Abstract
Background
Exposure to plastic additives, such as phthalates and bisphenols, has been associated with a higher risk of allergic conditions, but the evidence is inconsistent for children younger than five.
Objective
To examine the association between pre- and postnatal urinary phthalates and bisphenols, and allergic conditions, and potential effect modification by sex, in pre-school children, through a pooled analysis.
Methods
We pooled data from the Barwon Infant Study (Australia), the Canadian Healthy Infant Longitudinal Development Study (Canada), the Health Outcomes and Measures of the Environment (United States) and the Environmental Influences on Child Health Outcomes–wide cohorts (United States). Urinary phthalates and bisphenols were measured during pregnancy and early childhood. We estimated daily intakes from urinary concentrations, except for mono-(3-carboxypropyl) phthalate (MCPP). Outcomes, including asthma, wheeze, eczema, and rhinitis, were assessed up to five years of age through questionnaires and clinical assessments. We used generalised estimating equations for single compounds and quantile G-computation for the chemical mixtures.
Results
5306 children were included. A two-fold increase in prenatal dibutyl phthalates (DBP; risk ratio [RR] = 1.08; 95% confidence interval [CI]: 1.00–1.16) and benzyl butyl phthalate (BBzP; RR = 1.06; 95%CI: 1.00–1.12) increased the risk of asthma in children under five. Prenatal MCPP levels were associated with rhinitis (RR = 1.05; 95%CI: 1.01–1.09). Postnatal BBzP levels increased the risk of wheezing (RR = 1.05; 95%CI 1.01–1.09), as well as di(2-ethylhexyl) phthalate (DEHP; RR = 1.06; 95%CI: 1.01–1.11) and MCPP (RR = 1.09; 95%CI: 1.04–1.14). These were also inversely associated with eczema. A one-quartile increase in the postnatal chemical mixture increased the risk of wheezing (RR = 1.14; 95%CI: 1.02–1.26). There was limited evidence of effect modification by sex.
Impact
-
Phthalates and bisphenols are widespread and may contribute to allergic conditions in children. We pooled data from 5000 children across multiple birth cohorts, suggesting that early-life exposure to these chemicals is associated with increased risks of asthma, wheezing, and rhinitis by age five. We further investigated the timing of exposure, non-linear dose-response relationships, and effect measure modification by sex. This study provides a comprehensive assessment of early-life exposure to phthalates and bisphenols and strengthens the evidence for their role in the development of childhood allergic outcomes.
Similar content being viewed by others
Introduction
Endocrine-disrupting chemicals (EDCs) can alter the endocrine system by interfering with hormonal action and have been associated with a variety of adverse health outcomes [1, 2]. Phthalates and bisphenols are among the most common EDCs and are ubiquitous in the population [2, 3]. High-molecular-weight phthalates (HMWPs), such as benzyl-butyl phthalate (BBzP) and di-2-ethylhexyl phthalate (DEHP), increase the flexibility and durability of plastics and are added to products such as vinyl flooring, food packaging, and children’s toys [2]. Low-molecular-weight phthalates (LMWPs), such as dimethyl phthalate (DMP), diethyl phthalate (DEP), or di-iso-butyl phthalate (DiBP), are mainly found in personal care products [2]. Finally, bisphenols, such as bisphenol A (BPA), bisphenol S (BPS), and bisphenol F (BPF), are used in the manufacture of polycarbonate plastics and epoxy resins, and are mainly found in food packaging, thermal paper, and cans [3].
Phthalates and bisphenols have been suggested to induce a T helper (Th) 2 immune response, resulting in increased susceptibility to allergic inflammation [4, 5]. Potential mechanisms are numerous but include oxidative stress, epigenetic, and estrogenic modulations [4, 5]. Given that these chemicals can interact with estrogenic pathways, their effect on childhood allergic conditions may also differ between sexes [4]. Epidemiological studies have shown that phthalates and bisphenols can increase the risk of childhood asthma, wheezing, eczema, and rhinitis [6,7,8,9,10,11]. However, systematic reviews and meta-analyses have highlighted that findings in the literature are inconsistent and that the relationship between phthalates, bisphenols and childhood allergic conditions requires further study [12,13,14,15,16]. For instance, on study has shown an increase in the odds of developing eczema [11], while others have contradicted these findings [10]. Such variations in the literature can be attributed to variations in the timing of exposure, latency between exposure and health effects, variations in the exposure mixture, and non-monotonic dose-response relationships [17].
The prenatal period is a critical window of susceptibility, as foetal development is highly sensitive to environmental insults, which may disrupt organogenesis and the maturation of the immune and respiratory systems [18]. Furthermore, children up to five years old are particularly vulnerable to chemical exposure due to higher exposure relative to their body weight, immature metabolic pathways, and active physiological growth [18]. However, this age group remains underrepresented in studies investigating the association between phthalates, bisphenols, and allergic conditions. Moreover, previous studies were hindered by small sample sizes, potential residual confounding issues and exposure measurement error [4]. Finally, most studies have focused on specific chemicals, disregarding the potential for a combined effect of plasticisers.
We aimed to investigate associations between early-life urinary phthalates and bisphenols and the risk of allergic conditions in preschool-aged children using a pooled analysis from three high-income countries. We also aimed to assess whether the child’s sex modifies the association between phthalates, bisphenols, and allergic conditions. Finally, we estimated the association between chemical mixtures and childhood allergic conditions.
Methods
Data sources
This pooled analysis draws from four data sources: the Barwon Infant Study (BIS), the Canadian Healthy Infant Longitudinal Development (CHILD) Study, the Health Outcomes and Measures of the Environment (HOME) Study, and the Environmental Influences on Child Health Outcomes (ECHO)-wide cohorts. Details on the individual cohorts, such as the inclusion/exclusion criteria, have been published elsewhere [19,20,21,22]. Briefly, BIS enrolled 1074 mother-infant pairs from Victoria, Australia, recruited between 2010 and 2013, excluding very preterm deliveries (≤32 weeks), genetic diseases, major congenital malformations, or serious illnesses [19]. Ethical approval was provided by the Barwon Health Human Research Ethics Committee (HREC 10/24), and families provided informed consent. CHILD, established between 2008 and 2012, recruited 3624 pregnant mothers from four Canadian communities, excluding mothers with moderate preterm deliveries (≤35 weeks), respiratory distress syndrome, and in vitro fertilisation, resulting in 3542 infants in the inception cohort [20]. Each recruitment centre obtained approval from local Research Ethics Boards, and each participant provided signed informed consent. The HOME Study, a longitudinal cohort from Cincinnati, Ohio, recruited women from prenatal practices between 2003 and 2006 during their 2nd trimester of gestation, with inclusion criteria related to year residence was built and maternal health conditions, resulting in 401 women–infant pairs [21]. Ethical approval was provided by the Cincinnati Children’s Hospital Medical Centre and cooperating delivery hospitals, and all participants provided written informed consent. The ECHO-wide cohorts, encompassing 69 pregnancy and paediatric cohorts across the USA and Puerto Rico, started in the 1980s and continues to enrol participants, focusing on diverse populations and harmonising data collection through the ECHO-Wide Data Collection Protocol (ECWP) approved by the Western institutional review board in 2019 [22]. ECHO data was obtained through the Data and Specimen Hub provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with data locked as of 31st August 2022 [23]. The ECHO-wide cohorts, with their diverse designs, objectives, and data collection protocols, included 56 cohorts (30,904 children) that consented to the ECWP [22]. Local institutional review boards approved all cohorts, and de-identified data were used for this analysis [22].
Study participants
The study population included participants who had data on urinary phthalates or bisphenols during pregnancy or within the first five years of childhood and had at least one measure of childhood allergy within the same timeframe. We separated the study population into those with exposure measured during pregnancy (prenatal analysis) and those measured during childhood (postnatal analysis). We excluded cohorts in which more than 50% of participants had missing covariate data or those with a sample size below 30 [24]. We included nine ECHO-wide cohorts, BIS, CHILD and HOME studies. After applying the exclusion criteria, there were 3763 participants in the prenatal analysis and 1862 participants in the postnatal analysis (Fig. S1).
Exposure characterisation
In all cohorts, urinary phthalates and bisphenols were assessed using validated methods described elsewhere [25,26,27,28] (Table 1). All methods used metabolite deconjugation before analysis by high-performance liquid chromatography-tandem mass spectrometry. Within each cohort, phthalates with a detection rate > 50% and bisphenols with a detection rate >10% were included (Table 1). These thresholds allowed us to maximise the number of participants in the analyses while maintaining sufficient variability in the exposure to identify associations, in line with previous studies [29, 30]. The limits of detection for each metabolite are shown in Table S1. To maintain methodological continuity and comparability with previous work from BIS, HOME and CHILD, we applied the same approaches to handling values below the limit of detection (LOD) that had been previously validated [31,32,33].
BIS
Single spot urine samples were collected at 36 gestational weeks and analysed by the Queensland Alliance for Environmental Health Sciences [25, 29]. Measurements below the limit of detection (LOD) were imputed using LOD/√2 for phthalates and, due to lower detection rates, bisphenols were multiply imputed using a left-censored log-normal distribution with five datasets [29, 34]. The Levine-Fahey equation was used to correct for urine dilution using specific gravity: \({E}_{{Sg}}={E}_{0} \times [({{Sg}}_{{median}}-1)/({{Sg}}_{0}-1)]\), where Esg is the specific gravity-corrected analyte, E0 is the observed analyte, Sgmedian is the median of specific gravity values in the study sample, and Sg0 is the observed specific gravity value [35]. Phthalate and bisphenol measurements were weighted to account for batch effects [36], and corrected for the time of day at sampling using the residual method [37].
ECHO-wide cohorts
Spot urine samples were collected throughout pregnancy (4–40 gestational weeks) and analysed by the Division of Laboratory Sciences, National Centre for Environmental Health, Centres for Disease Control and Prevention (CDC), or the Wadsworth Laboratory, New York State Department of Health (Table 1) [26, 27]. The number of samples per pregnant woman ranged from one to eight, averaging two samples per mother. Values below the LOD were imputed with LOD/√2 for phthalates. Due to lower detection rates in some cohorts, bisphenols were multiply imputed using a left-censored log-normal distribution, as above. When available, we used specific gravity to standardise the sample for urine dilution, as described above. If specific gravity was unavailable for an entire cohort (n = 3 cohorts, 548 children), we used the Boeniger method for creatinine standardisation: \({E}_{{Cr}}={E}_{0}\times [({{Cr}}_{{median}})/({{Cr}}_{0})]\), where Ecr is the creatinine-corrected analyte, E0 is the observed analyte, Crmedian is the creatinine median in the study sample, and Cr0 is the observed creatinine value [38]. Missing urine dilution data (n = 120 samples, 2%) were imputed using linear regression with gestational age at sampling, maternal age, and pre-pregnancy weight. Standardisation for time of day at sampling was applied using the residual method [37].
CHILD
Spot urine samples were collected at three months, one year, and three years [32]. For nontoilet-trained children, urine was collected using Tegaderm plastic film and cotton pads, which were subsequently squeezed to extract urine [32]. AXYS Analytical Service analysed the samples using the Kato method [28]. We used previously described phthalate variables, standardised for specific gravity using the Levine-Fahey equation [32]. Values below the LOD were imputed with a log-normal distribution using NDexpo [32].
HOME
Spot urine samples were collected into polypropylene cups during pregnancy at 16 and 26 weeks and annually during childhood before analysis by the CDC [26, 27]. Surgical inserts were placed inside the diaper for non-toilet-trained infants, and urine was extracted into the specimen cups [39]. Specific gravity was used for urine dilution correction as described above [35]. Missing specific gravity data (n = 82 samples, 5%) were imputed using linear regression with creatinine, gestational age, maternal age, and body weight. Analytes with values below the LOD were imputed with LOD/√2 [34].
Outcome assessment
This study assessed four health outcomes from birth to five years of age: asthma, wheezing, eczema, and rhinitis.
BIS
Caregivers completed a validated questionnaire at 1, 3, 6, 9, 12, 18, 24, and 48 months [40]. Asthma was assessed during the 2- and 4-year reviews based on a positive response from parents to the question, “Has your child ever had asthma?”. Wheeze was evaluated at each time point by asking caregivers, “Has your child experienced wheezing or whistling from the chest since the last review?”. Eczema was identified at all time points using the modified UK Working Party criteria [41]. This included a history of itchy skin, plus at least three of the following: a history of dry skin, a family history of allergy, a history of skin rash affecting the flexures or outer surfaces of the limbs or the head or cheeks, visible dermatitis assessed during a study visit at either 1 month, 6 months, 1 year or 4 years. Rhinitis was determined at the 2- and 4-year reviews if parents responded affirmatively to the question, “Has your child ever had hay fever?”
ECHO-wide cohorts
Outcomes were assessed using the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire [42]. All outcomes were caregiver-reported episodes since the previous visit. The timing and number of outcome measurements varied across cohorts, with some children having their outcome measured at six months only, while others had repeated measures up to eight times, spanning from birth to five years of age (Table S2). Asthma was defined as a positive response to the question, “Has the child ever had asthma?”; wheeze as “Has the child ever had wheezing or whistling in the chest at any time in the past?”; eczema as “Has the child ever had eczema (also called atopic dermatitis)?”; and rhinitis as “Has the child ever had a problem with sneezing or a runny or blocked (stuffy) nose when he/she did NOT have a cold or the flu?”.
CHILD
Subspecialist paediatricians assessed allergic outcomes in children aged 1, 3 and 5 years, which were classified as “definite”, “possible”, or “no” [43]. Only those with a “definite” diagnosis were considered cases to mitigate the risk of outcome misclassification. Asthma and allergic rhinitis were assessed at the three- and five-year study visits [43]. Eczema was clinically assessed at 1, 3, and 5 years, using the modified UK Working Party criteria [41]. Data on wheezing collected at 3, 6, 12, 18, 24, 36, 48, and 60 months was defined as a positive response to “In the last X months, has your child had a wheezing noise (whistling sound) coming from his/her chest either WITH a cold or WITHOUT a cold?”.
HOME
Caregivers were surveyed biannually on their child’s allergic outcomes from six months to five years of age, with a questionnaire adapted from the National Health and Nutrition Examination Survey [44]. Asthma was defined from two to five years by the question, “Has a doctor or other health professional ever told you that [child] has asthma?”. Wheeze was assessed at all time points with the question, “Has [child] had wheezing or whistling in his/her chest since he/she was X months old?”. Eczema was evaluated by asking, “Has [child] had any symptoms of eczema since he/she was X months old?”. Rhinitis was defined by the question, “Since [child] was X months old, has he/she had any symptoms of hay fever or allergies, such as recurrent runny, itchy, or stuffy nose without a cold, or recurrent sneezing and itchy eyes?”.
Covariates
We identified potential confounders by reviewing the literature using a directed acyclic graph (Fig. S2). Most covariates were caregiver-reported, except those collected at birth, for which hospital records were used. Data harmonisation of categorical variables is shown in Table S3. To account for variability in exposure distributions, outcome prevalence and covariate distribution between cohorts, we adjusted all models for cohort membership, namely BIS, HOME, CHILD, and each of the nine ECHO-wide cohorts. In the prenatal analysis, covariates included offspring sex (male; female), maternal age at conception (years; continuous), ethnicity (Caucasian/White; Other), Marital status reported during pregnancy (Single/Not married; Other), the highest level of caregiver education reported during pregnancy (high school or under; bachelor’s degree; master or doctorate; Other), family history of asthma (yes; no), and season of birth (categorical; Spring, Summer, Autumn, Winter). Ethnicity was included to account for racial disparities in chemical exposure and prevalence of childhood allergies. We collapsed ethnicity categories given the differences in categories between cohorts (Table S3). In addition, prenatal exposure to tobacco smoke was assessed via questionnaires administered during pregnancy, while for the HOME Study, this was quantified using serum cotinine levels at 16 and 26 gestational weeks, with women exhibiting cotinine levels above the LOD (0.015 ng/ml) classified as exposed to tobacco smoke [45]. Further adjustments for the postnatal analysis were made for any breastfeeding duration (weeks, continuous), gestational age (weeks, continuous), postnatal tobacco smoke exposure (yes; no), and age at outcome assessment (years, continuous) in addition to the covariates above. Postnatal tobacco smoke exposure was determined based on caregiver-reported smoking inside the house or dwellings. In the HOME Study, child serum cotinine concentrations at each visit were used, with thresholds described by Mourino et al. [46]. The distribution of serum cotinine concentrations in HOME is shown in Fig. S3. All covariates were considered time-fixed except age at outcome assessment, which we considered time-varying.
Statistical analysis
Exposure processing
To normalise biomarker concentrations for body weight and facilitate comparison with regulatory reference values, we derived estimated daily intakes (EDI) from urinary metabolites, accounting for weight at sampling, average daily urine volume, fractional excretion, and the compound-to-metabolite molecular weight ratio:
Where Cadj is the standardised metabolite urinary concentration (µg/L), UV is the average daily urine volume (1.6 L during pregnancy), FUE is the metabolite fractional urinary excretion (unitless), MW is the molecular weight (g/mol), and W is the body weight at sampling (kg). In all cohorts, pre-pregnancy weight was self-reported. In BIS, weight at sampling was imputed using a linear model including pre-pregnancy weight, maternal weight at 28 weeks, birth weight, placental weight, maternal postnatal weight (4 weeks postpartum), and recalled pre-pregnancy weight gain at four years postpartum [31]. In other cohorts, it was estimated using the Institute of Medicine’s recommended weight gain during pregnancy based on pre-pregnancy BMI [47]. For postnatal exposure, UV/W was estimated from the child’s age at sampling [48]. Mono-methyl phthalate (MMP) was used to calculate dimethyl phthalate (DMP) daily intake, monoethyl phthalate (MEP) for diethyl phthalate (DEP), mono-isobutyl phthalate (MiBP) for di-isobutyl phthalate (DiBP), and mono-n-butyl phthalate (MnBP) for di-n-butyl phthalate (DnBP); we used the sum of DiBP and DnBP to derive di-butyl phthalates (DBP) daily intake. Monobenzyl phthalate (MBzP) was used to calculate benzyl butyl phthalate (BBzP). For di(2-ethylhexyl) phthalate (DEHP), we used the molar sum of mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP). In the HOME Study, MEHP was excluded from the 1–3-year time points due to external contamination, so only DEHP oxidative metabolites were considered for DEHP calculation to ensure consistency across maternal and child samples [39]. In BIS, MEHP was excluded due to external contamination [29]. In CHILD, we lacked data on MECPP; therefore, we used MEHP, MEOHP, and MEHHP for DEHP calculation. Since Mono (3-carboxypropyl) phthalate (MCPP) is a non-specific metabolite of multiple high-molecular-weight phthalates, we kept urinary MCPP concentrations rather than calculating EDI. Due to insufficient measurements and low detection rates, we did not include DMP, bisphenol S (BPS), or bisphenol F (BPF) in the postnatal analysis.
Single-compound associations
We first summarised cohort characteristics using descriptive statistics. Given the right-skewed distribution of exposure variables, we applied a log2 transformation. The correlation plots of exposures are shown in Fig. S4–S5. In a complete case analysis, we estimated pairwise longitudinal associations between single compounds and repeated outcome measures. We used generalised estimating equations with an exchangeable correlation matrix to account for repeated measures to estimate longitudinal associations. A Poisson model with log link function and robust standard errors was used to estimate risk ratios (RR), associated with a twofold increase in the exposure levels and 95% confidence intervals (CI) [49]. For the prenatal analysis, the average of each compound was used when multiple samples were available. For postnatal analysis, we estimated lagged associations. Specifically, future outcomes were regressed on repeated exposures, with outcome measures aggregated over each exposure time point (i.e., 12-month exposure aligned with outcomes from 12–24 months), as previously described elsewhere (Fig. S6) [50]. We then added smoothing terms with restricted cubic splines with three knots located at each exposure’s 10th, 50th and 90th percentiles to plot dose-response curves, and calculated p-values for non-linearity with Wald tests on spline terms [51]. To investigate effect measure modification by sex, we added an interaction term to each model, and stratum-specific estimates were obtained using the marginaleffects package (RStudio, version 0.17.0) [52]. To identify windows of vulnerability during pregnancy, we used the average exposure concentration for each trimester and stratified the analysis by trimester. We opted not to correct for multiple comparisons, given that we aimed to investigate underlying patterns of associations, thereby prioritising the detection of potential signals over the control of the Type I error rate [53].
Sensitivity analyses
First, we repeated our analysis on urinary biomarkers instead of estimated daily intakes to assess whether the uncertainty in estimating daily intakes influenced our results. Since a pooled analysis assumes a common treatment effect across cohorts, we then conducted a random-effects meta-analysis using restricted maximum likelihood on cohort-stratified effect estimates to evaluate this assumption and examine between-cohort heterogeneity [54]. Third, assuming a missing-at-random mechanism, we imputed missing covariates (n = 207 children; 3.9%) with multiple imputations by chained equations, using the same variables from the main analysis, generating five datasets with 20 iterations [24]. Fourth, to mitigate potential selection bias arising from systematic differences between the subjects included in the analysis and those excluded from the inception cohorts due to missing exposure or outcome data, we used stabilised inverse probability weights (IPWs) [55, 56]. The weights were based on the same covariates used in the main models. Fifth, to ensure that variability in DEHP metabolite availability did not influence our estimates, we repeated the analysis using only metabolites consistently measured across cohorts, excluding MEHP from pre- and postnatal analyses, and excluding MECPP from the postnatal analysis. Last, we derived E-values from our estimates to evaluate the potential for unmeasured confounding [57].
Overall mixture associations
Since 77.9% of children (n = 2930) did not have DMP, BPS, and BPF measured during pregnancy, these compounds were excluded from the mixture analysis. Similarly, BPA was excluded from the postnatal analysis as it was not measured in CHILD. Participants who did not have all exposures measured were excluded, resulting in 2402 children in the prenatal analyses and 1654 in the postnatal analyses. We employed Quantile G-computation to estimate the association of the chemical mixture with allergic conditions [58]. This method fits a marginal structural model with quantised exposure, allowing the estimation of the effect of increasing all exposures by one quantile simultaneously. We estimated RRs associated with a one-quartile increase in the chemical mixture, considering subjects as random intercepts, with 95% CI estimated via 1000 bootstrap replicates. Quantile G-computation also accommodates non-linear relationships between the chemical mixture and the outcome. We increased the number of quantiles to 20 to plot dose-response curves between the chemical mixtures and outcomes. We also added second- and third-order polynomials on top of restricted cubic splines and selected the models with the best Akaike Information Criterion (AIC) (Table S4). Finally, we used the qgcompint package (version 0.7.0) to assess the interaction between sex and the overall chemical mixture [59]. All statistical analyses were performed using R version 4.2.2 [60].
Results
Sample characteristics
The study population included 5306 children, with 3444 having exposure assessed during pregnancy, 1543 during childhood, and 319 during both periods (Table 2). Further details of characteristics for each of the included cohorts are provided in Table S5. The average maternal age was approximately 30 years for all cohorts. The study population was predominantly Caucasian/White, ranging from 53.9% in ECHO (n = 1376) to 76.7% in BIS (n = 633). Single parenthood was the least common in BIS (n = 24, 2.9%), and most common in ECHO (n = 619, 24.5%). BIS had the highest prevalence of a family history of asthma (n = 411, 49.4%), while the CHILD cohort had the lowest (n = 324, 21.1%). The proportion of children having asthma in the first five years ranged from 11.6% in CHILD (n = 132) to 21.7% in BIS (n = 140). The proportion of wheezing varied across cohorts, with ECHO showing the lowest rates (n = 712, 27.9%), and BIS showing the highest (n = 466, 67.2%). The proportion of children with eczema ranged from 36.5% (n = 790) in ECHO to 66.4% (n = 805) in CHILD. Finally, the prevalence of rhinitis ranged from 9.6% in CHILD (n = 125), to 63.1% in HOME (n = 238).
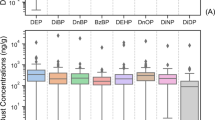
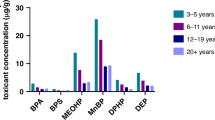
With the exception of DEP, children had higher urinary phthalates and bisphenols concentrations relative to body weight than pregnant women (Table 3). Average exposure levels ranged from 5 ng/kg/day for BPF during pregnancy to 6.6 µg/kg/day for DEHP during childhood. As expected, we found variations in exposure levels between cohorts, with BIS showing lower levels of bisphenols and HOME exhibiting the highest levels of DEHP (Tables S6–S7).
Single-compound associations
Prenatal analysis
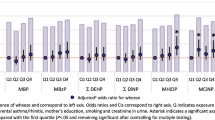
We found that each two-fold increase in prenatal DBP levels was associated with a higher risk of asthma (RR = 1.08; 95% CI: 1.00–1.16) and wheezing (RR = 1.04; 95% CI: 1.00–1.08) (Table 4). Similarly, a two-fold increase in prenatal BBzP levels was associated with a higher risk of asthma (RR = 1.06; 95% CI: 1.00–1.12). Additionally, MCPP levels were positively associated with the risk of wheezing (RR = 1.03; 95% CI: 1.00–1.05) and allergic rhinitis (RR = 1.05; 95% CI: 1.01–1.09), while DEHP showed an inverse relationship with eczema (RR = 0.97; 95% CI: 0.93–1.00). No other associations were observed between prenatal urinary phthalates, bisphenols, and health outcomes. Crude estimates are presented in Table S8. While most associations were linear, a few demonstrated non-linearity (Fig. 1). For instance, there were inverted U-shaped associations between prenatal urinary BPS and eczema, and between DEP and rhinitis. Furthermore, the association between DEP and eczema exhibited a U-shaped relationship.
Models obtained with generalised estimating equations. Dose-response relationships are displayed with continuous covariates held at their mean values and categorical covariates set to their reference category. P-values for non-linearity obtained with Wald tests on spline terms. Models adjusted for cohort membership, maternal age, ethnicity, parental education, marital status, family history of asthma, sex, prenatal tobacco smoke exposure, and season of birth. All exposures are modelled as estimated daily intakes, except MCPP, which is modelled using biomarker concentrations.
Postnatal analysis
We found some evidence that high-molecular-weight phthalates, such as BBzP (RR = 1.05; 95% CI: 1.01–1.09), DEHP (RR = 1.06; 95% CI: 1.01–1.11), and MCPP (RR = 1.09; 95% CI: 1.04–1.14), were associated with a higher risk of wheezing (Table 5). Conversely, high-molecular-weight phthalates were inversely associated with eczema, such as BBzP (RR = 0.98; 95% CI: 0.95–1.00), DEHP (RR = 0.95; 95% CI: 0.91–0.98), and MCPP (RR = 0.95; 95% CI: 0.92–0.98). Crude estimates are shown in Table S9. We found a U-shape dose-response relationship between DBP and eczema, and between MCPP and rhinitis (Fig. 2). Furthermore, the association between BPA and wheeze followed an inversed-U relationship.
Models obtained with generalised estimating equations. Dose-response relationships are displayed with continuous covariates held at their mean values and categorical covariates set to their reference category. P-values for non-linearity obtained with Wald tests on spline terms. Models adjusted for cohort membership, maternal age, ethnicity, parental education, marital status, family history of asthma, sex, prenatal tobacco smoke exposure, season of birth, breastfeeding duration, age at outcome assessment, postnatal smoke exposure and gestational age. All exposures are modelled as estimated daily intakes, except MCPP, which is modelled using biomarker concentrations.
Overall mixture associations
Using quantile G computation, there was no association between prenatal phthalates and bisphenols mixture and outcomes (Table 6). However, the postnatal chemical mixture was positively associated with wheezing (RR = 1.14; 95%CI: 1.02–1.26), with MCPP showing the highest weight (Fig. S7). Except for a linear association between the postnatal chemical mixture and wheeze, there was no clear pattern of association for other outcomes (Fig. 3).
Dose-response relationships are displayed with continuous covariates held at their mean values and categorical covariates set to their reference category. Prenatal analysis models adjusted for cohort membership, maternal age, ethnicity, parental education, marital status, family history of asthma, sex, prenatal tobacco smoke exposure, and season of birth. Postnatal analysis models were further adjusted for breastfeeding duration, age at outcome assessment, postnatal smoke exposure and gestational age.
Effect measure modification by sex
Although evidence of effect measure modification by sex was limited in single compound models, postnatal DEHP was associated with a higher risk of wheezing in females (RR = 1.11; 95%CI: 1.04–1.18; p = 0.04 for interaction; Tables S10–S11), and a lower risk of rhinitis in males (RR = 0.94; 95% CI: 0.87–1.00, p = 0.02 for interaction). Additionally, the association between pre- and postnatal DEP levels and asthma followed an inverted U-shape pattern in females, but not in males (pprenatal < 0.001 for interaction; ppostnatal = 0.04 for interaction; Fig. S8–S9). No evidence of effect measure modification by sex was found when using quantile G-computation (Table 7). However, the relationship between the postnatal chemical mixtures and eczema exhibited a U-shaped pattern only in females (Fig. 4).
Red circles: Females. Blue triangles: Male. Models obtained with quantile G-computation. Prenatal analysis models adjusted for cohort membership, maternal age, ethnicity, parental education, marital status, family history of asthma, prenatal tobacco smoke exposure, and season of birth. Postnatal analysis models were further adjusted for breastfeeding duration, age at outcome assessment, postnatal smoke exposure and gestational age.
Susceptible window of exposure during pregnancy
We found that the association of prenatal exposure to phthalates and bisphenols with childhood allergic conditions differed by trimester of exposure (Fig. S10–S13). There were stronger associations for first-trimester BPS (RR = 2.45; 95% CI: 1.42, 4.23) and BBzP (RR = 1.43; 95% CI: 1.20–1.71), with asthma, but also with first-trimester MCPP and eczema (RR = 1.12; 95% CI: 1.00–1.24). Second-trimester DBP levels (RR = 1.14; 95% CI: 1.03–1.26), BBzP (RR = 1.11; 95% CI: 1.02–1.21), and MCPP (RR = 1.13; 95% CI: 1.04–1.24) were associated with a higher risk of asthma. Furthermore, second-trimester BPF was positively associated with eczema (RR = 1.08; 95% CI: 1.01–1.15), while MCPP was associated with rhinitis (RR = 1.06; 95% CI: 1.01–1.12) and wheezing (RR = 1.05; 95% CI: 1.00–1.10). Regarding third-trimester exposures, only DEP levels were associated with eczema (RR = 1.05; 95% CI: 1.01–1.09).
Sensitivity analyses
Repeating the analysis using urinary biomarkers rather than estimated daily intakes did not substantially change our estimates (Table S12–S13). The heterogeneity between cohorts, measured by the I2 statistic, was generally low, below 40% in the prenatal analysis, indicating a reasonable degree of replication across cohorts (Fig. S14–S17). However, the postnatal analysis had higher heterogeneity, except for eczema (Fig. S18–S21). Point estimates remained generally unchanged when combining cohort-specific effect sizes in a random-effect meta-analysis. However, confidence intervals were wider in some cases due to moderate to high heterogeneity (Fig. S22–S23). The imputation of missing values or using IPW to account for potential selection bias did not substantially change our findings (Fig. S22–S23). Furthermore, restricting DEHP metabolites to those measured in all cohorts did not substantially change our estimates (Table S14). Last, an unmeasured confounder would need to be associated with both prenatal DBP exposure and childhood asthma by a risk ratio of at least 1.37 to fully explain the observed association. For the postnatal phthalate mixture and wheeze, the corresponding E-value was 1.54.
Discussion
This pooled analysis suggests that prenatal and postnatal levels of specific phthalates and bisphenols are marginally associated with asthma, wheezing and allergic rhinitis in children up to five. Additionally, phthalates and bisphenols chemical mixtures during childhood were associated with childhood wheezing. However, we found inverse associations between phthalates and eczema. In some cases, the associations were non-linear, exhibiting either a U-shape or an inverse U-shape dose-response relationship. Finally, the risk of childhood allergic conditions associated with prenatal phthalates and bisphenols might differ by trimester of exposure.
Many studies have investigated whether phthalates and bisphenols are associated with asthma and wheezing during childhood, yet results have been inconsistent. Our findings on prenatal DBP and BBzP with asthma align with those of the Infancia y Medio Ambiente (INMA) study [6] and the Columbia Centre for Children’s Environmental Health Cohort [61]. However, results from Berger et al. suggested no association between MiBP and MnBP during the second trimester of pregnancy and asthma at seven years of age [10]. While differences in exposure windows may explain different findings, the Raine study reported reduced odds of persistent asthma and higher odds for transient asthma associated with serum MBzP during the second and third trimesters of pregnancy [62], suggesting these chemicals may affect different asthma phenotypes. Overall, there was no strong evidence of effect measure modification by sex, as the magnitude of estimates remained similar across sexes, suggesting that the observed differences may be due to chance rather than true modification. While some studies have suggested effect measure modification by sex [10, 62], others have reported limited or no sex-specific effects [6, 63,64,65], indicating that the evidence for sex-specific effects of phthalates and bisphenols on childhood allergic outcomes remains limited.
Surprisingly, we found inverse associations between high-molecular-weight phthalates and eczema. Few studies reported similar findings between prenatal phthalates and childhood eczema [8, 63, 64, 66, 67]. Moreover, many studies investigating childhood exposure and eczema lacked a clear temporal relationship between exposure and outcome. Children with eczema may use more personal care products, such as creams and ointments, potentially leading to higher phthalate and bisphenol exposure and inflated estimates [68]. Only a few cohort studies have demonstrated temporal precedence between childhood measurements and outcomes. The Polish Mother and Child cohort reported an inverse association between longitudinal MEOHP measurements and atopic dermatitis at age nine [8]. In contrast, two other cohort studies found no association between phthalate exposure during early childhood and subsequent eczema [69, 70]. In our postnatal analysis, we ensured that exposure measurements preceded the outcome, mitigating reverse causation. Since most reported associations between childhood phthalate exposure and eczema are cross-sectional, further longitudinal studies maintaining exposure-outcome timing are necessary to confirm these findings.
Although our estimates were modest and unlikely to be clinically important, plastic chemical exposure is chronic and widespread [2, 3]. Thus, population-level strategies to reduce exposure could decrease the burden of asthma and wheezing in developed countries over time [71]. In our mixture analysis, dibutyl and HMW phthalates had the highest relative weights for asthma and wheezing. While these chemicals are primarily introduced through diet, a randomised study found that dietary interventions failed to reduce phthalate levels [72], suggesting that further studies are warranted to develop effective strategies for reducing plastic chemical exposure at a population level.
Human evidence on the susceptibility window of EDC exposure is limited. Consistent with our results, the INMA cohort study reported that first-trimester phthalate exposure were associated with a higher risk of asthma, wheezing, and eczema in children aged four to seven, whereas third-trimester exposure did not [6]. Since airway development is completed by 16–18 gestational weeks, this period may represent a critical window of susceptibility [73]. However, these results should be interpreted cautiously, as the estimates for different trimesters were based on different subsets of the study population, with small sample sizes for the first trimester.
Phthalates and bisphenols may induce childhood allergic conditions through multiple pathways, although mechanisms are not fully understood [4]. Experimental studies support the role of DBP and BBzP in the development of asthma. In mice, oral DBP administration increased Th2 and Th17 cytokine infiltration in lung tissue and elevated oxidative stress biomarkers [74], supporting its role in airway inflammation. Furthermore, gestational BBzP exposure in mice led to offspring airway inflammation via global DNA hypermethylation in CD4 cells [75], aligning with our findings. Phthalates with 8 carbon atoms on their side chains, such as DEHP, had the strongest immunostimulatory effects [76]. This aligns with our findings of postnatal exposure to these phthalates being associated with wheezing, suggesting that structural differences between phthalates may influence their biological activity. Regarding eczema, while most murine studies suggest that phthalates exacerbate eczema, these studies often used non-environmentally relevant doses and mainly focused on their adjuvant effects rather than direct eczema onset [4]. Interestingly, one study reported that subcutaneous injection of high-molecular-weight phthalates in ovalbumin-sensitised mice reduced IgE production [77], potentially supporting the inverse association we observed. Nonetheless, further studies are warranted to determine the relevance of these mechanisms in humans.
Strengths of this study include the integration of four different data sources from various populations combined with a uniform analytical strategy. Thus, decreasing the heterogeneity of our estimates and enhancing the generalisability of our results within high-income, English-speaking populations. By increasing sample size and exposure range, we captured relationships and dose responses that may have gone undetected in smaller cohorts. To our knowledge, this is the largest study investigating urinary phthalate and bisphenol exposure and childhood allergic conditions across both the prenatal and postnatal periods. We also estimated the overall effects of chemical mixtures through longitudinal associations. Finally, most cohorts had repeated urinary samples available, offering more reliable exposure assessments than single-spot urine samples [78].
Limitations include the large variations in outcome prevalence rates, mainly due to differences in assessment methods and age at evaluation. Similarly, exposure assessment methods varied across cohorts, further contributing to heterogeneity and potentially affecting the precision of our findings. However, the literature on this topic is often conflicting, and pooling multiple cohorts with diverse exposure and outcome assessment methods allowed us to evaluate the robustness of previously reported associations rather than relying on a single study. While clinical assessment is the gold standard for diagnosing outcomes, caregiver-reported outcomes might introduce potential misclassification. Nevertheless, validated surveys, such as the ISAAC questionnaire, have demonstrated 87% sensitivity and 100% specificity for asthma in children aged 6–7 years [79]. Similarly, the UK Working Party criteria for eczema, used in the BIS and CHILD cohorts, have shown 85% sensitivity and 96% specificity for childhood eczema [41]. Detection rates for bisphenols were lower than for phthalates, likely resulting in reduced exposure levels compared to other studies of bisphenols. This may have diminished exposure variability and limited our capacity to identify associations. Additionally, models for bisphenols also had smaller sample sizes, which may lack adequate statistical power to detect small effect sizes. Furthermore, our mixture approach did not specifically examine co-adjusted compounds or co-exposure multiplicative interactions. We cannot rule out the potential for residual confounding from maternal diet, household factors, or other correlated environmental chemicals that may have influenced our estimates and dose-responses. Furthermore, the aggregation of covariate categories, though necessary, may have led to some residual confounding. While the direction and extent of total unmeasured confounding remain uncertain, an unmeasured confounder would need a 1.37-fold association with both exposures and outcomes to nullify the association between prenatal DBP exposure and asthma [57].
In summary, this pooled analysis adds to the growing body of evidence that EDCs are associated with respiratory and allergic outcomes, with novel observations in children under five. Our findings on asthma align with previous research, reinforcing the potential risk that specific phthalates pose during critical developmental windows. Furthermore, the observed inverse relationship with eczema requires further investigation. Future studies should focus on longitudinal designs with repeated exposure measurements, ensure proper exposure-outcome timing precedence, and explore potential mechanisms.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due privacy and ethical reasons but are available from the corresponding author on reasonable request.
References
World Health O, United Nations Environment P, Inter-Organization Programme for the Sound Management of C, Bergman Å, Heindel JJ, Jobling S, et al. State of the science of endocrine disrupting chemicals 2012: summary for decision-makers. Technical documents. World Health Organization; 2013 2013.
Wang Y, Zhu H, Kannan K. A review of biomonitoring of phthalate exposures. Toxics. 2019;7:21.
Bousoumah R, Leso V, Iavicoli I, Huuskonen P, Viegas S, Porras SP, et al. Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review. Science Total Environ. 2021;783:146905.
Bølling AK, Sripada K, Becher R, Bekö G. Phthalate exposure and allergic diseases: Review of epidemiological and experimental evidence. Environ Int. 2020;139:105706.
Robinson L, Miller R. The impact of Bisphenol A and Phthalates on allergy, asthma, and immune function: a review of latest findings. Curr Environ Health Rep. 2015;2:379–87.
Gascon M, Casas M, Morales E, Valvi D, Ballesteros-Gómez A, Luque N, et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol. 2015;135:370–8.e7.
Berger K, Eskenazi B, Balmes J, Kogut K, Holland N, Calafat AM, et al. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatric Allergy Immunol. 2019;30:36–46.
Podlecka D, Gromadzińska J, Mikołajewska K, Fijałkowska B, Stelmach I, Jerzynska J. Longitudinal effect of phthalates exposure on allergic diseases in children. Annals Allergy, Asthma Immunol. 2020;125:84–9.
Stelmach I, Majak P, Jerzynska J, Podlecka D, Stelmach W, Polańska K, et al. The effect of prenatal exposure to phthalates on food allergy and early eczema in inner-city children. Allergy Asthma Proc. 2015;36:72–8.
Berger K, Eskenazi B, Balmes J, Holland N, Calafat AM, Harley KG. Associations between prenatal maternal urinary concentrations of personal care product chemical biomarkers and childhood respiratory and allergic outcomes in the CHAMACOS study. Environ Int. 2018;121:538–49.
Herberth G, Pierzchalski A, Feltens R, Bauer M, Röder S, Olek S, et al. Prenatal phthalate exposure associates with low regulatory T-cell numbers and atopic dermatitis in early childhood: Results from the LINA mother-child study. J Allergy Clin Immunol. 2017;139:1376–9.e8.
Jaakkola JJ, Knight TL. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic Review and Meta-analysis. Environ Health Perspect. 2008;116:845–53.
Li M-C, Chen C-H, Guo YL. Phthalate esters and childhood asthma: A systematic review and congener-specific meta-analysis. Environ Pollut. 2017;229:655–60.
Wu M, Wang S, Weng Q, Chen H, Shen J, Li Z, et al. Prenatal and postnatal exposure to Bisphenol A and Asthma: a systemic review and meta-analysis. J Thorac Dis. 2021;13:1684–96.
Zhang H, Chen S, Chen X, Zhang Y, Han Y, Li J, et al. Exposure to phthalate increases the risk of eczema in children: Findings from a systematic review and meta-analysis. Chemosphere. 2023;321:138139.
Xie M-Y, Ni H, Zhao D-S, Wen L-Y, Li K-S, Yang H-H, et al. Exposure to bisphenol A and the development of asthma: A systematic review of cohort studies. Reprod Toxicol. 2016;65:224–9.
Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocrine Rev. 2009;30:293–342.
Sly PD, Flack F. Susceptibility of children to environmental pollutants. Annals N. Y Acad Sci. 2008;1140:163–83.
Vuillermin P, Saffery R, Allen KJ, Carlin JB, Tang ML, Ranganathan S, et al. Cohort Profile: The Barwon Infant Study. Int J Epidemiol. 2015;44:1148–60.
Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax. 2015;70:998.
Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2017;46:24.
Knapp EA, Kress AM, Parker CB, Page GP, McArthur K, Gachigi KK, et al. The Environmental Influences on Child Health Outcomes (ECHO)-Wide Cohort. Am J Epidemiol. 2023;192:1249–63.
Gillman M. Environmental Influences on Child Health Outcomes (ECHO)-wide Cohort (Version 2). [Dataset]. NICHD Data and Specimen Hub. 2023; https://doi.org/10.57982/bg8s-9535.
Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials – a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162.
Heffernan AL, Gomez-Ramos MJ, Symeonides C, Hare DJ, Vijayasarathy S, Thompson K, et al. Harmonizing analytical chemistry and clinical epidemiology for human biomonitoring studies. A case-study of plastic product chemicals in urine. Chemosphere. 2020;238:124631.
Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, et al. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography–atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B. 2003;789:393–404.
Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–13.
Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 Phthalate metabolites in urine using automated sample preparation and on-line preconcentration/High-Performance Liquid Chromatography/Tandem Mass Spectrometry. Anal Chem. 2005;77:2985–91.
Eisner A, Gao Y, Collier F, Drummond K, Thomson S, Burgner D, et al. Cord blood immune profile: Associations with higher prenatal plastic chemical levels. Environ Pollut. 2022;315:120332.
Blaauwendraad SM, Gaillard R, Santos S, Sol CM, Kannan K, Trasande L, et al. Maternal phthalate and bisphenol urine concentrations during pregnancy and early markers of arterial health in children. Environ Health Perspect. 130:047007.
Sugeng EJ, Symeonides C, O’Hely M, Vuillermin P, Sly PD, Vijayasarathy S, et al. Predictors with regard to ingestion, inhalation and dermal absorption of estimated phthalate daily intakes in pregnant women: The Barwon infant study. Environ Int. 2020;139:105700.
Navaranjan G, Takaro TK, Wheeler AJ, Diamond ML, Shu H, Azad MB, et al. Early life exposure to phthalates in the Canadian Healthy Infant Longitudinal Development (CHILD) study: a multi-city birth cohort. J Expos Sci Environ Epidemiol. 2020;30:70–85.
Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33:558–66.
Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51.
Levine L, Fahy JP. Evaluation of urinary lead excretion for persons at work. J Ind Hyg Toxicol. 1946;28:98.
Engel SM, Villanger GD, Nethery RC, Thomsen C, Sakhi AK, Drover SSM, et al. Prenatal phthalates, maternal thyroid function, and risk of attention-deficit hyperactivity disorder in the Norwegian Mother and Child Cohort. Environ Health Perspect. 126:057004.
Mortamais M, Chevrier C, Philippat C, Petit C, Calafat AM, Ye X, et al. Correcting for the influence of sampling conditions on biomarkers of exposure to phenols and phthalates: a 2-step standardization method based on regression residuals. Environ Health. 2012;11:29.
Kuiper JR, O’Brien KM, Ferguson KK, Buckley JP. Urinary specific gravity measures in the U.S. population: Implications for the adjustment of non-persistent chemical urinary biomarker data. Environ Int. 2021;156:106656.
Etzel TM, Braun JM, Kuiper JR, Calafat AM, Cecil KM, Chen A, et al. Gestational and childhood phthalate exposures and adolescent body composition: The HOME study. Environ Res. 2022;212:113320.
Gray LEK, Ponsonby A-L, Collier F, O’Hely M, Sly PD, Ranganathan S, et al. Deserters on the atopic march: Risk factors, immune profile and clinical outcomes of food sensitized–tolerant infants. Allergy. 2020;75:1404–13.
Williams HC, Jburney PG, Pembroke AC, Hay RJ, Party ADDCW. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. British J Dermatol. 1994;131:406–16.
Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. European Respir J. 1995;8:483.
Dharma C, Lefebvre DL, Tran MM, Lu Z, Lou WYW, Subbarao P, et al. Diagnosing atopic dermatitis in infancy: Questionnaire reports vs criteria-based assessment. Paediatr Perinat Epidemiol. 2018;32:556–67.
Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994:1–407.
Prasodjo A, Pfeiffer CM, Fazili Z, Xu Y, Liddy S, Yolton K, et al. Serum cotinine and whole blood folate concentrations in pregnancy. Annals Epidemiol. 2014;24:498–503.e1.
Mourino N, Pérez-Ríos M, Santiago-Pérez MI, Lanphear B, Yolton K, Braun JM. Secondhand tobacco smoke exposure among children under 5 years old: questionnaires versus cotinine biomarkers: a cohort study. BMJ Open. 2021;11:e044829.
Medicine Io, Council NR. Weight Gain During Pregnancy: Reexamining the Guidelines. Rasmussen KM, Yaktine AL, editors. Washington, DC: The National Academies Press; 2009;868.
Heffernan AL, Aylward LL, Toms LML, Eaglesham G, Hobson P, Sly PD, et al. Age-related trends in urinary excretion of Bisphenol A in Australian children and adults: evidence from a pooled sample study using samples of convenience. J Toxicol Environ Health A. 2013;76:1039–55.
Liu X Chapter 9 - Generalized estimating equations (GEEs) models. In: Liu X, editor. Methods and Applications of Longitudinal Data Analysis. Oxford: Academic Press; 2016;281–308.
Spanier AJ, Kahn RS, Kunselman AR, Schaefer EW, Hornung R, Xu Y, et al. Bisphenol A exposure and the development of wheeze and lung function in children through age 5 years. JAMA Pediatr. 2014;168:1131–7.
Harrell FE General aspects of fitting regression models. In: Harrell JFE, editor., Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Cham: Springer International Publishing; 2015. pp. 13–44.
Arel-Bundock V (2023). marginaleffects: Predictions, Comparisons, Slopes, Marginal Means, and Hypothesis Tests. (0.17.0) Available from: https://CRAN.R-project.org/package=marginaleffects.
Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics Med. 2002;21:1539–58.
Metten M-A, Costet N, Multigner L, Viel J-F, Chauvet G. Inverse probability weighting to handle attrition in cohort studies: some guidance and a call for caution. BMC Med Res Methodol. 2022;22:45.
Petersen GL, Jørgensen TSH, Mathisen J, Osler M, Mortensen EL, Molbo D, et al. Inverse probability weighting for self-selection bias correction in the investigation of social inequality in mortality. Int J Epidemiol. 2024;53:dyae097.
Vanderweele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Annals Intern Med. 2017;167:268–74.
Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect.128:047004.
Keil A (2021). qgcompint: Quantile G-Computation Extensions for Effect Measure Modification. (0.7.0) Available from: https://CRAN.R-project.org/package=qgcompint.
R Core Team. (2023). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.R-project.org/.
Whyatt RM, Perzanowski MS, Just AC, Rundle AG, Donohue KM, Calafat AM, et al. Asthma in inner-city children at 5–11 years of age and prenatal exposure to phthalates: The Columbia Center for Children’s Environmental Health Cohort. Environ Health Perspect. 2014;122:1141–6.
Foong RE, Franklin P, Sanna F, Hall GL, Sly PD, Thorstensen EB, et al. Longitudinal effects of prenatal exposure to plastic-derived chemicals and their metabolites on asthma and lung function from childhood into adulthood. Respirology. 2022;28:236–46.
Jøhnk C, Høst A, Husby S, Schoeters G, Timmermann CAG, Kyhl HB, et al. Maternal phthalate exposure and asthma, rhinitis and eczema in 552 children aged 5 years; a prospective cohort study. Environ Health. 2020;19:32.
Ait Bamai Y, Miyashita C, Araki A, Nakajima T, Sasaki S, Kishi R. Effects of prenatal di(2-ethylhexyl) phthalate exposure on childhood allergies and infectious diseases: The Hokkaido Study on Environment and Children’s Health. Sci Total Environ. 2018;618:1408–15.
Casas M, Gascon M. Prenatal exposure to endocrine-disrupting chemicals and asthma and allergic diseases. J Investig Allergol Clin Immunol. 2020;30:215–28.
Smit LAM, Lenters V, Høyer BB, Lindh CH, Pedersen HS, Liermontova I, et al. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy. 2015;70:653–60.
Granum B, Oftedal B, Agier L, Siroux V, Bird P, Casas M, et al. Multiple environmental exposures in early-life and allergy-related outcomes in childhood. Environ Int. 2020;144:106038.
Bloom MS, Clark JM, Pearce JL, Ferguson PL, Newman RB, Roberts JR, et al. Impact of skin care products on phthalates and phthalate replacements in children: the ECHO-FGS. Environ Health Perspect. 132:097001.
Wang IJ, Lin C-C, Lin Y-J, Hsieh W-S, Chen P-C. Early life phthalate exposure and atopic disorders in children: A prospective birth cohort study. Environ Int. 2014;62:48–54.
Lin L-Y, Tsai M-S, Chen M-H, Ng S, Hsieh C-J, Lin C-C, et al. Childhood exposure to phthalates and pulmonary function. Sci Total Environ. 2018;615:1282–9.
Rodgers A, Ezzati M, Vander Hoorn S, Lopez AD, Lin R-B, Murray CJL, et al. Distribution of major health risks: findings from the global burden of disease study. PLOS Med. 2004;1:e27.
Sathyanarayana S, Alcedo G, Saelens BE, Zhou C, Dills RL, Yu J, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expos Sci Environ Epidemiol. 2013;23:378–84.
Vilcins D, Sly PD. Asthma and Allergy. Textbook of Children’s Environmental Health. 2nd ed: Oxford University Press; 2024. p. 650-60.
Zhou S, Han M, Ren Y, Yang X, Duan L, Zeng Y, et al. Dibutyl phthalate aggravated asthma-like symptoms through oxidative stress and increasing calcitonin gene-related peptide release. Ecotoxicol Environ Saf. 2020;199:110740.
Jahreis S, Trump S, Bauer M, Bauer T, Thürmann L, Feltens R, et al. Maternal phthalate exposure promotes allergic airway inflammation over 2 generations through epigenetic modifications. J Allergy Clin Immunol. 2018;141:741–53.
Larsen ST, Nielsen GD. Structure-activity relationship of immunostimulatory effects of phthalates. BMC Immunol. 2008;9:61.
Larsen ST, Hansen JS, Thygesen P, Begtrup M, Poulsen OM, Nielsen GD. Adjuvant and immuno-suppressive effect of six monophthalates in a subcutaneous injection model with BALB/c mice. Toxicology. 2001;169:37–51.
Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–8.
Valle SOR, Kuschnir FC, Solé D, Silva MAV, Silva RI, Da Cunha AJLA. Validity and Reproducibility of the Asthma Core International Study of Asthma and Allergies in Childhood (ISAAC) Written Questionnaire Obtained by Telephone Survey. J Asthma. 2012;49:390–4.
Acknowledgements
The authors thank the Barwon Infant Study participants for their valuable contribution. The establishment work and infrastructure for the BIS was provided by the Murdoch Children’s Research Institute, Deakin University and Barwon Health. Subsequent funding was secured from the National Health and Medical Research Council of Australia, the Jack Brockhoff Foundation, the Scobie Trust, the Shane O’Brien Memorial Asthma Foundation, the Our Women’s Our Children’s Fundraising Committee Barwon Health, the Shepherd Foundation, the Rotary Club of Geelong, the Ilhan Food Allergy Foundation, GMHBA Limited and the Percy Baxter Charitable Trust, Perpetual Trustees, and the Minderoo Foundation. In-kind support was provided by the Cotton On Foundation and CreativeForce. Research at Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program. Data collection for the HOME Study phases represented in this analysis was supported by funding from the National Institutes of Health P01 ES011261, R01 ES014575, R01 ES024381. The data used in this report is supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under the following awards: U24OD023382 [Data Analysis Center]; U2COD023375 [Coordinating Center]; U24OD023319 [Participant Reported Outcomes (PRO) Core]; UH3OD023251, UH3OD023320, UH3OD023332, UH3OD023287, UH3OD023253, UH3OD023248, UH3OD023313, UH3OD023328, UH3OD023318, UH3OD023279, UH3OD023289, UH3OD023282, UH3OD023290, UH3OD023365, UH3OD023244, UH3OD023275, UH3OD023271, UH3OD023285, UH3OD023347, UH3OD023389, UH3OD023344, UH3OD023342, UH3OD023288, UH3OD023349, UH3OD023286, UH3OD023348, UH3OD023272, UH3OD023249, UH3OD023305, UH3OD023268, UH3OD023337 [ECHO Cohorts]; and U2CES026544, U2CES030857, U2CES026542, U2CES026533, U2CES026561, U24ES026539, U2CES026555 [Human Health Exposure Analysis Resource Core]. These data are from the second public release from this ongoing study. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the ECHO Cohort investigators. We acknowledge NICHD DASH for providing the Environmental influences on Child Health Outcomes (ECHO)-wide Cohort - 2nd Release data that was used for this research. We thank the CHILD Cohort Study (CHILD) participant families for their dedication and commitment to advancing health research. CHILD was initially funded by CIHR and AllerGen NCE. Visit CHILD at childstudy.ca
Funding
TBON was supported by an Australian Government Research Training Program (RTP) Scholarship and a small grant award from the University of Queensland—Child Health Research Centre. PDS is a leadership Fellow (L3) of the National Health and Medical Research Council. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Consortia
Contributions
TBON, NL, PS, ALP, AC, and DV all contributed to the conceptualisation and methodology of this study. NL, PS, ALP, AC, and DV supervised the project. TBON conducted the formal analysis and drafted the original manuscript. All authors and consortia members were involved in data acquisition, interpretation of findings, and the review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Joseph Braun was compensated for serving as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water. All other authors declare they have no conflicts of interest related to this work to disclose.
Ethical approval
All participants in the included cohorts provided written informed consent before enrolment. Ethical approval for each study was obtained from the relevant institutional review boards: the Barwon Infant Study was approved by the Barwon Health Human Research Ethics Committee (HREC 10/24); the HOME Study was approved by the Institutional Review Boards of Cincinnati Children’s Hospital Medical Centre, cooperating delivery hospitals, and the U.S. Centre for Disease Control and Prevention (CDC); local Research Ethics Boards approved the CHILD Cohort Study at participating institutions; and the Western Institutional Review Board approved the ECHO-wide Cohort Protocol (version 1.2). All study procedures were conducted in accordance with applicable institutional and national ethical guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boissiere-O’Neill, T., Lazarevic, N., Sly, P.D. et al. Phthalates and bisphenols early-life exposure, and childhood allergic conditions: a pooled analysis of cohort studies. J Expo Sci Environ Epidemiol (2025). https://doi.org/10.1038/s41370-025-00790-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-025-00790-2