
Abbreviations
- APO:
-
Adverse pregnancy outcome
- HDP:
-
Hypertensive disorder of pregnancy
- DM:
-
Diabetes mellitus
- GDM:
-
Gestational diabetes mellitus
- MI:
-
Myocardial Infarction
- HF:
-
Heart Failure
- OR:
-
Odds Ratio
- CI:
-
Confidence Interval
- ACOG:
-
American College of Obstetrics and Gynecology
- AHA:
-
American Heart Association
- CVH:
-
Cardiovascular health
- CVD:
-
cardiovascular disease
- FUPEC:
-
Follow-up Preeclampsia Outpatient Clinic
- HbA1c:
-
Hemoglobin A1c
- BP:
-
Blood pressure
- RCT:
-
Randomized control trial
- DPP:
-
Diabetes Prevention Program
- DASH:
-
Dietary Approaches to Stop Hypertension
- U.S.:
-
United States
- SDOH:
-
Social drivers of health
- EMR:
-
Electronic medical record
References
O’Kelly, A. C. et al. Pregnancy and reproductive risk factors for cardiovascular disease in women. Circ. Res. 130, 652–672 (2022).
Parikh, N. I. et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation 143, e902–e916 (2021).
Wu, P. et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ: Cardiovasc. Qual. Outcomes 10, e003497 (2017).
Khosla, K. et al. Long-term cardiovascular disease risk in women after hypertensive disorders of pregnancy: recent advances in hypertension. Hypertension 78, 927–935 (2021).
Garovic, V. D. et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American heart association. Hypertension 79, e21–e41 (2022).
Lewey, J. et al. Opportunities in the postpartum period to reduce cardiovascular disease risk after adverse pregnancy outcomes: a scientific statement from the American Heart Association. Circulation 149, e330–e346 (2024).
Countouris, M. et al. Hypertension in pregnancy and postpartum: current standards and opportunities to improve care. Circulation 151, 490–507 (2025).
Mehta, L. S. et al. Call to action: maternal health and saving mothers: a policy statement from the American Heart Association. Circulation 144, e251–e269 (2021).
Brown, H. L. et al. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration With Obstetricians and Gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation 137, e843–e852 (2018).
Lumsden, R., Page, C. B., Phelan, M., Wheeler, S. & Pagidipati, N. Longitudinal Management of Cardiovascular Risk Factors Among Postpartum Women. J. Womens Health 33, 853–862 (2024).
Countouris, M. et al. Feasibility of utilizing telehealth in a multidisciplinary postpartum hypertension clinic. Womens Health Rep. 3, 877–886 (2022).
Mosca, L. et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation 123, 1243–1262 (2011).
Lloyd-Jones, D. M. et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation 146, e18–e43 (2022).
Chen, H. et al. Life’s Essential 8 and cardiovascular disease in women with a history of adverse pregnancy outcomes. Eur. J. Prev. Cardiol. zwaf021, https://doi.org/10.1093/eurjpc/zwaf021 (2025).
Crear-Perry, J. et al. Social and structural determinants of health inequities in maternal health. J. Womens Health 30, 230–235 (2021).
Rokicki, S. et al. Home visits and the use of routine and emergency postpartum care among low-income people: a secondary analysis of a randomized clinical trial. JAMA Netw. Open 7, e2451605 (2024).
Rokicki, S. et al. Impact of nurse home visiting on take-up of social safety net programs in a Medicaid population. Health Aff. Sch. 3, qxaf038 (2025).
Daubert, M. A. et al. Early postpartum blood pressure screening is associated with increased detection of cardiovascular risk factors in women with hypertensive disorders of pregnancy. Am. Heart J. 273, 130–139 (2024).
Cusimano, M. C., Pudwell, J., Roddy, M., Cho, C.-K. J. & Smith, G. N. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. Am. J. Obstet. Gynecol. 210, 438.e1–438.e9 (2014).
Janmohamed, R. et al. Cardiovascular risk reduction and weight management at a hospital-based postpartum preeclampsia clinic. J. Obstet. Gynaecol. Can. 37, 330–337 (2015).
Celi, A. C., Seely, E. W., Wang, P., Thomas, A. M. & Wilkins-Haug, L. E. Caring for women after hypertensive pregnancies and beyond: implementation and integration of a postpartum transition clinic. Matern. Child Health J. 23, 1459–1466 (2019).
van der Bijl, M. F. et al. FUPEC study, a prospective open-cohort on severe pre-eclampsia and cardiovascular risk factors based in the Netherlands. BMJ Open 14, e093423 (2024).
Sharma, G. et al. Optimizing psychological health across the perinatal period: an update on maternal cardiovascular health: ascientific statement from the American Heart Association. J. Am. Heart. Assoc. 14, 1–18 (2025).
Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 135, e237–e260 (2020).
ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstet. Gynecol. 131, e140–e150 (2018).
Hauspurg, A. & Jeyabalan, A. Postpartum preeclampsia or eclampsia: defining its place and management among the hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 226, S1211–S1221 (2022).
Kitt, J. et al. Long-term blood pressure control after hypertensive pregnancy following physician-optimized self-management: the POP-HT randomized clinical trial. JAMA 330, 1991–1999 (2023).
Cairns, A. E. et al. Self-management of postnatal hypertension: the SNAP-HT trial. Hypertension 72, 425–432 (2018).
Kitt, J. et al. Cardiac remodeling after hypertensive pregnancy following physician-optimized blood pressure self-management: the POP-HT randomized clinical trial imaging substudy. Circulation 149, 529–541 (2024).
Benschop, L. et al. Blood pressure profile 1 year after severe preeclampsia. Hypertension 71, 491–498 (2018).
Hirshberg, A., Zhu, Y., Smith-McLallen, A. & Srinivas, S. K. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet. Gynecol. 141, 1163–1170 (2023).
Lemon, L. S. et al. Clinical outcomes associated with a remote postpartum hypertension monitoring program. Obstet. Gynecol. 144, 377–385 (2024).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 71, 1269–1324 (2018).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104 (2018).
Echouffo Tcheugui, J. B., Guan, J., Fu, L., Retnakaran, R. & Shah, B. R. Association of concomitant gestational hypertensive disorders and gestational diabetes with cardiovascular disease. JAMA Netw. Open 5, e2243618 (2022).
ElSayed, N. A. et al. 15. Management of diabetes in pregnancy: standards of care in diabetes—2023. Diab. Care 46, S254–S266 (2023).
Ratner, R. E. An update on the diabetes prevention program. Endocr. Pr. 12, 20–24 (2006).
Ratner, R. E. et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J. Clin. Endocrinol. Metab. 93, 4774–4779 (2008).
Gómez, M. L., Hieronymus, L. B., Ashford, K. B., Barnett, J. M. & Renn, T. A. Linking postpartum and parenting women with a national diabetes prevention program: recruitment efforts, challenges, and recommendations. Diab. Spectr. 31, 324–329 (2018).
Montes, A. et al. Physiologic and supraphysiologic increases in lipoprotein lipids and apoproteins in late pregnancy and postpartum. Possible markers for the diagnosis of ‘prelipemia’. Arteriosclerosis 4, 407–417 (1984).
Agarwala, A. et al. Dyslipidemia management in women of reproductive potential: An Expert Clinical Consensus from the National Lipid Association. J. Clin. Lipido. 18, e664–e684 (2024).
Stuebe, A. M. & Rich-Edwards, J. W. The reset hypothesis: lactation and maternal metabolism. Am. J. Perinatol. 26, 81–88 (2009).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 139, e1082–e1143 (2019).
Grant, J. K. et al. Lipid-lowering therapy in women of childbearing age: a review and stepwise clinical approach. Curr. Cardiol. Rep. 24, 1373–1385 (2022).
Nanna, M. G. et al. Sex differences in the use of statins in community practice: patient and provider assessment of lipid management registry. Circ: Cardiovasc. Qual. Outcomes 12, e005562 (2019).
Klevmoen, M. et al. Loss of statin treatment years during pregnancy and breastfeeding periods in women with familial hypercholesterolemia. Atherosclerosis 335, 8–15 (2021).
Makarem, N. et al. Association of a Mediterranean diet pattern with adverse pregnancy outcomes among US women. JAMA Netw. Open 5, e2248165 (2022).
ACOG Committee Opinion No. 804: Physical Activity and Exercise During Pregnancy and the Postpartum Period: Correction. Obstet. Gynecol. 138, 683–683 (2021).
Tobacco and Nicotine Cessation During Pregnancy: ACOG Committee Opinion, Number 807. Obstet. Gynecol. 135, e221–e229 (2020).
Rich-Edwards, J. W. et al. Randomized trial to reduce cardiovascular risk in women with recent preeclampsia. J. Womens Health 28, 1493–1504 (2019).
Lewey, J. et al. Effectiveness of a text-based gamification intervention to improve physical activity among postpartum individuals with hypertensive disorders of pregnancy: a randomized clinical trial. JAMA Cardiol. 7, 591–599 (2022).
Kim, D. S., Eltahir, A. A., Ngo, S. & Rodriguez, F. Bridging the gap: how accounting for social determinants of health can improve digital health equity in cardiovascular medicine. Curr. Atheroscler. Rep. 27, 9 (2024).
Powell-Wiley, T. M. et al. Role of technology in promoting heart healthy behavior change to increase equity in optimal cardiovascular health: a scientific statement from the American Heart Association. Circulation 151, e972–e985 (2025).
Kókai, L. L. et al. Needs and preferences of women with prior severe preeclampsia regarding app-based cardiovascular health promotion. BMC Womens Health 22, 427 (2022).
Ogunwole, S. M., Zera, C. A. & Stanford, F. C. Obesity management in women of reproductive age. JAMA 325, 433–434 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 389, 2221–2232 (2023).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Sharma, G. Treating reproductive-aged women with glucagon-like peptide-1 receptor agonists: what are the clinical considerations?. Circulation 150, 1487–1489 (2024).
Drummond, R. F., Seif, K. E. & Reece, E. A. Glucagon-like peptide-1 receptor agonist use in pregnancy: a review. Am. J. Obstet. Gynecol. 232, 17–25 (2025).
Dao, K. et al. Use of GLP1 receptor agonists in early pregnancy and reproductive safety: a multicentre, observational, prospective cohort study based on the databases of six Teratology Information Services. BMJ Open 14, e083550 (2024).
Cesta, C. E. et al. Safety of GLP-1 receptor agonists and other second-line antidiabetics in early pregnancy. JAMA Intern. Med. 184, 144–152 (2024).
Imbroane, M. R., LeMoine, F. & Nau, C. T. Preconception glucagon-like peptide-1 receptor agonist use associated with decreased risk of adverse obstetrical outcomes. Am. J. Obstet. Gynecol. S0002937825000419; https://doi.org/10.1016/j.ajog.2025.01.019 (2025).
Schwarz, E. B. et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet. Gynecol. 113, 974–982 (2009).
Obstetric Care Consensus No. 8: Interpregnancy Care. Obstet Gynecol 133, e51–e72; (2019).
Lindley, K. J. et al. Contraception and reproductive planning for women with cardiovascular disease: JACC focus seminar 5/5. J. Am. Coll. Cardiol. 77, 1823–1834 (2021).
Miller, H. E. et al. Addressing postpartum contraception practices utilizing a multidisciplinary Pregnancy Heart Team approach. AJOG Glob. Rep. 2, 100100 (2022).
Murugappan, G., Alvero, R. J., Lyell, D. J., Khandelwal, A. & Leonard, S. A. Development and validation of a risk prediction index for severe maternal morbidity based on preconception comorbidities among infertile patients. Fertil. Steril. 116, 1372–1380 (2021).
Leonard, S. A., Kennedy, C. J., Carmichael, S. L., Lyell, D. J. & Main, E. K. An expanded obstetric comorbidity scoring system for predicting severe maternal morbidity. Obstet. Gynecol. 136, 440–449 (2020).
Powell-Wiley, T. M. et al. Social determinants of cardiovascular disease. Circ. Res. 130, 782–799 (2022).
Hurwitz, M., Bonomo, J., Spitz, J. & Sharma, G. Intersectionality and social drivers of health in cardiovascular care. Methodist Debakey Cardiovasc. J. 20, 98–110 (2024).
Keith, M. H. & Martin, M. A. Social determinant pathways to hypertensive disorders of pregnancy among nulliparous U.S. women. Womens Health Issues 34, 36–44 (2024).
Sharma, G. et al. Social determinants of suboptimal cardiovascular health among pregnant women in the United States. J. Am. Heart Assoc. 11, e022837 (2022).
Chen, M., Tan, X. & Padman, R. Social determinants of health in electronic health records and their impact on analysis and risk prediction: a systematic review. J. Am. Med. Inf. Assoc. 27, 1764–1773 (2020).
White-Williams, C. et al. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation 141, e841–e863 (2020).
Lindley, K. J. et al. Socioeconomic determinants of health and cardiovascular outcomes in women. J. Am. Coll. Cardiol. 78, 1919–1929 (2021).
Metlock, F. E. et al. Impact of social determinants of health on hypertension outcomes: a systematic review. Hypertension 81, 1675–1700 (2024).
Churchwell, K. et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation 142, e454–e468 (2020).
Caropreso, L., De Azevedo Cardoso, T., Eltayebani, M. & Frey, B. N. Preeclampsia as a risk factor for postpartum depression and psychosis: a systematic review and meta-analysis. Arch. Womens Ment. Health 23, 493–505 (2020).
Roberts, L., Henry, A., Harvey, S. B., Homer, C. S. E. & Davis, G. K. Depression, anxiety and posttraumatic stress disorder six months following preeclampsia and normotensive pregnancy: a P4 study. BMC Pregnancy Childbirth 22, 108 (2022).
Screening and Diagnosis of Mental Health Conditions During Pregnancy and Postpartum: ACOG Clinical Practice Guideline No. 4. Obstet. Gynecol. 141, 1232–1261 (2023).
ACOG Committee Opinion No. 757: Screening for Perinatal Depression. Obstet. Gynecol. 132, e208–e212 (2018).
Baattaiah, B. A. et al. The relationship between fatigue, sleep quality, resilience, and the risk of postpartum depression: an emphasis on maternal mental health. BMC Psychol. 11, 10 (2023).
Lewis, B. A., Gjerdingen, D., Schuver, K., Avery, M. & Marcus, B. H. The effect of sleep pattern changes on postpartum depressive symptoms. BMC Womens Health 18, 12 (2018).
Smolderen, K. G. et al. The role of the clinical psychologist in the care of adults with cardiovascular disease. JACC Adv. 3, 100910 (2024).
Sanghavi, M. et al. Telemedicine may increase visit completion rates in postpartum patients with preeclampsia. PLoS One 17, e0275741 (2022).
Hauspurg, A. et al. A postpartum remote hypertension monitoring protocol implemented at the hospital level. Obstet. Gynecol. 134, 685–691 (2019).
Ladeiras-Lopes, R. et al. Social media in cardiovascular medicine: a contemporary review. Eur. Heart J. Digit Health 1, 10–19 (2020).
Cameron, N. A. et al. An enhanced postpartum transition program to primary care. J. Womens Health 33, 1417–1422 (2024).
Author information
Authors and Affiliations
Contributions
S.M.M. – initial drafting, revision and editing; M.H. – initial drafting, revision and editing; Y.K. - initial drafting, revision and editing; E.B. - initial drafting, revision and editing; J.S. – revision and editing; L.D. – revision and editing; F.M. – revision and editing; A.S. – revision and editing; A.S. – revision and editing; A.K. – revision and editing; G.S. – revision and editing; S.M.M./M.H. – contributed equally (co-author).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
McCoy, S.M., Hurwitz, M., Kwapong, Y.A. et al. Advances in postpartum hypertension management: a review of current guidelines and interventions. npj Womens Health 3, 47 (2025). https://doi.org/10.1038/s44294-025-00095-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44294-025-00095-7