Abstract
Background
Development of bronchopulmonary dysplasia (BPD) in premature neonates is associated with infection and inflammation. Both Ureaplasma parvum and Ureaplasma urealyticum are associated with BPD. We examined whether there is a difference in pathogenicity between the two species
Methods
Tracheal aspirates of 25 preterm neonates were analyzed for bacterial presence and inflammatory mediators. Alveolar epithelial cells were infected with U. parvum and U. urealyticum strains to assess inflammatory mediators, cell death and oxidative stress.
Results
U. parvum was detected in 2/25 and U. urealyticum in another 3/25 neonates. E. coli was co-detected in 3/5 Ureaplasma-positive samples. U. parvum-positive samples contained high IL-6, IL-8 and CXCL5. U. urealyticum-positive samples also contained high IL-6 and IL-8, but low CXCL5, and high CXCL1 and CCL2. Five-to-ten-fold higher IL-6 and two-fold higher IL-8 levels were detected in U. parvum-infected cell cultures than U. urealyticum, whereas apoptotic cell death was detected in U. urealyticum-infected cultures. Infection with both species induced ROS.
Conclusion
Both Ureaplasma species may contribute to inflammation and cell damage, via oxidative stress, as observed in BPD, yet through different mechanisms. U. parvum infection induces a strong pro-inflammatory mediator response in alveolar epithelial cells while U. urealyticum infection results in cell death.
Impact
-
U. urealyticum and U. parvum can contribute to the inflammation and cell damage seen in chronic lung disease through the secretion of inflammatory mediators.
-
The two species differ in their mechanism of action: U. parvum infection induces a strong pro-inflammatory mediator response in alveolar epithelial cells while U. urealyticum infection results in epithelial cell death.
-
Our data provide new insights into the role of Ureaplasma in the development of chronic lung disease in premature infants.
Similar content being viewed by others
Introduction
Ureaplasma spp. are atypical bacteria that belong to the Mycoplasmatacae family, which members are marked by the lack of a cell wall.1 The absence of a cell wall has implications for treatment, as it makes Ureaplasma spp. insensitive to beta-lactam antibiotics. Human Ureaplasma has been separated into two species with 14 known serovars: U. parvum includes serovars 1, 3, 6, and 14, while U. urealyticum includes serovars 2, 4, 5, 7–13.2,3,4
Ureaplasma spp. commonly colonize the mucous membranes of the lower urogenital tracts in both asymptomatic men and women, with about 40% of genitourinary samples from these individuals testing positive.1,5 In pregnant women, Ureaplasma spp. can ascend and cause chorioamnionitis, preterm labor, and preterm birth.6,7,8,9,10 Both animal and clinical studies have shown that intra-amniotic Ureaplasma spp. infections can contribute to preterm labor and delivery. For example, intra-amniotic inoculation of U. parvum clinical isolates in pregnant mice and sheep resulted in preterm births.11,12,13 Similarly, in humans, the intra-amniotic presence of U. urealyticum strongly correlated with preterm labor and preterm delivery.14,15 Vertical transmission of U. urealyticum from mother to fetus can also occur in utero, with the fetus being exposed to the bacteria through aspiration of amniotic fluid, thereby directly exposing fetal lungs to Ureaplasma spp.11,16,17 This has been observed in animal studies, where Ureaplasma spp. were isolated from the lungs of newborn mice and lambs.11,12,13 Ureaplasma spp. have also been isolated from respiratory tract samples of human preterm newborns, such as tracheal aspirates and bronchoalveolar lavage samples.2,11,18,19 The rates of prenatal and perinatal transmission, as well as subsequent fetal colonization and infection, appear to be inversely proportional to gestational age.20
Ureaplasma spp. in neonatal lungs is linked to inflammation and disruptions in lung development, potentially leading to bronchopulmonary dysplasia (BPD), a common complication in premature infants and a major cause of life-long morbidity.21,22,23,24,25 BPD is characterized by diffuse structural lung damage, neutrophilic inflammation, and fibrosis of the parenchyma of the neonatal lungs.26 The pathogenesis of BPD suggests that lung injury is frequently initiated by intra-uterine infection and a dysregulated inflammatory response.11,16,27
It is hypothesized that the presence of Ureaplasma spp. in the neonatal lungs can result in an augmented inflammatory response that contributes to the development of BPD. Lambs born prematurely upon intra-uterine Ureaplasma spp. injection, showed Ureaplasma infection in the developing lung, with increased levels of IL-1β, IL-6, and IL-8, as well as neutrophil and monocyte infiltration.28,29,30 Similar neutrophil and monocyte/macrophage influx and increased levels of inflammatory TNFα were observed in the lungs of U. urealyticum-positive preterm neonates.31,32 Additionally, in vitro and in vivo studies demonstrated that Ureaplasma spp. induces apoptosis in human type II lung epithelial cells, human pulmonary microvascular endothelial cells, and macrophages, contributing to cellular damage and pulmonary fibrosis.24,33,34,35 Studies in preterm infants and animal models show evidence of interstitial fibrosis and cell apoptosis in Ureaplasma infected lungs, supporting the idea that Ureaplasma infection worsens the inflammatory environment, potentially exacerbating BPD.24,30,31,32,33
Although studies indicated an association between the presence of Ureaplasma spp. and BPD development36 a species-specific contribution remains unclear due to differences in study populations and methods. Some studies highlight U. parvum,18,22, others U. urealyticum,14,15,19, while others find no difference.37,38 In addition, limited in vitro data exist on qualitative and quantitative differences in host cell responses between Ureaplasma species. To address this, we compared the pulmonary pathogenicity and host responses of the two Ureaplasma species, U. urealyticum and U. parvum, using neonatal tracheal aspirates and a human lung epithelial cell infection model.
Materials and methods
Clinical background
Tracheal aspirate samples were collected randomly from intubated patients at the Neonatal Intensive Care Unit (NICU) of a tertiary care facility between April 2019 and May 2024. To optimally ventilate intubated neonates, removal of obstructive sputum by rinsing the tube and trachea with 1–2 mL NaCl 0.9% with subsequent aspiration of fluid is part of standard care. These tracheal aspirates were collected in a sterile tube and transported to the laboratory. Aspirates were processed immediately, and supernatants were stored in a freezer until further analysis. The study was approved by the hospital’s Medical Research Ethics Committee (ID MEC-2020-0159). Parents provided informed consent to use leftover materials, including tracheal aspirates.
Detection of pathogens in tracheal aspirates
Total DNA was extracted from 200 μL tracheal aspirates, that were spiked with a known concentration of seal herpesvirus 1 (PhHV-1) DNA to control for nucleic acid extraction efficiency. Presence of bacteria was assessed using a single-plex PCR with pan-bacterial 16S primers. Ureaplasma species, E. coli, K. pneumoniae, and S. agalactiae (Group B Streptococcus) were identified by multiplex quantitative real-time PCR using the following reaction mixture: 10 μL iQ multiplex powermix (Bio-Rad), 20x primer/probe mix, and 5 μL DNA template in a total volume of 20 μL. S. aureus was detected by quantitative real-time PCR single-plex: 10 μL Taqman universal master mix, 0.5 µM primers, 0.25 µM probe, and 1 µL DNA template were mixed in a total volume of 20 µL. The PCR cycling conditions were 2 min at 95 °C; 40 cycles of 15 s at 95 °C, 1 min at 60 °C. The primer and probe sequences are shown in Table S1.
U. urealyticum and U. parvum
U. urealyticum and U. parvum reference strains were obtained from the American Tissue Culture Collection (ATCC); the strains are as follows: U. urealyticum #1-ATCC 27618 (formerly 50782 T or 50912, serovar 8), U. urealyticum #2-ATCC 27817 (serovar 5), U. parvum #3-ATCC 27815 (serovar 3), U. parvum #2-ATCC 700970 (serovar 3). U. urealyticum #3-2983, U. parvum #1-2990, and U. parvum #4-3763 are clinical isolates from pregnant women (kind gift of Dr. Biernat Sudolska; Jagiellonian University Medical College, Krakow, Poland). Ureaplasma isolates were cultured in modified 10B broth (referred to as “m10B broth”) containing 2% PPLO (pleuropneumonia-like organism medium; Becton, Dickinson & Company, Franklin Lakes, NJ), 2.2% Yeast extract (Becton, Dickinson & Company, Franklin Lakes, NJ), 20% (v/v) heat-inactivated horse serum (Bio-West; Nuaillé, France), 0.4% L-Cysteine-HCl (Sigma; Steinheim, Germany), 4% urea (VWR; Solon), 1.6% Putrescine dihydrochloride (Sigma), 0.025% phenol red, 0.5% Isovitalex Enrichments (BD) and 105 U/100 mL Penicillin (Hospital Pharmacy, Erasmus MC) were dissolved in MilliQ; the medium was adjusted to pH 6.0. After filtration through a 0.22 μm membrane (Millipore 0.22 µM Vacuum filter system), the medium was adjusted to pH 6.5. Ureaplasma strains were cultivated in m10B broth at 37 °C with 5% CO2 until the medium changed from yellow to pink-red. The Ureaplasma were harvested, and aliquots were stored at \(-\)80 °C until use. The bacterial concentrations of the suspensions were confirmed with colony-forming units (CFU) onto mA8 agar plates. To generate mA8 agar plates, first mA8 basal medium was prepared: 7.5 g PPLO, 2.5 g tryptic soy broth, 2 g NaCl, 1 g KH₂PO₄, 7 g agar, and 385 mL Milli-Q water were mixed (pH 6.0). After sterilization, the basal broth was cooled to 55 °C, and the following supplements were added: 5 ml yeastolate (0.25 g/mL); 100 mL heat-inactivated horse serum; 0.5 mL filter-sterilized L-cysteine (0.1 g/mL); 1.25 mL filter-sterilized urea (0.4 g/mL); 2.5 mL isovitalex; 5 mL filter-sterilized putrescine (0.2 g/mL); 2.5 mL Penicillin (2×105 U/mL) and 0.5 mL filter-sterilized CaCl₂·2H₂O (0.16 g/mL).
Heat inactivation of Ureaplasma species
An aliquot of Ureaplasma stock was resuspended in PBS/2 mM MgCL2, diluted to 1 × 107 CFU/mL, and incubated at 56 °C for 45 min. After cells were cooled to RT, the Ureaplasma bacteria were centrifuged at 13,000 × g for 10 min. The supernatants were removed, and the pellets were washed twice with PBS, then resuspended in broth and homogenized. Heat-inactivated Ureaplasma bacteria were subsequently stored at –80 °C until further use.
Ureaplasma infection of human alveolar epithelial cells
Human alveolar epithelial cells (ATCC, CCL-185) were cultured at 37 °C and 5% CO2 in RPMI-1640 medium (Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum (RPMI/10% FCS) in the absence of antibiotics. The cells were seeded in a 96-well flat-bottom plate (50,000 cells/well; Greiner, Frickenhausen, Germany) and grown to 80% confluence at 37 °C and 5% CO2. The cells’ confluent monolayer was confirmed by observation with a Nikon TMS inverted microscope (Hicksville, NY). Aliquots of Ureaplasma bacteria were diluted in RPMI-1640 medium to a concentration of 5 × 105 and 5 × 106 CFU/mL to reach multiplicities infection (MOI) 1 and 10, respectively. The epithelial cells were initially washed with PBS and then infected with live U. urealyticum or U. parvum, as well as heat-inactivated U. urealyticum or U. parvum, at different MOI for 24 h at 37 °C and 5% CO2. Cells were incubated with medium or FSL-1 as negative and positive controls, respectively. All experimental conditions were performed in triplicate.
Ureaplasma infection of alveolar epithelial cells in the presence of E. coli LPS
Alveolar epithelial cells were cultured with indicated U. urealyticum or U. parvum strains at MOI 10 or MOI 100 in the absence or presence of indicated concentrations of LPS (LPS from Escherichia coli O5:B55, InvivoGen, France). After 24 h of culture, supernatants were harvested, and the levels of IL-6 and IL-8 were determined by ELISA. Additionally, after 48 h of culture, the cell damage was determined by quantifying the amounts of lactate dehydrogenase (LDH) released into the supernatants.
Determination of cytokine concentrations by ELISA and LEGEND-plex assay
Interleukin (IL)-6, IL-8, IL-1β, IL-10 and tumor necrosis factor (TNF)-α concentrations in the supernatants were measured by ELISA using specific antibody pairs (Thermo Fisher Scientific, Waltham, MA; BD Biosciences, Heidelberg, Germany), according to the manufacturer’s instructions. 3, 3′, 5, 5′-Tetramethybenzidine (Sigma-Aldrich) was used as a substrate. The optical density was measured at OD450nm using a microplate reader (SpectraMax iD3, Molecular Devices, San José, CA). A Human Proinflammatory Chemokine Panel 1 (BioLegend, San Diego, CA) was used to measure the concentration of MCP-1 (CCL2), RANTES (CCL5), IP-10 (CXCL10), Eotaxin (CCL11), TARC (CCL17), MIP-1α (CCL3), MIP-1β (CCL4), MIG (CXCL9), MIP-3α (CCL20), ENA-78 (CXCL5), GROα (CXCL1), I-TAC (CXCL11) and IL-8 (CXCL8) in the supernatants, according to the manufacturer’s instructions. The samples were acquired using a FACSCanto™ II flow cytometer (BD Bioscience, Heidelberg, Germany). Data were analyzed using LEGENDplex V8.0 Data Analysis Software (Biolegend, San Diego, CA).
Evaluation of cell death
Human alveolar epithelial cells were seeded in a 96-well flat-bottom plate at a density of 50,000 cells per well or on 8-well cell culture slides (MatTek, Ashland, MA) and grown to 80% confluence at 37 °C and 5% CO2. The confluent epithelial monolayers were gently washed with PBS, and live or heat-inactivated U. urealyticum or U. parvum were added to the cells at MOI 100. A549 cells incubated with medium or 10% DMSO were used as negative and positive control, respectively. After 24 h or 48 h, supernatants were harvested to determine the amounts of LDH released by damaged cells using the CyQUANT LDH Cytotoxicity Assay kit (Thermo Fisher Scientific). The percentage of damaged epithelial cells was calculated according to the manufacturer’s instructions. To evaluate induction of apoptosis, cells were stained with Annexin-V antibody (Thermo Fisher Scientific), imaged, and quantified with ImageJ. Additionally, cells were washed with PBS and incubated with the LIVE/DEAD fixable Violet Dead Cell Stain (Thermo Fisher Scientific) for 30 min at 4 °C, and the frequency of dead cells was assessed using Canto-II flow cytometry (BD Bioscience, Heidelberg, Germany). A minimum of 50,000 events was acquired and analyzed with FACSDiva 8.0.1 software (BD Bioscience).
Evaluation of oxidative stress markers–Reactive Oxygen Species (ROS), Glutathione Peroxidase (GSH-Px) activity, and Malondialdehyde (MDA) concentration
To assess ROS activity, A549 cells were seeded at 50,000 cells/well onto black, clear-bottom 96-well plates in phenol red free media and incubated for 24 h at 37 °C with 5% CO2. Cells were stained with DCFDA for 45 min. After washing with PBS, live or heat-inactivated U. urealyticum or U. parvum were added to the cells at MOI 10 for 3 h. As a positive control of ROS induction, cells were exposed to 200 µM hydrogen peroxide. ROS activity was detected by fluorescence spectroscopy with excitation/emission at 485/535 nm and expressed as fluorescence intensity.
GSH-Px activity and MDA concentration were assessed using commercial kits (Thermo Fisher Scientific). In brief, A549 cells were seeded at 50,000 cells/well in 96 well flat-bottom plates and incubated for 24 h at 37 °C with 5% CO2. U. urealyticum or U. parvum were added to the cells at MOI 100. After 24 h incubation, the cells were washed with PBS, incubated with lysis buffer, and GSH-Px and MDA were assessed according to manufacturer’s instructions. GSH-Px activity is expressed as U/mg protein.
Statistical analysis
Statistical analyses were performed using Prism 9.0 (GraphPad Software Inc., San Diego, CA). Data are presented as means ± standard error of the mean (SEM) or standard deviation (SD) from one experiment, as indicated with usually three technical repeats within each experiment. Experiments were repeated at least twice. Continuous variables are presented as mean and standard deviation, or median and interquartile range (IQR). Data were checked for normality, and statistically significant differences between groups were determined by one-way ANOVA with post-hoc Dunnett’s multiple comparisons test. Results were considered statistically significant when p < 0.05.
Results
Incidence of Ureaplasma in tracheal aspirates of preterm neonates
A total of 25 tracheal aspirate samples from 25 preterm infants (gestational age <36 weeks) admitted to the NICU were collected for this exploratory study. Demographic data are summarized in Table 1. The median gestational age of these patients was 26 weeks\(\,+\) 5 days (interquartile range (IQR) 25 weeks \(+\) 4 days – 29 weeks \(+\) 1 days) and birth weight was 910 g (IQR 670–1290 g). The median number of days on a ventilator was 10 days (IQR 5–21 days). Of the 25 neonates, 18 developed BPD. Bacteria were detected in all samples, with targeted analysis revealing the presence of Ureaplasma spp., Klebsiella pneumoniae, Staphylococcus aureus, and Escherichia coli. In none of the samples GBS was detected. Ureaplasma spp. were detected in five samples: two were positive for U. parvum, and three were positive for U. urealyticum (Table 1). K. pneumoniae and S. aureus were detected in six (33.3%) and eight (44.4%) samples respectively. More than one pathogen was detected in 13 (52%) samples (Fig S1). In Ureaplasma-positive infants, the median gestational age was 26 weeks\(\,+\) 5 days (IQR 26 weeks \(+\) 2 days − 30 weeks \(+\) 3 days) and birth weight was 950 g (IQR 577.5−1655 g). Only the number of days on ventilation was higher in neonates who were Ureaplasma-positive compared to all neonates (19 days; IQR 8.5–67.75 days versus 10 days; IQR 5–21 days). Notably, four out of five Ureaplasma-positive infants developed BPD. Of the investigated pathogens, only E. coli (60%, 3/5 cases) was detected in Ureaplasma-positive patients.
Inflammatory mediators in Ureaplasma-positive tracheal aspirates
Next, we aimed to determine whether the presence of U. parvum creates a distinct inflammatory environment compared to U. urealyticum. Here to comparative analysis of the levels of inflammatory cytokines and chemokines in the airway supernatants was performed. Among the five Ureaplasma-positive samples, the highest levels of IL-6 and IL-8 were detected in the samples from neonate #2 (U. urealyticum) and neonate #3 (U. parvum) (Fig. 1). The neutrophil chemoattractant CXCL1 and monocyte attractant CCL2 were highest in the U. urealyticum-positive samples neonate #2 and neonate #7, while the neutrophil-activating CXCL5 was highest in U. parvum-positive sample neonate #3. This small data set suggests that U. parvum and U. urealyticum may exist in different inflammatory microenvironments.
Heatmap showing changes in abundances of six quantified cytokines and chemokines in tracheal aspirates from five Ureaplasma-positive preterm neonates. Pro-inflammatory cytokines and chemokines were determined by ELISA and Legend-plex assay. Individual protein abundance per milliliter of tissue was z-score normalized. Blue indicates lower protein concentrations while yellow represents higher protein concentrations (see scale). Dark blue squares denote the presence of U. urealyticum in a patient sample; and green squares indicate the presence of U. parvum. Black boxes indicate whether a neonate developed BPD. One sample ran out (gray squares) and could not be tested.
U. parvum induces stronger alveolar epithelial immune cell responses than U. urealyticum
We observed differences in the inflammatory responses between U. urealyticum and U. parvum in clinical samples, potentially influenced by different cell types. Since respiratory epithelial cells are the first to encounter Ureaplasma spp., we compared the inflammatory responses of these cells to infection by U. urealyticum and U. parvum in a controlled in vitro model. Supernatants were collected from epithelial cells infected with three different isolates of each species.
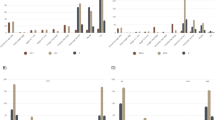
The production of IL-6 was 5- to 8-fold higher in the supernatants of epithelial cells infected with U. parvum strains compared with U. urealyticum strains at the same MOI (Figs. 2a, d and S2). Similar observations were made for IL-8, where cytokine production was higher following incubation with U. parvum compared to U. urealyticum, except in the case for U. parvum strain #3 at MOI 1 (Fig. 2b, e). Given that Ureaplasma infection of the respiratory tract strongly promotes neutrophil influx,39,40 we also determined the levels of neutrophil-attracting and -activating chemokines CXCL5 and CXCL1, in the supernatants. Both U. parvum and U. urealyticum strains, at MOI 1 and MOI 10, significantly increased the production of CXCL5 (Fig. 2c, f) and CXCL1 (Fig. 2g, j) by epithelial cells compared to uninfected control cells. However, the levels of these chemokines were not significantly different between U. urealyticum-inoculated and U. parvum-inoculated cultures. All U. parvum strains at MOI 10 significantly increased CCL2 production compared to uninfected controls, but this was only observed for strain #1 of the U. urealyticum strains (Fig. 2h, k). Infection with either U. parvum or U. urealyticum strains resulted in low levels of CCL20 (Fig. 2i, l). No differences were found between reference and clinical strains in their ability to induce pro-inflammatory cytokines in alveolar epithelial cells (Fig. 2). These data indicate that U. parvum and U. urealyticum upregulate expression of different sets of inflammatory mediators in human alveolar epithelial cells.
Alveolar epithelial cells were inoculated with three different strains of U. urealyticum (UU) or U. parvum (UP) at MOI 1 and MOI 10. Three strains of each species were tested: UP#1 = U. parvum clinical isolate 2990, UP#2 = U. parvum ATCC 700970, UP#3 = U. parvum ATCC 27815; UU#1 = U. urealyticum ATCC 27618, UU#2 = U. urealyticum ATCC 27817, UU#3 = U. urealyticum clinical isolate 2983. After 24 h, the levels of pro-inflammatory cytokines and chemokines in these supernatants were determined by a, d ELISA (IL-6) and b, c, e–l LEGEND-plex, respectively. Bars represent the mean ± SD of triplicate cultures. Representative results of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 (One-way ANOVA followed by Dunnett’s multiple comparison test).
U. urealyticum infection drives more alveolar cell death than U. parvum
We subsequently investigated whether the alveolar cell death observed in vivo in Ureaplasma-positive airways24,33 results from direct Ureaplasma infection. First, epithelial cell damage was assessed using an LDH assay. As shown in Fig. 3a, U. urealyticum induced more cell damage, leading to greater LDH release than U. parvum. The mean percentage of cytotoxicity in U. urealyticum-infected cultures was 10.6% ± 1.52, while in U. parvum-containing cultures it was 2.7% ± 0.58. LDH release induced by U. parvum was not significantly different from that of uninfected control cells. Similar results were obtained when using a viability dye and flow cytometry to assess cell death (Fig. 3b). Infection with U. urealyticum at MOI 100 resulted in an average of 13.8% (±1.07) dead cells, while infection with U. parvum resulted in 50% fewer dead cells, with an average of 6.5% (±2.39) dead cells.
Alveolar epithelial cells were inoculated with the indicated U. urealyticum (UU) or U. parvum (UP) strains at a MOI 100. After a 48 h incubation, cells and supernatants were harvested. a Supernatants were analyzed for the amounts of LDH release, which indicates cell death. Bars represent the mean ± SEM of triplicate cultures. b Cells were incubated with a LIVE/DEAD marker, and the frequency of dead cells was assessed using flow cytometry. Bars show the mean ± SEM of duplicate cultures. Representative results of three independent experiments. UP#1 = U. parvum clinical isolate 2990, UP#2 = U. parvum ATCC 700970, UP#3 = U. parvum ATCC 27815, UP#4 = U. parvum clinical isolate 3763; UU#1 = U. urealyticum ATCC 27618, UU#2 = U. urealyticum ATCC 27817, UU#3 = U. urealyticum clinical isolate 2983. * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 (One-way ANOVA followed by Dunnett’s multiple comparison test).
Differential effects of Ureaplasma species on inflammation and cytotoxicity are maintained and enhanced by E. coli-derived LPS
Since E. coli was co-detected in three of the five Ureaplasma-positive neonatal samples, we speculated that the presence of Ureaplasma might prime the innate immune system to mount a stronger response to other bacterial infections. To test this, we performed infection assays in the presence of LPS derived from E. coli. The concentration of LPS used in these assays reflected the mean number of E. coli bacteria detected in the neonatal samples. The administration of LPS to epithelial cells resulted in a concentration-dependent increase in IL-6 and IL-8 production (Fig. 4a). The presence of a low amount of LPS, i.e., 10 ng/mL, increased IL-6 levels induced by U. parvum by three-fold, while IL-8 increased only slightly (Fig. 4a). IL-8 was significantly increased when higher amounts of LPS, i.e., 500 ng/mL, were present. Co-activation of alveolar epithelial cells with U. urealyticum and LPS also increased IL-6 and IL-8 secretion, but the cytokine levels did not reach those induced by U. parvum. In fact, they remained 2.5 to 4.5-fold lower compared to the levels measured in U. parvum-infected cultures (Fig. 4a; using 10 ng/mL LPS, mean IL-6: 42.3 vs 152.6 pg/mL; and mean IL-8: 273.9 vs 1215.6 pg/mL. Using 100 ng/mL LPS, mean IL-6: 78.6 vs 200.5 pg/mL; and mean IL-8: 475.7 vs 1157.9 pg/mL). U. urealyticum-induced cell cytotoxicity was enhanced by LPS: a mean of 11.2% cytotoxicity was measured in the presence of LPS versus 7.4% in its absence (Fig. 4b). In contrast, addition of LPS to U. parvum-infected cultures did not increase cell cytotoxicity, with 1.7% compared to 4.1% without LPS (Fig. 4b). These data indicate that the presence of other microorganisms, such as E. coli, potentiates U. parvum-induced inflammation and U. urealyticum-induced cell death.
Alveolar epithelial cells were activated with UP#1 = U. parvum clinical isolate 2990 or UU#1 = U. urealyticum ATCC 27618 at a MOI 10 or MOI 100 in the absence or presence of indicated concentrations of LPS. a After 24 h culture at MOI 10, supernatants were harvested, and the levels of IL-6 and IL-8 were determined by ELISA. b After 48 h at MOI 100, cell damage was determined by quantifying the amounts of LDH released into the supernatants. Bars show the mean ± SEM of triplicate cultures. Representative results of two independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 (One-way ANOVA followed by Dunnett’s multiple comparison test).
Ureaplasma infection induced oxidative stress
Inflammation and cell death are closely linked to oxidative stress. Since oxidative stress is also frequently observed in BPD,41 we next examined the potential of U. parvum and U. urealyticum to induce oxidative stress by measuring the generation of ROS, the activity of MDA, and the concentration of the antioxidant GSH-Px. Infection of epithelial cells with either U. parvum or U. urealyticum resulted in robust generation of ROS (Fig. 5a). Vice versa, the levels of GSH-Px were reduced upon infection with U. parvum and U. urealyticum, with most strong effects observed in U. parvum infected cultures (Fig. 5b). GSH-Px activity is higher in U. urealyticum infected lung cells than in U.parvum infected cells. Interestingly, incubation with either Ureaplasma species did not result in lipid peroxidation, as no MDA could be detected (data not shown). This finding indicates that the cell death induced by U. urealyticum is not ferroptosis. Instead, evaluation of Annexin-V revealed that infection with U. urealyticum resulted in the induction of apoptosis. A two- to five-fold increase in Annexin-V positive events was observed in U. urealyticum containing cultures compared to control and U. parvum cultures (Fig. 5c). The limited occurrence of apoptotic events in U. parvum cultures is consistent with the observations made on LDH and cell death (Fig. 3a). Together, these data suggest that oxidative stress triggers the inflammation and cell death induced by U. parvum and U. urealyticum, respectively.
Alveolar epithelial cells were activated with indicated U. urealyticum (UU) or U. parvum (UP) isolates at a MOI 10 or 100. UP#1 = U. parvum clinical isolate 2990, UP#2 = U. parvum ATCC 700970, UP#3 = U. parvum ATCC 27815; UU#1 = U. urealyticum ATCC 27618, UU#2 = U. urealyticum ATCC 27817, UU#3 = U. urealyticum clinical isolate 2983. a To assess ROS generation, cells were stained with DCFDA, and after 3 h culture with Ureaplasma bacteria at MOI 10, ROS was detected by fluorescence spectroscopy with excitation/emission at 485/535 nm. b Cells were cultured with Ureaplasma bacteria at MOI 100, and after 24 h the activity of the antioxidant GSH-Px was determined using a commercial kit, and c the induction of apoptosis was determined by staining cells with Annexin-V antibody. Cells were analyzed by fluorescence microscopy and Annexin-V positive events were quantified using ImageJ. Representative results of two independent experiments. (One-way ANOVA followed by Dunnett’s multiple comparison test).
Oxidative stress, inflammation, and cell death in alveolar epithelial cells is triggered by a secreted factor from Ureaplasma
The inflammatory and cell-damaging effects observed in airway epithelial cells following Ureaplasma infection could be due to direct membrane-membrane interactions or the secretion of factors by Ureaplasma during proliferation in the epithelial cells.42,43 To test the latter, we heat-inactivated the Ureaplasma strains, making them incapable to secrete products. Infection of alveolar cells with either heat-inactivated U. parvum or heat-inactivated U. urealyticum did not lead to the generation of significant amounts of ROS (Fig. 6a, b). Similarly, infection of epithelial cells with heat-inactivated bacteria did not induce significant production of IL-6 (Fig. 6c, d) or IL-8 (Fig. 6e, f). The levels of ROS in the epithelial cells and the cytokines in the culture supernatants were comparable to those in the medium control cultures. Both inflammatory cytokines were only produced by alveolar epithelial cells in response to live, proliferating Ureaplasma spp., with the highest levels of IL-6 and IL-8 found in U. parvum-infected cultures (Fig. 6c, e). Analysis of supernatants for LDH release indicated that U. urealyticum-induced cytotoxicity was also dependent on factors released by live, active bacteria (Fig. 6h). While infection with live U. urealyticum at MOI 100 resulted in 15% dead cells, the frequency of dead cells in cultures with heat-inactivated bacteria was significantly reduced to the level of medium control wells (Fig. 6h). U. parvum strains did not induce cell death (Fig. 6g). Together, these findings suggest that the induction of inflammation and cell damage in epithelial cells is dependent on the proliferation and factors secreted by live U. parvum and U. urealyticum.
Alveolar epithelial cells were inoculated with heat-inactivated or live U. urealyticum or U. parvum strains at indicated MOI. Cells were stained with DCFDA to determine ROS generation after 3 h (a, b). Culture supernatants were harvested after 24 h and 48 h incubation. (c, d) IL-6 and (e, f) IL-8 levels in the culture supernatants were determined by ELISA. g, h Cytotoxicity was determined by quantifying the amount of LDH. Bars show the mean ± SD of triplicate cultures, and one representative of two independent experiments is shown. UP#1 = U. parvum clinical isolate 2990, UP#2 = U. parvum ATCC 700970, UP#3 = U. parvum ATCC 27815; UU#1 = U. urealyticum ATCC 27618, UU#2 = U. urealyticum ATCC 27817, UU#3 = U. urealyticum clinical isolate 2983. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 (One-way ANOVA followed by Dunnett’s multiple comparison test).
Discussion
In this study, we provide novel insights into the differences in immunomodulatory capacities between the two Ureaplasma species associated with the development of BPD in premature infants. A direct comparison of U. urealyticum and U. parvum in a controlled in vitro infection model demonstrated that both Ureaplasma species may contribute to inflammation and cell damage as can be observed in BPD, yet through different mechanisms. We observed a stronger pro-inflammatory mediator response in alveolar epithelial cells infected with U. parvum compared to U. urealyticum. Additionally, more epithelial cell death occurred after incubation with U. urealyticum than with U. parvum. These damaging effects appear to be triggered by oxidative stress and are largely dependent on soluble factors secreted by Ureaplasma. This is evidenced by the observation that heat-inactivated Ureaplasma isolates induced minimal alveolar epithelial cell responses compared to live isolates.
In our cohort of premature neonates with respiratory insufficiency and need for mechanical ventilation, 5 of 25 (20%) neonates had Ureaplasma-positive tracheal aspirates, and all but one eventually developed BPD. This frequency aligns with observational studies showing that Ureaplasma presence in neonatal airways is associated with BPD.20,44,45,46 Early life Ureaplasma spp. exposure and prolonged mechanical ventilation may contribute to BPD development,45 with factors such as serovar virulence, colonization duration, Ureaplasma load, and co-colonization with other bacteria, potentially influencing outcomes.44
Besides Ureaplasma spp., also K. pneumoniae, S. aureus, and E. coli were detected in the lungs of infants, but only E. coli was found in Ureaplasma-positive infants. GBS was not detected in any neonatal airway sample, possibly due to the small cohort. Alternatively, some newborns may have detectable gastro-intestinal carriage without oropharyngeal GBS presence.47 We cannot fully attribute this to a negative GBS carriage status in the mothers. Retrospective analysis indicated that maternal GBS carriage status was negative in 52%, and unknown in 48%, as routine maternal GBS screening is not conducted in the Netherlands.
Analysis of inflammatory mediators in the tracheal aspirates revealed distinct profiles in U. urealyticum-positive airways compared to U. parvum-positive airways, with the most notable differences observed in CXCL1, CXCL5, CCL20, and CCL2. The chemokines CXCL1, CCL20, and CCL2 were most elevated in U. urealyticum-positive samples, while CXCL5 was dominant in U. parvum-positive samples. These differences may reflect the recruitment and activation of different cell types at the sites of U. urealyticum versus U. parvum infection, and consequently, a distinct type of inflammation. At U. urealyticum infection sites, IL-8 and CCL2, derived from alveolar epithelial cells, mediate the recruitment of neutrophils and monocytes. These cells further amplify the inflammation process by secreting CXCL1 and CCL20, which recruit additional leukocytes.48 While CCL20 primarily recruits CCR6+ effector B and T cells, CXCL1 facilitates another wave of neutrophil recruitment and activation for microbial killing. These findings are consistent with previous studies in humans and experimental animal models, which showed that Ureaplasma-positive preterm newborns had higher concentrations of pro-inflammatory mediators, such as IL-8, IL-1β, IL-6, and CXCL1, as well as elevated numbers of neutrophils in their airway samples compared to Ureaplasma-negative newborns.11,28,39,40
In the U. parvum-positive airways, other than high IL-6 and IL-8, high levels of CXCL5 were detected, particularly in patient 3. During bacterial challenge in the airways, CXCL5 is secreted by activated alveolar type II epithelial cells to mediate optimal recruitment and activation of neutrophils at the infection site. However, it has been shown that CXCL5 can impede this process during severe airway infections with Gram-negative bacteria such as E.coli and K. pneumoniae.49,50 CXCL5 can regulate the sequestration in blood and here with bioavailability of CXCL1 and CXCL2, two other neutrophil attractants. In case of high CXCL5 levels, sequestration of CXCL1 and CXCL2 is prevented, resulting in high levels of these chemokines in blood and hereby inversing the chemokine gradient between blood and infected tissue. Consequently, recruitment and activation of neutrophils at the infection site are impaired. Interestingly, E. coli was co-detected with U. parvum in the tracheal aspirate of patient 3. Whether neutrophil influx into the airways of this patient was hampered via the above-described CXCL5 mechanism is unknown, as analysis of the immune composition of the tracheal aspirates was beyond the scope of our explorative study.
Using an in vitro infection model with human alveolar epithelial cells we determined whether differences between U. urealyticum and U. parvum pathogenicity already arise at the epithelial level, the first barrier for pulmonary pathogens. Infection with U. parvum resulted in five-to-eight-fold higher IL-6 and IL-8 production by human epithelial cells than with U. urealyticum. In contrast, no significant differences in CXCL1, CXCL5, and CCL2 levels as found in our neonatal airway samples were detected in vitro. In case of CXCL1, this is likely explained by the fact that this chemokine is mainly expressed by neutrophils and macrophages in response to Ureaplasma. These cells were not present in our in vitro infection model. Also, in vivo, other bacteria such as E. coli can be present. The in vitro experiments testing the influence of E. coli on Ureaplasma infection by adding E. coli-derived LPS indicated that the specific responses induced by either U. parvum (i.e., high levels of pro-inflammatory mediators) or U. urealyticum (i.e., cytotoxicity) were further enhanced. Comparable enhancement of LPS-induced pro-inflammatory cytokine production by Ureaplasma was shown in studies using neonatal monocytes.34,51 Here, co-stimulation of neonatal monocytes with Ureaplasma and LPS maintained and aggravated pro-inflammatory responses. In one of these in vitro studies, responses between a U. parvum serotype 3 strain and a U. urealyticum serotype 8 strain were compared. However, in contrast to our findings, no major overall differences in the cytokine responses induced by these two strains were observed. This could be related to the fact that a different host cell type (i.e., monocytes versus alveolar epithelium) was used than in our study. Alternatively, it may be due to the fact that only one strain of each species was tested. In our study, we tested three different isolates of each species and observed that for certain inflammatory mediators, one of the U. urealyticum isolates (e.g., U. urealyticum #3-2983) induced higher levels than the other isolates tested. Nevertheless, our data obtained ex vivo in neonatal tracheal aspirates and in vitro infection model are in line with previous speculations that Ureaplasma infection might affect immunity to a second microorganism by exacerbating inflammation and promote tissue injury by inducing cell cytotoxicity. Of note, Ureaplasma-positive neonate #2 was the one patient in whom E. coli was not co-detected. Despite that, high levels of pro-inflammatory mediators were measured in the tracheal aspirate. Of the five Ureaplasma-positive neonates, neonate #2 had been longest on a ventilator (i.e., 84 days versus 5-19 days, respectively). There is accumulating evidence that the sensitivity of the neonatal lung to stressors such as mechanical ventilation is increased by pulmonary infection and inflammation.52,53 Indeed, in preterm infants, prolonged ventilation additive to colonization with Ureaplasma spp. has been shown to confer an increased risk of severe chronic lung injury and/or development of BPD.23,32,54,55
Epithelial cultures infected with U. urealyticum showed more cell death than those infected with U. parvum. Ureaplasma presence may damage host cells through inflammation, either via membrane-associated molecules or via soluble factors.4,56,57 A key membrane-associated protein in Ureaplasma spp., multiple-banded antigen (MBA), triggers production of inflammatory mediators by binding to TLR2/1 and TLR2/6 on alveolar epithelial cells and macrophages, leading to IL-1β and TNF production, which are linked with cell damage and lung fibrosis.58 However, the observation that heat-inactivation of the Ureaplasma strains abolished their ability to induce inflammation in alveolar epithelial cells suggests that soluble factors, rather than MBA, are the primary contributors to pathogenicity. The factors in question comprise ammonia, hydrogen peroxidase, and phospholipases. Ammonia is produced from urease metabolism by Ureaplasma spp., which can cause toxicity to host tissues due to a shift in pH. Altered pH and increased ammonia concentrations of the amniotic fluid have been linked to intra-uterine Ureaplasma infections,59 and the ammonia liberated by Ureaplasma may contribute to chronic tissue damage in the fetal lung.60 Moreover, it has been proposed that ammonia can react with water to form ammonium hydroxide, which could in turn promote injury and inflammation of the mucosa. Membrane peroxidation and phospholipid degradation by hydrogen peroxidase and phospholipases also play a role in cell damage. The results obtained on oxidative stress markers suggest that the inflammatory and cytotoxic effects on human lung cells following infection with, respectively, U. parvum and U. urealyticum might be mediated by ROS. However, the involvement of this pathway might be to a different extent in U. parvum than U. urealyticum infections, as shown by the higher activity of the antioxidant GSH-Px in U. urealyticum-infected cells compared to U. parvum infected cells. The higher GSH-Px activity was observed despite a seemingly equal induction of ROS. Further investigation is required to ascertain the precise molecular pathways that lead to oxidative stress-induced inflammation and apoptosis by various U. parvum and U. urealyticum isolates.
Our analysis of the clinical samples has some limitations. First, we lack information on the duration of Ureaplasma exposure in the respiratory tract of preterm neonates, both intra-uterine and after birth. Longer exposure is likely to induce immune cell influx, leading to a cascade of responses to Ureaplasma. This exposure time likely varies among neonates, challenging the comparison of analyses. The decision to collect tracheal aspirate samples was made by the treating physician, and the obtained tracheal aspirate volume was not standardized, varying depending on the treating physician. Lastly, the number of subjects included in this explorative study was small.
In summary, U. parvum and U. urealyticum species exhibit distinct effects on the immune responses and apoptosis of alveolar cells in vitro. While alveolar epithelial cells infected with U. parvum resulted in a stronger pro-inflammatory mediator response compared to U. urealyticum, more epithelial cell death occurred after incubation with U. urealyticum than with U. parvum. These Ureaplasma-induced inflammatory responses appear to be mediated via ROS and driven by secreted factors as responses induced by heat-inactivated bacteria were significantly lower than those by live bacteria. The development of BPD in infants associated with the presence of Ureaplasma spp. may result from various etiologies, as different cytokine and chemokine profiles were also observed in clinical tracheal samples. These findings contribute to the understanding of the inflammatory processes and cell death at the site of Ureaplasma infections, providing insights for developing targeted therapies or preventive strategies for BPD development.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
20 October 2025
The original online version of this article was revised: Due to a typesetting mistake, in Figures 2, 4 and 6 the abbreviation to denote UU has been replaced by UP, which was incorrect. The publishers would like to apologize for this mistake. The figure have been corrected.
28 October 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41390-025-04547-3
References
Sweeney, E. L., Dando, S. J., Kallapur, S. G. & Knox, C. L. The human ureaplasma species as causative agents of chorioamnionitis. Clin. Microbiol. Rev. 30, 349–379 (2017).
Kim, M. et al. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J. Perinat. Med. 31, 146–152 (2003).
Robertson, J. A. et al. Proposal of Ureaplasma Parvum Sp. Nov. and emended description of Ureaplasma Urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int. J. Syst. Evol. Microbiol. 52, 587–597 (2002).
Schelonka, R. L. & Waites, K. B. Ureaplasma infection and neonatal lung disease. Semin. Perinatol. 31, 2–9 (2007).
Kasprzykowska, U., Elias, J., Elias, M., Mączyńska, B. & Sobieszczańska, B. M. Colonization of the lower urogenital tract with Ureaplasma parvum can cause asymptomatic infection of the upper reproductive system in women: a preliminary study. Arch. Gynecol. Obstet. 289, 1129–1134 (2014).
Senthamaraikannan, P. et al. Intra-amniotic Ureaplasma parvum-induced maternal and fetal inflammation and immune responses in rhesus macaques. J. Infect. Dis. 214, 1597–1604 (2016).
Plummer, E. L. et al. Are Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum associated with specific genital symptoms and clinical signs in nonpregnant women?. Clin. Infect. Dis. 73, 659–668 (2021).
Deguchi, T. et al. Association of Ureaplasma urealyticum (Biovar 2) with Nongonococcal Urethritis. Sex. Transm. Dis. 31, 192–195 (2004).
Romero, R. et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J. Perinat. Med. 43, 19–36 (2015).
Pararas, M. V., Skevaki, C. L. & Kafetzis, D. A. Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur. J. Clin. Microbiol. Infect. Dis. 25, 562–569 (2006).
Motomura, K. et al. Intra-amniotic infection with ureaplasma parvum causes preterm birth and neonatal mortality that are prevented by treatment with clarithromycin. mBio 11 e00797–20 (2020).
Ruma, M. et al. Maternal periodontal disease, systemic inflammation, and risk for preeclampsia. Am. J. Obstet. Gynecol. 198, 389 e381–385 (2008).
Boggess, K. A. et al. Fetal immune response to oral pathogens and risk of preterm birth. Am. J. Obstet. Gynecol. 193, 1121–1126 (2005).
Yoon, B. H., Chang, J. W. & Romero, R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet. Gynecol. 92, 77–82 (1998).
Gerber, S., Vial, Y., Hohlfeld, P. & Witkin, S. S. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J. Infect. Dis. 187, 518–521 (2003).
Pavlidis, I. et al. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat. Commun. 11, 199 (2020).
Tantengco, O. A. G. et al. Modeling ascending Ureaplasma parvum infection through the female reproductive tract using vagina-cervix-decidua-organ-on-a-chip and feto-maternal interface-organ-on-a-chip. FASEB J. 36, e22551 (2022).
Tadera, K. et al. Prevalence of Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum detection in urine and respiratory tract samples in Hiroshima, Japan. Heliyon 9, e14543 (2023).
Beeton, M. L. et al. Isolation of separate Ureaplasma species from endotracheal secretions of twin patients. Pediatrics 138, e20160565 (2016).
Silwedel, C., Laube, M., Speer, C. P. & Glaser, K. The role of Ureaplasma species in prenatal and postnatal morbidity of preterm infants: current concepts. Neonatology 121, 627–635 (2024).
Chun, J., Chun, S. H., Han, Y. S. & Sung, T. J. Different degrees of maternal Ureaplasma colonization and its correlation with bronchopulmonary dysplasia in <32 weeks’ preterm infants. Pediatr. Neonatol. 60, 441–446 (2019).
Gobec, K. et al. Association between colonization of the respiratory tract with Ureaplasma species and bronchopulmonary dysplasia in newborns with extremely low gestational age: a retrospective study. Croat. Med. J. 64, 75–83 (2023).
Viscardi, R. M. & Hasday, J. D. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr. Res. 65, 84R–90R (2009).
Li, Y. H. et al. Ureaplasma Urealyticum induces apoptosis in human lung epithelial cells and macrophages. Biol. Neonate 82, 166–173 (2002).
Jensen, E. A. et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am. J. Respir. Crit. Care Med. 200, 751–759 (2019).
Schmidt, A. R. & Ramamoorthy, C. Bronchopulmonary dysplasia. Paediatr. Anaesth. 32, 174–180 (2022).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Moss, T. J. et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am. J. Obstet. Gynecol. 198, 122 e121– e128 (2008).
Kallapur, S. G., Kramer, B. W. & Jobe, A. H. Ureaplasma and Bpd. Semin. Perinatol. 37, 94–101 (2013).
Collins, J. J. et al. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma Parvum. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L852–L860 (2010).
Viscardi, R. M., Manimtim, W. M., Sun, C. C., Duffy, L. & Cassell, G. H. Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr. Dev. Pathol. 5, 141–150 (2002).
Viscardi, R. et al. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma Urealyticum pneumonitis. Pediatr. Dev. Pathol. 9, 143–151 (2006).
Hargitai, B. et al. Apoptosis in various organs of preterm infants: histopathologic study of lung, kidney, liver, and brain of ventilated infants. Pediatr. Res. 50, 110–114 (2001).
Glaser, K. et al. Ureaplasma species differentially modulate pro- and anti-inflammatory cytokine responses in newborn and adult human monocytes pushing the state toward pro-inflammation. Front. Cell Infect. Microbiol. 7, 484 (2017).
Cultrera, R., Seraceni, S., Germani, R. & Contini, C. Molecular evidence of ureaplasma urealyticum and Ureaplasma parvum colonization in preterm infants during respiratory distress syndrome. BMC Infect. Dis. 6, 166 (2006).
Stol, K., Jans, J., Ott de Bruin, L., Unger, W. & van Rossum, A. Perinatal Infections with Ureaplasma. Pediatr. Infect. Dis. J. 40, S26–S30 (2021).
Sung, T. J. et al. Frequency of Ureaplasma serovars in respiratory secretions of preterm infants at risk for bronchopulmonary dysplasia. Pediatr. Infect. Dis. J. 30, 379–383 (2011).
Katz, B. et al. Characterization of Ureaplasmas isolated from preterm infants with and without bronchopulmonary dysplasia. J. Clin. Microbiol. 43, 4852–4854 (2005).
Baier, R. J., Loggins, J. & Kruger, T. E. Monocyte chemoattractant protein-1 and interleukin-8 are increased in bronchopulmonary dysplasia: relation to isolation of ureaplasma urealyticum. J. Investig. Med. 49, 362–369 (2001).
Groneck, P., Goetze-Speer, B. & Speer, C. P. Inflammatory bronchopulmonary response of preterm infants with microbial colonisation of the airways at birth. Arch. Dis. Child Fetal Neonatal Ed. 74, F51–F55 (1996).
Wang, J. & Dong, W. Oxidative stress and bronchopulmonary dysplasia. Gene 678, 177–183 (2018).
Venturelli, N., Zeis, A., De Beritto, T. & Hageman, J. R. Ureasplasma and its role in adverse perinatal outcomes: a review. Neoreviews 22, e574–e584 (2021).
Zheng, X. et al. Small repeating units within the Ureaplasma urealyticum mb antigen gene encode serovar specificity and are associated with antigen size variation. Infect. Immun. 63, 891–898 (1995).
Yada, Y., Honma, Y., Koike, Y., Takahashi, N. & Momoi, M. Y. Association of development of chronic lung disease of newborns with neonatal colonization of Ureaplasma and cord blood interleukin-8 level. Pediatr. Int. 52, 718–722 (2010).
Glaser, K. et al. Perinatal Ureaplasma exposure is associated with increased risk of late onset sepsis and imbalanced inflammation in preterm infants and may add to lung injury. Front. Cell Infect. Microbiol. 9, 68 (2019).
van Waarde, W. M., Brus, F., Okken, A. & Kimpen, J. L. Ureaplasma urealyticum colonization, prematurity and bronchopulmonary dysplasia. Eur. Respir. J. 10, 886–890 (1997).
Berardi, A. et al. Group B streptococcal colonization in 160 mother-baby pairs: a prospective cohort study. J. Pediatr. 163, 1099–1104.e1091 (2013).
Koltsova, E. K. & Ley, K. The mysterious ways of the chemokine Cxcl5. Immunity 33, 7–9 (2010).
Craig, A., Mai, J., Cai, S. & Jeyaseelan, S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 77, 568–575 (2009).
Mei, J. et al. Cxcl5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity 33, 106–117 (2010).
Manimtim, W. M. et al. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect. Immun. 69, 3906–3915 (2001).
Van Marter, L. J. et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J. Pediatr. 140, 171–176 (2002).
Speer, C. P. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol. Neonate 79, 205–209 (2001).
Polglase, G. R. et al. Ventilation-mediated injury after preterm delivery of Ureaplasma parvum colonized fetal lambs. Pediatr. Res. 67, 630–635 (2010).
Inatomi, T. et al. Antenatal exposure to Ureaplasma species exacerbates bronchopulmonary dysplasia synergistically with subsequent prolonged mechanical ventilation in preterm infants. Pediatr. Res. 71, 267–273 (2012).
Silwedel, C. et al. Differential modulation of pulmonary caspases: is this the key to Ureaplasma-driven chronic inflammation?. PLoS ONE 14, e0216569 (2019).
Amabebe, E. et al. Ureaplasma parvum infection induces inflammatory changes in vaginal epithelial cells independent of sialidase. Mol. Biol. Rep. 50, 3035–3043 (2023).
Sprong, K. E., Mabenge, M., Wright, C. A. & Govender, S. Ureaplasma species and preterm birth: current perspectives. Crit. Rev. Microbiol. 46, 169–181 (2020).
Robinson, J. W. et al. Ureaplasma parvum serovar 3 multiple banded antigen size variation after chronic intra-amniotic infection/colonization. PLoS ONE 8, e62746 (2013).
Biernat-Sudolska, M., Bilska-Wilkosz, A., Rojek-Zakrzewska, D., Zawilińska, B. & Kosz-Vnenchak, M. Evaluation of urease activity by the human ureaplasma species. Folia Biol. 65, 142–147 (2017).
Acknowledgements
We thank all patients and their caregivers for participating in this study. HZ was supported by the Chinese Scholarship Council (202003250071). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
WU and KS conceived the project. RK collected patient samples. HZ, POJ, GH, LvL, and ACJMB performed experiments and analysed data. HZ, POJ, GH, MV, AvR, KS, and WU interpreted data and drafted the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the hospital’s Medical Research Ethics Committee (ID MEC-2020-0159). Parents provided informed consent to use left-over materials, including tracheal aspirates.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to a typesetting mistake, in Figures 2, 4 and 6 the abbreviation to denote UU has been replaced by UP, which was incorrect. The publishers would like to apologize for this mistake. The figure have been corrected.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, H., Oliveras-Julià, P., Hasperhoven, G.F. et al. Ureaplasma parvum and Ureaplasma urealyticum induce distinct types of inflammation in neonates and human epithelial cell models. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04415-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04415-0
This article is cited by
-
The role of Ureaplasma in preterm lung disease: does species matter?
Pediatric Research (2026)