Abstract
Metabolic Dysfunction-Associated Steatohepatitis (MASH), the advanced stage of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), is a leading cause of chronic liver disease, driving cirrhosis, liver cancer, and mortality. With metabolic, genetic, and environmental factors at play, early detection and treatment are critical. This review highlights the latest breakthroughs in MASH pathophysiology, cost-effective noninvasive diagnostics, and emerging therapies, emphasizing the urgency of accessible solutions to combat this growing global health crisis.
Similar content being viewed by others
Introduction
Metabolic dysfunction-associated liver diseases, including Metabolic Dysfunction-Associated Steatohepatitis (MASH) and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), are a growing global health concern. According to the most recent meta-analysis by Younossi et al., approximately one third of the global adult population are afflicted with MASLD, making it the leading cause of chronic liver disease. MASLD can advance to MASH in about 5% of the cases1. This prevalence is significantly higher among individuals with central obesity, visceral fat, and type 2 diabetes mellitus (T2DM). Over 75% of MASH cases occur in these subpopulations with the pooled prevalence ranging from 44–66.4%2,3. MASLD is also known to affect lean individuals and children with the global pooled prevalence of 11.2% and 13% respectively in the general population4,5.
MASLD and MASH are not confined to liver pathology but represent systemic metabolic disorders with profound implications across multiple organ systems. In particular, cardiovascular disease (CVD) has emerged as the leading cause of mortality among patients with MASLD and MASH, surpassing liver-related complications6. The pathophysiological nexus between MASLD and CVD is driven by shared metabolic risk factors such as insulin resistance, dyslipidaemia, and chronic low-grade inflammation which collectively promote endothelial dysfunction, atherogenesis, and myocardial remodeling7. Beyond cardiovascular sequelae, MASLD/MASH is independently associated with an increased risk of chronic kidney disease (CKD), likely via overlapping mechanisms involving systemic inflammation, altered adipokine profiles, and activation of the renin-angiotensin system8. Moreover, compelling evidence has linked MASLD to elevated risks of extrahepatic malignancies, including colorectal, breast, and pancreatic cancers, suggesting that hepatic steatosis and its metabolic milieu may exert carcinogenic effects beyond the liver9. Together, these findings firmly position MASLD and MASH as multisystem disorders with a complex clinical spectrum that extends well beyond liver-related morbidity.
MASH pathophysiology involves a complex interplay of metabolic, genetic, and environmental factors10. Central to this process is hepatic steatosis, marked by excessive lipid accumulation in hepatocytes due to increased free fatty acid (FFA) influx, impaired beta-oxidation, and enhanced de novo lipogenesis. Insulin resistance exacerbates this, promoting lipotoxicity and FFA delivery to the liver11. Mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and oxidative stress contribute to hepatocyte injury and inflammation, triggering fibrosis through Kupffer and hepatic stellate cell activation12. These combined factors emphasize the need for an integrated approach to managing MASLD and MASH.
Current diagnostic approaches for MASLD and MASH remain heavily reliant on invasive liver biopsy, which, despite providing detailed histological information, is associated with significant risks and limitations, such as high cost, dependence on trained personnel, and risks of complications that limit its widespread use13. Noninvasive alternatives, including blood tests, imaging techniques, and predictive models, have emerged as promising tools for diagnosing and staging liver diseases. However, the high cost and need for specialized equipment limit their implementation in routine clinical practice.
Point-of-care (PoC) testing allows clinical analysis near the site of patient care, eliminating the need for advanced laboratory equipment14. PoC tests provide rapid results, enabling timely patient management and improved clinical and economic outcomes15. In MASLD and MASH management, PoC diagnostic technologies leverage biomarkers like patatin-like phospholipase domain containing 3 (PNPLA3) gene mutations, microRNAs, and long non-coding RNAs to offer a noninvasive, fast, and cost-effective alternative to liver biopsy and imaging. Requiring minimal sample volumes, these devices deliver results within minutes, making them ideal for resource-limited settings. Their affordability enhances accessibility, enabling large-scale screening and early diagnosis for timely intervention.
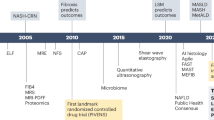
In the therapeutic landscape, resmetirom (Rezdiffra), approved in 2024, is the first pharmacotherapy for non-cirrhotic MASH with moderate-to-advanced fibrosis16. It reduces intrahepatic triglycerides and improves fibrosis, though concerns about side effects persist. Other emerging therapies targeting inflammation, metabolic dysfunction, and fibrosis include glucagon-like peptide-1 receptor agonists (GLP1-RA), peroxisome proliferator-activated receptor (PPAR) agonists, fibroblast growth factors (FGF) analogs, and fatty acid synthase (FASN) inhibitors17,18,19. The multifactorial nature of MASLD and MASH demands multi-targeted treatment that balances efficacy and safety.
Recent advancements in MASLD and MASH have improved understanding of their pathophysiology, diagnosis, and treatment. This review provides a comprehensive overview, linking disease mechanisms to the latest diagnostic innovations and emerging therapies. Emphasis is given on affordable, noninvasive diagnostic solutions for early detection, personalized care, and prevention, particularly in underserved and low-income regions.
Pathophysiology of metabolic liver disease
Pathophysiology of hepatic steatosis
Hepatic steatosis refers, the accumulation of triglycerides (TG) in hepatocytes, is a key histological feature of MASLD. Increased TG accumulation induces hepatic inflammation, leading to MASH. Development of MASH is caused by excessive lipid deposition into hepatocytes that exceeds the liver’s metabolic capacity20. The outdated “two-hit” hypothesis has been replaced by the “multiple-hit” model, which identifies genetic predisposition, insulin resistance, adipose-derived hormones, gut microbiota changes, and environmental factors as the major contributors to MASH progression (Fig. 1)21. Hepatic steatosis induces organelle dysfunction, oxidative stress, and lipotoxicity, which accelerate disease progression. Increased fatty acid flux and deposition, together with insulin resistance and mitochondrial dysfunction, are central to this process10. Obesity further exacerbates lipid accumulation, disrupting metabolism and inducing gut dysbiosis and inflammation, promoting MASH development22.
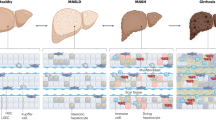
The illustration of the sequential progression of liver disease from a healthy liver to HCC through intermediate stages including MASLD, MASH, and cirrhosis. MASLD is characterized by >5% fat accumulation in hepatocytes and can progress to MASH, which involves steatosis, inflammation, hepatocyte ballooning, and varying degrees of fibrosis (30–40%). Without intervention, MASH can advance to cirrhosis (15–25%) and eventually HCC (5–10%), potentially resulting in death or requiring liver transplantation. Early stages of MASLD and MASH are potentially reversible. Recurrence or de novo development of MASLD/MASH after liver transplantation is common, influenced by factors such as post-transplant diabetes mellitus (Post LT-DM), BMI, and genetics. Key risk factors for disease onset include obesity, T2DM, insulin resistance, elevated cholesterol, genetic predisposition, gut microbiota dysbiosis, and lifestyle factors. Note this illustration created with BioRender.com.
The accumulation of hepatic free cholesterol plays a significant role in lipotoxicity and MASH progression23. The liver-adipose tissue interplay is critical for lipid uptake and metabolism. FA transport proteins (FATP2 and FATP5) mediate lipid uptake into hepatocytes, as shown in mouse models with FATP deficiencies that reduce hepatic FA accumulation24. Elevated FA translocase CD36 levels in MASH patients and increased de novo lipogenesis (DNL) increased enzymes such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) highlight their roles in lipid accumulation25. Increased FA oxidation exacerbates mitochondrial dysfunction, producing reactive oxygen species (ROS) and worsening liver injury26. Systemic insulin resistance is crucial for hepatic steatosis. In insulin resistance, disrupted signalling promotes DNL through transcription factors like sterol regulatory element-binding protein 1c (SREBP-1c) and carbohydrate response element binding protein (ChREBP), leading to steatosis27. Lipotoxicity drives hepatocyte damage through endoplasmic reticulum (ER) stress, inflammation, and cell death28.
Organelle dysfunction and oxidative stress
The unfolded protein response (UPR) is a protective mechanism for maintaining ER homeostasis, but prolonged activation leads to apoptosis, causing hepatocyte damage29. Key pathways, including activating transcription factor-6 (ATF6), eukaryotic initiation factor 2 alpha (eIF2α), and X-box-binding protein 1 (XBP1), regulate MASLD progression by modulating lipid synthesis and activating C/EBP-homologous protein (CHOP), a mediator of ER stress30. Oxidative stress, driven by excessive ROS, accelerates MASLD progression by activating hepatic stellate cells (HSCs), promoting fibrosis and inflammation via NF-κB31. Oxidized phospholipids (OxPLs) contribute to mitochondrial dysfunction, with neutralization alleviating oxidative stress and fibrosis32. Elevated forkhead box A3 (FOXA3) links ER stress to MASLD progression33.
Mitochondrial dysfunction in MASH is linked to both genetic and epigenetic factors. Excess FFAs overwhelm mitochondrial capacity, leading to oxidative stress and ROS accumulation26. Peroxisomal ω-oxidation and mitochondrial CYP2E1 further increase ROS production34. Mitochondrial dysfunction is characterized by reduced mitochondrial DNA, ATP depletion, and lysosomal enzyme leakage, which activate NF-κB and TNF-α, leading to cell death35.
Immune system and inflammatory pathways
Insulin resistance promotes adipose tissue lipolysis, increasing FFAs that activate proinflammatory pathways, including NF-κB, contributing to hepatic lipotoxicity36. Hepatocyte injury and inflammation are central to MASH pathogenesis. Injured hepatocytes release cytokines and chemokines (e.g., CCL2, TNF-α, IL-6) that exacerbate inflammation and injury37. Kupffer cells, the liver’s resident macrophages, play a pivotal role. Activated proinflammatory M1 Kupffer cells contribute to fibrogenesis, while monocyte-derived macrophages enhance inflammation and fibrosis by secreting CCL2 and IL-1β. Fibrosis in MASH results from the activation of HSCs. Proinflammatory Ly-6Chi macrophages secrete CCL2 and IL-1β, promoting HSC activation, while pro-restorative Ly-6Clo macrophages aid extracellular matrix degradation38,39. Toll-like receptor 4 (TLR4) signalling recruits Ly-6Chi macrophages through CCL2/CCR2 interactions, further driving MASLD and MASH progression40.
Genetic and epigenetic factors
Genome-wide association studies (GWAS) have identified several risk alleles for MAFLD and MASH, with a heritable component estimated at 35–61%41. The most strongly associated genetic variant is I148M in the PNPLA3 gene, which encodes a lipid droplet protein involved in lipolysis42. The I148M variant resists degradation, accumulating on lipid droplets, which leads to steatosis, fibrosis, and hepatocellular carcinoma (HCC) by impairing lipid catabolism. This variant also exacerbates lipid accumulation by disrupting proteasomal degradation of PNPLA343. Recent studies show that PNPLA3-I148M enhances the profibrogenic and proinflammatory characteristics of HSCs, amplifying liver disease progression44. The transmembrane 6 superfamily member 2 (TM6SF2) rs58542926 variant, resulting in the E167K substitution, increases the risk of progressive MASH while reducing cardiovascular disease risk. The E167K variant impairs very-low-density lipoproteins (VLDL) secretion, promoting hepatic triglyceride accumulation and advanced fibrosis. Silencing TM6SF2 increases triglyceride retention in hepatocytes, underscoring its role in hepatic lipid metabolism45,46. The hydroxysteroid 17-beta-dehydrogenase 13 (HSD17B13) gene, linked to reduced liver inflammation and fibrosis, is protective against MASH47. This protection is attributed to its loss of retinol dehydrogenase activity, altering retinol metabolism and modulating fibrosis48. The MBOAT7 gene, involved in lipid-driven fibrosis, is another important factor. The rs641738 variant is associated with reduced MBOAT7 protein levels, altered phospholipid remodelling, and increased fibrosis risk49. Rare variants such as protein phosphatase 1 regulatory subunit 3B (PPP1R3B), Immunity-related GTPase family M protein (IRGM), and lipin-1 (LPIN1) have been identified in specific populations. The PPP1R3B rs4240624 variant is linked to liver glycogenosis and increased alanine aminotransferase (ALT) levels50. While IRGM variants (e.g., rs10065172) are associated with mild steatosis, their role in disease progression remains unclear51.
Current methods for the diagnosis of MASLD and MASH
The diagnosis of MASLD and MASH remains a significant clinical challenge due to limited patient awareness, the nonspecific (or none) nature of clinical symptoms, and the lack of specific diagnostic tests and approved effective therapeutic interventions. Community-level screening reveals that over two-thirds of MASLD cases go undiagnosed, with only 3% of high-risk patients referred for specialist care52. Identifying and diagnosing at-risk individuals with MASLD and MASH is essential to preventing complications and guiding clinical management.
Current diagnostic methods for MASLD and MASH can be classified into invasive or noninvasive, broadly categorized into three main approaches: (i) Blood tests, which assess liver enzyme levels, lipid profiles, and markers of inflammation or viral hepatitis; (ii) Imaging tests, including conventional modalities like ultrasound, computed tomography (CT), and magnetic resonance imaging (CAP), as well as newer techniques such as transient elastography, which quantifies liver fat and stiffness; (iii) Liver biopsy, the gold standard for confirming MASH and assessing fibrosis stages. Each approach offers distinct advantages and limitations, which influence its applicability in routine clinical practice and resource-limited settings.
Currently available blood-based biomarker scores
Noninvasive biomarkers represent a promising tool for MASLD diagnosis, with many focused on detecting hepatic steatosis (Fig. 2). Commonly used scoring systems include: (i) Fatty Liver Index (FLI): Incorporates body mass index (BMI), waist circumference, triglyceride levels, and gamma-glutamyl transferase (GGT). FLI is cost-effective and simple, but its sensitivity for mild steatosis and ability to grade disease severity are limited53. (ii) Hepatic Steatosis Index (HSI): Combines BMI, diabetes status, and the AST/ALT ratio. While validated in biopsy-proven MASLD cohorts, its diagnostic specificity remains suboptimal54. (iii) SteatoTest: A biomarker panel comprising 12 parameters (e.g., ALT, glucose, A2M, ApoA1, BMI) for predicting liver fat content. Despite its potential, cost and complexity hinder widespread use55. (iv) NAFLD Liver Fat Score (LFS): Incorporates metabolic syndrome, fasting insulin, AST, and the AST/ALT ratio. Its reliance on fasting insulin limits feasibility in routine clinical settings56. While these tools offer cost-efficient and accessible screening options, their diagnostic accuracy and ability to stage steatosis remain constrained, limiting their integration into clinical practice.
This figure illustrates a range of noninvasive diagnostic strategies for MASLD and MASH. These approaches include conventional blood-based biomarkers such as liver function tests, lipid profile, fibrosis markers as well as imaging techniques like ultrasound, CT, and MRI. Advancements in artificial intelligence and machine learning are increasingly integrated to enhance diagnostic accuracy and risk stratification. Innovative biomarker platforms—particularly those based on genetic markers and extracellular vesicles (e.g., circulating miRNAs and lncRNAs)—are emerging as powerful tools for early detection and disease monitoring. While imaging offers diagnostic value, it remains costly and resource-intensive. Biomarker-based tests may suffer from limited sensitivity and specificity, and machine learning applications face challenges related to data quality and availability of skilled specialists. Illustration created with BioRender.com.
Imaging techniques
Imaging techniques have improved the diagnosis of MASLD by allowing direct measurement of liver fat, which provides greater accuracy and sensitivity compared to blood tests. Ultrasonography is the most commonly used imaging method due to its wide availability, low cost, and safety. While effective in detecting moderate to severe fat buildup in the liver, it is less reliable for diagnosing early-stage disease or advanced fibrosis57. CT offers detailed structural images and measures liver fat, but its use is limited by radiation exposure and lower sensitivity for detecting mild fat accumulation58. MRI, particularly advanced techniques such as magnetic resonance imaging proton density fat fraction (MRI-PDFF) and controlled attenuation parameter (CAP), offers far better sensitivity for detecting mild steatosis. CAP combines fat measurement with fibrosis assessment using transient elastography, while MRI-PDFF provides quick and accurate fat quantification across all stages of the disease59. MRI stands out as the most precise noninvasive imaging method for diagnosing MASLD, achieving 100% sensitivity in some studies60. However, its high cost and limited availability make it less feasible for use in low-resource settings.
Liver biopsy
Liver biopsy remains the definitive diagnostic tool for MASH, providing accurate detection of hepatic fibrosis and identifying at-risk individuals. However, this invasive procedure carries inherent risks, including pain, bleeding, and, in rare cases, mortality. Additionally, the high cost and dependence on pathologist expertise introduce further barriers. Sampling errors and inter-observer variability in pathological assessments also compromise diagnostic reliability. The logistical requirements for peri- and post-procedural monitoring restrict biopsy availability in remote or resource-constrained regions, where much of the global population resides. Consequently, liver biopsy is typically reserved for patients with advanced disease stages or unclear clinical presentations13. This underscores the urgent need for noninvasive diagnostic tools with high predictive accuracy to enable routine screening and risk stratification. Advances in noninvasive methods could bridge this gap, facilitating broader implementation of effective diagnostic algorithms in real-world settings.
Biopsy sampling and validation as the histological reference standard
Despite its well-established role as the reference standard, liver biopsy is increasingly recognized as an imperfect comparator for the diagnosis and staging of MASLD and MASH. Biopsy-based histological evaluation is subject to two key limitations: (1) sampling variability arising from the heterogeneous distribution of steatosis, inflammation, and fibrosis across the liver parenchyma, and (2) observer-dependent interpretation, particularly for subjective features such as hepatocyte ballooning and lobular inflammation61,62. A typical liver biopsy samples only ~1/50,000 of the total hepatic mass, introducing a significant risk of under- or misclassification, particularly in early or regionally confined disease62,63. Evidence consistently supports the link between specimen adequacy and diagnostic reliability. A minimum core length of 2.0 cm, ideally containing ≥11 complete portal tracts, is now considered optimal for accurate staging and grading. Biopsy cores shorter than 1.5 cm are associated with reduced sensitivity for detecting MASH, and single-pass biopsies may miss critical lesions altogether13,64.
The use of appropriately gauged needles (e.g., 16 G for percutaneous sampling) and multi-pass strategies (≥3 samples) significantly improves diagnostic yield, particularly for identifying fibrosis stage and activity grade63. To ensure consistency in the validation of noninvasive diagnostics, calibrated histologic thresholds must be employed. Hepatic steatosis is typically defined as involving ≥5% of hepatocytes, while fibrosis staging adheres to the F0–F4 framework outlined by both the NASH Clinical Research Network (CRN) and the steatosis–activity–fibrosis (SAF) scoring system65. Deviations from these definitions such as using a 0% steatosis threshold or simplified ordinal fibrosis scales can introduce significant discrepancies in test performance metrics, undermining the reproducibility and translational value of novel biomarker platforms. Moreover, to reduce interobserver variability, especially in borderline cases, we strongly advocate for standardized protocols that include blinded dual or consensus readings by expert hepatopathologists. Emerging digital pathology platforms and machine learning–assisted histological analysis may further enhance reproducibility and enable high-throughput assessment in future validation studies66. Taken together, while liver biopsy remains indispensable in MASLD/MASH diagnosis and as a benchmark for emerging noninvasive diagnostics, it must be interpreted with caution. Acknowledging and mitigating its limitations through standardized sampling protocols, rigorous threshold calibration, and advanced interpretive tools will be essential to ensuring that biopsy-based validation reflects true biological disease states rather than artefacts of procedural variability.
Novel noninvasive tools for diagnosing MASH
The progression of MASLD to MASH is marked by fibrosis, a key predictor of mortality. Early detection is vital to preventing disease progression. Recent efforts focus on serum biomarkers and predictive models: (i) Cytokeratin-18 (CK-18): a marker of hepatocyte apoptosis and necrosis. CK-18 shows moderate diagnostic performance, with sensitivity and specificity of 75% and 81%, respectively (Fig. 2)54. Combining CK-18 with other markers (e.g., adiponectin, IL-6) enhances detection accuracy67; (ii) Pro-inflammatory cytokines: markers like C-X-C motif chemokine ligand 10 (CXCL10), IL-8, and TNF-α demonstrate moderate accuracy for differentiating MASH from simple steatosis68; (iii) Fibroblast Growth Factor 21 (FGF21): shows potential when combined with CK-18 but is less reliable as a standalone marker69. Several predictive models, including the HAIR model, oxNASH, Palekar score, and NashTest, have also been developed70,71,72,73. The HAIR model, for instance, demonstrated high diagnostic performance with an area under the receiver operating characteristic curve (AUROC 0.90, sensitivity 80%, and specificity 89%)70. However, most models are validated in small, highly selected cohorts, limiting their generalizability to diverse populations and the newly defined MASLD and MASH criteria. Larger, multicentred studies are needed to establish their utility in clinical practice.
While conventional ultrasound has traditionally been viewed as a limited tool for hepatic fat quantification- particularly in detecting mild steatosis or distinguishing between simple steatosis and steatohepatitis- recent studies and meta-analyses have demonstrated that incorporating structured scoring systems like Ultrasonographic Fatty Liver Indicator (US-FLI) substantially enhances its diagnostic sensitivity and clinical relevance74,75. US-FLI evaluates multiple sonographic parameters including liver-kidney contrast, vessel blurring, and deep attenuation, allowing for a reproducible, semi-quantitative assessment of steatosis severity. Notably, it has been shown to correlate with histological features of steatohepatitis, thereby offering indirect insight into MASH risk74. Importantly, US-FLI and related ultrasound-based scoring systems are highly amenable to POC ultrasound (POCUS), making them practical tools for frontline screening in both primary and resource-limited settings76. The potential for rapid, noninvasive, and repeatable assessment aligns well with current public health needs for early detection of MASLD/MASH in the context of rising global prevalence. That said, it is critical to acknowledge the limitations. The accuracy of ultrasound-based modalities, including US-FLI, tends to decline in individuals with high BMI due to acoustic attenuation and poor sonographic window, which can obscure hepatic echotexture and vascular landmarks57. Furthermore, while US-FLI may suggest inflammatory activity through indirect signs, it remains insufficiently specific to replace biopsy or emerging noninvasive biomarkers for definitive MASH diagnosis.
Recent advances underscore the value of combining noninvasive serum-based fibrosis markers- such as the NAFLD Fibrosis Score (NFS) and Fibrosis-4 Index (FIB-4) with imaging modalities like POCUS to enhance risk stratification in patients with MASLD and MASH. While traditionally employed to estimate hepatic fibrotic burden, elevated NFS and FIB-4 scores have also been independently associated with increased cardiovascular morbidity and mortality, suggesting their broader systemic relevance77. Large-scale prospective cohorts demonstrate that these scores retain predictiveFIB value even after adjustment for classical cardiovascular risk factors, positioning them as dual-purpose tools that reflect both hepatic and extrahepatic disease processes. When combined with semi-quantitative ultrasonographic approaches such as the US-FLI, this dual-modality framework allows for real-time visualization of hepatic steatosis and steatohepatitis alongside biomarker-driven estimation of fibrosis severity and cardiometabolic risk78,79. This integrated approach is particularly well suited for use in primary care and resource-limited settings, where it may facilitate early triage, targeted diagnostic escalation, and timely intervention. Taken together, this synergy highlights the need for a more holistic, systems-oriented diagnostic paradigm in MASLD, one that bridges hepatology and cardiometabolic medicine.
Noninvasive tests for at-risk MASH
Circulating biomarkers for at-risk MASH
Developing noninvasive biomarker tests to assess the presence and severity of MASH is critical for identifying individuals at higher risk of adverse clinical outcomes. Several circulating biomarker tests have been recently introduced, including the Fibrotic NASH Index (FNI), NIS4®, NIS2 + ™, NASH ClinLipMet score, proteomics-based approaches, and the Metabolomics-Advanced StEatohepatitis Fibrosis (MASEF) score (Table 1)80,81,82,83. The FNI, derived from an Italian cohort of biopsy-confirmed patients, integrates high-density lipoprotein (HDL), aspartate aminotransferase (AST), and HbA1c to evaluate fibrotic MASH. In validation cohorts, it demonstrated an AUROC of 0.80–0.95 for identifying at-risk MASH80. Similarly, NIS4® (NASHnext™), a blood-based panel comprising α2-macroglobulin, HbA1c, YKL-40, and miR-34a, achieved an AUROC of 0.80 with 87.1% specificity for detecting at-risk MASH (NAFLD activity score ≥4 and fibrosis stage ≥2)81. However, both FNI and NIS4® primarily target advanced MASH and are less effective for identifying early-stage disease. Furthermore, NIS4® has limited adoption due to its recent marketing approval and lack of widespread validation. NIS2 + ™, an optimized successor to NIS4®, combines miR-34a-5p and YKL-40 and has shown improved accuracy in detecting at-risk MASH, regardless of patient characteristics such as age, sex, BMI, or type 2 diabetes mellitus (T2DM). While NIS2 + ™ represents a robust diagnostic tool, its validation remains confined to the RESOLVE-IT dataset, necessitating external validation in broader populations82. The NASH ClinLipMet score integrates metabolomic, lipidomic, and clinical data with genetic biomarkers (e.g., PNPLA3 genotyping) to predict MASH with high accuracy (AUROC 0.86–0.88)83. Proteomics-based approaches have identified numerous candidate proteins for diagnosing advanced MASH, including extracellular vesicle-derived proteomic signatures84. Meanwhile, metabolomics-based tools, such as the MASEF score, utilize lipids, alanine aminotransferase (ALT), AST, and BMI to evaluate MASH, with validation cohorts showing an AUROC of 0.79, 78% sensitivity, and 65% specificity85. However, the complex methodologies and limited accessibility of metabolomics, lipidomics, and proteomics restrict their routine clinical application, compounded by a lack of external validation.
Imaging biomarkers for at-risk MASH
MRI-based techniques have emerged as promising tools for identifying at-risk MASH. MRI-derived iron-corrected T1 (cT1) has been validated for distinguishing at-risk MASH with an AUROC of 0.78 in biopsy-confirmed MASLD patients. Combining cT1 with MRI-derived steatosis metrics slightly improved performance (AUROC 0.79)86. Integration of cT1 with serological markers and vibration-controlled transient elastography (VCTE) further enhanced positive predictive value (PPV) and classification accuracy for advanced fibrosis. Hyperpolarized (HP) 13 C MRI represents an emerging modality for assessing hepatic metabolic changes in real time. Early studies using HP α-ketobutyrate in animal models demonstrated potential for detecting metabolic alterations in early MASH87. However, its relevance to human disease and underlying contrast mechanisms requires further investigation.
Combining imaging and circulating biomarkers
Combining imaging and circulating biomarkers has yielded superior diagnostic performance for at-risk MASH. Models such as the MRI-AST (MAST), FibroScan-AST (FAST), and FIB-4 (MEFIB index) integrate these modalities to enhance diagnostic accuracy88,89. The FAST score, which combines VCTE-based liver stiffness measurement (LSM) and controlled attenuation parameters with AST, achieved an AUROC of 0.90 and PPVs of 33–81% in validation cohorts88. The MAST score, which incorporates MRI elastography-based LSM, MRI proton density fat fraction, and AST, demonstrated a high negative predictive value (96.5–98.1%) but lower PPV89. The MEFIB index, which combines Magnetic Resonance Enterography (MRE) and FIB-4, addresses the PPV limitations of earlier models and has outperformed the FAST score in predicting at-risk MASH and fibrosis stage ≥2 in biopsy-proven MASLD cohorts90. Despite their promise, these models rely on advanced imaging techniques that are expensive, time-consuming, and limited to specialized centers, highlighting the need for validation in general clinical settings to ensure broader implementation.
Machine learning approaches
Machine learning (ML), an area of artificial intelligence, uses algorithm and patients’ data to enable computers to analyse data and make predictions or decisions without pre-programing to do so91. ML models leveraging clinical and laboratory data are being explored for diagnosing MASLD and MASH. Early ML models using AST, ALT, cholesterol, and other routine parameters showed limited accuracy due to a restricted number of classifiers. Recent advancements utilizing supervised learning techniques and expanded classifiers have improved prediction accuracy for MASH (81.3%), sensitivity (86%) and specificity (70.5%)92. However, these approaches face challenges such as small dataset sizes, data quality dependence, and limited expertise, which hinder their integration into clinical practice.
Practical applicability of noninvasive diagnostic tools for MASLD and MASH
The clinical adoption of noninvasive diagnostic tools for MASLD and MASH is influenced not only by their diagnostic accuracy but also by their real-world practicality. Key considerations include cost-effectiveness, infrastructure requirements, operator training, and ease of integration into existing care pathways- factors that often dictate test uptake, especially in resource-constrained settings. Among first-line assessments, the FIB-4 index remains one of the most accessible tools globally. It uses age and routinely collected blood tests (AST, ALT, platelet count), requiring neither additional infrastructure nor specialist interpretation93. Owing to its simplicity and negligible cost, FIB-4 is particularly well suited for broad implementation in primary care as a triage tool. Studies have demonstrated that combining FIB-4 with second-line confirmatory tests enhances diagnostic performance and reduces unnecessary specialist referrals. For example, a UK-based health-economic evaluation found that the FIB-4/ELF and FIB-4/VCTE pathways reduced referrals by 33.7% and 40.6%, respectively, while achieving per-patient cost savings compared to standard of care (SoC) pathways (£983.37 for FIB-4/VCTE and £993.15 for FIB-4/ELF, versus £1014.15 for SoC)94. The ELF test, which quantifies serum fibrosis-related biomarkers (hyaluronic acid, PIIINP, TIMP-1), offers higher specificity for advanced fibrosis but requires centralized laboratory processing. Nevertheless, it remains operator-independent and has been associated with a 15.1% reduction in average cost per diagnosed case compared to liver biopsy (£8059 vs. £9487)95.
Imaging-based modalities such as (VCTE; FibroScan), MRE, and LiverMultiScan offer higher diagnostic precision and can noninvasively assess steatosis and fibrosis. FibroScan is increasingly used at the point-of-care and is portable, but it requires moderate operator training and quality-controlled acquisition96. MRE, while more accurate in staging fibrosis, is significantly constrained by the need for MRI-compatible elastography software and skilled radiologists. A U.S.-based analysis demonstrated that MRE remains cost-effective, with an incremental cost-effectiveness ratio (ICER) of $7048 per quality-adjusted life year (QALY) gained compared to VCTE97. LiverMultiScan, a multiparametric MRI-based tool that characterizes liver tissue using T1 mapping and iron correction algorithms, has also shown favourable cost-effectiveness. In a European multi-country analysis, it demonstrated an ICER of €4968/QALY versus standard care. However, its deployment is currently restricted by elevated short-term costs (€1300 per patient vs. €830 for SoC) and dependency on high-end imaging infrastructure and radiology expertise98. Emerging tools such as the NIS4 and NIS2+ panels, which integrate circulating biomarkers and clinical variables (e.g., BMI, HbA1c), show high translational promise, especially in primary care or low-resource environments. These panels require no specialized equipment or operator training and are being validated in real-world and community-based cohorts82. (Table 2) summarizes the infrastructure and operator requirements of key noninvasive tests. Collectively, these findings underscore the importance of balancing diagnostic precision with contextual feasibility. While blood-based and composite scoring systems remain vital for early detection and triage, imaging modalities may be reserved for confirmatory diagnosis or specialist care settings. A tiered diagnostic approach tailored to health system capacity and population risk profiles is likely to yield the greatest clinical and economic benefit.
Diagnostic accuracy: accounting for population spectrum, uncertainty, and calibration effects
The evaluation of diagnostic tools for MASLD and MASH must extend beyond conventional measures of discrimination such as the AUROC. While widely used, AUROC reflects overall diagnostic accuracy without accounting for critical real-world variables, including population heterogeneity, disease prevalence, and operational thresholds99. Consequently, a more comprehensive approach is required- one that integrates “intention to diagnose”100, addresses the spectrum effect, and utilizes a broader array of metrics to quantify diagnostic performance and uncertainty. The principle of “intention to diagnose” underscores the importance of validating diagnostic tests within the population in which they are intended to be applied. Many studies reporting high diagnostic performance for noninvasive fibrosis or steatosis markers have been conducted in secondary or tertiary care settings, where patients often present with advanced disease or known risk factors. These populations have elevated pre-test probabilities, which can inflate sensitivity, specificity, and AUROC estimates. In contrast, primary care or population-based screening environments typically involve a broader clinical spectrum, often with a lower disease prevalence and higher variability in risk profiles. This shift in population characteristics introduces spectrum bias, a well-established phenomenon described by Obuchowski, in which test performance varies systematically across subgroups with differing disease severities, comorbidities, or demographic traits101. For instance, elastography-based tools such as VCTE demonstrate reduced sensitivity for detecting advanced fibrosis in individuals with obesity or hepatic steatosis102. Similarly, age-weighted indices like FIB-4 may underperform in younger populations due to suppressed score inflation93. These examples illustrate how performance metrics derived from enriched cohorts may not extrapolate to more diverse or asymptomatic populations, thus emphasizing the need for spectrum-aware diagnostic validation.
In addressing these limitations, reliance on AUROC alone is insufficient. We advocate for the incorporation of threshold-based metrics such as Youden’s index (J = sensitivity + specificity – 1), which identifies optimal cutoff points that balance false positives and false negatives103. Additionally, positive and negative predictive values (PPV and NPV) offer practical insights into test utility within specific prevalence contexts, particularly when aligned with pre-test probabilities in clinical decision-making. To enhance reproducibility and transparency, all reported performance estimates should be accompanied by 95% confidence intervals, acknowledging inherent statistical uncertainty104. Another often-overlooked dimension is the effect of calibration thresholds on diagnostic accuracy. The standard histological criterion for hepatic steatosis ≥5% fat accumulation forms the basis for many noninvasive tool validations. However, some studies employ a 0% threshold as a reference point, particularly in advanced fibrosis where steatosis may regress. This divergence introduces threshold misalignment, leading to systematic errors in sensitivity and specificity estimation. For example, a diagnostic tool trained to detect ≥5% steatosis will inherently appear less sensitive when tested against a 0% calibration threshold, given the broader inclusion of “disease-negative” individuals. Moreover, modern imaging techniques and machine learning classifiers may detect trace hepatic lipid content that is not clinically meaningful, further complicating diagnostic calibration105. To address these challenges, we propose an interpretive framework that combines spectrum-adjusted validation, threshold calibration, and decision-analytic metrics. This includes stratifying diagnostic performance across risk deciles, reporting calibration curves to assess agreement between predicted and observed outcomes, and utilizing decision-curve analysis to evaluate net clinical benefit at varying threshold probabilities. Together, these refinements will foster the development and deployment of diagnostic tools that are not only statistically robust but also clinically relevant, population-sensitive, and tailored for real-world integration.
Advancing MASLD and MASH diagnosis through point-of-care
PoC test are detection technologies that uses portable devices to analyse clinical samples and generates on-the-spot rapid results, leading to early diagnosis and management of the patients. Emerging blood-based portable PoC devices are particularly impactful for remote clinics and regions with limited healthcare infrastructure106,107. By integrating multiple diagnostic processes into compact, portable platforms, these devices require minimal sample volumes such as small blood or saliva samples-reducing patient discomfort while delivering results within minutes. The ability to detect MASLD and MASH biomarkers at the earliest stages allows for immediate interventions, bridging critical gaps in healthcare accessibility. Deployed in primary care settings or mobile health units, these portable blood based PoC technologies provide a lifeline for underserved communities. Rapid results enable healthcare providers to initiate timely treatments, potentially averting irreversible liver damage. These devices are especially valuable for high-risk populations, such as individuals with obesity or diabetes, by supporting personalized treatment strategies tailored to genetic and metabolic profiles. Incorporating genetic susceptibility testing, such as PNPLA3 gene variations, into these platforms further enhances their utility for precision medicine. However, despite these promising advancements, significant challenges remain. Validating these emerging PoC devices across diverse populations, integrating them into existing healthcare systems, and maintaining stringent quality control standards will be critical for their widespread adoption. Continued research and innovation are essential to refine these technologies for broader clinical use.
Advancing a PoC test technologies transform the landscape of MASLD and MASH diagnostics by providing rapid, cost-effective, and noninvasive alternatives to traditional methods, such as liver biopsies and advanced imaging. These cutting-edge devices could detect a wide range of biomarkers, including genetic markers like the PNPLA3 gene, which is strongly associated with liver fat accumulation and disease progression, as well as mRNA and non-coding RNAs such as microRNAs and lncRNAs. A suite of innovative PoC diagnostic methods such as Point-of-Care Ultrasound (POCUS) has emerged, enabling earlier detection and improve management of MASLD108.
SomaSignal Tests exemplify this progress by offering noninvasive blood protein profiling capable of predicting all four major components of MASH-steatosis, inflammation, fibrosis, and ballooning, thereby providing comprehensive disease monitoring109. Similarly, the NIS2 + ™ Test employs a two-biomarker blood analysis to identify patients at risk for MASH, facilitating timely interventions82. NordicPRO-C3™ focuses on detecting both MASH and liver fibrosis, offering clinicians a powerful tool to individualize disease management strategies110.
Noninvasive imaging technologies, such as FibroScan® and VCTE, are advancing fibrosis detection by assessing liver stiffness. FibroScan® further enhances diagnostic accuracy by incorporating additional serum biomarkers to identify advanced fibrosis more effectively. Blood-based tests, including the Enhanced Liver Fibrosis (ELF) Test and MASEF, integrate biomarker and metabolomic profiling to evaluate the risk of advanced fibrosis with remarkable precision. Meanwhile, simpler indices, like the FIB-4 Index and AST-to-Platelet Ratio Index (APRI), provide cost-effective options using readily available clinical data. Complementing these tools, Point-of-Care Ultrasound (POCUS) enables bedside assessment of liver steatosis, particularly in moderate-to-severe cases, making it an invaluable asset in remote and resource-constrained settings. Together, these PoC technologies are redefining MASLD and MASH diagnostics, delivering rapid, accurate, and accessible solutions that hold immense potential for scaling early disease intervention.
For resource-limited populations, portable PoC tests are not merely a convenience- they are a necessity. By eliminating the need for long-distance travel to healthcare facilities, these tools bring diagnostic capabilities directly to underserved communities. Empowering healthcare workers with portable diagnostic devices reduces delays, facilitates early interventions, and improves patient outcomes, particularly in rural and remote regions. Early diagnosis and treatment enabled by PoC technologies have the potential to halt or slow disease progression, significantly alleviating the global burden of liver disease. These transformative tools underscore the importance of equitable access to cutting-edge diagnostic innovations, ultimately paving the way for a future where liver disease is detected and managed proactively across all populations.
Current treatments for MASLD and MASH
A major challenge in optimizing therapeutics is individual variation in treatment response. This is particularly relevant for MASLD and MASH, where diverse pathophysiological pathways, metabolic comorbidities, and disease progression have hindered the discovery of a targeted drug largely due to host genetic factors. A personalized treatment approach, tailored to a patient’s lifestyle, metabolic status, genetic predisposition, and environment, could help mitigate disease progression.
Non-pharmacological treatments
Lifestyle modifications remain the cornerstone of MASLD and MASH management, targeting weight reduction, dietary interventions, and physical activity. A weight loss of 3–5% can mitigate hepatic steatosis, while 7% and 10% weight reductions have been shown to resolve MASH and improve fibrosis after one year, respectively111,112. Dietary approaches such as Mediterranean and low-carbohydrate diets outperform traditional low-fat regimens and are recommended for MASH resolution, with the Mediterranean diet particularly effective and accessible, including for paediatric patients113,114. Physical activity (e.g., 30 min walking five days per week) complements dietary changes by improving serum lipid profiles, hepatic enzyme levels, and intrahepatic lipid content115. Intermittent fasting has emerged as a promising low-cost intervention, targeting metabolic drivers of MASH pathogenesis and reducing disease progression116. Combined lifestyle interventions also enhance overall cardiometabolic health, including improvements in glycemic control, blood pressure, and cardiovascular risk117. When lifestyle modifications are insufficient, bariatric surgery offers a viable alternative for patients with obesity (e.g., BMI ≥ 35 kg/m2 plus medical complications or BMI ≥ 40 kg/m2) and MASLD and MASH. It significantly reduces steatosis and achieves long-term MASH resolution in up to 84% of cases after five years118. However, it is contraindicated for patients with established cirrhosis due to elevated perioperative risks119.
Pharmacological treatments
Despite two decades of clinical trials, no pharmacotherapy had been approved for MASLD and MASH until 2024, when the Food and Drug Administration (FDA) approved Rezdiffra (resmetirom) for noncirrhotic MASH with moderate to advanced fibrosis. Rezdiffra, a thyroid hormone receptor-beta (THRβ) agonist, reduces intrahepatic triglycerides, achieving significant MASH resolution and fibrosis improvement in clinical trials. However, it carries risks of liver toxicity, gallbladder side effects, and potential drug interactions, particularly with statins120,121. The heterogeneity of MASLD and MASH, driven by complex genetic and environmental factors, underscores the need for multi-mechanism therapies. Current trials focus on two endpoints: MASH resolution without fibrosis worsening and fibrosis regression without worsening MASH.
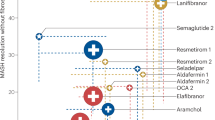
Emerging pharmacological approaches on clinical trials target inflammation, metabolic dysfunction, and fibrosis (Table 3). (i) PPAR agonists: Peroxisome proliferator-activated receptor (PPAR) agonists showed promise in modulating lipid metabolism, insulin sensitivity, and inflammation. Saroglitazar17 (PPARα/γ agonist) reduce liver fat and cholesterol in phase II trials, while lanifibranor122 demonstrated fibrosis improvement in a phase IIb trial. However, elafibranor failed to meet efficacy endpoints in phase III trials123; (ii) Thyroid Hormone Receptor (THR) Agonists: VK2809 and resmetirom target THRβ, which is crucial for lipid metabolism, and have demonstrated efficacy in improving liver histology and resolving MASH124; (iii) Fibroblast Growth Factor (FGF) analogues: Pegozafermin18, a glycopegylated FGF21 analog, significantly reduced liver fat, while pegbelfermin125 and efruxifermin126 improved hepatic fat fraction and fibrosis, respectively; (iv) Fatty Acid Synthase (FASN) Inhibitors: Denifanstat and TVB-2640 block de novo lipogenesis (DNL), reducing lipotoxicity, inflammation, and fibrosis. Denifanstat demonstrated significant MASH resolution in phase IIb trials and received FDA breakthrough designation19; (v) FXR agonists: Farnesoid X receptor (FXR) agonists like obeticholic acid (OCA) and tropifexor target inflammation and fibrosis127,128. However, OCA, though promising in fibrosis regression, has yet to gain FDA approval due to safety concerns. (vi) GLP-1RAs, originally developed for glycaemic control in T2DM, exert multifaceted metabolic effects. Upon binding to the GLP-1 receptor (GLP-1R)- a G protein–coupled receptor expressed not only in pancreatic β-cells but also in hepatocytes, adipose tissue, and immune cells- GLP-1 activates cAMP and protein kinase A signaling pathways, enhancing glucose-dependent insulin secretion, inhibiting glucagon release, and promoting satiety. Beyond these classical effects, GLP-1RAs exhibit anti-inflammatory and antifibrotic actions in the liver by attenuating hepatic stellate cell activation and reducing oxidative stress and pro-fibrotic cytokine production129. Notably, recent trials such as GLP-1R and Dual Agonists: Semaglutide and dual agonists like cotadutide and efinopegdutide target glucagon-like peptide-1 receptor (GLP1R) pathways, demonstrating efficacy in resolving MASH and improving fibrosis in clinical trials highlighting GLP-1RAs as a promising disease-modifying therapy for MASH130,131,132.
SGLT2 inhibitors, which block glucose reabsorption in the proximal renal tubules, lead to glucosuria, weight loss, and improved insulin sensitivity. Their metabolic benefits extend to reductions in hepatic steatosis and inflammation, potentially through enhanced adipose tissue lipolysis, decreased ectopic fat accumulation, and modulation of hepatic AMP-activated protein kinase activity133. Several preclinical and early-phase clinical studies, including those on dapagliflozin and empagliflozin, report improvements in liver enzymes (e.g., ALT, GGT), hepatic fat content, and fibrosis-related biomarkers134. While the phase III trial of dapagliflozin for MASH (NCT03723252) has completed enrollment, its results are pending publication. Importantly, both GLP-1RAs and SGLT2i have demonstrated significant reductions in major adverse cardiovascular events (MACEs), heart failure hospitalizations, and renal endpoints in large cardiovascular outcomes trials135. This dual hepatic and cardiometabolic benefit makes these agents particularly attractive for patients with MASH, who often present with overlapping metabolic syndrome, T2DM, and elevated cardiovascular risk. While these advancements mark significant progress, further studies are necessary to refine treatment strategies, enhance long-term safety profiles, and address MASLD and MASH’s multifaceted pathogenesis.
Challenges and future directions
Diagnosing and managing MASLD and MASH present significant clinical challenges and opportunities for future advancements. A major hurdle lies in the lack of awareness and nonspecific clinical symptoms, leading to over two-thirds of cases remaining undiagnosed at the community level. To address this, public health campaigns and routine screening programs (e.g. waist circumference) targeted at high-risk groups- such as individuals with stage 2–3 obesity, type II diabetes, and metabolic syndrome- are critical. These initiatives should be integrated into primary care to standardize early detection practices, ensuring more timely interventions.
The current gold standard for diagnosing MASH, liver biopsy, is invasive, expensive, and prone to sampling errors and inter-observer variability, making it inaccessible for many, especially in resource-limited settings. Transitioning toward noninvasive diagnostic tools such as advanced imaging and blood-based biomarkers could revolutionize the field. However, existing biomarker-based scores and imaging techniques lack sensitivity for early detection or are cost-prohibitive for routine use. To overcome these limitations, future research must prioritize the discovery of novel biomarkers, refinement of affordable imaging technologies, and large-scale validation in diverse and resource-constrained populations. Emerging diagnostic methods, including metabolomics and proteomics-based biomarkers, show promise but face obstacles like high costs, complex methodologies, requirement for specialized equipment, and limited clinical applicability. Simplifying these technologies and conducting rigorous validation studies are essential for broader adoption. Similarly, advanced imaging techniques such as MRI-derived biomarkers offer high accuracy but remain financially and technically inaccessible for widespread use. Innovations that reduce costs and combine these imaging modalities with noninvasive biomarkers could dramatically improve diagnostic accuracy and scalability. Artificial intelligence (AI) and machine learning (ML) offer transformative potential for MASLD and MASH diagnosis by integrating diverse datasets to enhance diagnostic precision. However, barriers such as limited data quality, small datasets, and the absence of user-friendly tools for clinicians hinder their practical application. Expanding datasets through collaborative research, standardizing data collection, and developing intuitive AI tools will be essential for embedding AI-driven diagnostics into clinical practice.
Novel PoC diagnostic devices can represent a game-changing approach for MASLD and MASH management. By enabling noninvasive, rapid testing through small samples like blood or saliva, PoC devices address key limitations of traditional diagnostics such as liver biopsies and advanced imaging. These affordable, portable tools are particularly valuable in resource-constrained settings, enabling large-scale screening campaigns and rapid, actionable results. Integrating PoC devices into primary care workflows can empower healthcare providers to deliver timely interventions, reducing disease progression and improving outcomes.
Effective management of MASLD and MASH also requires addressing challenges in both non-pharmacological and pharmacological treatments. Lifestyle interventions, though foundational, are difficult to sustain due to behavioural, social, and economic factors. Developing culturally tailored, socioeconomically appropriate programs, coupled with digital tools like mobile apps and wearable devices, can enhance adherence. For paediatric cases, family-centred and tailored strategies are necessary to ensure sustainability. On the pharmacological front, the limited availability of approved drugs highlights the need for accelerated therapeutic development. Combination therapies targeting inflammation, fibrosis, and metabolic dysfunction, along with innovative drug classes like GLP-1R agonists and FASN inhibitors, offer promising avenues. Refining clinical trial designs with robust biomarkers and stratified patient cohorts will further expedite drug approvals.
A holistic, multi-pronged strategy is essential to tackle the MASLD and MASH epidemic. Combining advanced imaging, biomarker-based tools, and AI-driven diagnostics with robust public health campaigns and personalized treatment approaches integrating lifestyle, genetics, biomarkers, and pharmacology will significantly improve diagnostic accuracy and therapeutic outcomes. The broadening of diagnostic access through PoC technologies holds the potential to enhance global health equity, transforming the landscape of MASLD and MASH care.
Data availability
No datasets were generated or analysed during the current study.
References
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatol. 77, 1335–1347 (2023).
Quek, J. et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 8, 20–30 (2023).
Younossi, Z. M. et al. The Global Epidemiology of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis among Patients with Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 22, 1999–2010.e8 (2024).
Lee, E. J. et al. Prevalence of nonalcoholic fatty liver disease in pediatrics and adolescents: a systematic review and meta-analysis. World J. Pediatr. 20, 569–580 (2024).
Young, S. et al. Prevalence and profile of nonalcoholic fatty liver disease in lean adults: systematic review and meta-analysis. Hepatol. Commun. 4, 953–972 (2020).
Targher, G., Byrne, C. D., Lonardo, A., Zoppini, G. & Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 65, 589–600 (2016).
VanWagner, L. B. & Rinella, M. E. Extrahepatic manifestations of nonalcoholic fatty liver disease. Curr. hepatol. rep. 15, 75–85 (2016).
Musso, G. et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 11, e1001680 (2014).
Mantovani, A. et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut 71, 778–788 (2022).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018).
Sakuma, T. et al. A diet-induced murine model for non-alcoholic fatty liver disease with obesity and insulin resistance that rapidly develops steatohepatitis and fibrosis. Lab. Invest. 102, 1150–1157 (2022).
An, P. et al. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. commun. 11, 2362 (2020).
Neuberger, J. et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 69, 1382–1403 (2020).
O’Kelly, R. et al. A survey of point of care testing in Irish hospitals: room for improvement. Ir. J. Med. Sci. 180, 237–240 (2011).
Nichols, J. H. Utilizing point-of-care testing to optimize patient care. Ejifcc 32, 140 (2021).
Chen, V. L. et al. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatol. 81, 312–320 (2025).
Gawrieh, S. et al. Saroglitazar, a PPAR-α/γ agonist, for treatment of NAFLD: a randomized controlled double-blind phase 2 trial. Hepatol. 74, 1809–1824 (2021).
Loomba, R. et al. Safety, pharmacokinetics, and pharmacodynamics of pegozafermin in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 1b/2a multiple-ascending-dose study. Lancet Gastroenterol. Hepatol. 8, 120–132 (2023).
Loomba, R. et al. Denifanstat for the treatment of metabolic dysfunction-associated steatohepatitis: a multicentre, double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 9, 1090–1100 (2024).
Schweiger, M. et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat. Commun. 8, 14859 (2017).
Stefan, N., Yki-Järvinen, H. & Neuschwander-Tetri, B. A. Metabolic dysfunction-associated steatotic liver disease: heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 13, 134–148 (2024).
Kang, G. G., Trevaskis, N. L., Murphy, A. J. & Febbraio, M. A. Diet-induced gut dysbiosis and inflammation: Key drivers of obesity-driven NASH. Iscience 26, (2023).
Liang, J. Q. et al. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat. Commun. 9, 4490 (2018).
Enooku, K. et al. Hepatic FATP5 expression is associated with histological progression and loss of hepatic fat in NAFLD patients. J. Gastroenterol. 55, 227–243 (2020).
Zhao, L. et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J. Hepatol. 69, 705–717 (2018).
Moore, M. P. et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatol. 76, 1452–1465 (2022).
Smith, G. I. et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Inves. 130, 1453–1460 (2020).
Dong, J. et al. Hepatocyte-specific IL11 cis-signaling drives lipotoxicity and underlies the transition from NAFLD to NASH. Nat. Commun. 12, 66 (2021).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Liu, L. et al. Activation of the PERK-CHOP signaling pathway during endoplasmic reticulum stress contributes to olanzapine-induced dyslipidemia. Acta Pharm. Sinic. 45, 502–516 (2024).
Sharma, P. et al. Reactive oxygen species (ROS)-mediated oxidative stress in chronic liver diseases and its mitigation by medicinal plants. Am J. Transl. Res. 15, 6321 (2023).
Sun, X. et al. Neutralization of oxidized phospholipids ameliorates non-alcoholic steatohepatitis. Cell Metab. 31, 189–206. e188 (2020).
Liu, C. et al. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J. Hepatol. 75, 150–162 (2021).
Emery, M. G. et al. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatol. 38, 428–435 (2003).
Li, Z., Berk, M., McIntyre, T. M., Gores, G. J. & Feldstein, A. E. The lysosomal-mitochondrial axis in free fatty acid–induced hepatic lipotoxicity. Hepatol. 47, 1495–1503 (2008).
Pal, S. C. & Mendez-Sanchez, N. Insulin resistance and adipose tissue interactions as the cornerstone of metabolic (dysfunction)-associated fatty liver disease pathogenesis. World J. Gastroenterol. 29, 3999–4008 (2023).
Koyama, Y. & Brenner, D. A. Liver inflammation and fibrosis. J. Clin. Inves. 127, 55–64 (2017).
Cha, J.-Y., Kim, D.-H. & Chun, K.-H. The role of hepatic macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Lab. Anim. Res. 34, 133–139 (2018).
Kazankov, K. et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159 (2019).
Miura, K., Yang, L., van Rooijen, N., Ohnishi, H. & Seki, E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1310–G1321 (2012).
Chen, Y. et al. Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease. Nat. Genet. 55, 1640–1650 (2023).
Anstee, Q. M. et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort(☆). J. Hepatol. 73, 505–515 (2020).
BasuRay, S., Smagris, E., Cohen, J. C. & Hobbs, H. H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatol. 66, 1111–1124 (2017).
Bruschi, F. V. et al. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatol. 65, 1875–1890 (2017).
Pirola, C. J. & Sookoian, S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatol. 62, 1742–1756 (2015).
Borén, J. et al. Effects of TM6SF2 E167K on hepatic lipid and very low-density lipoprotein metabolism in humans. JCI insight 5, e144079 (2020).
Pirola, C. J. et al. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease [S]. J. lipid Res. 60, 176–185 (2019).
Ma, Y. et al. 17-Beta Hydroxysteroid Dehydrogenase 13 Is a Hepatic Retinol Dehydrogenase Associated With Histological Features of Nonalcoholic Fatty Liver Disease. Hepatol. 69, 1504–1519 (2019).
Thangapandi, V. R. et al. Loss of hepatic Mboat7 leads to liver fibrosis. Gut 70, 940–950 (2021).
Stender, S. et al. Relationship between genetic variation at PPP1R3B and levels of liver glycogen and triglyceride. Hepatol. 67, 2182–2195 (2018).
Bellini, G., Del Giudice, E. M., Nobili, V. & Rossi, F. The IRGM rs10065172 variant increases the risk for steatosis but not for liver damage progression in Italian obese children. J. Hepatol. 67, 653–655 (2017).
Blais, P. et al. Nonalcoholic Fatty Liver Disease is Underrecognized in the Primary Care Setting. Am. Colleg. Gastroenterol. 110, 10–14 (2015).
Kouvari, M. et al. Liver biopsy-based validation, confirmation and comparison of the diagnostic performance of established and novel non-invasive steatotic liver disease indexes: Results from a large multi-center study. Metab. 147, 155666 (2023).
Lee, J.-H. et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 42, 503–508 (2010).
Poynard, T. et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp. Hepatol. 4, 1–14 (2005).
Castera, L., Friedrich-Rust, M. & Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterol. 156, 1264–1281. e1264 (2019).
Hernaez, R. et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatol. 54, 1082–1090 (2011).
Çam, İ, Koc, U., Genez, S. & Güneş, A. Computed tomography measurements of hepatic steatosis in cholelitihiasis and cholecystectomy cases using unenhanced images. J. Med. Imaging Radiat. 51, 137–144 (2020).
Caussy, C. et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatol. 67, 1348–1359 (2018).
Bannas, P. et al. Quantitative magnetic resonance imaging of hepatic steatosis: validation in ex vivo human livers. Hepatol. 62, 1444–1455 (2015).
Kleiner, D. E. & Makhlouf, H. R. Histology of NAFLD and NASH in adults and children. Clin. liver Dis. 20, 293 (2015).
Ratziu, V. et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterol. 128, 1898–1906 (2005).
Vuppalanchi, R. et al. Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 481–486 (2009).
Bedossa, P. & Carrat, F. Liver biopsy: the best, not the gold standard. J. Hepatol. 50, 1–3 (2009).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatol. 41, 1313–1321 (2005).
Taylor-Weiner, A. et al. A machine learning approach enables quantitative measurement of liver histology and disease monitoring in NASH. Hepatol. 74, 133–147 (2021).
Grigorescu, M. et al. A novel pathophysiological-based panel of biomarkers for the diagnosis of nonalcoholic steatohepatitis. J. Physiol. Pharmacol. 63, 347–353 (2012).
Zhang, X. et al. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J. Hepatol. 61, 1365–1375 (2014).
Shen, J. et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J. Hepatol. 56, 1363–1370 (2012).
Dixon, J. B., Bhathal, P. S. & O’brien, P. E. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterol. 121, 91–100 (2001).
Palekar, N. A., Naus, R., Larson, S. P., Ward, J. & Harrison, S. A. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 26, 151–156 (2006).
Feldstein, A. E. et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis [S]. J. Lipid Res. 51, 3046–3054 (2010).
Poynard, T. et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 6, 1–16 (2006).
Ballestri, S. et al. Ultrasonographic fatty liver indicator detects mild steatosis and correlates with metabolic/histological parameters in various liver diseases. Metab. 72, 57–65 (2017).
Ballestri, S. & Lonardo, A. Ultrasonographic fatty liver indicator (US-FLI): a reliable biomarker for non-invasive NAFLD stratification. Metab. Target Organ Damage 3, 13 (2023).
Premkumar, M., Karvellas, C. J., Kulkarni, A. V., Bhujade, H. & Reddy, K. R. Role of point-of-care ultrasound (POCUS) in clinical hepatology. Hepatol. 10, 1097 (2024).
Barbosa, J. V. et al. Fibrosis-4 index can independently predict major adverse cardiovascular events in nonalcoholic fatty liver disease. Am. Colleg. Gastroenterol. 117, 453–461 (2022).
Liu, X. et al. Association Between Noninvasive Liver Fibrosis Scores and Heart Failure in a General Population. J. Am Heart Assoc. 13, e035371 (2024).
Schonmann, Y., Yeshua, H., Bentov, I. & Zelber-Sagi, S. Liver fibrosis marker is an independent predictor of cardiovascular morbidity and mortality in the general population. Dig. Liver Dis. 53, 79–85 (2021).
Tavaglione, F. et al. Development and Validation of a Score for Fibrotic Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 21, 1523–1532.e1521 (2023).
Harrison, S. A. et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 5, 970–985 (2020).
Harrison, S. A. et al. NIS2+, an optimisation of the blood-based biomarker NIS4(R) technology for the detection of at-risk NASH: A prospective derivation and validation study. J. Hepatol. 79, 758–767 (2023).
Zhou, Y. et al. Noninvasive Detection of Nonalcoholic Steatohepatitis Using Clinical Markers and Circulating Levels of Lipids and Metabolites. Clin. Gastroenterol. Hepatol. 14, 1463–1472 e1466 (2016).
Povero, D. et al. Characterization and proteome of circulating extracellular vesicles as potential biomarkers for NASH. Hepatol. Commun. 4, 1263–1278 (2020).
Noureddin, M. et al. Serum identification of at-risk MASH: The metabolomics-advanced steatohepatitis fibrosis score (MASEF). Hepatol. 79, 135–148 (2024).
Andersson, A. et al. Clinical utility of magnetic resonance imaging biomarkers for identifying nonalcoholic steatohepatitis patients at high risk of progression: a multicenter pooled data and meta-analysis. Clin. Gastroenterol. Hepatol. 20, 2451–2461. e2453 (2022).
von Morze, C. et al. Detection of early-stage NASH using non-invasive hyperpolarized (13)C metabolic imaging. Sci. Rep. 14, 14854 (2024).
Newsome, P. N. et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 5, 362–373 (2020).
Noureddin, M. et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J. Hepatol. 76, 781–787 (2022).
Kim, B. K. et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J. Hepatol. 77, 1482–1490 (2022).
Richens, J. G., Lee, C. M. & Johri, S. Improving the accuracy of medical diagnosis with causal machine learning. Nat. Commun. 11, 3923 (2020).
Ma, H., Xu, C.-F., Shen, Z., Yu, C.-H. & Li, Y.-M. Application of machine learning techniques for clinical predictive modeling: a cross-sectional study on nonalcoholic fatty liver disease in China. BioMed. Res. Int. 2018, 4304376 (2018).
McPherson, S. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. Colleg. Gastroenterol. 112, 740–751 (2017).
Younossi, Z. M. et al. Economic evaluation of non-invasive test pathways for high-risk metabolic dysfunction-associated steatotic liver disease (MASLD) in the United Kingdom (UK). Ann. Hepatol. 30, 101789 (2025).
Srivastava, A. et al. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 19, 122 (2019).
Berzigotti, A. et al. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis–2021 update. J. Hepatol. 75, 659–689 (2021).
Sangha, K., Chang, S. T., Cheung, R. & Deshpande, V. S. Cost-effectiveness of MRE versus VCTE in staging fibrosis for nonalcoholic fatty liver disease (NAFLD) patients with advanced fibrosis. Hepatol. 77, 1702–1711 (2023).
Shumbayawonda, E. et al. Utility and cost-effectiveness of LiverMultiScan for MASLD diagnosis: a real-world multi-national randomised clinical trial. Commun. Med. ((Lond)). 5, 74 (2025).
Pepe, M. S., Longton, G. & Janes, H. Estimation and comparison of receiver operating characteristic curves. Stata J. 9, 1–16 (2009).
Bossuyt, P. M. et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 4, 41–44 (2003).
Goehring, C., Perrier, A. & Morabia, A. Spectrum bias: a quantitative and graphical analysis of the variability of medical diagnostic test performance. Stat Med. 23, 125–135 (2004).
Castéra, L. et al. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J. Hepatol. 52, 191–198 (2010).
Ruopp, M. D., Perkins, N. J., Whitcomb, B. W. & Schisterman, E. F. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biometrical J.: J. Math. Methods Biosci. 50, 419–430 (2008).
McHugh, L. C., Snyder, K. & Yager, T. D. The effect of uncertainty in patient classification on diagnostic performance estimations. PLoS One. 14, e0217146 (2019).
Starekova, J., Hernando, D., Pickhardt, P. J. & Reeder, S. B. Quantification of liver fat content with CT and MRI: state of the art. Radiol. 301, 250–262 (2021).
Sima, N. et al. SHERLOCK4HAT: A CRISPR-based tool kit for diagnosis of Human African Trypanosomiasis. EBioMed. 85, 104308 (2022).
Abbot. A Portable Blood Analysis System That Delivers Lab-Quality Diagnostic Results in Minutes., https://www.globalpointofcare.abbott/us/en/product-details/apoc/i-stat-system-us.html.
Fraleigh, C. D. & Duff, E. Point-of-care ultrasound: An emerging clinical tool to enhance physical assessment. Nurse Pract. 47, 14–20 (2022).
Sivakumar, P., Saul, M., Robinson, D., King, L. E. & Amin, N. B. SomaLogic proteomics reveals new biomarkers and provides mechanistic, clinical insights into Acetyl coA Carboxylase (ACC) inhibition in Non-alcoholic Steatohepatitis (NASH). Sci. Rep. 14, 17072 (2024).
Erhardtsen, E. et al. Determining a healthy reference range and factors potentially influencing PRO-C3–a biomarker of liver fibrosis. JHEP Rep. 3, 100317 (2021).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterol. 149, 367–378. e365 (2015).
Younossi, Z. M., Corey, K. E. & Lim, J. K. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterol. 160, 912–918 (2021).
Shi, X. et al. A systematic review and meta-analysis of randomized controlled trials: effects of mediterranean diet and low-fat diet on liver enzymes and liver fat content of NAFLD. Food Funct. 15, 8248–8257 (2024).
Della Corte, C. et al. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: The results of an Italian Study. Nutr. 39, 8–14 (2017).
Wang, S. -t. et al. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 20, 1–12 (2020).
Minciuna, I., Gallage, S., Heikenwalder, M., Zelber-Sagi, S. & Dufour, J.-F. Intermittent fasting—the future treatment in NASH patients?. Hepatol. 78, 1290–1305 (2023).
Haase, C. L. et al. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: evidence from a UK primary care database. Int. J. Obes. 45, 1249–1258 (2021).
Lassailly, G. et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterol. 159, 1290–1301. e1295 (2020).
Aminian, A. et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy-proven nonalcoholic steatohepatitis. Jama. 326, 2031–2042 (2021).
FDA, U. S. F. a. D. A. FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease, https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease (2024).
Harrison, S. A. et al. Effects of resmetirom on noninvasive endpoints in a 36-week phase 2 active treatment extension study in patients with NASH. Hepatol. Commun. 5, 573–588 (2021).
Francque, S. M. et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. New Eng. J. Med. 385, 1547–1558 (2021).
Nakajima, A. et al. Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 54, 1263–1277 (2021).
Harrison, S. A. et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. New Eng. J. Med. 390, 497–509 (2024).
Sanyal, A. et al. A PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 392, 2705–2717 (2019).
Harrison, S. A. et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 8, 1080–1093 (2023).
Rinella, M. E. et al. Non-invasive evaluation of response to obeticholic acid in patients with NASH: Results from the REGENERATE study. J. Hepatol. 76, 536–548 (2022).
Sanyal, A. J. et al. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat. Med. 29, 392–400 (2023).
Zheng, Z. et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct. Target Ther. 9, 234 (2024).
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. New Eng. J. Med. 384, 1113–1124 (2021).
Parker, V. E. et al. Cotadutide promotes glycogenolysis in people with overweight or obesity diagnosed with type 2 diabetes. Nat. Metab. 5, 2086–2093 (2023).
Healio. FDA grants fast track designation to Merck’s efinopegdutide for treatment of NASH, https://www.healio.com/news/gastroenterology/20230615/fda-grants-fast-track-designation-to-mercks-efinopegdutide-for-treatment-of-nash (2023).
Lee, P., Ganguly, S. & Goh, S. Y. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes. Rev. 19, 1630–1641 (2018).
Shimizu, M. et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 21, 285–292 (2019).
Lee, M. M., Ghouri, N., McGuire, D. K., Rutter, M. K. & Sattar, N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in Asian versus white patients with and without type 2 diabetes. Diabetes Care. 44, 1236–1241 (2021).
Sumida, Y. et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J. Gastroenterol. 46, 257–268 (2011).
Schattenberg, J. M. et al. NASHmap: clinical utility of a machine learning model to identify patients at risk of NASH in real-world settings. Sci. Rep. 13, 5573 (2023).
McPherson, S., Stewart, S. F., Henderson, E., Burt, A. D. & Day, C. P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 59, 1265–1269 (2010).
Vali, Y. et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J. Hepatol. 73, 252–262 (2020).
Abdelmalek, M. et al. FIBROSpect® NASH serum test identifies advanced liver fibrosis in patients with nonalcoholic steatohepatitis: Results of a validation study. J. Hepatol. 68, S571–S572 (2018).
Boyle, M. et al. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Rep. 1, 188–198 (2019).
Selvaraj, E. A. et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 75, 770–785 (2021).
Imajo, K. et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterol. 150, 626–637.e627 (2016).
Lindvig, K. P. et al. Development, validation, and prognostic evaluation of LiverPRO for the prediction of significant liver fibrosis in primary care: a prospective cohort study. Lancet Gastroenterol. Hepatol. 10, 55–67 (2025).
Naderi Yaghouti, A. R., Zamanian, H. & Shalbaf, A. Machine learning approaches for early detection of non-alcoholic steatohepatitis based on clinical and blood parameters. Sci. Rep. 14, 2442 (2024).
Gruneau, L., Kechagias, S., Sandstrom, P., Ekstedt, M. & Henriksson, M. Cost-effectiveness analysis of noninvasive tests to identify advanced fibrosis in non-alcoholic fatty liver disease. Hepatol. Commun. 7, e00191 (2023).
Younossi, Z. M., Paik, J. M., Henry, L., Stepanova, M. & Nader, F. Pharmaco-Economic Assessment of Screening Strategies for High-Risk MASLD in Primary Care. Liver Int. 45, e16119 (2025).
Imajo, K. et al. Head-to-head comparison among FAST, MAST, and multiparametric MRI-based new score in diagnosing at-risk MASH. Eur. Radiol. 35, 3599–3609 (2024).
Zhang, X. et al. A blood-based biomarker panel for non-invasive diagnosis of metabolic dysfunction-associated steatohepatitis. Cell. Metab. 37, 59–68.e53 (2025).
Loomba, R. et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. New Eng. J. Med. 389, 998–1008 (2023).
Ratziu, V. et al. Aramchol in patients with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 27, 1825–1835 (2021).
Loomba, R. et al. TVB-2640 (FASN inhibitor) for the treatment of nonalcoholic steatohepatitis: FASCINATE-1, a randomized, placebo-controlled phase 2a trial. Gastroenterol. 161, 1475–1486 (2021).
Loomba, R. et al. Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis with Liver Fibrosis. New Eng. J. Med. 391, 299–310 (2024).
Sanyal, A. J. et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. New Eng. J. Med. 391, 311–319 (2024).
Sanyal, A. J. et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat. Med. 30, 1–12 (2024).
Lawitz, E. J. et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 16, 1983–1991. e1983 (2018).
Acknowledgements
This work was funded by a grant from the Commonwealth of Australia, represented by the Department of Health (Grant Activity 4-DGEJZ1O/4-CW7UT14). All figures used in this manuscript have been created in BioRender. Tantu, M. (2025) https://BioRender.com/uutv6z3.
Author information
Authors and Affiliations
Contributions
M.T.T., A.G.R. and M.J.A.S. conceptualized the manuscript. M.T.T. and M.J.A.S. drafted the original manuscript. F.Z.F., F.H., K.M.K. A.G.R. and L.Q. critically revised the manuscript for important intellectual content. M.T.T. prepared the figures. M.T.T., A.G.R. and M.J.A.S. revised the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tantu, M.T., Farhana, F.Z., Haque, F. et al. Pathophysiology, noninvasive diagnostics and emerging personalized treatments for metabolic associated liver diseases. npj Gut Liver 2, 18 (2025). https://doi.org/10.1038/s44355-025-00030-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44355-025-00030-2
This article is cited by
-
Association between atherogenic index of plasma (AIP) and all-cause and cardiovascular mortality in patients with metabolic dysfunction-associated steatotic liver disease (MASLD): a cohort study based on NHANES 1999–2018
Cardiovascular Diabetology (2025)
-
Metabolic and hepatic biomarkers associated with MASLD in the Chinese population
Scientific Reports (2025)