Abstract
Previous preclinical studies suggested that Shexiang Tongxin Dropping Pill (STDP) would be effective for coronary slow flow phenomenon (CSFP), but its clinical efficacy and safety remain uncertain. A multicenter, randomized, controlled phase IV trial was implemented to enroll 200 participants diagnosed with angina and CSFP between July 2016 and August 2020 to examine the drug’s effectiveness and safety. The analysis revealed that corrected TIMI frame count (CTFC) values in left anterior descending (LAD) and left circumflex (LCX) arteries in STDP group could be decreased, respectively (both p < 0.01), whereas no significant changes were seen with placebo; between group differences were significant for both LAD (p = 0.008) and LCX (p = 0.044). Furthermore, STDP was well tolerated. Taken together, the results demonstrated STDP could significantly improve coronary blood flow and maintain an excellent safety profile, making it a favorable option in clinic for angina patients with CSFP. Trial registration: The trial was registered in Chinese Clinical Trial Registry (ID: ChiCTR-IPR-16008950).
Similar content being viewed by others
Introduction
The coronary slow flow phenomenon (CSFP) was first reported by Tambe et al.1 in 1972, who observed delayed vascular opacification during coronary angiography. CSFP is characterized by the presence of delayed distal perfusion visualized on coronary angiography, even in the absence of significant coronary lesions (stenosis < 40%)2,3. The clinical burden of CSFP is considerable; its incidence among patients undergoing conventional coronary angiography ranges from 1 to 7%, and among those with clinically suspected angina, it varies between 5.5 and 34.0%4,5,6,7.
A substantial body of research has established a significant association between slow coronary blood flow and poor clinical outcomes8,9,10. CSFP can precipitate severe adverse cardiovascular events, including myocardial infarction and sudden death. The management of CSFP is particularly challenging due to the absence of standardized treatment guidelines and the limited efficacy of current medications. Presently, therapeutic strategies are primarily aimed at enhancing microcirculatory function through a multimodal approach.
In recent years, the integration of modern medical technology and traditional Chinese medicine has brought remarkable advance in the management of CSFP. Shexiang Tongxin Dropping Pill (STDP), a traditional Chinese medicine approved by the Chinese FDA in 2008, is composed of Salvia miltiorrhiza Bunge, Moschus, Bovis Calculus Artifactus, Bufonis Venenum, Borneolum Syntheticum, total ginsenoside of ginseng stems and leaves, and Fel Ursi. Based on the retention time and MS spectra, forty-one constituents were identified in STDP, including bile acids, salvianolic acids, triterpene saponins, bufadienolides, and tanshinones11. STDP has been widely used in China for the treatment of stable coronary heart disease. Recent investigations have demonstrated that STDP offers beneficial effects such as endothelial and vascular protection, plaque stabilization, and lipid metabolism modulation, in addition to reducing the expression of serum inflammatory and oxidative stress factors12,13,14.
Based on these mechanistic insights, a pilot trial involving 22 CSFP patients was conducted, revealing rapid improvements in coronary blood flow as early as five minutes following the sublingual administration of STDP15. Therefore, we performed a randomized, double-blind, placebo-controlled clinical trial to rigorously evaluate the long-term efficacy and safety of STDP for angina patients with CSFP in real-world clinical settings.
Results
Patient characteristics
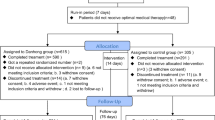
A total of 199 patients were randomly assigned to either the STDP group (n = 100) or the placebo group (n = 99), with one patient withdrew consent (Fig. 1). The mean age was 60.30 years in STDP group and 61.37 years in placebo group, with no significant difference between the two group (p = 0.435). The duration of chronic angina pectoris history was comparable between the two groups (11.00 [95%CI:1.00, 36.00] vs. 12.00 [95%CI:1.00, 36.50], p = 0.519). A significantly higher proportion of patients in the placebo group had diabetes compared to the STDP group (22.47% vs. 8.99%, p = 0.014). Other demographic and baseline characteristics, including etiological factors of CSF such as smoking, hypertension, and hyperlipidemia, as well as angiographic features, were balanced between the groups (p > 0.05) (Table 1, Fig. 2).
This diagram illustrates the screening, randomization, allocation, follow-up, and analysis processes of the randomized, double-blind, placebo-controlled, multicenter trial. A total of 200 participants were enrolled and randomly assigned to either the STDP group or the placebo group. The final analysis included 199 participants who completed the study. V1, screening baseline phase (from hospital admission to the first coronary angiography) V2, medication phase 1 (sublingual medication to the second coronary angiography). Abbreviations: CTFC corrected TIMI frame count, STDP Shexiang Tongxin Dropping Pill, FAS full analysis set, PPS per protocol set, SS Safety Set.
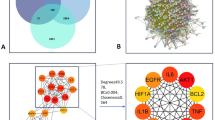
Venn-based jvenn visualization of CTFC improvements in coronary branches for the Placebo (A) and STDP (B) group. The improvements of CTFC in different coronary branches of patients in two groups were shown in the Venn images (A, B). Abbreviations: LAD left anterior descending coronary artery, LCX left circumflex artery, RCA right coronary artery, CTFC corrected TIMI frame count, STDP Shexiang Tongxin Dropping Pill.
Furthermore, no statistically significant differences were observed in baseline oral medication usage between the groups. The proportions of patients using calcium channel blockers, beta-blockers, statins, ACE inhibitors, ARBs, antiplatelet agents, and long-acting nitroglycerin were comparable between two groups (p > 0.05) (Table 2).
Primary outcomes
Overall, a statistically significant difference in the average CTFC was observed between the two groups (p = 0.003). In the LAD branch, patients in the STDP group demonstrated a marked reduction in CTFC values, declining from 47.06 (95% CI: 39.08–58.24) at baseline to 42.94 (95% CI: 34.12–55.29) after post-treatment. The mean change was −4.12 (95% CI: −10.59–0.59), which was statistically significant (p < 0.001). In contrast, the placebo group exhibited no significant change in LAD CTFC (p = 0.540). The between-group difference in LAD CTFC improvement was also statistically significant (p = 0.008). For the LCX branch, the STDP group showed a significant decrease in CTFC from 54.00 (95% CI: 40.71–66.00) at baseline to 48.00 (95% CI: 40.71–61.00) post-treatment, yielding a mean change of −4.00 (95% CI: −14.00–4.00; p = 0.001). The placebo group, however, did not exhibit a meaningful change (p = 0.343). A statistically significant difference was observed between the two groups in terms of LCX CTFC reduction (p = 0.044). In the case of the RCA branch, although a downward trend was observed in the STDP group (from 53.50 [95% CI: 39.00–72.86] to 53.00 [95% CI: 38.71–66.43]), the change of −1.00 (95% CI: −13.29–7.00) did not reach statistical significance (p = 0.082). Similarly, the placebo group exhibited a slight, non-significant increase in CTFC (p = 0.563), and the difference in change between groups was not statistically significant (p = 0.073) (Figs. 3 and 4). The CTFC values can be found in Supplementary Table 1.
Error plots show the median CTFC values including LAD (A), LCX (B), RCA (C) and average changes (D) from baseline to post-treatment for both the STDP and placebo groups. *p < 0.05, **p < 0.01, ***p < 0.001. Abbreviations: LAD Left anterior descending coronary artery, LCX Left circumflex artery, RCA right coronary artery, STDP Shexiang Tongxin Dropping Pill, CTFC corrected TIMI frame count.
Radar chart illustrating the differences in CTFC improvement across LAD, LCX, and RCA branches between the STDP (A) and placebo (B) groups. Abbreviations: LAD Left anterior descending coronary artery, LCX Left circumflex artery, RCA right coronary artery, STDP Shexiang Tongxin Dropping Pill, CTFC corrected TIMI frame count.
In addition, in the comparison of the distribution of delayed coronary perfusion, the performance of the Placebo group and the STDP group showed the following characteristics: LAD (left anterior descending artery) was the most commonly affected vessel in both groups, with the incidence in the Placebo group reaching 97.98% (97/99 cases), slightly higher than the 94.95% (94/99 cases) in the STDP group; the LCX (left circumflex artery) involvement rate was 93.94% (93 cases) in the STDP group and 89.90% (89 cases) in the Placebo group; the RCA (right coronary artery) involvement rate had the smallest difference between the two groups, with 74.75% (74 cases) in the STDP group and 72.73% (72 cases) in the Placebo group.
Secondary outcomes
Significant within-group improvements were observed in both the STDP and placebo groups from baseline to 4-week after treatment across SAQ, including physical limitation, angina stability, angina frequency, treatment satisfaction, and illness perception (p < 0.001). However, the magnitude of improvement did not differ significantly between the two groups (p > 0.05) (Fig. 5).
Error plots display the scores for summary score (a), physical limitation (b), angina stability (c), angina frequency (d), treatment satisfaction (e), and illness perception (f) in both the STDP and placebo groups. ***p < 0.001. Abbreviations: SAQ Seattle angina questionnaire, STDP Shexiang Tongxin Dropping Pill.
Regarding ECG parameters, no significant changes in ST segment depression were observed in either group (p = 0.351), with no significant difference between the groups (p = 0.702). In contrast, T-wave inversion significantly decreased in the placebo group at 4-week after treatment (p = 0.025), whereas the STDP group showed a non-significant increase (p = 0.105); notably, the between-group difference for T-wave inversion was statistically significant (p = 0.035). The SAQ scores and ECG parameters are presented in Supplementary Table 2.
Safety and tolerability
No MACE events occurred in all patients. The proportion of patients reporting at least one AE was similar between the STDP and placebo groups (22 [22.00%] vs. 25 [25.25%], respectively; p = 0.589). The most common adverse events were hyperlipidemia, circulatory and urinary-related symptoms and gastrointestinal symptoms (Table 3); none of these were considered treatment-related. Additionally, a total of 9 serious AEs occurred during the study period, with 7 in the placebo group and 2 in the STDP group. Among these, 2 serious AEs in the placebo group (drug allergy and multiple lacunar cerebral infarctions) were not definitively attributed to the study treatments, while the other 7 events (micronodular cirrhosis, bilateral cataracts, allergic dermatitis, sick sinus syndrome, pre-excitation syndrome, acute lower extremity arterial thrombosis, and benign nasopharyngeal tumors) were considered unrelated to treatment. Also, the post-administration vital signs did not differ significantly between the two groups (Tables 4 and 5).
Discussion
This study demonstrated that STDP could significantly improve coronary blood flow in patients with CSFP, particularly in LAD and LCX arteries, as evidenced by a significant reduction in CTFC compared with placebo. Also, no treatment-related AEs were identified, and the incidence of serious AEs was lower in the STDP group than in the placebo group. These findings suggest that STDP may offer potential benefits in improving coronary microvascular perfusion in angina patients with CSFP without compromising safety.
Our study showed a significant reduction in CTFC of both LAD and LCX arteries within the STDP group, compared with the placebo group. Although CTFC of RCA branch did not exhibit a statistically significant improvement, a positive trend was nonetheless observed in the treatment group compared to the control group. Moreover, both groups showed enhancements across the SAQ. These findings align with previous studies. The pilot study12 showed that 22 patients with slow coronary artery blood flow experienced a decrease in thrombolytic therapy frame counts for myocardial infarction after taking STDP, as evidenced by coronary angiography performed five minutes later (p < 0.05). Additionally, another study discovered that the microcirculatory resistance index was lower in the observation group treated with STDP compared to the control group, and both SAQ and Canadian Cardiovascular Society (CCS) scores were superior (p < 0.05)16. Collectively, these results reinforce the efficacy of STDP in CSFP treatment, thereby providing robust support for clinical interventions.
The pathogenesis of CSFP remains incompletely understood, while multiple studies have suggested potential associations with coronary microvascular dysfunction (CMD), endothelium-dependent vasodilatory abnormalities, abnormal blood cell morphology and function, and inflammatory responses17,18. It has been established that STDP treatment significantly decreases the pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β), and oxidative stress markers such as malondialdehyde (MDA)13,14,19. Moreover, STDP enhances angiogenesis by upregulating of the PI3K/Akt/mTORC1 signaling pathway, which increases the release of vascular endothelial growth factor (VEGF-A) and facilitates endothelial cell proliferation and migration20. In CMD models, STDP improves endothelial function by inhibiting the Dectin-1/Syk/IRF5 and S1PR2/RhoA/ROCK signaling pathways, ultimately enhancing endothelial barrier function and reducing microvascular leakage21,22. Furthermore, studies in cerebral microvasculature have demonstrated that STDP both suppresses the TXNIP/NLRP3 inflammasome—restoring oxidative balance and blood–brain barrier integrity—and enhances endothelial cystathionine-γ-lyase (CSE) expression with H₂S production, contributing to the inactivation of P66shc, thereby restoring mitochondrial respiration and alleviating cerebral microvascular dysfunction23,24. Collectively, these mechanisms are closely linked to the observed improvements in CTFC and relief of angina symptoms in this study, thereby providing robust theoretical support for the further exploration and optimization of clinical treatment strategies.
In this study, we observed a significant imbalance in the baseline prevalence of diabetes between the STDP and placebo groups, with a higher proportion of diabetic patients in the placebo group. Given the known influence of diabetes on coronary microvascular function, this raised potential concerns regarding confounding. To address this, we performed rigorous multivariate analyses adjusting for diabetes status, baseline CTFC, and center effects (Supplementary Table 3). The results confirmed that the beneficial effects of STDP on coronary blood flow, particularly in the LAD and overall coronary circulation, remained significant after accounting for diabetes (Supplemental Table 3). However, the effects in LCX and RCA branches were not statistically significant after adjustment. This suggests that the therapeutic efficacy of STDP is not solely driven by differences in diabetes prevalence and may exert direct beneficial effects on coronary microvascular function in CSFP patients regardless of diabetic status. Nonetheless, it is important to acknowledge that the relatively small number of diabetic patients limited the power to fully explore interaction effects and subgroup analyses stratified by diabetes. Future larger-scale trials with pre-specified stratification based on diabetic status are warranted to further elucidate the interplay between diabetes and STDP efficacy. Moreover, the observed imbalance reflects the pragmatic, real-world nature of our trial and underscores the external validity of our findings, providing clinically relevant insights into the management of CSFP patients with diverse metabolic profiles.
We noted a statistically significant difference in baseline high-density lipoprotein (HDL) levels between the two groups. Since this study was conducted using a randomized design and no significant differences were found in other baseline characteristics, the HDL disparity may be attributable to random variation. However, HDL is known to influence cardiovascular health and endothelial function, which raises the possibility that this baseline imbalance could impact outcomes. We acknowledged this limitation and suggest that future studies should include larger sample sizes and incorporate multivariate adjustments for lipid profiles, including HDL, to more accurately assess the independent efficacy of STDP.
Our study has strong strengths. Firstly, it is a randomized, double-blind clinical trial, which can reduce the selection biases. Secondly, not only the immediate coronary flow changes assessed by angiography were compared, but also the angina symptoms and ECGs parameters were evaluated after administration of the drug for 2 months, which could give some added information of the safety and efficacy of the STDP.
This study has several limitations. First, the quantitative assessment of angina symptoms at enrollment was limited, and more precise symptom evaluation tools should be incorporated in future research to better stratify patient risk. Second, the trial design included angina patients randomized to placebo, which could potentially compromise patient safety. However, this risk was mitigated by strict safety protocols, including continuation of baseline standard therapies, prohibition of certain antianginal medications during the study period, use of nitroglycerin only for acute relief, and continuous inpatient monitoring. These measures effectively protected all participants, including those in the placebo arm, and no serious adverse events related to the placebo or trial design occurred. It is important to note that the quantitative assessment of angina symptoms used in the inclusion criteria had some limitations, which we aim to refine in future studies. Finally, the study population was limited to Chinese patients, so generalizability to other ethnic groups remains to be established.
Methods
Study design and participants
This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the central Institutional Review Board of Shanghai Changzheng Hospital affiliated with Naval Medical University (Approval No. STDP-2016-V2.0) and by local ethics committees of each participating center. Written informed consent was obtained from all participants prior to enrollment.
A randomized, double-blind, multicenter, placebo-controlled trial was conducted across ten centers in China between July 2016 and August 2020. The trial was registered prospectively in the Chinese Clinical Trial Registry (ChiCTR-IPR-16008950) on 1 July 2016. The reporting of this trial follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines, and the completed CONSORT checklist is provided in the Supplementary Materials. A detailed overview of the study design is presented in the flowchart (Fig. 1). The trial was performed at the following sites: (1) Shanghai Changzheng Hospital, Shanghai; (2) Shanghai Xinhua Hospital, Shanghai; (3) Yueyang Hospital of Integrated of Traditional Chinese and Western Medicine, Shanghai; (4) Ningbo First Hospital, Ningbo; (5) Fujian Provincial Hospital, Fuzhou; (6) Jiaxing Second Hospital, Jiaxing; (7) The Third Xiangya Hospital of Central South University, Changsha; (8) Renmin Hospital of Wuhan University, Wuhan; (9) Puai Hospital of Wuhan City, Wuhan; (10) Northern Jiangsu People’s Hospital, Yangzhou (Fig. 6).
Patients aged 18 to 75 years who presented with angina or angina-like symptoms were screened for eligibility via coronary angiography. CSFP was diagnosed when at least one major coronary artery exhibited delayed perfusion on angiography, with the findings confirmed by both investigator and coordinator. The main basis for judging CSFP is the filling speed of the contrast agent at the end of the blood vessels during coronary angiography, using the corrected TIMI blood flow frame count (CTFC). Coronary angiography is performed, and CSF is diagnosed after corrected TIMI frame count (the average frame number of LAD divided by 1.7 is used as the corrected TIMI frame number, and the average TIMI frame number of the three coronary arteries> 27 frames is defined as slow coronary blood flow). Key exclusion criteria included poorly controlled hypertension, severe cardiac dysfunction or arrhythmia, severe infections, moderate to severe anemia, and severe primary diseases of the hepatic, renal, or hematopoietic systems. Additionally, pregnant or lactating women, patients with a history of recent surgery, those with bleeding tendencies within past four weeks were excluded (Table 6).
Reporting in this article followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines. The protocol was approved by the central Institutional Review Board of Shanghai Changzheng Hospital affiliated to the Naval Medical University (approval number: STDP-2016-V2.0), as well as by the local institutional review boards of each site if required. Written informed consent was obtained from all participants before enrollment. The coordinating investigator ensured effective oversight by regularly convening meetings— via teleconference or face-to-face — to review the status of the trial with all investigators. Data supporting the findings of this study are available from the corresponding author (L.C.) upon reasonable request.
CSF definition and analysis
At least four views of the left coronary artery and two views of the right coronary artery (RCA) were performed during coronary angiography. Coronary blood flow was quantitatively assessed using the thrombolysis in myocardial infarction frame count (TFC) technique on cineangiographic sequences recorded at 30 frames per second. The first frame count was defined as the frame in which concentrated dye completely occupied the proximal coronary artery lumen–making contact with both vessel borders–and exhibited forward motion. The last frame was designated when the leading edge of the contrast column initially reached the distal end. The distal end was defined as follows: for the left anterior descending artery (LAD), it was the distal bifurcation; for the left circumflex artery (LCX), it was the distal bifurcation of the segment with the longest total distance; and for the right coronary artery (RCA), it was the first branch of the posterolateral artery. CSFP was diagnosed when TFC > 27 in at least one coronary artery. The LAD frame counts were corrected by dividing by 1.7 to obtain the corrected TFC (CTFC)25.
All angiograms were analyzed in the Angiographic Core Laboratory at Shanghai Changzheng Hospital. Two reviewers, along with a senior reviewer were blinded to clinical outcomes, simultaneously determined the frame counts. If the discrepancy between reviewers was ≤5 frames, the senior reviewer’s determination was accepted. In cases where the variation >5 frames among any of the three readings, a consensus reading was performed by all three reviewers. If consensus could not be reached, the final frame count was determined by the director of the core laboratory.
Randomization
The random allocation sequence indicating assignment to treatment or placebo group was generated in SAS ([Strategic Applications Software], version 9.1.3) by statisticians from Department of Statistics, Navy Medical University. The sequence was sealed in envelopes and stored in the major study unit and statistic unit. These envelopes kept closed until the study ended.
Intervention
Angiography was performed immediately upon arrival at the cardiac catheterization laboratory and repeated five minutes following the administration of four pills of STDP or placebo. Subsequently, patients in the STDP group received a regimen consisting of two pills (35 mg per pill; Inner Mongolia Conba Pharmaceutical Group, Co., Ltd., Inner Mongolia, China) administered three times daily over a period of four weeks. The placebo, identical in appearance and smell, was administered following the same schedule.
During the trial, traditional Chinese medicine related to the treatment of coronary heart disease cannot be taken orally, intravenously; for the treatment of coronary heart disease combined with hypertension, hyperlipidemia, diabetes, etc., aspirin, ACEI, ARB, statins, and beta-blockers can be used in combination. After signing the informed consent form, the original treatment plan is maintained, and the original treatment plan is not allowed to be changed during the treatment process; during the study, only nitroglycerin is used to relieve acute angina attacks. The specification of nitroglycerin is: 0.5 mg/tablet, which is uniformly provided by the sponsor. In clinical trials, it is prohibited to use medium- and long-acting nitrates, calcium ion antagonists (verapamil, diltiazem), nicorandil, trimetazidine, etc. to treat angina pectoris and its complications of coronary heart disease.
During the study, if the patient’s condition worsens and other critical conditions occur, medium- and long-acting nitrates and calcium ion antagonists can be considered, but the drugs, usage, dosage, causes and relief must be recorded truthfully, but the cases in this case will be treated as invalid cases and only enter the safety analysis.
All patients in this study were hospitalized and the researchers closely monitored them to ensure their safety.
The entire clinical trial is divided into two phases (Fig. 1). The first phase is the screening/baseline phase, which is from hospital admission to the first coronary angiography; the second phase is the medication follow-up phase, which requires observation and recording of relevant indicators before and after medication. (1) V1: screening baseline phase (from hospital admission to the first coronary angiography) (2) V2: medication phase 1 (sublingual medication to the second coronary angiography) (3) V3: medication phase 1 (the first day after surgery) (4) V4: medication phase 2 (28 ± 7 days) (5) V5: follow-up phase (56 ± 7 days).
Study outcomes
The primary outcome was the change in CTFC values in the LAD, LCX, and RCA before and after sublingual drug administration. Secondary endpoints included angina-specific health status, as measured by Seattle Angina Questionnaire (SAQ), as well as alterations in electrocardiogram (ECG) parameters.
Adverse events (AEs) were documented in Case Report Forms throughout the trial, and their potential association with the study medication was carefully evaluated by the investigators. Major adverse cardiovascular events (MACE) were defined as a composite outcome consisting of all-cause mortality, non-fatal myocardial infarction, and non-fatal stroke. Additionally, vital signs such as heart rate and respiratory rate were recorded.
Statistical analysis
The sample size calculation was based on mean CTFC values from a prior pilot study. Assuming SD of 9 for the change in CTFC values attributable to the intervention, it was estimated that 44 participants each group would be required to achieve 80% statistical power to detect a significant difference using a two-tailed test with an alpha level of 0.05. To accommodate an anticipated dropout rate of approximately 20%, the total sample size was increased to 108 participants.
Statistical analyses were conducted according to the intent-to-treat (ITT) principle and were performed on the full analysis set (FAS). The FAS comprised all randomized participants who had completed baseline assessments and at least one post-baseline efficacy evaluation. An analysis of covariance (ANCOVA) was conducted for post-treatment CTFC while adjusting for pre-treatment CTFC. Means and standard deviations were used to summarize continuous data with a normal distribution, while medians and interquartile ranges were used for data with an un-normal distribution. Group comparisons for continuous variables were conducted by two-sample t-tests or Wilcoxon’s Rank Sum tests for data depending on whether the data followed a normal distribution. Differences between pre- and post-treatment values within groups were assessed using paired t-tests or Wilcoxon’s signed rank tests depending on whether the data followed a normal distribution. Categorical data comparisons were conducted using Chi-square tests, with Fisher’s exact test applied when expected frequencies in any cell count below five. The angiographic features of CSFP were also compared between groups using the jvenn visualization tool26. All data analyses were performed using SAS software (version 9.4, SAS Institute, Inc., Cary, NC), and a p-value of less than 0.05 was considered statistically significant.
Data availability
The data that support the findings of this study are available from Shanghai Changzheng hospital but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Shanghai Changzheng hospital.
References
Tambe, A. A., Demany, M. A., Zimmerman, H. A. & Mascarenhas, E. Angina pectoris and slow flow velocity of dye in coronary arteries–a new angiographic finding. Am. Heart J. 84, 66–71 (1972).
Chalikias, G. & Tziakas, D. Slow coronary flow: pathophysiology, clinical implications, and therapeutic management. Angiology 72, 808–818 (2021).
Ghunaim, A. H. et al. Disparities in therapies for coronary artery disease with reduced left ventricular ejection fraction. Vessel Plus 7, 29 (2023).
Kodama T., Kondo T., Oida A., Fujimoto S., Narula J. Computed tomographic angiography-verified plaque characteristics and slow-flow phenomenon during percutaneous coronary intervention. JACC Cardiovasc. Interv. 5, 636–643 (2012).
Hawkins, B. M., Stavrakis, S., Rousan, T. A., Abu-Fadel, M. & Schechter, E. Coronary slow flow–prevalence and clinical correlations. Circ. J. 76, 936–942 (2012).
Simone, F. ezzi et al. Coronary physiology in the catheterization laboratory: current practices, historical insights, and future directions. Cardiol. Plus 10, 217–234 (2025).
La, S., Tavella, R., Pasupathy, S. & Beltrame, J. F. Clinico-pathophysiological considerations in coronary microvascular disorders. Vessel Plus 6 (2022).
Mareai, R. M. et al. Prognostic implication of coronary slow flow assessed by cTFC in patients with myocardial infarction with Non-obstructive coronary arteries. Eur. J. Intern. Med. 108, 74–80 (2023).
Montone, R. A. et al. Coronary slow flow is associated with a worse clinical outcome in patients with Takotsubo syndrome. Heart 106, 923–930 (2020).
Wang, X. & Nie, S. P. The coronary slow flow phenomenon: characteristics, mechanisms and implications. Cardiovasc. Diagn. Ther. 1, 37–43 (2011).
Chen, D. et al. Qualitative and quantitative analysis of the major constituents in Shexiang Tongxin dropping pill by HPLC-Q-TOF-MS/MS and UPLC-QqQ-MS/MS. Molecules 20, 18597–18619 (2015).
Lin, Y. J., Jiao, K. L., Liu, B., Fang, L. & Meng, S. Antiplatelet and myocardial protective effect of Shexiang Tongxin Dropping Pill in patients undergoing percutaneous coronary intervention: A randomized controlled trial. J. Integr. Med. 20, 126–134 (2022).
Liu, H. et al. Shexiang Tongxin dropping pill protects against sodium laurate-induced coronary microcirculatory dysfunction in rats. J. Tradit. Chin Med. 41, 89–97 (2021).
Xiong, M. et al. Shexiang Tongxin dropping pill attenuates atherosclerotic lesions in ApoE deficient mouse model. J. Ethnopharmacol. 159, 84–92 (2015).
Wang, S. H. et al. Effect of Shexiang Tongxin Dropping Pills () on the Immediate blood flow of patients with coronary slow flow. Chin. J. Integr. Med. 25, 360–365 (2019).
Zhao, Y. Q. et al. Shexiang Tongxin dropping pill improves stable angina patients with phlegm-heat and blood-stasis syndrome: a multicenter, randomized, double-blind, placebo-controlled trial. Chin. J. Integr. Med. 31, 685–693 (2025).
Huang, Z. & Sun, A. Metabolism, inflammation, and cardiovascular diseases from basic research to clinical practice. Cardiol. Plus 8, 4–5 (2023).
Karauzum, K. et al. The systemic immune-inflammation index may predict the coronary slow flow better than high-sensitivity C-reactive protein in patients undergoing elective coronary angiography. Cardiol. Res. Pract. 2022, 7344639 (2022).
Bai, Y. et al. Efficacy of Shexiang Tongxin dropping pills in a swine model of coronary slow flow. Front. Physiol. 13, 913399 (2022).
Lu, X. et al. Shexiang Tongxin dropping pills promote macrophage polarization-induced angiogenesis against coronary microvascular dysfunction via PI3K/Akt/mTORC1 Pathway. Front. Pharmacol. 13, 840521 (2022).
Sun, Y. et al. Shexiang Tongxin dropping pills ameliorate myocardial ischemia-reperfusion injury progression via the S1PR2/RhoA/ROCK pathway. J. Tradition. Chin. Med. Sci. 12, 31–43 (2025).
Cui, L. et al. Shexiang Tongxin Dropping Pill alleviates M1 macrophage polarization-induced inflammation and endothelial dysfunction to reduce coronary microvascular dysfunction via the Dectin-1/Syk/IRF5 pathway. J Ethnopharmacol. 316, 116742 (2023).
Zhu, L. et al. Shexiang Tongxin Dropping Pills attenuate ischemic microvascular dysfunction via suppressing P66Shc-mediated mitochondrial respiration deficits. J. Ethnopharmacol. 346, 119664 (2025).
Zhu, L. et al. Shexiang Tongxin dropping pills protect against ischemic stroke-induced cerebral microvascular dysfunction via suppressing TXNIP/NLRP3 signaling pathway. J. Ethnopharmacol. 322, 117567 (2024).
Jespersen, L., Abildstrøm, S. Z., Peña, A., Hansen, P. R. & Prescott, E. Predictive value of the corrected TIMI frame count in patients with suspected angina pectoris but no obstructive coronary artery disease at angiography. Clin. Res. Cardiol. 103, 381–387 (2014).
Bardou, P., Mariette, J., Escudié, F., Djemiel, C. & Klopp, C. jvenn: an interactive Venn diagram viewer. BMC Bioinform. 15, 293 (2014).
Acknowledgements
We thank all patients and their families for participating in this study and all of the centers and investigators who devoted their effort and time to this study. We especially thank Professor Keji Chen from Xiyuan Hospital, China Academy of Chinese Medical Sciences, and Professor Zonggui Wu for his help in protocol design and supervision of this trial. This work was supported by National Key R&D Program of China (2022YFC3501701), National Natural Science Foundation of China (82120108004, 82574796), Clinical Collaboration Program of Traditional Chinese and Western Medicine for Major and Intractable Diseases (ZDYN-2024-A-154), Shanghai Collaborative Grant for combination of traditional Chinese and Western medicine (2018-2020-FWTX-1102), Shanghai Wumengchao Medical Science Foundation (LCYX20161017-003), Shanghai foundation for supporting the military and families (E3F2A20102) to C.L.
Author information
Authors and Affiliations
Contributions
C.L.: Conceptualization, supervision, investigation, project administration, funding acquisition, reviewing, and editing. W.W.: Study design and supervision. N.L. and Z.H.: Data curation, original draft writing. Y.C.: Visualization and investigation. L.W., Y.G., S.W., Y.Z., M.F., B.Y., J.X., Y.S.G., and Y.Cao.: Investigation, reviewing, and editing. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, N., He, Z., Chen, Y. et al. Efficacy and safety of Shexiang Tongxin pill for coronary slow flow in angina patients. npj Cardiovasc Health 3, 2 (2026). https://doi.org/10.1038/s44325-025-00098-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44325-025-00098-y