Abstract
Ionic liquids (ILs) have been advantaged and promising candidates for flexible ionic conductors. This review comprehensively summarizes ionic liquid-based ionic conductors (ILICs), including ionic conductive hydrogels, ionogels, and liquid-free ionic conductive elastomers. Furthermore, recent strategies for improving the performance of ILICs, and their applications, including energy harvesting, flexible sensors, and others are overviewed. Finally, an outlook is provided on the challenges and opportunities for ILICs in ionotronic applications.
Similar content being viewed by others
Introduction
Considering that biological systems primarily rely on ions as charge carriers rather than electrons, ionotronics have burgeoned to manipulate ion transport in materials to develop ionic counterparts with functionalities of electronics. Ionotronic applications refer to the response of ions as charge carriers to stimuli. As carriers for ion transport, quasi-solid ionic conductors formed by trapping liquid ionic media within a polymeric network hold promises for translating external stimuli into ion migration and redistribution, thus serving as the core functional materials for ionotronic applications. Ionic liquids (ILs) possess intrinsic ionic conductivity and unique characteristics and tunable properties, couple with that they can be synthesized into poly(ionic liquids) (PILs) for creating liquid-free ionic conductive materials, making them the most advantageous candidates for quasi-solid ionic conductors. Consequently, ionic liquid-based ionic conductors (ILICs) whose ionic conductivity is derived from the IL components are highly favored.
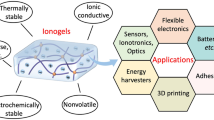
In this review, we comprehensively summarize recent progress of ILICs in ionotronic applications. Firstly, the development of ionotronics and quasi-solid ion conductors is introduced. Subsequently, the evolution and advantages of ILs are presented, followed by a comprehensive overview of the three categories of ILICs: ionic conductive hydrogels, ionogels, and liquid-free ionic conductive elastomers, discussing their respective features and preparation methods. Furthermore, recent strategies for improving the performance of ILICs are summarized. Their ionotronic applications are then reviewed, primarily including energy harvesting, as well as flexible and stretchable sensors (Fig. 1). Finally, an outlook is provided on the challenges and opportunities for ionotronic applications, offering an overview of the advancement of ILICs.
Ionotronics
Flexible and wearable electronics represent a fast-evolving emerging field of research across various applications, including sensing or perception1, healthcare monitoring2, soft robotics3, human-machine interactions4, and energy harvesting and storage5. The research progress of this multidisciplinary field depends on advancements at multiple levels, from revealing molecular mechanisms, fabricating high-performance materials, to innovating device design, system integration, and novel applications. The development of flexible conductive functional materials is crucial for the progression of flexible and wearable electronics.
In organism, information is transmitted using ions instead of electrons as charge carriers (Fig. 2a), and thereby, ionotronics, a field at the intersection of ion transport and electronic systems, has emerged to manipulate ionic charge carriers in materials and to develop ionic counterpart with functionalities of electronics6,7, such as ionic diodes8,9,10 and ionic transistors11 (Fig. 2b)12. The concept of ionotronics (also called iontronics) builds in contrast to traditional electronics, which primarily relies on the flow of electrons for functionality. In contrast, ionotronics incorporate ionic charge carriers, enabling devices to respond to ionic signals in addition to electronic ones. This duality allows for a greater range of functionalities, making ionotronic devices particularly suited for applications that require interaction with biological systems or complex environmental conditions. In particular, flexible and stretchable ionotronics offer unique benefits for bio-integration, creating numerous opportunities in emerging bio-related fields such as wearable bioelectronics13, neuromodulation devices7, and medical implants14,15.
a Information transmission in organisms using ions as carriers. Reproduced with permission11. Copyright 2024 American Chemical Society. b The npn-type ionotronic transistor composed of hydrogel droplets. Reproduced with permission12. Copyright 2024 American Association for the Advancement of Science. c Ionoelastomer junction composed of polyanion and polycation. Reproduced with permission8. Copyright 2020 American Association for the Advancement of Science. d Ion-selective transport confined by pores. Reproduced with permission16. Copyright 2024 The Author(s). e Bio-inspired tactile perception system. Reproduced with permission27. Copyright 2022 American Association for the Advancement of Science. f Polyelectrolyte-confined fluidic memristor. Reproduced with permission28. Copyright 2023 American Association for the Advancement of Science.
Ion transport occurs through various mechanisms, including diffusion, migration under an electric field, gradient, or stimuli-responsive activations. In flexible ionotronics, ion control is primarily achieved through polyelectrolytes and nanopore confinement. The main chains of polyelectrolytes are fixed, allowing only counterions to be transported under stimulation, and the charged main chains repel ions with the same charges, permitting only ions with opposite charges to pass through (Fig. 2c)8. Nanopore materials can achieve selectivity in ion transport through spatial confinement and the electric double layer on the pore walls (Fig. 2d)16,17,18,19. Therefore, materials or composites such as polyelectrolytes, ionic conductive gels, and nanostructured materials are at the forefront of research in flexible ionotronics, as they exhibit tunable ionic transport properties15.
Ionotronics leverages ionic conduction to develop devices that can interact with ionic systems, environmental factors, and electronic interfaces. The integration of ionic and electronic functionalities enables the development of sensors and ionic skin20,21,22, tactile perception systems (Fig. 2e)23,24,25,26,27, neuromorphic computing, and memristors (Fig. 2f)28. Due to factors such as the arbitrary diffusion of ions, ion equilibrium, and low ion mobility, ion transport is more challenging to fully control than electrons. So far, ionotronics struggle to achieve high efficiency and complex functionality found in electronics. However, investigation in ionotronics benefits our understanding of how the brain processes information and stores memories through selective ion transport, opening up a path for the development of low-power ionic machines and efficient energy conversion29. Therefore, ionotronic application, which refers to a response to stimuli with ions as charge carriers, holds great promise and deserves greater attention.
Ionic conduction is a critical aspect of ionotronics, as it governs how effectively ions move through materials30. The mechanisms governing ionic conduction typically involve the migration of ions through a solid or liquid medium, influenced by factors such as temperature, composition, and structural characteristics. Inspired by the working mechanism of organisms that use ions as charge carriers, bio-inspired ionic conductors that conduct current by mobile ions have been developed. To distinguish from solid-state ionic conductors in ionic crystals31,32, the ionic conductors loading ionic media with a polymeric matrix are referred to as quasi-solid ionic conductors33. Leveraging numerous bionic characteristics, including flexibility, softness, and ionic conductivity, bio-inspired quasi-solid ionic conductors develop diverse functionalities and are applied across a wide range of fields, such as sensors, actuators, and power supplies22,33,34,35. The rapid development of flexible and stretchable ionic conductors has greatly expanded ionotronics in a variety of applications, including artificial skin, muscles, axons, ionotronic touchpads, and stretchable luminescence. So far, based on the ionic medium, quasi-solid ionic conductors can be categorized into three types: ionic conductive hydrogels, ionogels, and liquid-free ionic conductive elastomers (Fig. 3)36.
Ionic liquids and poly(ionic liquids)
Ionic liquids (ILs) are molten salts that remain liquid near room temperature, typically defined as having a melting point below 100 °C. Composed entirely of cations and anions, they exhibit unique characteristics, including low vapor pressure, high thermal and chemical stability, intrinsic ionic conductivity, nonflammability, and easy recovery. Due to their ionic nature, ILs can dissolve a variety of organic and inorganic compounds, making them valuable as green solvents, electrolytes, and electrochemistry37. ILs have undergone significant evolution through several generations, each marked by advancements in composition and application (Fig. 4a)38.
The first generation of ILs emerged in the early 1990s, primarily featuring simple organic cations like dialkylimidazolium and alkylpyridinium paired with conventional anions such as chloride ions. These ILs showcased low volatility and excellent thermal stability, making them suited as solvents and electrolytes in energy storage applications like batteries and supercapacitors. The second generation focused on ambient stability and marked the beginning of the era of ILs as green solvents. The representatives of the extended cations are phosphoniums and ammoniums, and typical anions are tetrafluoroborate ([BF4]−), hexafluorophosphate ([PF6]−). The third generation emphasized biocompatible and biodegradable ILs. The aim was to reduce the ecological footprint of ILs by designing greener alternatives that still maintained desirable properties. Moreover, the diverse cation-anion combinations were explored to tailor properties for task-specific applications, thereby broadening the scope of applications, including gas absorption, catalysis, pharmaceuticals, and biomaterials. At present, the fourth generation introduced functionalized ILs by mixing with a second component, and the distinctive nature of these mixtures relies on the synergy of interactions between the ions and the second species, broadening the realm of ILs and expanding their potential applications39. Among them, compounds formed through hydrogen bonding are known as deep eutectic solvents (DESs), while products formed through Lewis acid-base interactions are referred to as liquid coordination complexes (LCCs)37.
Furthermore, ionic liquid monomers can undergo polymerization to obtain poly(ionic liquids) (PILs) (Fig. 4b)40. PILs are composed of ionically charged polymer chains and counterions, providing a versatile platform for numerous applications. Based on the ions carried by the chains, PILs can be classified into polycations, polyanions, and polyzwitterions (polyampholytes). PILs combine the unique characteristics of ILs with the structural advantages of polymers. PILs exhibit tunable ionic conductivity, nonvolatility, remarkable thermal stability, electrochemical stability, and universal compatibility, making them suitable for use in electrochemical devices. In PIL, only the counterion behaves as a charge carrier while the motion of the ionic polymer backbone is negligible. Therefore, the ion conductivity comes from the diffusion of counterion affiliated with the ionic polymer backbone, and the ion diffusivities in such quasi-single-ion conductors can be three orders of magnitude lower than those in monomeric IL. Additionally, by altering the combinations of anions and cations, as well as the polymer backbone, the properties of PILs can be customized for specific applications. This adaptability extends to mechanical properties, thermal behavior, and solubility. PILs also demonstrate significant potential in fields such as separation technologies, where they can be used as membranes to selectively filter ions. Due to the various interactions formed by PILs in composites, such as hydrogen bonds, electrostatic interactions, PILs can be used to impart ionic selectivity functionality to these composites41,42,43,44,45,46. Their ability to form gels and films further enhances their utility in ionotronic applications.
Ionic liquid-based ionic conductors (ILICs)
Ionic liquid (IL) is rising as a dazzling star material to construct ionic conductors due to its attractive advantages. Moreover, the tunable physical and chemical properties of ILs make them an ideal building block for intelligent ionic conductors47. Therefore, IL-based composites formed through encapsulating ILs within solid materials have been widely used for energy harvesting and energy storage systems48,49,50,51. Yao’s group developed a moisture electricity generator by an IL-based film which supported by rigid defect-engineered metal–organic frameworks (MOF)/graphene oxide membranes (Fig. 5a)50. In the same year, Yao and co-workers reported that IL infused into nanochannels of chemically modified aluminum oxide (AAO) for energy harvesting from ubiquitous humidity gradients (Fig. 5b)51. Despite the high ionic conductivity and tailorable ion transport by regulating the IL/host interactions, such rigid IL-based systems suffer from problems such as the complexity of encapsulation, inflexibility, and leakage risks which impose limitations on the practical applications and long-term stability of the resultant devices. In contrast, flexible IL-based ionic conductors (ILICs) such as IL-based gels would promote safety, stability, and mechanical properties. To date, flexible ILIC have been widely developed based on hydrogels, ionogels, and liquid-free elastomers52.
Ionic conductive hydrogels
Hydrogel is a three-dimensional, hydrophilic polymer network that swells in water, retaining plenty of water while preserving its structural integrity53. Hydrogels are characterized by their high water content, which imparts unique properties such as softness, resilience, and resembling natural tissue, thereby enabling a wide range of applications. Incorporating conductive fillers, such as carbon nanotubes (CNT), graphene, polyaniline (PANI), and poly(3,4-ethylenedioxythiophene) (PEDOT), can impart conductivity to hydrogels, which enhances their functionality54,55,56,57,58,59. It is noteworthy that the conductive hydrogels or semiconducting hydrogels comprise of carbon-based nanomaterials or conductive polymers rely on electrical properties of the conductive fillers rather than ionic conductivity capability60,61, which limits their compatibility and adaptability for real-time interaction with soft ionic systems. The ionic conduction in hydrogels arises from the presence of mobile ionic species, which can be achieved through incorporating water-soluble salts or electrolyte ions into hydrogels. This unique combination allows ionic conductive hydrogels (ICHs) to exhibit ionic conductivity, usually in the range of 10−3 to 10 S/m, while maintaining desirable mechanical properties such as stretchability and resilience61,62. ICHs combine the merits of hydrogels with ionic conductivity to mimic biological tissues, making them particularly suited for various applications in wearable bioelectronics63, energy harvest or storage devices, and soft robotics.
ILs can incorporate into the liquid phase of hydrogels to enhance the ionic conductivity of hydrogels64. Hu and co-workers developed a cellulose (Cel)-IL hydrogel by designing hydrogen-bond topological network65. The fabricated Cel-IL hydrogels exhibit Turing-pattern microstructure and multiply tunable and reversible properties. Another IL-based hydrogel is PIL hydrogels, where ILs are polymerized to form PILs or copolymerized with other monomers to produce polymeric matrices of the hydrogels66,67,68. Qian and co-workers proposed a PIL hydrogel with high performance mechanical and electrical properties via designing a phenylboronic acid (PBA)-IL with multiple roles (Fig. 6a)69. The hydrogel with semi-interpenetrating network is obtained through a facile one-step method by incorporating CNFs into the PBA-IL/acrylamide (AAm) crosslinked network. The boronic ester bonds and physical interactions of the crosslinked network including hydrogen bonds and electrostatic interactions endow the hydrogels with remarkable properties such as elongation of 1810%, toughness of 2.65 MJ m−3, self-healing ability, adhesiveness, and good transparency.
IL-based hydrogels can usually absorb water and mitigate the common issues caused by water evaporation. Except water loss, the stability of hydrogel at harsh environments such as at subzero temperatures is important for their applications. It was reported that polyzwitterions can be employed to develop anti-freezing hydrogels due to the strong hydration effect of the ions which can break the formation of crystal lattices of the ice. Yan and co-workers developed an anti-freezing hydrogel based on a zwitterionic PIL for application of multimodal artificial skin which can function at low temperatures (Fig. 6b)70.
Ionogels
Ionogels (also named ion gels, ion-gels, or ionic liquid gels) are a class of hybrid materials formed by incorporating ILs into a gel matrix through non-covalent interactions. Due to the inherent conductivity of ILs, they serve both as the liquid phase and as the source of ions, while the polymeric network forms a gel matrix that maintains mechanical flexibility71. Ionogels combine the ionic conductivity of ILs with the structural integrity of gels, making them suitable for various applications in electrochemical devices, energy harvesting devices, sensors, and wearable electronics35,72,73,74,75. Their unique properties stem from the synergistic interactions between the IL and the gel network, enabling tunable mechanical and electrochemical performance38,76,77. Furthermore, because ILs possess numerous advantages, ionogels inherit these benefits, making ionogels an ideal alternative to hydrogels in ionotronic applications.
The widely used strategy to fabricate ionogels is conducted by dissolving polymerizable monomers in IL or in the mixture of IL and cosolvent. Cost-effective, simple, and controllable photopolymerization is the most popular synthetic approach. For instance, Yan and co-workers demonstrated fully physically crosslinked Poly(N-isopropylacrylamide) (PNIPAM) ionogels through in situ polymerization of NIPAM in the H2O/IL binary solvent system via UV irradiation (Fig. 7a)78. Moreover, PIL-based ionogel can be prepared by photopolymerization of the polymerizable IL in presence of another IL. For instance, Lu and co-workers fabricated an ionogel by a one-step photopolymerization of 1-vinyl-3-butylimidazolium tetrafluoroborate in another IL of 1-butyl-3-methylimidazolium tetrafluoroborate (BMIMBF4)79. Thermal polymerization also can be used to construct ionogels, which is commonly proceeded by thermal initiator of ammonium persulfate (APS)80.
Except free radical polymerization, non-covalent interactions such as solvophobic interactions, π–π bond interactions81, metal–ligand coordination82,83, H-bonding, and ion-dipole interactions can also be employed to prepare ionogels52,84,85,86,87,88. For instance, Jiang and co-workers fabricated a transparent, mechanical robust, and highly stable ionogel via H-bonding between poly(ethyl acrylate)-based elastomers and IL of 1-ethyl-3-methylimidazolium bis-(trifluoromethylsulfonyl)imide (EMIMTFSI) (Fig. 7b)85. The ionogel showed high stability, and a skin-like strain sensor was constructed using this stable ionogel which showed outstanding durability and the ability to work under various harsh environmental conditions. By solvophobic interactions, Moon and co-workers synthesized an ionogel by statistical copolymers of poly(methyl methacrylate-co-butyl acrylate) (P(MMA-co-BA))89. Compared with similar copolymers with poly(styrene) as solvophobic component, the low glass transition temperature of the IL-phobic PBA regions allows the ionogel to effectively dissipate the stress and results in higher stretchability. By π–π interactions between the graphene oxide nanoplatelets and the imidazolium ring of cations from IL, Crump and co-workers developed a ionogel by mixed graphene oxide, BMIMBF4, and hydrazine90.
Liquid-free ionic conductive elastomer
Besides hydrogels and ionogels, liquid-free ionic conductive elastomers (ICEs) represent a type of material that merges the mechanical flexibility of elastomers with ionic conductivity. Since ICEs do not contain a liquid phase, they are designed to overcome the limitations of traditional ionic conductors, particularly issues related to solvent volatility, leakage associated with liquid media, and environmental stability. ICEs are typically composed of a polymer matrix that incorporates ionic conductive additives, such as organic salts or ionic polymers. ICEs are generally fabricated by incorporating salts such as choline chloride (ChCl) or lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) into a polymer matrix91,92,93,94,95,96,97, or by polymerizing IL monomers to form copolymers (Fig. 8a)98. IL-based ICEs, notwithstanding containing IL components, are obtained by the polymerization of IL into solid elastomeric networks instead of liquid phases, thus fundamentally differing from ionogels that are swelled by ILs.
PILs which composed of polymeric backbones and IL species in each repeating unit can be used to fabricate IL-based ICE99. The mobile counterion of the polycation or polyanion is responsible for ionic conduction. Suo and co-workers fabricated the polyanionic and polycationic elastomers through polymerization of IL monomers8. The excellent properties enabled the ICEs to be integrated to fabricate stretchable and transparent ionotronic diodes and transistors. To introduce self-healing ability in ICE, Liu and co-workers developed a PIL-based ICE through simultaneous incorporation of flexible ethoxy chains and H-bonding sites into the imidazole-based PIL main chains100. The reversible electrostatic interactions and H-bonding interactions enable the ICE with self-healing ability to recover mechanical and electrical performances at ambient temperature. Zhu and co-workers developed an all-solid-state ionic conductor by intrinsically conductive fluorinated PIL copolymer with strong ion–dipole interactions (Fig. 8b)101. Furthermore, zwitterionic PIL is also a promising ionic material for the preparation of ICE. For instance, Pu and co-workers developed a recyclable, adhesive and self-healable ICE using a single-component zwitterionic PIL102. The ICE exhibited excellent mechanical performance, ionic conductivity, high transparency, fast self-healing rate, strong adhesion, and impressive recyclability.
Strategies for improving IL-based ionic conductors
Practical applications in ionotronics pose various requirements for the properties of ILICs, such as mechanical strength, modulus, stretchability, elasticity, and ionic conductivity. In addition to ILs, the polymeric network is crucial in determining the overall performance of ILICs. Currently, to meet application demands, a variety of materials, besides polymers, have been used as gel matrices for ILICs, such as nanocellulose, supramolecular gelators, organosilane networks, and silica nanoparticles76.
The multiple outstanding properties of nanocellulose, such as mechanical properties, low density, thermal stability, and high specific surface area, make it an ideal matrix for the fabrication of mechanically robust ionic conductors. The incorporation of nanocellulose enhances the structural integrity of ionic conductors, improving their mechanical properties while maintaining their ionic conductivity. For instance, Feng et al. prepared ionogels with high thermoelectric properties by loading ILs into a bacterial cellulose (BC) nanofibrous network (Fig. 9a)103. Jiang et al. achieved an ultrastretchable (up to 10250% stretchability) IL-based gel with all-around performances through water-induced hydration interactions (Fig. 9b)104. Yu et al. developed a scalable BC ionogels through the in-situ molecularization of cellulose using IL [Bmim]Cl (Fig. 9c)105. It is noteworthy that [Bmim]Cl has good dissolution on cellulose, making it frequently used in the preparation of cellulose-based ionic conductors106,107. Yao’s group proposed a bio-inspired strategy to develop superstrong nanofibril-based ionogel through complex coacervation between surface-charged cellulose nanofiber (CNF) and synthesized PIL (Fig. 9d)108. PIL binds the nanofibrils to assemble the reticular nanofibrous structure, and thus the incorporation of PIL can simultaneously improve the mechanical properties and ionic conductivity of the ionogels. Besides, Qian et al. constructed super stretchable, self-healing, adhesive, ionic conductive hydrogels by integrating phenylboronic acid-ionic liquid (PBA-IL) with CNF69.
a Free-standing bacterial cellulose-based ionogels (BCIGs). Reproduced with permission103. Copyright 2021 Wiley-VCH GmbH. b PAA-CNF-IL-H2O ionogels. Reproduced with permission104. Copyright 2022 Wiley-VCH GmbH. c Bacterial cellulose ionogel. Adapted with permission under a Creative Commons105. Copyright 2022 Geyuan Jiang et al. d CNF-based superstrong ionogel enabled by coacervation-induced nanofibril assembly. Reproduced with permission108. Copyright 2024 American Chemical Society.
In addition, numerous strategies have been proposed to enhance the performance of ILICs. On the one hand, polymeric matrices can be designed through polymer engineering strategies. For instance, Yan et al. proposed introducing covalent-network microspheres into the entangled linear segments to reduce stress concentration at the crack tip, thereby enabling the ionogel to achieve tear and crack-propagation resistance, self-healing, and adhesion (Fig. 10a)109. Dickey et al. developed strong and tough ionogels, with strengths reaching up to 12 MPa, utilizing in situ phase separation of copolymers in ILs (Fig. 10b)110. Yan et al. proposed a nanoconfinement polymerization strategy induced by covalent organic frameworks (COF) to achieve hysteresis-free, crack-propagation resistant gels (Fig. 10c)111. On the other hand, the interactions within ILICs can be regulated to improve their properties. For example, Dickey et al. proposed a solvent crosslinking toughening strategy using IL to fabricate stiff and strong glassy gels, which integrate excellent Young’s modulus (1 GPa), fracture strength (42 MPa), yield strength (73 MPa), and toughness (110 MJ m−3) (Fig. 10d)112. Yan et al. constructed ultra-tough (toughness is 78 MJ m−3), fatigue-resistant, self-healing, and recyclable ionogels using halometallic ILs as coordinated supramolecular solvents (Fig. 10e)113,114. Wu et al. achieved a low-hysteresis, fatigue-resistant, tough ionogel through low-energy-dissipating cross-linking by supramolecular interactions of 2-ureido-4-pyrimidone (UPy) groups, which can be fully resilient at 400% strain (Fig. 10f)115. Besides, one of the crucial conditions to obtain highly conductive and robust ionogels is the effective gelation by judiciously designing the gelators (e.g., copolymers), for example, double-network, physically crosslinked gels116, and physical-co-chemical cross-linked network gels82,83 have been reported to improve the performances of ILICs117. In summary, their mechanical properties can be enhanced through strategies, such as designing IL or gel matrices, adjusting the interactions between IL and the matrix, adding fillers, and controlling the preparation process.
a Microspheres entangled ionogel. Reproduced with permission109. Copyright 2022 Wiley-VCH GmbH. b Microphase-separated ionogel. Reproduced with permission110. Copyright 2022 The Author(s), under exclusive licence to Springer Nature Limited. c Nanoconfined polymerization. Reproduced with permission111. Copyright 2023 The Author(s), under exclusive licence to Springer Nature Limited. d Solvent crosslinking toughening. Reproduced with permission112. Copyright 2024 The Author(s), under exclusive licence to Springer Nature Limited. e Halometallate ILs ionogel. Reproduced with permission113. Copyright 2022 Wiley-VCH GmbH. f Supramolecular ionogel. Reproduced with permission115. Copyright 2024 Wiley-VCH GmbH.
Emerging applications of IL-based ionic conductors
The synergy between ILs and polymer matrices endows flexible ILICs with unique properties, such as ionic conductivity, stretchability, elasticity, and enables ILICs to exhibit ion transport properties under stimulation, making them promising candidates in ionotronic applications. Additionally, their tunable mechanical properties allow for the development of materials that can withstand various environmental conditions. The tunable properties and ion transport allow them to be widely used in flexible strain sensors84,118,119,120,121,122, energy harvesting and storage devices123,124,125, soft actuators126,127,128,129, and electroluminescent devices130,131,132.
Energy harvesting and storage
ILICs can be used to convert various energies such as mechanical energy, waste heat energy, osmotic energy into electricity. In energy storage, ILICs are primarily utilized as quasi-solid-state electrolytes for batteries and supercapacitors97,133. Furthermore, ILICs are applied in electricity generation, such as thermocells or ionic thermoelectric generator134,135,136, heat harvesting103, piezoelectric nanogenerators (PENG), and triboelectric nanogenerators (TENG)137,138. For instance, ionic-based TENG can harvest mechanical energy based on the triboelectrification and electrostatic induction (Fig. 11a). You and co-workers developed a high-performance TENG by ionogel which can work in a wide range of temperatures139. The TENG can also serve as a self-powered sensor to detect human motions. Wang and co-workers developed a self-powered contact-separation TENG with high transparency and stretchability by a double-network ionogel140.
a The work mechanism and electrical performance of the ionogel-based TENG. Reproduced with permission137. Copyright 2021 Elsevier Ltd. b PIL membrane for high performance osmotic energy harvesting. Reproduced with permission144. Copyright 2022, Elsevier Ltd. c Self-healable and stretchable electrolytes with ionic bonds for lithium batteries. Reproduced with permission146. Copyright 2019 Elsevier B.V. d Moisture-enabled electricity generation of CNF-based ionogel through ion transmembrane selective migration. Reproduced with permission108. Copyright 2024 American Chemical Society.
ILICs have received significant attention for harvesting heat energy due to their much higher ionic Seebeck coefficient than electronic thermoelectric materials by several orders141. For instance, Sajid and co-workers developed a chemically crosslinked ionogel as a high-performance thermoelectric material142. PIL is also a promising ion contributor for ionic thermocells. Compared with IL, the diffusion mobility difference between cations and anions in PIL is larger than that in IL due to the immobile polymer metrices, making PIL-based materials have a comparable Seebeck coefficient with that of IL-based materials143.
Free-standing ILICs can be used as separation membranes or electrolytes in ion separation systems such as osmotic energy harvesting. These applications highly rely on the selective ion transport through ILICs. For instance, Wen and co-workers developed a bioinspired PIL membrane to efficiently harvest salinity gradient energy (Fig. 11b)144. The numerous nanochannels and the surface functional groups of the PIL membrane enable high-performance osmotic energy harvesting. Wang and co-workers developed PIL-based hierarchically porous carbon membranes for seawater desalination145.
In electrochemical energy storage systems, traditional organic solvent-based electrolytes suffer from safety risks caused by the high flammability and volatility of the organic solvents. In contrast, ILICs can promote the safety of batteries with features of non-volatility, high thermal and electrical stability, and ionic conductivity. For instance, Yang and co-workers prepared flexible solid-state lithium batteries using self-healing electrolytes (Fig. 11c)146. The electrolytes were constructed by a di-cationic polymerized IL and an IL that was impregnated in the interspaces among chain segments. The polymer backbone of the electrolyte formed a sea-island system which can increase its ionic conductivity and electrochemical stability. Except lithium batteries, the flexible ILICs can also be used as electrolytes in new-type batteries such as aluminum-ion batteries, magnesium-ion batteries and sodium-ion batteries147.
Additionally, the moisture-absorbing ILIC can be applied in moisture-enabled electricity generation (MEG). For example, Yao research group has utilized CNF-based ionogel for MEG based on the selective ion migration across the ionogel membranes (Fig. 11d)108. The ionogel-based generator can be applied in energy-harvesting window and wearable power generation.
Stretchable strain sensors
ILICs have the characteristic of dimension-dependent conductivity which allows them to be exploited for stretchable strain sensors for diverse applications. Such sensors can respond to a variety of external forces such as compression and extension to sense pressure and strain, respectively. The strain sensors based on flexible ILICs can be used in various applications such as monitoring human motions148, human-machine interaction149,150, real-time health monitoring151, ionic skin, and soft robotics152. For the sensing mechanism, ILICs can be utilized in strain sensors based on resistance or capacitance36,78,89,93,153. For the resistive-type strain sensors, the mechanical forces applied on the ionic conductors can cause their dimensional change, leading to a change in resistance. Resistive-type strain sensors using ILICs can be applied to stably monitor diverse human motions, such as finger bend, and movement of elbow154,155. Yue and co-workers fabricated a solvent-resistant, resilient, and self-healing ionogel by polymerization of 2,2,2-trifluoroethyl acrylate and AAm in a hydrophobic IL of EMIMTFSI. The fluorine-rich polymeric network endows the ionogel to respond to various pressure sensitively and the resultant sensor can detect tiny yet complex muscle movements such as speaking and pulse156. Moon and co-worker reported a dynamically switchable dual-mode wearable sensory platform through a simple bilayer electronic-ionic bimodal conductor integrating silver nanowires (AgNWs) and ionogels157. The working principle of the capacitive-type sensors are based on change in the thickness and area of the sensors when they are compressed or stretched, which cause change in capacitance of the device36,158,159. For instance, Kim and co-workers developed a capacitive device to detect various pressures by a TPU ionogel72.
Furthermore, ILICs have been applied in self-powered sensors through a piezoionic principle inspired by skin piezo 2 protein21,27,122,160. The mechanism for piezoionic sensors is that the cations and anions from IL would have different interactions with the polymer matrices which can yield uneven ion transport upon mechanical stimulus. Therefore, different from resistive-type and capacitive-type sensors, the piezoionic sensors are self-powered without an external energy supply. For instance, Yao research group developed a microphase-separated bicontinuous ionogel using poly(vinylidene fluoride-co-chlorotrifluoroethylene) (PVDF-co-CTFE) and EMIMTFSI for ionic skin, enabling bio-inspired multimodal sensing integrating piezoionic sensing and temperature response (Fig. 12a). Wu et al. reported a piezoionic elastomer with tunned microphase-separated structure by phase and interface engineering strategy, achieving an extraordinary piezoionic coefficient for energy harvesting ionotronics (Fig. 12b)13. Additionally, ILICs can also enable a self-powered sensor using ionotronic triboelectric nanogenerator (iTENG) mechanism95,161,162. Moreover, ionic conductors can be applied for ionotronic flexoelectricity, achieving mechanoelectric conversion through strain gradient-induced ion transport, thereby serving as flexoelectric sensors163. Furthermore, Pu et al. reported the enhancement of mechano-ionotronic transduction of a piezoionic hydrogel through synergizing bio-inspired multi-gradient structures164.
In addition, the high deformability and relatively low Young’s modulus of the gel-based ionic conductors make it possible for them to develop electrically driven actuators which can convert electrical signals into mechanical responses such as contraction, expansion, and movement35. Flexible ILICs, especially ionogels with high transparency and stretchability, can be used to fabricate electroluminescent devices and touchpads130,165.
In summary, the applications of ILICs primarily rely on the ion transport under stimuli, including stretching, pressure, temperature, humidity, ionic gradients, or chemical potential gradients.
Outlook
The advancement of ionotronics demands innovation in flexible and functional materials. Ionotronic applications refers to a response to stimuli with ions as charge carriers, bridging the gap between ionic systems and electronics. Quasi-solid ionic conductors can conduct ions and have the capability to respond ionically to stimuli. By virtue of their unique advantages, ILs have been the most promising ionic media. Therefore, IL-based ionic conductors (ILICs) hold significant promises for various ionotronic applications. Investigating the synergistic effects of ILs and matrices will be essential to enhance the performance of ILICs and to unlock new capabilities in ionotronics. Nevertheless, the applications of ILICs in ionotronics remain quite limited, and several challenges currently hinder their widespread adoption. On one hand, task-specific applications impose increasing requirements on the properties of ILICs, starving for comprehensive improvements in their performance, such as mechanical robustness, ionic conductivity, biocompatibility, and environmental stability, and others. On the other hand, the relatively low responsivity to stimuli and controllability of ions in ILICs compared to electrons restrict the efficiency and functionality of ionotronic applications. The key characteristics for ideal ILICs include excellent mechanical properties and flexibility, high ionic conductivity, environmental stability, and durability. Furthermore, the ions in ILICs efficiently respond to stimuli such as strain, temperature, and humidity, enabling them to generate sensing signals or be applied for energy harvesting.
To enhance the performance and functionality of ILICs, a potential strategy involves the design of hybrid materials that incorporate nanomaterials. For example, incorporating nanofiber such as CNF can strengthen the mechanical properties of ILICs; introducing nanoporous structures can enhance ion transport selectivity, thereby providing efficient functionality. Moreover, leveraging advanced fabrication techniques, such as 3D printing or layer-by-layer assembly, could enable the production of complex structures or metastructures. Additionally, many organisms and natural materials have been endowed with exceptional properties and functions through the process of evolution, providing inexhaustible inspiration for scientific research at various scales166,167, so bio-inspired strategies that draw inspiration from nature present a promising avenue to improving the performance and functionality of materials or devices.
Advancements of ILICs in ionotronic applications require continuous innovation at various scales, including mechanisms, material development, device design, system integration, and applications. In terms of mechanism exploration, more advanced characterization techniques are needed to “visualize” ionic transport and theoretically quantify ionic migration, enabling more precise control over ions. Regarding material development, it is necessary to optimize the composition and fabrication processes of ILICs to enhance their performance under diverse conditions. Besides, the preparation of materials requires standardized and scalable methods to ensure stability in applications. Regarding device design, it is essential to consider both the performance of the device itself and the interactions at the interfaces between various components to ensure reliable and optimal performance. In terms of system integration, it is necessary to integrate ionotronic devices or electronic devices with different functions to achieve complex systems, contributing to the development of next-generation wearable electronics. For example, combining energy harvesting devices with sensors creates self-sustaining systems for real-time monitoring. Additionally, new application scenarios for ionotronics need to be explored, and it is necessary to overcome practical challenges to optimize the reported materials or devices for real-world applications. These advancements will inevitably enable ionotronics to achieve more efficient, controllable, and complex functionalities, potentially surpassing electronics in certain aspects.
Recent developments have also explored the use of sustainable and eco-friendly materials like nanocellulose in the fabrication of ILICs, reflecting a growing emphasis on environmental considerations in material science. Besides, when applying ILICs in wearable electronics or bio-related applications, the biocompatibility of the materials is indispensable. Therefore, in the future, more natural biocompatible materials will be used to construct ILICs, such as bio-ILs and biomass-based matrices, even leading to the development of all-natural ionic conductors168, such as bio-gels and eutectogels169,170,171.
In summary, ILICs possess significant potential for a variety of applications, and continued investigation into their composition, processing methods, performance, and applications will be vital for unlocking their full capabilities in next generation ionotronics.
Data availability
Code and data is provided within the manuscript.
References
Hong, S. J. et al. Bio-inspired artificial mechanoreceptors with built-in synaptic functions for intelligent tactile skin. Nat. Mater. 24, 1100–1108 (2025).
Gong, S., Lu, Y., Yin, J., Levin, A. & Cheng, W. Materials-driven soft wearable bioelectronics for connected healthcare. Chem. Rev. 124, 455–553 (2024).
Lee, J. Y. et al. Variable-stiffness-morphing wheel inspired by the surface tension of a liquid droplet. Sci. Robot. 9, eadl2067 (2024).
Lin, W. et al. Imperceptible liquid metal based tattoo for Human-Machine interface on hairy skin. Chem. Eng. J. 490, 151595 (2024).
Tang, W., Sun, Q. & Wang, Z. L. Self-powered sensing in wearable electronics horizontal line a paradigm shift technology. Chem. Rev. 123, 12105–12134 (2023).
Chen, W. et al. Cascade-heterogated biphasic gel iontronics for electronic-to-multi-ionic signal transmission. Science 382, 559–565 (2023).
Chen, W. et al. Intercellular ion-gradient piezoheterogated biphasic gel for ultrahigh iontronic generation. J. Am. Chem. Soc. 147, 3283–3292 (2025).
Kim, H. J., Chen, B., Suo, Z. & Hayward, R. C. Ionoelastomer junctions between polymer networks of fixed anions and cations. Science 367, 773–776 (2020).
Guo, Z. H. et al. Flexible ionic diodes with high rectifying ratio and wide temperature tolerance. Adv. Funct. Mater. 32, 2112432 (2022).
Jiang, F. et al. Ion rectification based on gel polymer electrolyte ionic diode. Nat. Commun. 13, 6669 (2022).
Mei, T. et al. Ionic transistors. ACS Nano 18, 4624–4650 (2024).
Zhang, Y., Tan, C. M. J., Toepfer, C. N., Lu, X. & Bayley, H. Microscale droplet assembly enables biocompatible multifunctional modular iontronics. Science 386, 1024–1030 (2024).
Zhu, W., Wu, B., Lei, Z. & Wu, P. Piezoionic elastomers by phase and interface engineering for high-performance energy-harvesting ionotronics. Adv. Mater. 36, e2313127 (2024).
Harikesh, P. C., Tu, D. & Fabiano, S. Organic electrochemical neurons for neuromorphic perception. Nat. Electron. 7, 525–536 (2024).
Yang, C. & Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 3, 125–142 (2018).
Wang, A. et al. Selective ion transport through hydrated micropores in polymer membranes. Nature 635, 353–358 (2024).
Yang, K. et al. Ion-selective mobility differential amplifier: enhancing pressure-induced voltage response in hydrogels. Angew. Chem. Int. Ed. Engl. 64, e202415000 (2025).
Xu, R., Kang, Y., Zhang, W., Pan, B. & Zhang, X. Two-dimensional MXene membranes with biomimetic sub-nanochannels for enhanced cation sieving. Nat. Commun. 14, 4907 (2023).
Teng, Y. et al. Bioinspired nervous signal transmission system based on two-dimensional laminar nanofluidics: from electronics to ionics. Proc. Natl. Acad. Sci. USA 117, 16743–16748 (2020).
Zhang, Z. et al. A 10-micrometer-thick nanomesh-reinforced gas-permeable hydrogel skin sensor for long-term electrophysiological monitoring. Sci. Adv. 10, eadj5389 (2024).
Lv, D. et al. Microphase-separated elastic and ultrastretchable ionogel for reliable ionic skin with multimodal sensation. Adv. Mater. 36, e2309821 (2024).
Xiao, K., Wan, C., Jiang, L., Chen, X. & Antonietti, M. Bioinspired ionic sensory systems: the successor of electronics. Adv. Mater. 32, e2000218 (2020).
Chen, L. et al. Spike timing-based coding in neuromimetic tactile system enables dynamic object classification. Science 384, 660–665 (2024).
Chen, S. et al. Artificial organic afferent nerves enable closed-loop tactile feedback for intelligent robot. Nat. Commun. 15, 7056 (2024).
Liu, J. et al. Multidimensional free shape-morphing flexible neuromorphic devices with regulation at arbitrary points. Nat. Commun. 16, 756 (2025).
Yao, Y. et al. An organic electrochemical neuron for a neuromorphic perception system. Proc. Natl. Acad. Sci. USA 122, e2414879122 (2025).
Dobashi, Y. et al. Piezoionic mechanoreceptors: force-induced current generation in hydrogels. Science 376, 502–507 (2022).
Xiong, T. et al. Neuromorphic functions with a polyelectrolyte-confined fluidic memristor. Science 379, 156–161 (2023).
Bocquet, L. Concluding remarks: Iontronics, from fundamentals to ion-controlled devices - Random access memories. Faraday Discuss 246, 618–622 (2023).
Bisri, S. Z., Shimizu, S., Nakano, M. & Iwasa, Y. Endeavor of iontronics: from fundamentals to applications of ion-controlled electronics. Adv. Mater. 29, 1607054 (2017).
Knauth, P. Ionic conductor composites: theory and materials. J. Electroceram 5, 111–125 (2000).
Aniya, M., Sadakuni, H. & Hirano, E. Ionic conductors: effect of temperature on conductivity and mechanical properties and their interrelations. Crystals 11, 1008 (2021).
Lei, Z. & Wu, P. Bioinspired quasi-solid ionic conductors: materials, processing, and applications. Acc. Mater. Res. 2, 1203–1214 (2021).
Keplinger, C. et al. Stretchable, transparent, ionic conductors. Science 341, 984–987 (2013).
Cao, Y. et al. A transparent, self-healing, highly stretchable ionic conductor. Adv. Mater. 29, 1605099 (2017).
He, X. et al. Highly conductive and stretchable nanostructured ionogels for 3D printing capacitive sensors with superior performance. Nat. Commun. 15, 6431 (2024).
Matuszek, K. et al. Unexpected energy applications of ionic liquids. Adv. Mater. 36, e2313023 (2024).
Fan, X. et al. Ionogels: recent advances in design, material properties and emerging biomedical applications. Chem. Soc. Rev. 52, 2497–2527 (2023).
Gomes, J. M., Silva, S. S. & Reis, R. L. Biocompatible ionic liquids: fundamental behaviours and applications. Chem. Soc. Rev. 48, 4317–4335 (2019).
Yuan, J. Y., Mecerreyes, D. & Antonietti, M. Poly(ionic liquid)s: an update. Prog. Polym. Sci. 38, 1009–1036 (2013).
Shao, Y. et al. Crosslinking of a single Poly(ionic liquid) by water into porous supramolecular membranes. Angew. Chem. Int. Ed. Engl. 59, 17187–17191 (2020).
Zhang, S. Y. et al. Poly(ionic liquid) composites. Chem. Soc. Rev. 49, 1726–1755 (2020).
Zou, Y. H. et al. Porous metal-organic framework liquids for enhanced CO(2) adsorption and catalytic conversion. Angew. Chem. Int. Ed. Engl. 60, 20915–20920 (2021).
Han, Y. et al. Preparation of poly(ionic liquid@MOF) composite monolithic column and its application in the online enrichment and purification of tectochrysin in medicinal plants. Anal. Methods 14, 401–409 (2022).
Li, Q. et al. Poly(Ionic Liquid) double-network elastomers with high-impact resistance enhanced by cation-pi interactions. Adv. Mater. 36, e2311214 (2024).
Yi, M. et al. Poly(ionic liquid)-armored mxene membrane: interlayer engineering for facilitated water transport. Angew. Chem. Int. Ed. Engl. 61, e202202515 (2022).
Cui, J., Li, Y., Chen, D., Zhan, T. & Zhang, K. Ionic liquid-based stimuli-responsive functional materials. Adv. Funct. Mater. 30, 2005522 (2020).
Jia, H., Tao, X. & Wang, Y. Flexible and self-healing thermoelectric converters based on thermosensitive liquids at low temperature gradient. Adv. Electron. Mater. 2, 1600136 (2016).
Hu, Y. et al. Confined ionic-liquid-mediated cation diffusion through layered membranes for high-performance osmotic energy conversion. Adv. Mater. 35, e2301285 (2023).
Lv, D. et al. Defect-enhanced selective ion transport in an ionic nanocomposite for efficient energy harvesting from moisture. Energy Environ. Sci. 15, 2601–2609 (2022).
Zheng, S. et al. Continuous energy harvesting from ubiquitous humidity gradients using liquid-infused nanofluidics. Adv. Mater. 34, e2106410 (2022).
Gao, Y. et al. Ionic liquid-based gels for biomedical applications. Chem. Eng. J. 452, 139248 (2023).
Zhang, Y. S. & Khademhosseini, A. Advances in engineering hydrogels. Science 356, eaaf3627 (2017).
Shin, Y. et al. Low-impedance tissue-device interface using homogeneously conductive hydrogels chemically bonded to stretchable bioelectronics. Sci. Adv. 10, eadi7724 (2024).
Li, P. et al. N-type semiconducting hydrogel. Science 384, 557–563 (2024).
Liang, C. et al. Stiff and self-healing hydrogels by polymer entanglements in co-planar nanoconfinement. Nat. Mater. 24, 599–606 (2025).
Li, Z., Yun, H., Yan, Y., Zhao, Y. & Zhao, F. Boosting electronic charge transport in conductive hydrogels via rapid ion-electron transduction. Angew. Chem. Int. Ed. Engl. 64, e202506560 (2025).
Dai, Y. et al. Soft hydrogel semiconductors with augmented biointeractive functions. Science 386, 431–439 (2024).
Lao, J. et al. Intrinsically adhesive and conductive hydrogel bridging the bioelectronic-tissue interface for biopotentials recording. ACS Nano 19, 7755–7766 (2025).
Zhang, C. W. et al. Hydrogel-based soft bioelectronics for personalized healthcare. Med-X 2, 20 (2024).
He, Q. et al. Conductive hydrogel for flexible bioelectronic device: current progress and future perspective. Adv. Funct. Mater. 34, 2308974 (2023).
Roy, A. et al. A highly stretchable, conductive, and transparent bioadhesive hydrogel as a flexible sensor for enhanced real-time human health monitoring. Adv. Mater. 36, e2404225 (2024).
Mo, F., Zhou, P., Lin, S., Zhong, J. & Wang, Y. A review of conductive hydrogel-based wearable temperature sensors. Adv. Health. Mater. 13, e2401503 (2024).
He, Z. & Yuan, W. Highly stretchable, adhesive ionic liquid-containing nanocomposite hydrogel for self-powered multifunctional strain sensors with temperature tolerance. ACS Appl. Mater. Interfaces 13, 53055–53066 (2021).
Zhao, D. et al. A dynamic gel with reversible and tunable topological networks and performances. Matter 2, 390–403 (2020).
Zhou, Y., Fei, X., Tian, J., Xu, L. & Li, Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J. Colloid Interface Sci. 606, 192–203 (2022).
Bu, X., Wu, L., Ma, X., Diao, W. & Lu, D. Ultratough and reversibly stretchable Zwitterionic poly (ionic liquid) copolymer hydrogel with high ionic conductivity for high-performance flexible and cold-resistant supercapacitor. Int. J. Electrochem. Sci. 15, 2070–2088 (2020).
He, X. et al. Self-healing polymeric ionic liquid hydrogels with high mechanical strength and ionic conductivity. J. Mater. Sci. 56, 10231–10248 (2021).
Yao, X. et al. Super stretchable, self-healing, adhesive ionic conductive hydrogels based on tailor-made ionic liquid for high-performance strain sensors. Adv. Funct. Mater. 32, 2204565 (2022).
Liu, Z. Y. et al. Poly(ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper. Mater. Horiz. 7, 919–927 (2020).
Hopson, C. et al. Cellulose ionogels, a perspective of the last decade: a review. Carbohydr. Polym. 274, 118663 (2021).
Jin, M. L. et al. An ultrasensitive, visco-poroelastic artificial mechanotransducer skin inspired by Piezo2 protein in mammalian merkel cells. Adv. Mater. 29, 1605973 (2017).
Jin, M. L. et al. An ultrastable ionic chemiresistor skin with an intrinsically stretchable polymer electrolyte. Adv. Mater. 30, e1706851 (2018).
Seol, K. H., Lee, S. J., Cho, K. G., Hong, K. & Lee, K. H. Highly conductive, binary ionic liquid–solvent mixture ion gels for effective switching of electrolyte-gated transistors. J. Mater. Chem. C. 6, 10987–10993 (2018).
Cao, Y. et al. Self-healing electronic skins for aquatic environments. Nat. Electron. 2, 75–82 (2019).
Tome, L. C., Porcarelli, L., Bara, J. E., Forsyth, M. & Mecerreyes, D. Emerging iongel materials towards applications in energy and bioelectronics. Mater. Horiz. 8, 3239–3265 (2021).
Luo, Z., Li, W., Yan, J. & Sun, J. Roles of ionic liquids in adjusting nature of ionogels: a mini review. Adv. Funct. Mater. 32, 2203988 (2022).
Xu, S., Wu, S., Zhu, R., Qiu, Z. & Yan, Y. Fully physically crosslinked PNIPAM ionogels with high mechanical properties and temperature-managed adhesion achieved by H2O/ionic liquid binary solvents. Adv. Funct. Mater. 34, 2405965 (2024).
Xiang, S., Zheng, F., Chen, S. & Lu, Q. Self-healable, recyclable, and ultrastrong adhesive ionogel for multifunctional strain sensor. ACS Appl. Mater. Interfaces 13, 20653–20661 (2021).
Zhang, S., Wang, F., Peng, H., Yan, J. & Pan, G. Flexible highly sensitive pressure sensor based on ionic liquid gel film. ACS Omega 3, 3014–3021 (2018).
Kim, Y. M., Yu, K. S. & Moon, H. C. Regulating the π–π interactions of copolymer gelators: effective molecular design of highly stretchable and tough ionogels for wearable ionotronics. Chem. Eng. J. 480, 147947 (2024).
Hong, S. H., Kim, Y. M. & Moon, H. C. Dynamic metal–ligand coordination-assisted ionogels for deformable alternating current electroluminescent devices. ACS Appl. Mater. Interfaces 15, 28516–28523 (2023).
Kwon, J. H., Hong, S. H., Lee, G. R., Kim, J. C. & Moon, H. C. Synergistic dual-cross-linking gelation: exploring the impact of metal–ligand complexation on ionogel performance. ACS Appl. Mater. Interfaces 16, 61115–61122 (2024).
Ding, Y. et al. Preparation of high-performance ionogels with excellent transparency, good mechanical strength, and high conductivity. Adv. Mater. 29, 1704253 (2017).
Cao, Z., Liu, H. & Jiang, L. Transparent, mechanically robust, and ultrastable ionogels enabled by hydrogen bonding between elastomers and ionic liquids. Mater. Horiz. 7, 912–918 (2020).
Li, S., Li, Y., Wang, Y., Pan, H. & Sun, J. Highly stretchable, elastic, healable, and ultra-durable polyvinyl alcohol-based ionic conductors capable of safe disposal. CCS Chem. 4, 3170–3180 (2022).
Wang, P., Li, G., Yu, W., Meng, C. & Guo, S. color-customizable, stretchable, self-healable and degradable ionic gel for variable human-motion detection via strain, pressure, and torsion. Adv. Mater. Interfaces 9, 2102426 (2022).
Mantravadi, R., Chinnam, P. R., Dikin, D. A. & Wunder, S. L. High conductivity, high strength solid electrolytes formed by in situ encapsulation of ionic liquids in nanofibrillar methyl cellulose networks. ACS Appl. Mater. Interfaces 8, 13426–13436 (2016).
Kim, Y. M. & Moon, H. C. Ionoskins: nonvolatile, highly transparent, ultrastretchable ionic sensory platforms for wearable electronics. Adv. Funct. Mater. 30, 1907290 (2019).
Crump, M. R. et al. Monolithic 3D printing of embeddable and highly stretchable strain sensors using conductive ionogels. Nanotechnology 30, 364002 (2019).
Yiming, B. et al. Elastic, strong and tough ionically conductive elastomers. Nat. Commun. 16, 431 (2025).
Luo, C. et al. A fully self-healing and highly stretchable liquid-free ionic conductive elastomer for soft ionotronics. Adv. Funct. Mater. 33, 2304486 (2023).
Zhang, X. et al. Tough liquid-free ionic conductive elastomers with robust adhesion and self-healing properties for ionotronic devices. Adv. Funct. Mater. 34, 2307400 (2023).
Li, M. et al. Superstretchable, yet stiff, fatigue-resistant ligament-like elastomers. Nat. Commun. 13, 2279 (2022).
Zhang, P. et al. Dynamically crosslinked dry ion-conducting elastomers for soft iontronics. Adv. Mater. 33, 2101396 (2021).
Zhang, P. et al. Stretchable, transparent, and thermally stable triboelectric nanogenerators based on solvent-free ion-conducting elastomer electrodes. Adv. Funct. Mater. 30, 1909252 (2020).
Yin, L. et al. A dual-bond crosslinking strategy enabling resilient and recyclable electrolyte elastomers for solid-state lithium metal batteries. Angew. Chem. Int. Ed. 63, e202404769 (2024).
Li, Q. et al. Strong, spontaneous, and self-healing Poly(Ionic Liquid) elastomer underwater adhesive with borate ester dynamic crosslinking. Adv. Mater. 36, e2413901 (2024).
Guterman, R., Ambrogi, M. & Yuan, J. Harnessing Poly(ionic liquid)s for sensing applications. Macromol. Rapid Commun. 37, 1106–1115 (2016).
Qu, X. et al. Solid-state and liquid-free elastomeric ionic conductors with autonomous self-healing ability. Mater. Horiz. 7, 2994–3004 (2020).
Ming, X. et al. All-solid-state self-healing ionic conductors enabled by ion-dipole interactions within fluorinated Poly(Ionic Liquid) copolymers. ACS Appl. Mater. Interfaces 13, 41140–41148 (2021).
Li, L. et al. A recyclable, adhesive and fast self-healable ionic conducting elastomer based on a poly-zwitterionic liquid for soft iontronics. J. Mater. Chem. A 10, 24581–24589 (2022).
Liu, K. et al. Flexible and robust bacterial cellulose-based ionogels with high thermoelectric properties for low-grade heat harvesting. Adv. Funct. Mater. 32, 2107105 (2021).
Ye, Y. et al. Ultrastretchable Ionogel with extreme environmental resilience through controlled hydration interactions. Adv. Funct. Mater. 33, 2209787 (2022).
Jiang, G. et al. A scalable bacterial cellulose ionogel for multisensory electronic skin. Research 2022, 9814767 (2022).
Swatloski, R. P., Spear, S. K., Holbrey, J. D. & Rogers, R. D. Dissolution of cellulose [correction of cellose] with ionic liquids. J. Am. Chem. Soc. 124, 4974–4975 (2002).
Zhu, Y. et al. A general strategy for synthesizing biomacromolecular ionogel membranes via solvent-induced self-assembly. Nat. Synth. 2, 864–872 (2023).
Li, X. et al. Superstrong ionogel enabled by coacervation-induced nanofibril assembly for sustainable moisture energy harvesting. ACS Nano 18, 12970–12980 (2024).
Li, W. et al. Recyclable, healable, and tough ionogels insensitive to crack propagation. Adv. Mater. 34, e2203049 (2022).
Wang, M. et al. Tough and stretchable ionogels by in situ phase separation. Nat. Mater. 21, 359–365 (2022).
Li, W. et al. Nanoconfined polymerization limits crack propagation in hysteresis-free gels. Nat. Mater. 23, 131–138 (2024).
Wang, M. et al. Glassy gels toughened by solvent. Nature 631, 313–318 (2024).
Li, L. et al. Ultra-tough and recyclable ionogels constructed by coordinated supramolecular solvents. Angew. Chem. Int. Ed. Engl. 61, e202212512 (2022).
Li, W. et al. Supramolecular Ionogels tougher than metals. Adv. Mater. 35, e2301383 (2023).
Sun, B., Liu, K., Wu, B., Sun, S. & Wu, P. Low-hysteresis and tough ionogels via low-energy-dissipating cross-linking. Adv. Mater. 36, e2408826 (2024).
Seo, D. G. & Moon, H. C. Mechanically robust, highly ionic conductive gels based on random copolymers for bending durable electrochemical devices. Adv. Funct. Mater. 28, 1706948 (2018).
Ma, C. et al. Highly processable ionogels with mechanical robustness. Adv. Funct. Mater. 33, 2211771 (2023).
Qiu, Z. et al. Ionic skin with biomimetic dielectric layer templated from calathea zebrine leaf. Adv. Function. Mater. 28, 1802343 (2018).
Chen, B. et al. Highly stretchable and transparent ionogels as nonvolatile conductors for dielectric elastomer transducers. ACS Appl. Mater. Interfaces 6, 7840–7845 (2014).
Ren, Y. et al. Electric-field-induced gradient ionogels for highly sensitive, broad-range-response, and freeze/heat-resistant ionic fingers. Adv. Mater. 33, e2008486 (2021).
Shen, Z., Zhu, X., Majidi, C. & Gu, G. Cutaneous ionogel mechanoreceptors for soft machines, physiological sensing, and amputee prostheses. Adv. Mater. 33, 2102069 (2021).
Chen, C. et al. A self-healing and ionic liquid affiliative polyurethane toward a piezo 2 protein inspired ionic skin. Adv. Funct. Mater. 32, 2106341 (2022).
Park, S., Parida, K. & Lee, P. S. Deformable and transparent ionic and electronic conductors for soft energy devices. Adv. Energy Mater. 7, 1701369 (2017).
Mackanic, D. G., Kao, M. & Bao, Z. Enabling deformable and stretchable batteries. Adv. Energy Mater. 10, 2001424 (2020).
Shao, G., Yu, R., Chen, N., Ye, M. & Liu, X. Y. Stretchable supercapacitors: from materials and structures to devices. Small Methods 5, e2000853 (2021).
Kim, O., Kim, H., Choi, U. H. & Park, M. J. One-volt-driven superfast polymer actuators based on single-ion conductors. Nat. Commun. 7, 13576 (2016).
Liu, X. et al. Tough nanocomposite ionogel-based actuator exhibits robust performance. Sci. Rep. 4, 6673 (2014).
Benito-Lopez, F., Antonana-Diez, M., Curto, V. F., Diamond, D. & Castro-Lopez, V. Modular microfluidic valve structures based on reversible thermoresponsive ionogel actuators. Lab Chip 14, 3530–3538 (2014).
Fukushima, T., Asaka, K., Kosaka, A. & Aida, T. Fully plastic actuator through layer-by-layer casting with ionic-liquid-based bucky gel. Angew. Chem. Int. Ed. Engl. 44, 2410–2413 (2005).
Wang, J. et al. Extremely stretchable electroluminescent devices with ionic conductors. Adv. Mater. 28, 4490–4496 (2016).
Shi, X. et al. Large-area display textiles integrated with functional systems. Nature 591, 240–245 (2021).
Tan, Y. J. et al. A transparent, self-healing and high-kappa dielectric for low-field-emission stretchable optoelectronics. Nat. Mater. 19, 182–188 (2020).
Yu, L. & Chen, G. Z. Ionic liquid-based electrolytes for supercapacitor and supercapattery. Front. Chem. 7, 272 (2019).
Lei, Z., Gao, W. & Wu, P. Double-network thermocells with extraordinary toughness and boosted power density for continuous heat harvesting. Joule 5, 2211–2222 (2021).
Yang, M. et al. Chaotropic effect-boosted thermogalvanic ionogel thermocells for all-weather power generation. Adv. Mater. 36, e2312249 (2024).
Ho, D. H. et al. Zwitterionic polymer gel-based fully self-healable ionic thermoelectric generators with pressure-activated electrodes. Adv. Energy Mater. 13, 2301133 (2023).
Dang, C., Shao, C., Liu, H., Chen, Y. & Qi, H. Cellulose melt processing assisted by small biomass molecule to fabricate recyclable ionogels for versatile stretchable triboelectric nanogenerators. Nano Energy 90, 106619 (2021).
Han, J. H., Kim, S. Y. & Moon, H. C. Unveiling the impact of tailoring ionic conductor characteristics on the performance of wearable triboelectric nanogenerators. ACS Appl. Mater. Interfaces 16, 27778–27784 (2024).
Sun, L. et al. Ionogel-based, highly stretchable, transparent, durable triboelectric nanogenerators for energy harvesting and motion sensing over a wide temperature range. Nano Energy 63, 103847 (2019).
Zhao, G. et al. Transparent and stretchable triboelectric nanogenerator for self-powered tactile sensing. Nano Energy 59, 302–310 (2019).
Liu, Z., Cheng, H., He, H., Li, J. & Ouyang, J. Significant enhancement in the thermoelectric properties of ionogels through the solid network engineering. Adv. Funct. Mater. 32, 2109772 (2021).
Sajid, I. et al. Synthesis and characterization of novel p-type chemically cross-linked ionogels with high ionic seebeck coefficient for low-grade heat harvesting. Electrochim. Acta 320, 134575 (2019).
Matsumoto, M. et al. Exceptionally high electric double layer capacitances of oligomeric ionic liquids. J. Am. Chem. Soc. 139, 16072–16075 (2017).
Hu, Y. et al. Bioinspired poly (ionic liquid) membrane for efficient salinity gradient energy harvesting: Electrostatic crosslinking induced hierarchical nanoporous network. Nano Energy 97, 107170 (2022).
Shao, Y. et al. All-Poly(ionic liquid) membrane-derived porous carbon membranes: scalable synthesis and application for photothermal conversion in seawater desalination. ACS Nano 12, 11704–11710 (2018).
Tian, X. et al. Self-healing and high stretchable polymer electrolytes based on ionic bonds with high conductivity for lithium batteries. J. Power Sour. 450, 227629 (2020).
Liu, Z. et al. Low-cost gel polymer electrolyte for high-performance aluminum-ion batteries. ACS Appl. Mater. Interfaces 13, 28164–28170 (2021).
Wang, Z. et al. 3D printable, highly stretchable, superior stable ionogels based on poly (ionic liquid) with hyperbranched polymers as macro-cross-linkers for high-performance strain sensors. ACS Appl. Mater. Interfaces 13, 5614–5624 (2021).
Li, R. et al. Supercapacitive iontronic nanofabric sensing. Adv. Mater. 29, 1700253 (2017).
Amoli, V. et al. A bioinspired hydrogen bond-triggered ultrasensitive ionic mechanoreceptor skin. Nat. Commun. 10, 4019 (2019).
Sun, J. et al. Robust physically linked double-network ionogel as a flexible bimodal sensor. ACS Appl. Mater. Interfaces 12, 14272–14279 (2020).
Villa, S. et al. Soft piezoionic/piezoelectric nanocomposites based on ionogel/BaTiO3 nanoparticles for low frequency and directional discriminative pressure sensing. ACS Macro Lett. 8, 414–420 (2019).
Zhang, M. et al. Bionic artificial skin based on self-healable ionogel composites with tailored mechanics and robust interfaces. Adv. Mater. 36, e2405776 (2024).
Li, T., Wang, Y., Li, S., Liu, X. & Sun, J. Mechanically robust, elastic, and healable ionogels for highly sensitive ultra-durable ionic skins. Adv. Mater. 32, e2002706 (2020).
Kim, Y. M., Kwon, J. H., Kim, S., Choi, U. H. & Moon, H. C. Ion-cluster-mediated ultrafast self-healable ionoconductors for reconfigurable electronics. Nat. Commun. 13, 3769 (2022).
Xu, L. et al. A transparent, highly stretchable, solvent-resistant, recyclable multifunctional ionogel with underwater self-healing and adhesion for reliable strain sensors. Adv. Mater. 33, e2105306 (2021).
Kwon, J. H. & Moon, H. C. Dynamically switchable dual-mode wearable sensory platforms based on electrical-ionic bimodal conductors. Adv. Funct. Mater. 35, 2503935 (2025).
Sun, J. Y., Keplinger, C., Whitesides, G. M. & Suo, Z. Ionic skin. Adv. Mater. 26, 7608–7614 (2014).
Zhao, C. et al. Ionic flexible sensors: mechanisms, materials, structures, and applications. Adv. Funct. Mater. 32, 2110417 (2022).
Cheng, Y. et al. Aquatic skin enabled by multi-modality iontronic sensing. Adv. Funct. Mater. 32, 2205947 (2022).
Zhang, M. et al. Mechanically robust and highly conductive ionogels for soft ionotronics. Adv. Funct. Mater. 33, 2208083 (2022).
Pu, X. et al. Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci. Adv. 3, e1700015 (2017).
Jia, L. et al. Giant iontronic flexoelectricity in soft hydrogels induced by tunable biomimetic ion polarization. Adv. Mater. 36, 2403830 (2024).
Li, L. et al. A d33 piezoionic hydrogel with bioinspired multi-gradient structure for enhanced mechano-iontronic transduction. Nano Energy 133, 110477 (2025).
Lee, J. I. et al. Visco-Poroelastic electrochemiluminescence skin with piezo-ionic effect. Adv. Mater. 33, e2100321 (2021).
Li, X. et al. Bio-inspired spontaneous splitting of underwater bubbles along a superhydrophobic open pathway without perturbation. Droplet 1, 70–80 (2022).
Li, X. et al. Fog collection on bio-inspired topological alloy net with micro/nanostructures. ACS Appl. Mater. interfaces 12, 5065–5072 (2019).
Lan, L. et al. Skin-inspired all-natural biogel for bioadhesive interface. Adv. Mater. 36, e2401151 (2024).
Li, T. et al. Robust skin-integrated conductive biogel for high-fidelity detection under mechanical stress. Nat. Commun. 16, 88 (2025).
Jiang, Y. et al. Mechanically robust eutectogels enabled by precisely engineered crystalline domains. Nat. Commun. 16, 7417 (2025).
Liu, T. et al. Eutectogel skin electrodes with superior adhesion, sweat resistance, and long-term stability for electrophysiological signal monitoring. Adv. Function. Mater. e03568 https://doi.org/10.1002/adfm.202503568 (2025).
Acknowledgements
This work was supported by the Research Grant Council of Hong Kong (CityU 11307721), Guangdong Basic Research Program (2025A1515011479), Shenzhen Basic Research Program (JCYJ20240813153107010), and IDM Project (9229501-14-YX) from City University of Hong Kong.
Author information
Authors and Affiliations
Contributions
X.Y. supervised the project and proposed the theme of the review. X.L. conducted literature research, organized the figures, and wrote the original manuscript. All the authors discussed the framework of the review and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Yao, X. Recent progress of ionic liquid-based ionic conductors for ionotronic applications. npj Soft Matter 2, 1 (2026). https://doi.org/10.1038/s44431-025-00013-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44431-025-00013-6