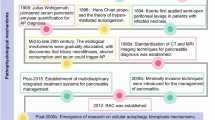
Abstract
Biorepositories with validated collection and preservation of biospecimens are crucial for biomarker and therapeutic discovery. Presently, multiple US federally funded, multicenter pancreatitis consortia studies maintain active biorepositories. The current study provides a comprehensive comparison of their collection methods and intended usage. Beyond each sample’s intended usage, these samples hold potential for novel applications based on emerging methodologies in the field. We anticipate that this review will provide useful information to help guide a consensus among pancreatitis consortium studies on optimal specimen collection and storage methods, which will further accelerate the tempo of discovery platforms in the pancreatitis space.
Similar content being viewed by others
In the rapidly evolving landscape of preclinical and clinical research, biorepositories emerge as a cornerstone in advancing our understanding of complex diseases. These repositories are essential for collecting, cataloging, and storing biological samples for various applications, including the identification of novel disease biomarkers and the support of translational studies. International collaborative consortia for pancreatic disease, such as the Pancreatic Cancer Early Detection Consortium (PRECEDE) and the PANcreatic Disease ReseArch Consortium (PANDoRA), have accumulated tens of thousands of biospecimens, provided novel insights into disease nature and mechanisms, and contributed to the development of official standards of care and guidelines for diseases and patient care1,2. As a result, the implementation of standardized protocols for biorepositories becomes imperative to ensure that scientific progress is substantiated by reliable and high-quality materials.
Pancreatitis is a life-threatening inflammatory disorder for which there has been growing interest in developing clinical consortia studies3. There are currently several existing protocols within and beyond the pancreatitis field on how to collect and organize a biorepository. However, a comprehensive comparison and analysis of biorepositories within emerging multicenter pancreatitis consortia studies in the US has not been conducted. This paper aims to highlight the vast biorepositories established over the past decade by US federally funded clinical consortia studies for pancreatitis disease, review the similarities and differences in sample acquisition methods between studies, and assess the potential for the scientific community to leverage this valuable resource for investigating important research questions.
Currently existing pancreatitis consortia studies
As of 2024, the largest and most comprehensive biorepositories are housed within multicenter consortia studies in the US and funded by federal agencies. These studies are noteworthy for the highly diverse biospecimens they maintain that support a wide range of research applications. We specifically focus on US federally funded, multicenter pancreatitis consortia studies as subjects of comparison and analysis. The seven studies that meet these criteria include SVI, PROCEED, INSPPIRE 2, POST, SHARP, ACCESS-AP, and DREAM (Table 1).
The Stent vs Indomethacin Study (SVI) was one of the earliest studies that initiated the development of a multicenter biorepository for pancreatitis patient samples, beginning in 2015. Its main objective was to ascertain whether indomethacin treatment alone could prevent post-ERCP pancreatitis (PEP) without the need for a prophylactic pancreatic duct stent placement (PSP). The study collected samples from patients at elevated risk for PEP to develop a foundation for future translational research initiatives using biorepository samples4,5.
In 2017, the PROspective Evaluation of Chronic Pancreatitis for EpidEmiologic and Translational StuDies (PROCEED) study was initiated under the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC). PROCEED aimed to examine a well-phenotyped study population at different stages of pancreatitis to accurately define disease progression and associated complications. In addition to its primary research objectives, the longitudinal study also created an extensive biorepository to support translational studies related to diagnostic, predictive, and prognostic biomarker testing6.
Also founded as an ancillary study to the CPDPC cohort during 2017, the INternational Study Group of Pediatric Pancreatitis: In Search for a CuRE Cohort Study (INSPPIRE 2). INSPPIRE 2 was created as a prospective cohort to study risk factors, natural history, and outcomes of acute recurrent pancreatitis (ARP) and chronic pancreatitis (CP) in children. This study similarly generated a biorepository focused on identifying biomarkers for early diagnosis of CP in children with progressive ARP, as well as predictors of long-term complications7.
To assess patient selection and timing for total pancreatectomy with islet auto transplantation (TPIAT) as a treatment for ARP, CP, and diabetes, the Prospective Observational Study of Total Pancreatectomy with Islet Autotransplantation (POST) was developed in 2017. Patients at any age undergoing TPIAT were enrolled to develop a risk model for guiding informed decisions related to the timing of a TPIAT intervention, and a biorepository was established to facilitate further ancillary research8.
The SpHincterotomy for Acute Recurrent Pancreatitis Randomized Trial (SHARP) was initiated in 2018 to evaluate the efficacy of two treatment approaches in managing ARP and pancreas divisum: endoscopic ultrasound with endoscopic retrograde cholangiopancreatography (ERCP) and minor papilla endoscopic sphincterotomy (miES) versus endoscopic ultrasound with a sham procedure. The study established the creation of a biological biorepository for future exploratory analyses of genetic and laboratory associations with patient outcomes9.
By 2020, A Case-CrossovEr study deSign to inform tailored interventions to prevent disease progression in Acute Pancreatitis (ACCESS-AP) was developed to investigate the impact of short-term changes in alcohol consumption on the risk of acute pancreatitis (AP) or ARP. This study aimed to provide data on covariates such as drinking patterns and behavioral changes prior to and following AP or ARP. ACCESS-AP further established a biorepository to generate preclinical data on biomarkers that have predictive potential or actionable targets for therapeutic agents10,11.
The most recent cohort in 2022 was the Diabetes RElated to Acute Pancreatitis and Its Mechanisms (DREAM) Study, created to investigate the incidence, etiology, and pathophysiology of diabetes following AP. Established under the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC), the study’s primary objective is to define the cumulative incidence and clinical characteristics associated with the development of diabetes, pathophysiology, and immunological mechanisms of diabetes after one or more episodes of AP. A biorepository was established to allow continued investigation of covariate influences contributing to diabetes onset following AP12,13,14.
Overview of the biospecimen collection process
Biospecimen preservation involves several essential steps to preserve the viability and quality of samples over a prolonged period of time (Fig. 1). In this paper, the term “biospecimens” is synonymous with “samples” and may be used interchangeably. The process begins by collecting samples from patients of interest, including blood, urine, saliva, stool, and other bodily fluids or biological tissues. Following collection, the samples undergo laboratory processing to prepare for long-term storage and potential use in subsequent research studies. After processing, they are transported to a central repository and stored under highly controlled conditions. These storage facilities preserve samples and associated data until they are necessary for future research usage.
Diagram of the four main steps in the biospecimen handling process employed by consortia. The process begins with Collection, where biospecimen samples are obtained during ambulatory visits, research visits, hospitalization, or before, during, or after surgery. Following collection, the samples undergo Processing, which includes processing within a maximum time frame after collection, sample addition to specified tubes as outlined in each study’s SOP, aliquoting the samples, and placing them in short-term storage. All samples are eventually Transported to the central repository of each consortium. Note that the Transportation step may occur concurrently with or after processing, depending on the specific protocol. Finally, the samples are placed in Storage, typically by freezing, to maintain their quality for future use.
To protect the integrity of these valuable samples, standardized operating procedures (SOPs) must be implemented. Thus, it is imperative that all pancreatitis consortia studies with an established biorepository impose detailed SOPs that maximize the preservation of the collected biospecimens to ensure consistency and minimize variability in future research outcomes.
Biospecimen types collected
The biorepository process begins with the collection of biospecimens from patients of interest, which typically occurs during hospitalization, ambulatory visits, research-only visits, or surgery, depending on each study’s objective. The biospecimens collected by the consortia in this review fall under four main sample types: blood, fluids, stool, and pancreatic tissue.
All seven studies collect blood samples for biorepository use. Several different forms of blood samples can be subsequently processed and stored, including serum, plasma, peripheral blood mononuclear cells (PBMCs), DNA or RNA, and whole blood (Table 2). Six out of the seven studies collect non-blood-derived fluid sample types, including urine, saliva, duodenal fluid, and pure pancreatic juice (Table 3). Only four studies collect stool samples (Table 4).
Finally, only the POST consortium study is documented to have collected pancreas tissue samples. For pancreatic tissue, one to two samples of approximately 300 micrograms each are collected, contingent upon patient consent. One set of samples is preserved through paraffin embedding for histological analysis, while a separate sample of similar size is snap-frozen and then stored at −80 °C for various downstream analyses, such as RNA extraction and mass spectrometry.
Timing and frequency of collection
The exact timing and frequency of biospecimen collection contribute to variability among samples, especially when they are collected at different stages of disease progression. Given that definitions of pancreatitis stages remain ambiguous and vary across studies, it is crucial for studies storing pancreatitis samples to have clear guidelines on the specific time points for sample collection. We utilize the PROCEED guidelines of definitive characteristics for each stage to classify pancreatitis disease types15. Five consortia studies collect samples at multiple time points to support longitudinal studies dedicated to delineating disease progression. They are: PROCEED, INSPPIRE 2, POST, ACCESS-AP, and DREAM (Fig. 2). Furthermore, while data related to the history of patients with AP and CP at the time of sample procurement may be available upon request, access to the metadata requires approval from a formal ancillary studies committee.
Timeline that provides an overview of the pancreatitis disease types from which each consortium study collected biospecimens as pancreatitis progresses from acute to chronic stages. The consortia studies listed at each main stage (Acute Pancreatitis, Acute Recurrent Pancreatitis, and Chronic Pancreatitis) represent the studies involved in biospecimen collection at those disease stages. Consortia studies conducting longitudinal studies have multiple collection points across different stages of pancreatitis.
Biospecimen processing time
After the biospecimens are collected, the duration of processing time can affect sample quality and viability. Variability in this time frame is evident across the SOPs of each study, as several studies permit a wide range of intervals between collection and processing.
For blood samples, some consortia studies, including PROCEED, POST, SHARP, and ACCESS-AP, specify that serum, plasma, and PBMC processing must occur within 4 h of collection16,17. In contrast, SVI specifies that processing should occur within 24 h of collection, and INSPPIRE 2 does not impose a strict time frame for blood sample processing (Table 2). Moreover, DREAM specifies that serum and plasma processing must occur within 4 h of collection, while PBMC processing can occur within 12 h of collection, and whole blood processing does not have a maximum time frame. Finally, PROCEED specifies that whole blood (RNA) processing should occur within 72 h, and whole blood (DNA) processing does not have a maximum time frame.
A similar time restriction applies to urine samples; PROCEED, INSPPIRE 2, POST, SHARP, and ACCESS-AP all require urine collection and processing to be completed within 4 h, and SVI mandates only 2 h between collection. For other fluid samples, INSPPIRE 2 and POST do not specify a maximum time frame between collection and processing of saliva samples, whereas PROCEED consistently requires processing of all samples within 4 h, and SVI requires processing of saliva and duodenal fluid within 24 h (Table 3)16.
For stool samples, the time frame is generally longer, as most samples are procured through home collection kits (Table 4). PROCEED, INSPPIRE 2, and DREAM specify a maximum time frame of 14 days after collection in stabilizer tubes before samples are frozen at the central repository16. SVI collects stool samples from a digital rectal examination during indomethacin administration and immediately places samples in short-term storage at −20 °C or −70 °C (preferred).
Overall, the variations in time frames and additional steps prior to processing demonstrate how each consortium has taken different, subtle approaches to preserve sample quality and produce reliable analytical results.
Methods of biospecimen processing and storage before transportation
Biorepository consortia studies have largely standardized the processing techniques for blood samples. Each study’s SOPs cover major steps such as centrifugation and aliquoting. For blood samples, the first step is typically centrifugation, which separates the blood into layers (e.g., serum, plasma, PBMCs) before further processing occurs (Table 2). Moreover, the type of additive used is consistent among consortia, depending on the blood sample type being processed. Following processing, samples are aliquoted, with slight variations among consortia studies in the minimum volume required for each sample layer.
After processing, a number of consortia studies also stipulate certain short-term storage conditions before being transported to long-term storage. For instance, the SVI consortium mandates that all blood samples be stored in a freezer at temperatures between −20 °C and −70 °C as soon as they are aliquoted and transferred to long-term storage within 24 h of processing. The PROCEED consortium offers additional specifications for every blood sample type. Before freezing, plasma aliquots can be kept on ice or in the refrigerator at 4 °C for up to 4 h. In contrast, whole blood (RNA) aliquots should be kept at ambient temperature for 2–72 h and then frozen at −20 °C for at least 24 h before transferring to long-term storage. Both PROCEED and ACCESS-AP also require PBMC aliquots to be frozen at −80 °C for 4–96 h before being moved to long-term storage16. The POST cohort allows all blood aliquots (serum, plasma, and PBMCs) to be kept on ice or in the refrigerator for up to 4 h prior to freezing.
The processing of fluid samples has greater variation across consortia studies. Four of the six studies that collect urine samples (PROCEED, INSPPIRE 2, POST, and ACCESS-AP) list centrifugation as a processing step where only the supernatant layer is aliquoted (Table 3). Furthermore, PROCEED and INSPPIRE 2 require that the supernatant must be separated into three aliquots: one with no additives, one with an RNase inhibitor, and one with a protease inhibitor. Afterward, aliquots with specified minimum volumes are prepared for long-term storage16.
Out of the four studies that collected saliva samples, each consortium had varying procedures upon sample collection. The SVI protocol allows saliva samples to be directly aliquoted without any additives and requires samples to be placed in a −20 °C to −70 °C freezer immediately after aliquoting before being moved to long-term storage conditions within 24 h of processing. The POST protocol, on the contrary, instructs saliva samples to be mixed with additives upon collection in the ORAcollect Dx kit. The PROCEED SOP varies depending on the volume of saliva collected. If the samples collected are under 2 mL, they can be aliquoted without centrifugation or added media; if the samples are over 2 mL, 1 mL should be mixed with an RNase inhibitor without centrifugation before aliquoting16. Based on its intended use for DNA collection, the INSPPIRE 2 cohort adheres to a distinct protocol. After being collected in the Oragene DISCOVER kit and transported to the central repository, the saliva samples undergo DNA purification before being placed into long-term storage.
The least amount of processing is needed for stool samples, and protocols again differ according to the intended use. The SVI network stores stool samples in a −20 °C or −70 °C freezer immediately after collection without additional processing requirements (Table 4). PROCEED, INSPPIRE 2, and DREAM all use the OMNIgene•GUT kit for microbiome profiling, and DREAM further uses OMNImet•GUT for metabolome profiling16.
The processing of pancreas tissue samples by the POST study varies depending on the intended use, either for histopathological evaluation or gene expression analysis. The first set of samples intended for histology is stored at ambient temperature for 24–72 h and washed twice with a 70% alcohol solution. These tissues were later shipped at ambient temperature and embedded in paraffin blocks at the central repository. The second set of samples intended for transcriptome analysis using RNA is refrigerated at 4 °C for 24–72 h to allow the RNA-Later solution to penetrate the tissue. Subsequently, RNA-later tissues were shipped on dry ice to the central repository and stored at −80 °C until RNA extraction and library preparation.
Although most key biospecimen processing steps are generally consistent across studies, some variation remains within particular procedural steps. These variations draw attention to the different procedures required for distinct intended uses and may indicate the need for standardized procedures to guarantee even more uniformity in research involving biospecimens.
Biospecimen transportation conditions to the central repository
All biorepository samples from each study are eventually shipped and stored long-term at a designated central repository. The responsibilities of each study’s central repository vary. Some repositories, such as PROCEED, allow individual satellite centers to process all samples before shipping them to the central repository16. Other repositories, such as INSPPIRE 2, handle all processing of blood and saliva samples after they arrive at the central repository.
The majority of studies mandate that samples be sent to the central repository either at ambient temperature or frozen on dry ice. All studies, except INSPPIRE 2, require blood samples to be shipped on dry ice, and DREAM requires its cryopreserved PBMC samples to be shipped in liquid nitrogen16,17. All studies also require urine samples to be shipped frozen on dry ice. While INSPPIRE 2 and POST allow saliva samples to be shipped at ambient temperature to the central repository, SVI and PROCEED require saliva samples to be shipped on dry ice. Lastly, SVI requires stool samples to be shipped on dry ice, while PROCEED, INSPPIRE 2, and DREAM require stool samples to be shipped at ambient temperature from home collection to the clinical center before being shipped to the central repository16,17. Despite having differing responsibilities, all central repositories are essential for the effective operation of biorepositories.
Storage requirements
The final step in the biospecimen process is placing samples in long-term storage to preserve them indefinitely. Each study has a designated data coordinating center responsible for the governance of data storage, handling, and protection. Moreover, all data are de-identified in accordance with the guidelines for privacy and confidentiality of human research participants, and the associated clinical information for each biorepository is available upon request.
In general, all studies require samples to be frozen and stored in a freezer. For blood samples, most studies specify storage in a −80 °C freezer. A few studies, such as SVI and POST, allow specimens to be stored at −70 °C. Additionally, consortia studies that collect PBMC samples, including PROCEED, ACCESS-AP, and DREAM, specify the placement of PBMC samples in a liquid nitrogen freezer16,17. Similarly, most studies generally require urine, saliva, stool, and tissue samples to be stored in a −80 °C freezer, while SVI and POST allow urine samples to be stored at −70 °C16,17. Overall, these variations in storage practices further highlight the opportunity to align protocols for biospecimens, depending on their intended use.
Cited and potential other uses of biospecimens
In addition to providing detailed SOPs for the collection and processing of biorepository samples, each study lists several envisioned uses for the sample types collected (Table 5). Several common uses for blood samples include serum immune profiling, whole blood transcriptomic analyses, PBMC immune profiling, plasma proteomics, autoantibody and neo-antigen screening, genomics, and other functional assays. Examples of usages for fluid samples include utilizing urine samples for toxicology panels and saliva samples for genomics. Stool samples are mostly used for microbiome and metabolomic profiling.
Beyond each sample’s intended purpose, additional novel assays and metrics could be applied to the biorepository collections based on emerging methodologies in the field. Specifically, several promising applications include the detection of extracellular vesicles, T-cell receptor sequencing, blood microsampling, and further multi-omics approaches18,19,20. With the help of these novel techniques, a deeper understanding of disease mechanisms, patient stratification, and therapeutic interventions can ultimately be achieved.
Moreover, numerous publications have emerged from the consortia studies reporting various research outcomes associated with utilizing the collected biospecimens. For instance, the PROCEED consortium leveraged its samples for advanced applications, including serum immune profiling to better understand immune responses in pancreatitis disease continuum and mass cytometry by time-of-flight (CyTOF) analysis for biomarker discovery in CP21,22. These techniques are examples of versatile expansions on the conventional use of biorepository samples, and they illuminate the potential for addressing broader health outcomes in a way that is highly useful to a clinical setting.
Discussion
The current review analyzes the biorepositories established over the past decade through seven US federally funded, multicenter pancreatitis consortia studies, which together collect and store a wide array of biospecimens, along with detailed phenotypic metadata. These biorepositories, which contain several biospecimen types from differing pancreatitis patients, provide a solid basis for multimodal research that can help us deepen our understanding of pancreatitis. By choosing to analyze methods produced by well-established, major contributors within the realm of pancreatitis biorepository consortia, we can develop a greater understanding of the most time-tested and effective methods for biorepository collection, processing, and storage. We aimed to address three important objectives: (1) provide knowledge of the immense biorepositories established in the last decade through US federally funded, multicenter clinical consortia studies for pancreatitis, (2) understand the similarities and differences between consortia studies as they relate to sample acquisition, and (3) emphasize the potential for the scientific community to leverage the biorepositories to investigate additional research questions.
Several similarities were identified in the biorepository processing methods across studies, including the types of biospecimens collected, major processing steps, and long-term storage conditions. Additional comparison of INSPPIRE 2 and POST pediatric protocols with adult protocols from other consortia studies revealed no differences in the written protocols between the two age groups in terms of volume or other pediatric-specific requirements. Moreover, we noted important differences across consortia studies primarily related to the biospecimen collection time points, processing times, short-term storage conditions, central repository roles, and cited uses for various sample types. These differences can influence the viability and stability of samples; for instance, recent studies suggest that even brief processing delays could impact the stability of important analytes, such as immunological markers, in blood samples23. As such, current biospecimen protocols that allow for delays in processing may affect the chemical composition of samples, which could introduce variability in further analyses. Yet ultimately, existing protocol variations among consortia studies, such as collection time points and sample type diversity, allow for a wider range of available specimens that can be used for many distinct research interests.
Due to the observed variability between biorepository protocols, the question of the most effective practices for pancreatitis biorepositories subsequently arises. However, we recognize that each study has distinct initial objectives, and variability in sample protocols arises from these differing purposes. Researchers planning to conduct specific experiments using such biospecimens should take this variability into account. For this reason, a singular consensus practice cannot be reasonably implemented as each protocol is tailored to the specific needs and objectives of the study. Nonetheless, the generalized best practices include a maximally detailed protocol, continuous reevaluation of the workflow, and a multidisciplinary team of clinicians and researchers convened early on and throughout the process, as well as broad availability to the scientific community once the biorepositories have matured.
The potential of these biorepositories extends beyond the original applications for which these samples were intended. These samples offer a plethora of opportunities for novel assays and research applications, especially when coupled with an abundance of emerging methodologies. The current review emphasizes how the scientific community can leverage these valuable biorepositories to address additional research questions and advance their related fields.
In conclusion, although this paper focuses on pancreatitis disease and US federally funded multicenter consortia studies, we anticipate the current review will promote greater awareness of the existing landscape of current biorepository consortia and provide a direct comparison of the similarities and differences between consortia studies as they relate to sample acquisition. Furthermore, by offering a framework for consensus among pancreatitis biorepositories, our findings may help to optimize specimen collection and storage practices. Finally, not only are these insights relevant to pancreatitis research, but they can be widely applicable to biorepository disease consortia across various fields, thus demonstrating the potential for biorepository consortia to drive scientific breakthroughs and deepen our comprehension of human diseases worldwide.
Data availability
No datasets were generated or analyzed during the current study.
References
Zogopoulos, G. et al. The PRECEDE consortium: a longitudinal international cohort study of individuals with genetic risk or familial pancreatic cancer. J. Clin. Oncol. 40, https://doi.org/10.1200/jco.2022.40.16_suppl.e16239 (2022).
Campa, D. et al. The Pancreatic Disease Research (PANDoRA) Consortium: ten years’ experience of association studies to understand the genetic architecture of pancreatic cancer. Crit. Rev. Oncol. Hematol. 186, 104020 (2023).
Uc, A. & Husain, S. Z. Pancreatitis in children. Gastroenterology 156, 1969–1978 (2019).
Elmunzer, B. J. et al. Rectal indomethacin alone versus indomethacin and prophylactic pancreatic stent placement for preventing pancreatitis after ERCP: study protocol for a randomized controlled trial. Trials 17, 120 (2016).
Elmunzer, B. J. et al. Indomethacin with or without prophylactic pancreatic stent placement to prevent pancreatitis after ERCP: a randomised non-inferiority trial. Lancet 403, 450–458 (2024).
Yadav, D. et al. PROspective Evaluation of Chronic Pancreatitis for EpidEmiologic and Translational StuDies: rationale and study design from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 47, 1229–1238 (2018).
Uc, A. et al. INternational Study Group of Pediatric Pancreatitis: In Search for a CuRE (INSPPIRE 2) Cohort Study: design and rationale from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 47, 1222–1228 (2018).
Bellin, M. D. et al. A multicenter study of total pancreatectomy with islet autotransplantation (TPIAT): POST (Prospective Observational Study of TPIAT). Pancreatology 18, 286–290 (2018).
Coté, G. A. et al. SpHincterotomy for Acute Recurrent Pancreatitis Randomized Trial: rationale, methodology, and potential implications. Pancreas 48, 1061–1067 (2019).
Jeon, C. Y. et al. A Case-CrossovEr study deSign to inform tailored interventions to prevent disease progression in Acute Pancreatitis (ACCESS-AP)—study design and population. Pancreatology 21, 1231–1236 (2021).
Jeon, C. Y. et al. Differential impact of recent heavy drinking on first and recurrent acute pancreatitis. Alcohol Clin. Exp. Res. 49, 1053–1063 (2025).
Hart, P. A. et al. Rationale and Design for the Diabetes RElated to Acute Pancreatitis and Its Mechanisms Study: a prospective cohort study from the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Pancreas 51, 568–574 (2022).
Dungan, K. et al. Assessing the pathophysiology of hyperglycemia in the diabetes related to acute pancreatitis and its mechanisms study: from the Type 1 Diabetes in Acute Pancreatitis Consortium. Pancreas 51, 575–579 (2022).
Casu, A. et al. Evaluating the Immunopathogenesis of Diabetes After Acute Pancreatitis in the Diabetes RElated to Acute Pancreatitis and Its Mechanisms Study: from the Type 1 Diabetes in Acute Pancreatitis Consortium. Pancreas 51, 580–585 (2022).
Yadav, D. et al. Diagnostic and prognostic biomarkers of chronic pancreatitis. Gastroenterology 166, 957–962.e3 (2024).
Fisher, W. E. et al. Standard operating procedures for biospecimen collection, processing, and storage: from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 47, 1213–1221 (2018).
Wasserfall, C. et al. Standard operating procedures for biospecimen collection, processing, and storage: from the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Pancreas 51, 593–597 (2022).
Bockorny, B. et al. A large-scale proteomics resource of circulating extracellular vesicles for biomarker discovery in pancreatic cancer. eLife 12, RP87369 (2024).
Lee, B. et al. Single-cell sequencing unveils distinct immune microenvironments with CCR6-CCL20 crosstalk in human chronic pancreatitis. Gut. 71, 1831–1842 (2022).
Shen, X. et al. Multi-omics microsampling for the profiling of lifestyle-associated changes in health. Nat. Biomed. Eng. 8, 11–29 (2024).
Lee, B. et al. Distinct Serum Immune Profiles Define the Spectrum of Acute and Chronic Pancreatitis From the Multicenter Prospective Evaluation of Chronic Pancreatitis for Epidemiologic and Translational Studies (PROCEED) Study. Gastroenterology 165, 173–186 (2023).
Gumpper-Fedus, K. et al. Systemic neutrophil gelatinase-associated lipocalin alterations in chronic pancreatitis: a multicenter, cross-sectional study. Clin. Transl. Gastroenterol. 15, e00686 (2024).
Gottfried-Blackmore, A. et al. Effects of processing conditions on stability of immune analytes in human blood. Sci. Rep. 10, 17328 (2020).
Acknowledgements
The authors would like to sincerely thank the principal investigators and leaders of each consortium, including Drs. Dhiraj Yadav (PROCEED, SHARP), Aliye Uc and Mark Lowe (INSPPIRE 2), Melena Bellin (POST, Division of Pediatrics Endocrinology, University of Minnesota Medical School), Joseph Elmunzer (SVI, Division of Gastroenterology and Hepatology, Medical University of South Carolina), Stephen Pandol (ACCESS-AP, Division of Gastroenterology and Hepatology, Cedars-Sinai Medical Center), and Phil Hart (DREAM, Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center), for their contribution of detailed SOP protocols and willingness to answer inquiries related to the protocols of their biorepositories.
Author information
Authors and Affiliations
Contributions
C.L. conducted the literature review, analyzed and compared the SOPs for each biorepository consortium, and drafted the original manuscript. S.Z.H. and B.L. conceptualized, provided overall guidance and supervision, and critically revised the manuscript. C.L., D.Y., M.E.L., A.U., M.A., W.G.P., B.L., and S.Z.H. reviewed, edited, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, C., Yadav, D., Lowe, M.E. et al. The emerging landscape of biorepositories among pancreatitis consortia studies in the US. npj Gut Liver 3, 2 (2026). https://doi.org/10.1038/s44355-025-00048-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44355-025-00048-6