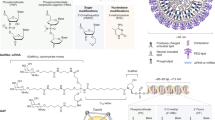
Abstract
The ability of small nucleic acids to modulate gene expression via a range of processes has been widely explored. Compared with conventional treatments, small nucleic acid therapeutics have the potential to achieve long-lasting or even curative effects via gene editing. As a result of recent technological advances, efficient small nucleic acid delivery for therapeutic and biomedical applications has been achieved, accelerating their clinical translation. Here, we review the increasing number of small nucleic acid therapeutic classes and the most common chemical modifications and delivery platforms. We also discuss the key advances in the design, development and therapeutic application of each delivery platform. Furthermore, this review presents comprehensive profiles of currently approved small nucleic acid drugs, including 11 antisense oligonucleotides (ASOs), 2 aptamers and 6 siRNA drugs, summarizing their modifications, disease-specific mechanisms of action and delivery strategies. Other candidates whose clinical trial status has been recorded and updated are also discussed. We also consider strategic issues such as important safety considerations, novel vectors and hurdles for translating academic breakthroughs to the clinic. Small nucleic acid therapeutics have produced favorable results in clinical trials and have the potential to address previously “undruggable” targets, suggesting that they could be useful for guiding the development of additional clinical candidates.
Similar content being viewed by others
Introduction
Gene therapies were initially used to treat diseases during the 1960s and early 1970s, and major biological and technological breakthroughs led to the development of several safe and effective platforms.1 Nucleic acid therapeutics use engineered sequences of nucleotides to selectively modulate gene expression.2 The most prominent nucleic acid drugs are those based on antisense oligonucleotides (ASOs), aptamers, short interfering RNAs (siRNAs) and microRNAs (miRNAs), which enhance the effects and delivery of drug materials, revolutionize precision medicine and increase the efficacy of existing pharmaceuticals.3,4 Oligonucleotides can be designed to target specific mRNAs and are capable of treating or managing a broad spectrum of diseases.5 The mechanism of action of these drugs differs from that of traditional drugs, which in some cases helps to prevent drug resistance, especially in cancer treatment. The efficacy of these drugs can be precisely controlled by altering their sequences, structures, or chemical modifications, and their flexibility allows the medication to be tailored according to the specific needs of the disease. In recent years, researchers have developed various delivery platforms to improve the stability and bioavailability of oligonucleotide drugs, thereby enhancing their therapeutic effects.6 Currently approved nucleic acid therapeutics and drugs assessed in clinical trials are valuable for patients who previously had limited treatment options (Fig. 1).
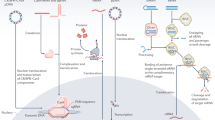
Translating small nucleic acid-based pharmacotherapy from bench to clinic and back. The development of small nucleic acid drugs begins with the design of potential nucleic acid sequences tailored to target specific diseases. Researchers then screen these sequences to identify the most effective candidates. Once optimal sequences are identified, they undergo chemical modifications to increase their stability and reduce their toxicity. These modifications optimize the drug’s pharmacokinetic properties, increasing the viability of the drug for therapeutic use. The modified small nucleic acid sequences are then encapsulated in delivery vectors. This step is crucial, as it further increases the drug’s stability in vivo and ensures that it reaches the specific target organ effectively. Extensive in vitro and in vivo evaluations have been conducted to assess the efficacy, safety, and pharmacodynamics of the drug. These evaluations help refine drug candidates before they proceed to clinical trials. Promising drug candidates then enter clinical trials, where many patients are recruited. Medical staff meticulously recorded the responses of different subjects to the candidate drugs, noting any therapeutic effects or adverse reactions. The clinical data obtained are analyzed by researchers, often with the assistance of artificial intelligence, to obtain deeper insights into the drug’s performance. This analysis helps researchers understand the drug’s efficacy, safety profile, and potential areas for improvement. The interpreted data are fed back to the laboratory for further optimization of the drug. Researchers may tweak the chemical structure, modify the delivery vector, or make other adjustments to enhance the drug’s performance. This iterative process continues, ensuring that each cycle brings the drug closer to its optimal form. This cycle of design, testing, feedback, and optimization is repeated until the drug meets the desired standards of efficacy and safety, ultimately leading to its approval and use in clinical settings
ASO technology, which originated from a 1978 study, uses synthetic strands of 15 or 20 nucleotides to complement a short RNA sequence of the Rous sarcoma virus directly.7 Based on Watson–Crick base pairing, the concept of treating a target RNA as a receptor for an ASO was proposed. Following this important finding, commercial companies focused on developing antisense therapeutics in the late 1980s to work against ‘undruggable’ receptors. High-throughput screening systems were established to identify optimal RNA binding sites for ASOs, leading to advances in ASO design. Currently, ASO drugs have been designed using this strategy, and further chemically modified ASOs have been used to enhance their pharmacological properties and passive tissue-targeting abilities.8 Additionally, RNA interference (RNAi) was first described in 1998 as a process of sequence-specific gene silencing initiated by double-stranded RNA (dsRNA), sparking great interest from drug developers for its potential to suppress the expression of specific genes.9,10,11 In 2001, Elbashir et al. were the first to use siRNAs to silence the expression of different genes in mammalian cell lines. These researchers generated siRNAs against sea pansy (Renilla reniformis, RL) luciferase and siRNAs against two sequence variants of firefly (Photinus pyralis, GL2 and GL3) luciferase.12 The first proof-of-principle experiment in which siRNAs could be utilized for the treatment of a disease in an in vivo model was performed in 2003. Research has shown that an intravenous injection of a Fas siRNA specifically reduces Fas mRNA levels and protects mice from fulminant hepatitis.13 The Nobel Prize in Medicine and Physiology was awarded to Andrew Fire and Craig Mello in 2006 for their discovery of RNA interference (RNAi). Since then, the development of delivery systems has broadened the potential applications of RNAi, particularly in rare diseases and cancers. The first human trial started in 2008, and the data were published in 2011. A phase 1 study (NCT00689065) was designed for the systemic administration of an siRNA to patients with solid tumors via a targeted nanoparticle delivery system, and the results revealed specific gene inhibition.14 However, drug developers have struggled to place siRNA therapies on the market quickly for clinical use, and the first globally approved siRNA drug for marketing was the therapeutic patisiran (ONPATTRO™), which is used for the treatment of hereditary transthyretin (hATTR)-mediated amyloidosis.15 Since then, interest in siRNA therapies has increased, even surpassing interest in ASO therapies.
Compared with traditional small-molecule and protein drugs, nucleic acid drugs have unique advantages. Fundamentally, the main targets of traditional small-molecule drugs and antibody drugs are proteins, but only 1.5% of the human genome can encode proteins, 80% of which are undruggable targets for traditional drugs. Unlike traditional drugs, nucleic acid drugs regulate target expression by binding to related nucleic acids, which means that nucleic acid drugs are theoretically applicable to any therapeutic target. Furthermore, designing a nucleic acid drug that binds to the target gene is relatively easy once the base sequence of the target gene is known. Finally, because of the simple nucleotide synthesis process and low cost, the research and development of nucleic acid drugs are quite accessible.
In recent years, small nucleic acid-based therapeutic modalities have expanded the platforms available for clinical oligonucleotide drug development. ASO drugs were first used on the market to control the expression of target genes. Chemical modification of ASOs is necessary for their stability and efficacy; however, the phosphorothioate or polyethylene glycol linkage in the backbone of ASOs can increase their binding affinity to unintended proteins, which is associated with toxicity. siRNA drugs with less modified linkages in the backbones can be developed as alternatives to ASO drugs. The first siRNA drug, patisiran, was approved by the FDA in 2018, and the second siRNA drug, givosiran, was approved in 2019.15,16 siRNA drugs have entered a rapid stage of development. After 20 years of research, the commercial potential and clinical value of small nucleic acid drugs have been proven (Fig. 2). In this review, we bridge the gap between laboratory research and the clinical implementation of small nucleic acid therapies, emphasizing how these technologies have evolved and are now poised for practical therapeutic use. Moreover, we focus on summarizing the latest advancements in drug delivery systems, particularly in the areas of nucleic acid chemical modification and nanoparticle carrier systems, highlighting how these improvements increase delivery efficiency. We also seek to identify the challenges and future directions in the field, discussing how these advancements could revolutionize the treatment of previously untreatable conditions.
Timeline of milestones from the discovery of small nucleic acid drugs to their clinical use. The development of nucleic acid therapeutics has been marked by considerable technological advances, pivotal drug approvals, and notable setbacks. A timeline highlighting crucial breakthroughs depicts the evolution from foundational research to clinical application. As the performance of antisense oligonucleotides (ASOs) has improved, the scope of therapeutic opportunities has broadened, now encompassing both rare and common diseases and virtually any delivery route. The bar graph illustrates the number of clinical trials for small activating RNAs (saRNAs), microRNAs (miRNAs), small interfering RNAs (siRNAs), and antisense oligonucleotides (ASOs) over the years. Each bar represents the beginning of enrollment recorded in the ClinicalTrials.gov database, providing a clear picture of the growing momentum in nucleic acid therapeutic research (up to the middle of 2024). RSV respiratory syncytial virus, RNA RNA interference, HCV hepatitis C virus, LNA locked nucleic acid, GalNAc N-acetylgalactosamine
Therapeutically relevant nucleic acids
Classification of therapeutically relevant nucleic acids
According to their molecular mechanisms, therapeutically relevant nucleic acids can be roughly categorized into eight categories: aptamer, siRNA, miRNA, ASO, small activating RNA (saRNA), PIWI-interacting RNA (piRNA), messenger RNA (mRNA) and plasmid DNA (pDNA). In this review, we focus on small nucleic acid therapeutics, in which mRNA and pDNA are not included (Fig. 3).
The functional mechanisms of four small nucleic acids. a ASOs: ASOs are short synthetic nucleic acid sequences that bind to complementary mRNA molecules. This binding can block the translation process or promote the degradation of the mRNA, thereby reducing the expression of the targeted protein. b Aptamers: Aptamers are short, single-stranded nucleic acids that bind specific targets with high affinity and specificity. They function by folding into unique three-dimensional structures, allowing them to interact with proteins, small molecules, or cells effectively. c siRNAs: siRNAs are double-stranded RNA molecules that guide the RNA-induced silencing complex (RISC) to complementary mRNA targets. This interaction leads to the cleavage and degradation of mRNAs, resulting in the silencing of specific genes. d miRNAs: miRNAs are endogenous, single-stranded RNA molecules that regulate gene expression posttranscriptionally. They bind to complementary sequences in the 3’ untranslated regions (UTRs) of target mRNAs, typically causing translational repression or mRNA degradation, thus modulating the expression of multiple genes. e PIWI-interacting RNAs (piRNAs) are small, single-stranded RNA molecules, approximately 24–30 nucleotides long. They interact with PIWI proteins to regulate gene expression, silence transposable elements, and maintain genome stability in germline cells. f saRNAs: saRNAs are synthetic short RNA molecules designed to upregulate gene expression. They bind to specific promoter regions of target genes, recruiting transcriptional activators or altering the chromatin structure to increase transcriptional activity, thereby increasing the production of specific proteins
ASOs
ASOs are short synthetic single-stranded oligonucleotides of 18–30 nucleotides in length and regulate gene expression by blocking the function of RNAs. Two major mechanisms for ASOs to exert their functions have been identified: RNase H1-dependent cleavage and steric hindrance.17 In the RNase H1-dependent mechanism, the ASO always contains a central contiguous sequence of 8–10 deoxynucleotides sandwiched between two RNA flanking regions, termed the ‘gapmer’ pattern.6,18 After binding, RNase H1 cleaves the target RNA strand, including the mRNA and pre-mRNA strands, approximately 7–10 nucleotides from the 5′-end of the duplex region since RNase H1 is robustly active in both the nucleus and the cytoplasm.19,20 In the RNase H1-independent mechanism, ASOs bind to target RNAs with high affinity, causing steric hindrance to alter gene expression by masking specific sequences rather than cleaving RNA.5 After the ASO binds to the initiation codon of an mRNA, steric interference prevents ribosome binding, thus inhibiting mRNA translation. Additionally, ASOs are also used to increase target gene expression.21 When multiple open reading frames (ORFs) exist in an mRNA, binding of the ASO to the initiation codon of the upstream ORF can alleviate translation inhibition of the main ORF, thereby activating the expression of the target protein, which is typically suppressed by the ORF.22 Moreover, ASOs can activate gene expression by masking premature termination codons or the exon junction complex, thereby leading to target mRNA decay.23,24 Importantly, ASOs can also alter the biological properties of proteins by binding to pre-mRNAs to make the splicing signal invisible to the spliceosome, thereby modulating splicing decisions such as exon skipping and exon inclusion.25,26
Single-stranded ASOs are unstable against intracellular nuclease degradation and are strictly complementary to target RNAs. These properties indicate that the ability to optimize the properties of ASOs via sequence variation is limited. Thus, chemically modified nucleotides or specific sequences are incorporated to increase the binding affinity and resistance to nuclease degradation of the ASOs.27 In addition, ASOs tolerate more extensive chemical modifications in the gapmer without abrogating the activity of RNase H1.28 The detailed chemical modifications of ASOs will be introduced in the next section.
Aptamers
Aptamers are short single-stranded nucleotides with unique tertiary structures, typically 20 to 100 nucleotides of DNA or RNA.29 Aptamers have diverse secondary structures, such as hairpins, internal loops, bulges, pseudoknots, and G tetramers.30 Aptamers can recognize, bind and subsequently block target molecules with high affinity, working like antibodies.31 Compared with in vivo synthesized antibodies, aptamers have the advantages of a low cost, low immunogenicity, a short synthesis time and high specificity.32 Additionally, due to their flexible nature, aptamers can be designed to bind even hidden epitopes, which is impossible for antibodies.33 Generally, high-affinity aptamers are selected from random libraries using an in vitro procedure called systematic evolution of ligands by exponential enrichment (SELEX).34 The basic process of SELEX is a repeated cycle of four steps: incubation, binding, elution of the bound target and amplification. First, a random oligonucleotide sequence library is incubated with the target molecule. During incubation, some sequences bind to the target molecule, whereas others bind weakly or do not react. Then, through partitioning and washing, lower affinity aptamers and unbound sequences are removed from the solution, whereas bound aptamer molecules are eluted. The eluted sequences are subsequently amplified for the next round of selection. After 5–15 rounds, a rich pool of aptamer candidates can be obtained for experimental evaluation and further optimization to obtain the final mature aptamers. However, traditional black box approaches such as SELEX run the risk of being unable to develop the best aptamer for a specific target.34 Thus, researchers have developed more advanced methods for consistently producing high-performance aptamers. For example, Gotrik et al. designed the microfluidic device SELEX and high-throughput sequencing SELEX, and the results revealed that after these alterations, higher-affinity aptamers can be obtained after far fewer rounds of selection relative to conventional methods.35 Capillary electrophoresis allows separation in free solution, and its participation in SELEX can greatly reduce the selection time and selection cost.36 Ishida et al. presented a novel method, RNA aptamer Ranker (RaptRanker), to identify optimal aptamers from HT-SELEX data by scoring and ranking. Through an evaluation, they proved that the performance of RaptRanker was superior to that of conventional methods, such as frequency, enrichment and MPBind.37
siRNAs
siRNAs are double-stranded RNA molecules consisting of 20–25 base pairs, with two nucleotides overhanging at the 3’ ends. Each molecule possesses a 5’ phosphate and a 3’ hydroxyl group.12,38 There are two strands of siRNA, one is guide strand (also called antisense strand), which can guide nuclease to cut the target gene; the other one is passenger strand (also known as sense strand). After the introduction of double-stranded siRNA into cytoplasm, the Argonaute 2 protein (AGO2) clears the passenger strand, releasing the guide strand, forming an RNA-induced silencing complex (RISC) with Dicer and transactivation response RNA binding protein (TRBP).39,40,41,42,43 A success RNAi initiation is owing to the correct selection of guide sense by Dicer, possessing ability of asymmetrical recognition, since the passenger sense is incapable in RNAi activation.40,44 TRBP maintains a proper orientation for the guide strand to bind to AGO2 because of its high flexibility. After the guide strand binding with specific mRNA, the target mRNA is cleaved, a process mediated by AGO2 that occurs between the 10th nucleotide and the 11th nucleotide from the 5′-most of paired sequences.45,46,47,48 Broken mRNA is unable to maintain the structural stability and subsequently degraded, resulting in target gene downregulation.
Initially, siRNA sequence selection was based on experimental insights, but bioinformatic methods are now used to aid in siRNA design. Key principles in siRNA design include targeting the coding sequence (CDS) or 3’ untranslated regions (UTR) and avoiding the 5’ UTR; ensuring no complementarity with nontarget mRNAs or homology with sequences from other species49; preferring 21-nt siRNAs with 2-nt 3′ overhangs and necessary 5′ phosphates50,51; avoiding overly long dsRNAs to prevent mRNA degradation failure and apoptosis52,53,54; preventing secondary structure formation in the guide strand55; maintaining a G-C content between 30% and 52%56; ensuring strand asymmetry, where the 5′ end of the guide strand is less stable and having U or A57,58; avoiding U-rich or GU-rich sequences in the guide strand to reduce immunogenicity59,60,61,62; and considering deoxyribonucleotide (TT) substitutions for 3′ overhangs to lower the synthesis costs.47
miRNA
The mechanism by which miRNAs silence gene expression is similar to that of siRNAs. Several steps are needed for endogenous miRNAs to progress from primary miRNAs (pri-miRNAs) to mature miRNAs. First, pri-miRNAs are transcribed from miRNA genes with RNA polymerase II in the nucleus.63 DGCR8 helps cleave pri-miRNAs at approximately 11 bp from the junction with Drosha, creating hairpin pre-miRNAs with 60 to 70 nucleotides, called pre miRNAs.64 Then, these pre-miRNAs are exported to the cytoplasm by the EXP5 complex and further processed into shorter double-stranded duplexes by Dicer.65 One strand (miRNA*) of the duplexes is stabilized in Argonaute to form the miRISC and the other strand (miRNA) is expelled.63,66,67,68 Finally, miRISC targets mRNAs, typically at the 3′-UTR, through seed sequence hybridization, leading to mRNA translational inhibition or decay. On the one hand, guide strand miRNAs that have perfect complementary pairing with their mRNA targets induce mRNA cleavage and subsequent degradation; on the other hand, miRNAs that have imperfect complementary pairing with their mRNA target repress mRNA translation through steric hindrance, resulting in target gene silencing.69 Unlike rigid siRNAs, a single miRNA duplex can simultaneously bind to different mRNAs, regulating the expression of multiple proteins, as more than half of the human genome contains numerous miRNA binding sites.70 Although abundant microRNAs are considered negative regulatory noncoding RNAs (ncRNAs) that function in the cytoplasm, emerging evidence shows that there are also some miRNAs in the nucleus.71,72,73 More unexpectedly, Xiao et al. reported a set of miRNAs located in the nucleus possessing gene-activating function. In their research, they found that miR-24-1, a member of theses miRNAs, can bind to enhancers of RNA transcripts, increasing expression of the target gene.74 The most commonly used miRNA drugs in the clinic are still miRNA mimics, whose sequences mirror those of natural endogenous miRNAs to downregulate the expression of target genes.
Since the sequences of miRNA mimics are exactly the same as those of natural miRNAs, modifying their sequences is challenging. However, key principles in miRNA mimic design have been identified. First, the mimics should involve two incompletely paired strands, as double-stranded miRNA mimics exhibit significantly greater efficacy in gene silencing than single-stranded mimics do, by approximately 100–1000-fold.75 Additionally, variations in the length of pre-miRNAs may affect Dicer cut site selection, potentially altering the miRNA seed sequence and influencing guide strand selection.76 Ideal length for miRNA mimics is 22 base pairs, matching that of mature endogenous miRNA duplexes. This length not only enhances the ease of delivery and reduces costs but also ensures optimal efficacy. Moreover, the seed and 3′ regions of synthesized miRNAs should be enriched with AU sequences to prevent strong binding between the target RNAs and the 3′ regions of guide strands.77,78
piRNAs
piRNAs are a class of single-stranded small noncoding RNAs (ncRNAs) with a length of 26–31 nucleotides.79 The concept of piRNAs was introduced in July 2006. Several research groups almost simultaneously reported that a new class of ncRNAs typically binds to PIWI proteins, resulting in the silencing of transposable elements, which is distinct from the mechanisms for siRNAs and miRNAs. PIWI, a subgroup of Argonaute proteins, is expressed mainly in the reproductive system.80,81,82,83 Endogenous piRNAs are processed from piRNA-coding sequences, which are predominantly grouped into 0.4–73.5 kilobase clusters occurring in the intergenic regions of chromosomes with highly uneven distributions.84 piRNA clusters are processed into mature piRNAs through several steps. The piRNA clusters are transcribed to primary piRNAs (pri-piRNAs) by RNA polymerase II in the nucleus, similar to the process of miRNAs. Single-stranded pri-piRNAs are subsequently transported to the Yb body in the cytoplasm for further slicing with Zucchini (Zuc).85 The sliced 5′ fragment subsequently binds to the PIWI proteins, forming an intermediate-piRNA-PIWI complex. After the 3′ end is trimmed to the optimal length and the 2′-hydroxy group at the 3′-end is methylated, the mature piRNA-PIWI complex migrates back into the nucleus to block target gene transcription.86 In addition to the primary processing pathway described above, mature piRNAs can be amplified through a “ping-pong” cycle. In addition to PIWI proteins, mature piRNAs can also bind to AGO3 (sense transcription) or AUB (antisense transcription). The resulting piRNA-AGO3/AUB complex can target pri-piRNAs and cut them into new mature piRNAs for the next cycle.87 Numerous studies have shown that piRNAs can regulate the differentiation and development of germ cells through transposon inhibition.88,89,90,91,92,93 In general, three major pathways by which piRNAs regulate gene expression have been identified. piRNAs can form a piRNA-induced silencing complex (piRISC) with PIWI proteins, resulting in transcriptional gene silencing in the nucleus and posttranscriptional gene silencing in the cytoplasm.94 In the PIWI cleavage process, mismatches to any target nucleotide can be tolerated, including those flanking the scissile phosphate.95 Additionally, piRISC can also bind to proteins to promote interactions with multiple proteins.96
Although most studies of piRNAs are focused on mechanistic research and rarely on gene regulation therapies in preclinical studies or clinical trials, growing evidence indicates that piRNA predominantly regulates the occurrence and progression of cancer cells, particularly in terms of proliferation, migration, metastasis, and apoptosis.97 piRNAs act as oncogenes or tumor suppressors by regulating factors in tumor-related signaling pathways. For example, piR-651 is upregulated in various human solid cancers, where its overexpression promotes cell proliferation and invasion, suppresses apoptosis, and decreases G0/G1 phase arrest, accompanied by increased expression of oncogenes such as MDM2, CDK4, and Cyclin D1.98 In contrast, piR-823 is expressed at significantly lower levels in gastric cancer tissues compared to non-cancerous tissues. Additionally, piR-823 interacts with PINK1, enhancing its ubiquitination and proteasomal degradation, thereby suppressing mitophagy during tumor development.99,100 Thus, targeting piRNAs presents a promising therapeutic strategy. Emerging studies suggest that piRNAs could be leveraged to modulate pathways that contribute to tumorigenesis, presenting new avenues for therapeutic intervention. For example, the downregulation of PIWIL1 and piR-DQ593109 enhanced BTB permeability via the MEG3/miR-330-5p/RUNX3 axis, providing valuable insights for glioma therapy.101 Another successful application is the upregulated expression of piRNA-36712, which shows a synergistic anticancer effect when combined with chemotherapeutic agents in breast cancer cells.102 However, delivering piRNA in a manner that is specific to organs remains a challenge to minimize off-target effects. Further research is needed to fully characterize the mechanisms through which piRNAs operate in different cancer types. Exploring the therapeutic potential of piRNA-based treatments, including the design of piRNA mimics or inhibitors, could lead to innovative cancer therapies with improved specificity and reduced side effects. Collectively, piRNAs offer a promising new frontier in cancer research, with the potential to be developed into targeted therapies that address both the prevention and treatment of cancer by leveraging their unique role in maintaining genomic stability and regulating key oncogenic pathways.
saRNAs
saRNAs are double-stranded RNAs consisting of 21 nucleotides, with 2 nucleotides overhanging at the 3’ end, which can increase the expression of target genes by RNA activation (RNAa).103,104 After introduction into the cytoplasm, the saRNA duplex binds to AGO2 and unwinds under the action of RNA helicase A (RHA), following an import into the nucleus mediated by importin-8.105,106,107,108,109 The guide strand then leads the complex to bind with target promoters, recruiting the polymerase-associated factor 1 (PAF1) complex to assemble the RNA-induced transcriptional activation (RITA) complex.110 Finally, the RITA complex initiates transcription and productive elongation by RNA polymerase II, thus increasing the expression of target proteins.111
The selection of genomic regions is vital in the sequence design of saRNAs. There should be sequences in the saRNAs which can complementary with the promoters or 3’ terminus of target genes.112 The target region should not be identical to other sequences to avoid off-target effects (OTEs) caused by sequences; the target region should not contain CpG islands or DNA hypermethylation to avoid OTEs caused by special nucleotides.113 Furthermore, every nucleotide is highly important for saRNAs since mutation of the seed region can influence RNAa levels and can even lead to complete dysfunction.114 Additionally, single-stranded saRNAs have no effect on gene expression activation, indicating that the presence of an intact duplex RNA is essential.115
Compared with the downregulation of genes triggered by siRNAs and miRNAs, the activation of gene expression induced by saRNAs lasts much longer, approximately 2 weeks, than that induced by other agents and is 7 days long.113 However, a greater concentration (nM) of saRNA therapeutics is required to achieve therapeutic effects, whereas siRNAs can act at a relatively low concentration (pM–nM).116 Compared with mRNAs, saRNAs offer advantages in mass production because of their lower nucleotide count, resulting in substantially lower costs. Moreover, the smaller size of saRNAs may pose fewer delivery challenges than those posed by the larger size of mRNA molecules. Additionally, saRNAs tap into the gene regulatory machinery of endogenous cells to activate gene expression, reduces the risk of detrimental overexpression and immunogenicity, a common challenge encountered with mRNAs.117
In summary, although some differences exist among the six types of small nucleic acid therapeutics, they all make great efforts to rescue the disordered gene expression of targets that were previously considered undruggable. Although the rational design of a nucleic acid sequence can increase its potency, chemical modification is a much more powerful strategy.
Modification of nucleic acids
Since unmodified naked nucleic acids that are intravenously injected into the target tissue can be rapidly degraded by RNases and cleared from the blood, chemical modification is crucial for generating effective nucleic acid drugs.118 The key goals for chemical modification include increasing the binding of small nucleic acids with target sequences, enhancing nuclease stability, optimizing pharmacokinetic characteristics, and minimizing side effects.119,120 To date, a lot of effort has been made in modification of small nucleic acids (Fig. 4).121
Chemical modifications used for small nucleic acids. Common modifications can be divided into three categories: ribosome modifications, nucleobase group modifications and backbone modifications. Modifications of ribosomes are always performed on the 2’ ends of ribose sugars (blue box) and sugar chains (orange box). In modifications of the base group, uracil, adenine and cytosine are the common objects (purple box), and the ribose sugar can be completely replaced (red box). The PO linkage of the backbone can be changed, including the nonbridging O atom (cyan box), 3’-bridging O atom (green box) and total PO linkage (golden box), to reduce the net anionic charge of small nucleic acids
Structural modification of nucleic acids
The structure of ribonucleotides can be altered by chemically modifying nucleotide bases, sugar moieties, or nucleosides. Widely used 2’-sugar modifications include 2ʹ-fluoro (2ʹ-F), 2ʹ-O-methyl (2ʹ-OMe) and 2ʹ-O-(2-methoxyethyl) (2ʹ-O-MOE), all of which have been proven to be effective and accessible to the market.122,123,124,125,126 In addition to adding modifications at the 2’ position of ribose, changing its structure is also feasible. RNAs with ribose structural modifications include constrained ethyl (cEt), glycol nucleic acids (GNAs), locked nucleic acids (LNAs; also known as 2′,4′-bridged nucleic acids, BNAs), nucleosides and tricyclo-DNA (tcDNA) and unlocked nucleic acids (UNAs). cEt nucleosides are similar to LNAs in structure with an additional methyl group.127 GNAs are acyclic nucleic acid analogs, which can result in greater thermal destabilization of the duplex structure.128 There is a methylene bridge to connect the 4′-carbon with 2′-oxygen of LNAs, which can both increase the serum stability and reduce the off-target effects.129 UNAs are 2′,3′-seco-RNAs that always used to disrupt the stability of double-stranded RNA.130 Another common strategy involves modifying the base group of nucleotides, such as replacing uracil with pseudouridine (Ψ),131 N1 methylpseudouridine (m1 Ψ),132 5-methyluridine (m5U),133 5-methoxyuridine (5moU)134 or 2-thiouridine (s2U)135; using N1-methyladenosine (m1 A)136 or N6-methyladenosine (m6 A)137 instead of adenine; and methylating cytosine to 5-methylcytidine (m5C).138 Moreover, the base group can be directly replaced with a nitroindole, an universal base to decrease undesired off-target effects.139 Through the addition of oligonucleotide analogs to nucleic acids, such as tcDNA, a conformationally constrained oligonucleotide analog140; PMO, which replaces the pentose sugar with a morpholine ring; phosphate141; peptide nucleic acids (PNAs), enhance both the selectivity and thermal stability of the compounds to increase the binding to target RNAs.142
Backbone modifications
Reducing the net anionic charge of oligonucleotide drugs and increase the delivery efficiency.143 A common strategy is to replace one of the nonbridging oxygen atoms of the phosphodiester bond (PO linkage) with a nonionic modification group,137 such as phosphorothioate (PS),144 methylphosphonate (MP),145 methoxypropyl phosphonate (MOP),146 phosphorodithioate (PS2),147 phosphoramidate (PA),148,149 phosphoroselenoate (PSe),150 phosphotriester (PTE)151 or boranophosphate (BS).152 Likewise, the 3′-bridging oxygen atoms of the PO linkage can be replaced with carbon or nitrogen to form 3′-methylene phosphonate or N3′-phosphoramidate, respectively.153,154 In fact, the PO linkage can be completely replaced. The trans isomers of amides preorganize sugars into C3′-endo conformations; thus, the C3-amide modification (3’-CH2-CO-NH-5’) increases the biological activity of nucleic acids.155 Methylene (methylimino) (MMI) is another nitrogen that contains an achiral four-atom linkage (3’-CH2N(CH3)-O-5’) and is completely resistant to nucleases.156 Apparently, the hydrophobic formacetal linkage (3’-O-CH2-O-5’) exhibited increased stability in all the RNA duplexes, whereas it strongly destabilized the DNA helix.157 Thioformacetal, which replaces the 3′-sided oxygen atom with a sulfur (3’-S-CH2-O-5’), increases both the stability of nucleic acids and the affinity for target RNA.158 Among these modifications, PS is the most commonly used. The PS linkage has chemical properties similar to those of the PO linkage, but it can greatly increase the metabolic stability of small nucleic acids and protect them from rapid degradation.159 In addition, there are two isomers of PS linkage, including right-handed (Rp) and left-handed (Sp), possessing different biological properties.160 With the development of synthetic methodologies, stereopure PSs with only one isomer are possible. However, whether nucleic acid drugs with stereopure PSs possess superior potency, efficiency and durability is still unclear since this topic has been highly debated for several years.161,162,163,164
In the early days of the chemical modification of nucleic acid drugs, primary agents were synthesized, and recent studies have shown that their combination with nucleic acids and the backbone has excellent potency. Wu et al. combined PS2 with 2’-OMe in a same nucleotide (MePS2), results showed enhanced resistance to nucleases degradation for siRNAs.165 Baker et al. suggested that introducing LNAs into splice-switching oligonucleotides, which have been modified with phosphorothioates, increases the activity of nucleic acids due to their reduced charge.166 Notably, though these chemical modifications showed robust efficiency and well tolerance in a broad of researches, there are some side effects occurred in the clinic.167 For example, PS-modified oligonucleotides lead to unexpected binding to platelets, thus forming thrombus.168 Moreover, LNAs can induce hepatotoxicity consistent with acute liver injury in as little as 4 days, which might be caused by two trinucleotide motifs, TCC and TGC.169,170 Safe and efficient chemical strategies should be a focus for the development of future nucleic acid therapeutics.
Nucleic acid delivery system
Although rational sequence design and chemical modification of nucleic acids can increase their stability and reduce OTEs, targeted delivery to specific cells and organs remains a great challenge. Ideal delivery systems are expected to possess high drug loading efficiency and sufficient safety, protect nucleic acids from rapid degradation and specific targeting. In the following sections, we describe the major nonviral delivery systems used for small nucleic acids (Fig. 5).
Technologies for the delivery of small nucleic acids. a The six types of common nonviral delivery vectors include lipid-based nanoparticles (LNPs), polymers, peptides, exosomes, conjugates (GalNAc) and inorganic nanoparticles. b Schematic diagram of small nucleic acid drug uptake pathways by target cells. There are four major endocytosis pathways, including macropinocytosis, caveolin-mediated endocytosis, clathrin-mediated endocytosis and direct membrane fusion. c Schematic diagram of endosomal escape. After internalization, endocytic vesicles develop into early endosomes and late endosomes and subsequently fuse with lysosomes to induce lysosomal degradation. Only a small fraction (usually less than 1%) of small nucleic acids can escape from the endosome to the cytoplasm
Lipid-based nanoparticles (LBNPs)
LBNPs are spherical nanoparticles with favorable biocompatibility and degradability and are currently the most promising delivery carrier. LBNPs can be divided into five categories according to their compositions: liposomes, lipid nanoparticles (LNPs), lipid nanoemulsions (LNEs), solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs).
Liposomes
Classic liposomes consist of cholesterols and amphiphilic phospholipid bilayers encapsulating an aqueous inner compartment, with sizes ranging from 100 nm to 5 μm, which is quite similar to the size of the natural cell membrane.171,172 The amphiphilic phospholipid has a hydrophobic tail group and a hydrophilic head group, which are oriented to the outer and inner sides in the composition of liposomes. Liposomes can be divided into 2 types according to their basic structures: unilamellar liposomes with single bilayer membranes and multilamellar liposomes containing multiple bilayer membranes.173 Liposomes were first discovered in 1961. Bangham and Horne reported that phospholipids dispersed in excess water could form closed vesicles composed of bimolecular membranes, and images captured with an electron microscope were published in 1964.174 In 1971, Gregoriadis et al. first constructed liposomes composed of phosphatidylcholine, cholesterol and diacetyl phosphate as enzyme drug carriers. The results revealed no measurable leakage of the encapsulated albumin, and most of the albumin accumulated in the liver.175 Since then, liposomes have been widely used as drug delivery systems.176,177,178 Yano et al. complexed siRNA-B717, which targets the human Bcl-2 oncogene, with a novel cationic liposome, LIC-101, containing 2-O-(2-diethylaminoethyl)-carbamoyl-1,3-O-dioleoylglycerol and egg phosphatidylcholine and intravenously injected into mice with liver metastasis and subcutaneously injected into prostate cancer-bearing mice (inoculated under the skin). In both models, the complex showed strong antitumor activity.179 However, cationic nanocarriers are reported to induce cellular necrosis, especially in the lungs, upon systemic administration.180 Changing the electric charge of liposomes could be a promising strategy. Qian et al. employed hyaluronan (HA) to negate the positive charge of liposomes since HA is an anionic polysaccharide with a negative charge. Through in vitro and in vivo tests, HA-modified liposomes were shown to exhibit reduced toxicity and a comparable transfection efficiency to unmodified liposomes.181 In addition, Villares et al. constructed neutral liposomes with neutral 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC), which can simultaneously improve the tumor uptake efficiency of nucleic acid‒liposome complexes. Their results first revealed the effective inhibition of melanoma growth and metastasis in melanoma-bearing mice after systemic treatment with the protease-activated receptor-1 siRNA-DOPC complex.182
Lipid nanoparticles (LNPs)
LNPs are the most advanced clinical delivery vehicles,183,184,185 currently showing strong potential for nucleic acids delivery in gene therapy.186 In addition to GalNAc-modified siRNAs, LNPs are the only small nucleic acid carriers approved by the FDA for the targeted delivery of siRNAs.187 The unique property of LNPs is their neutrally charged surface, which enables them to avoid extensive adsorption by serum proteins and prevent poor penetration into the target tissue.188,189 Unlike liposomes, LNPs contain multiple reverse micelle nuclei composed of nucleic acids and lipids, which are surrounded by a monolayer lipid membrane.190 Four primary lipids are used to form the shells of LNPs, namely, ionizable lipids, polyethylene glycol (PEG)-modified lipids, cholesterol and helper phospholipids, which are present in suitable proportions (commonly known as molar ratios of 50, 1.5, and 38.5/10, respectively).191 The composition of the LNP shell is quite important in the physicochemical properties and functions of LNPs, and the optimal ratio can vary under different conditions. Each lipid in the LNP shell plays a different but similarly essential role in effective small nucleic acid delivery. Ionizable lipids are the major component of LNPs because of their potency. Ionizable lipids can ensure that the surface of LNPs remains neutral at physiological pH, given their precise and appropriate pKa, which is between 6.2 and 6.5, different from previous cationic lipids.192,193 The ionizable lipids can respond to different pH values, remaining neutral at pH 7 but becoming positively charged at lower pH values.193 Therefore, for neutral LNPs, tolerability and safety are further increased by preventing reactions with negatively charged biomacromolecules, which can trigger severe side effects that are unavoidable when permanently charged cationic lipids are used.194 Moreover, a PEGylated lipid is a hydrophilic PEG polymer conjugated to a hydrophobic lipid anchor, with the lipid domain within the polymer structure and the PEGylated domain extending out from the polymer surface.195 PEG can prolong the half-life of LNPs in the circulation, since it can adsorb the serum proteins in blood.196,197,198 However, it can also reduce the proximity of LNPs to cell membranes and inhibit fusion between LNPs and cell membranes, leading to a decrease in nucleic acid uptake efficiency.199 Research has shown that a higher PEGylated lipid content results in a smaller particle size.200 However, the reduced delivery efficiency is driven primarily by an increase in the PEG content rather than by a decrease in the particle size.201 Thus, the optimal dose of PEGylated lipids should be selected to maintain a balance between stability and transfection competency and safety. Cholesterol maintains the stability of the LNP surface while increasing its delivery efficiency since it can fill the gaps between different lipids, reduce the possibility of immune clearance and bind Apo E and LDL receptors to aid in LNP uptake.202,203 The addition of cholesterol analogs and C-24 alkyl phytosterols not only increases the cellular uptake of nucleic acids but also prolongs the retention time after endosomal escape.204 Helper phospholipids, such as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), are always zwitterionic, which can increase the stability and delivery efficiency of LNPs. Cone-shaped DOPE forms an inverted hexagonal (HII) phase, and cylinder-shaped DSPC can provide greater bilayer stability.205
The most commonly used technology for LNP synthesis is the ethanol dilution method, which uses a microfluidic device. Generally, by mixing a lipid solution dissolved in ethanol and a small nucleic acid solution dissolved in aqueous buffer (pH 4), positively charged ionizable lipids bind to negative nucleic acids and subsequently encapsulate RNAs to form LNPs with other lipids.206 The residual ethanol is then removed from the pH 4 buffer through dialysis, followed by increasing the pH (7.4) for long-term storage and subsequent use.191 The particle size of LNPs is associated with the speed of ethanol dilution with a buffer solution, where more rapid dilution produces smaller LNPs, suggesting that the desired microfluidic device should be able to undergo rapid and controllable mixing.200,207 Detection methods have also improved, thereby improving the quality control of LNPs. For example, Kim et al. developed a robust high-performance liquid chromatography with charged aerosol detector (HPLCCAD), which can quantify the composition of LNPs using the designed approach.208
Research has shown that LNPs enter cells through clathrin-mediated endocytosis and micropinocytosis.209 After internalization, the nucleic acid–LNP complex is transported through early endosomes, late endosomes, and lysosomes, all of which have pH values less than 7.210 Small nucleic acid release occurs mainly in a moderately acidic early endocytic compartment before transport to late endosomes and lysosomes.
However, several hurdles limit the application of LNPs, the majority of which are safe and associated with LNP formulations. Although PEGylated lipids attribute a lot to LNP delivery systems, they are also the causes of various adverse reactions induced by nucleic acid-LNP drugs.211 First, accumulating evidence indicates that PEG can induce hypersensitivity reactions by stimulating the complement system.212,213 Moreover, PEGylated lipids could lead to accelerated blood clearance (ABC) phenomenon, reducing their therapeutic efficacy.214,215,216,217 Furthermore, another disadvantage of PEGylated lipids is their nonbiodegradability, suggesting that PEGs should have a relatively low molar mass, generally less than 20 kDa.218,219,220 Additionally, the molar mass should be greater than 400 Da since a lower mass is toxic in humans.221 Many efforts have been made to reduce the side effects of LNP treatment. Judge et al. evaluated LNPs the stabilities of LNPs with different PEGylated lipids, results showed that PEGylated lipids with shorter alkyl chains (C14) are more stable.222 To date, new PEGylated lipids have been preferentially designed, which can disengage from the LNPs after injection via a process called PEG shedding, thus maintaining the advantages of PEGylated lipids and simultaneously avoiding their side effects.223,224 For example, Hatakeyama et al. developed PEG–peptide–DOPE (PPD), showed higher efficiency of cellular uptake and endosomal escape.225 The long-chain PEG 5000 polymer O’-methyl polyethylene glycol (omPEG), which is a pH-sensitive PEG shed from LNPs, has low toxicity to blood and noncancerous intestinal cells.226 In addition, the fate of the nucleic acid–LNP complex is also a noteworthy issue, as nucleic acid–LNP complexes accumulate in large amounts in the liver when they are administered intravenously.227,228,229 The target organs of LNPs can be changed by altering the lipids used to form the LNP shell. For example, DSPC-containing LNPs preferentially accumulate in the spleen, whereas DOPE-containing LNPs preferentially accumulate in the liver, suggesting that different helper lipids should be chosen for different uses of LNPs.230 Additionally, adding a target molecule into an LNP can affect its delivery endpoint. For example, Lee et al. incorporated a Glu-urea-Lys PSMA-targeting ligand into the LNP system, resulting in increased LNP accumulation at tumor sites.231 Ramishetti et al. constructed tLNPs that can specifically deliver nucleic acids to CD4( + ) T lymphocytes by chemically conjugating mAbs against CD4 to the LNPs. After intravenous injection, particles were accumulated in lymph nodes, blood, and bone marrow, and effectively endocytosed by CD4( + ) T lymphocytes.232 Younis et al. designed ultrasmall lipid nanoparticles with a novel pH-sensitive lipid and a targeting peptide, exhibiting excellent tumor accumulation and gene silencing in vivo.233 In addition, by adding polyelectrolyte layers to LNPs, poly-L-arginine and hyaluronan (HA) can cross the BBB, targeting glioblastoma multiforme (GBM) cells more effectively than unmodified LNPs in vitro and delaying tumor growth in vivo after siRNA and miRNA transfection.234 Altering the administration route might also be a viable option. Bai et al. developed an inhalable and mucus-penetrating LNP system that can significantly diminish fibrosis development.235 Wang et al. constructed lipidoid (lipid-like) nanoparticles to deliver a VEGF siRNA into the eyes through intravitreal administration and treat retinal angiogenic diseases.236 In summary, LNPs show great potential in small nucleic acid delivery.
Lipid nanoemulsions (LNEs)
LNEs are nanosized spherical particles composed of surfactants and phospholipids and are approximately 200 nm in size. The structure of LNEs not only is similar to that of liposomes but also remains biodegradable, biocompatible and nonimmunogenic.237 Three dispersions of LNEs are commonly used—oil in water (O/W), water in oil (W/O) and water in oil in water (W/O/W)—of which O/W is the most commonly used in nucleic acid drug delivery.238 The oil phase of LNEs can protect nucleic acids from degradation by nucleases. Although no proteins are components of LNEs, apolipoproteins, such as ApoA, ApoC and ApoE, can be incorporated into LNEs through contact with plasma in the blood circulation. ApoE can be recognized by the low-density lipoprotein (LDL) receptor, which is overexpressed in some tumors; thus, LNEs can deliver nucleic acids to tumor cells with high LDL receptor expression.239 Changing the proportions of components or directly changing the composition of LNEs could alter their physicochemical characteristics and in vivo biodistribution. By increasing the weight ratio of medium-chain triglycerides to soybean oil from 1:4 to 3:2, Jiang et al. reported that the LNE size decreased from 126.4 ± 8.7 nm to 44.5 ± 9.3 nm. The addition of PEGylation increased the accumulation of LNEs in the tumor area by 6–7-fold.240 Kaneda et al. formulated LNEs consisting of DOTAP, DOPE and cholesterol. These siRNA-LNE complexes can be internalized through lipid raft transport, bypassing the usual endosomal nanoparticle uptake pathway and increasing drug availability.241 Compared with liposomes, the manufacture of LNEs is less expensive. Gehrmann et al. investigated the preparation of LNEs with disposable materials. By changing different syringe filters and pharmaceutically relevant emulsifiers, they finally developed a stable protocol to quickly and easily produce LNEs with narrow particle size distributions.242 Unfortunately, LNEs are more commonly used in the delivery of poorly water-soluble, highly lipophilic drugs but not hydrophilic nucleic acid drugs, as their oil phase can solubilize these drugs.243
Solid lipid nanoparticles (SLNs)
SLNs are spherical nanoparticles composed of biodegradable physiological lipids and surfactants with a drug-containing solid lipid core, and thus they are less toxic than other nanodelivery systems.244 The melting points of the employed lipids, such as monostearin, stearyl alcohol, stearic acid, glycerol monostearate and Precirol® ATO5, are above room temperature.245 Unlike LNEs, organic solvents are not used in the preparation of SLNs, which may introduce potential toxicity.246 Interestingly, Shirvani et al. produced a novel type of SLN using natural beeswax and propolis wax, both of which are considered food grade.247 SLNs are stable in biological fluids and during storage for at least one week at room temperature and 1 month at 4 °C.248 Lyophilization can further improve the storage stability of the solid forms of SLNs and retard unfavorable chemical degradation.249 In addition, by coating the surface of SLNs with chitosan, the residence time in the focal area of the drug–SLN complex can be further improved via mucoadhesive interactions.250 The size of LNEs may influence their in vivo distribution. By comparing SLNs with different particle sizes (120–480 nm), Huang et al. reported that a larger particle size led to a longer lung retention time.251 In addition, SLNs are quite easy to sterilize and mass produce.252,253
However, some obstacles prevent SLNs from delivering nucleic acids. For example, during the solidification process, the inner loaded nucleic acids might be excluded from the surface of SLNs, which are surrounded by an aqueous phase, resulting in an insufficient loading efficiency.254 In addition, during storage, SLNs are prone to aggregate and gelatinize.254
Nanostructured lipid carriers (NLCs)
NLCs contain a lipophilic core formed by a mixture of one solid lipid (in a greater quantity) with one liquid lipid (in a lower quantity).255 NLCs are recognized as second-generation SLNs, which address their limitations. First, liquid lipids are added to the solid lipid matrix of SLNs to disrupt the perfect crystal order, thereby stabilizing the LNEs and preventing loaded nucleic acid leakage during storage.256 By adjusting the ratio of liquid lipids to solid lipids, NLCs can also maintain a solid skeleton structure at room temperature for a long period, which is conducive to the controlled release of loaded nucleic acids.257 In addition, the payload of NLCs is increased, and the bioavailability of loaded nucleic acids is further improved.258 Because of these alterations, NLCs are more suitable for carrying biologically active ingredients.259 However, relatively few studies have investigated the use of NLCs for small nucleic acid delivery. Oner et al. successfully prepared 13 NLCs with characteristics similar to those of their precursor SLNs, but not all of these NLCs could form complexes with siRNAs.260 More detailed information about the LBNP system mentioned in this section is listed in Table 1.
Peptides
Cell-penetrating peptides (CPPs) and homing peptides consist of 5–30 amino acids and interact with biomolecules and cells to increase nucleic acid delivery efficiency.261 Compared with viral carriers, peptides of varying sizes and structural features have the advantages of a greater payload capacity and biocompatibility and have broad applications.262 Moreover, rationally designed peptides can overcome a series of biological obstacles, such as endosomal escape, cell internalization, and interactions with biomolecules and cells, to improve nucleic acid delivery efficiency. The side chains of these materials can carry various active functional groups that can be chemically modified to enhance the drug delivery systems. In addition, peptides can also be integrated as functional fragments into drug delivery systems.
CPPs and intracellular targeting peptides are of particular interest because they can improve the delivery efficiency of gene therapies into cells. CPP also known as a protein transduction domain (PTD), is a kind of membrane-active peptide that typically contains 5–30 amino acids. CPPs were initially designed to mimic the transactivator of transcription (TAT) protein transduction domain,263 penetratin,263 and Pvec,263 which can cross cellular membranes via energy-dependent or -independent mechanisms without interacting with specific receptors.264 Nucleic acids are attached to CPPs via covalent bonds or noncovalent complex formation.265 Noncovalent complex formation depends on electrostatic and/or hydrophobic interactions between negatively charged cargoes and positively charged CPPs, protecting bioactive conjugates from proteases or nucleases.265,266 Moreover, peptides can covalently interact with nucleic acids to produce conjugated molecules, and this method is suitable for generating neutral nucleic acids.267 Additionally, functional peptide fragments can be coupled to nucleic acids for delivery through chemical bonds (such as ester bonds, disulfide bonds, and thiol maleimide bonds). Peptide‒nucleic acid coupling molecules have specific structures, molecular weights, and high stability, with an excellent degree of reproducibility.268 CPPs are classified into three major categories according to their physicochemical characteristics: cationic, amphiphilic, and hydrophobic. Numerous preclinical evaluations of CPP-derived therapeutics have been performed to address major unmet medical needs (Table 2).
Cationic CPPs are typically short (up to 30 amino acids) and rich in arginine and lysine residues that have positive net charges.269 The positive charge of cationic peptides is attracted to negatively charged membrane constituents, enhancing the interaction of CPPs with the cell membrane to initiate transport.270 The TAT protein transduction domain was first identified in the HIV genome and is the first and most studied type of CPP. After the discovery of the TAT peptide, additional CPPs, such as Pep-1, polyarginine, and penetratin, were discovered.271,272 The structural prerequisites for the cellular uptake of cationic CPPs have been widely examined in various studies. For example, after cholesterol modification, the DP7 peptide (DP7-C) has shown significant potential because of its unique properties and effectiveness in facilitating cellular uptake.273,274,275,276,277 It can be conjugated with various nucleic acids, including siRNAs and miRNAs, enhancing the stability and bioavailability of nucleic acids.275,276,277 This broad applicability allows DP7-C to be used in a variety of therapeutic settings, treating numerous diseases and conditions.275,276,277 However, the precise mechanisms by which cationic CPPs enter the cell membrane are complex and debated. Arginine-rich CPPs are taken up by lipid raft-dependent macropinocytosis, independent of caveolar, clathrin-mediated endocytosis, and phagocytosis.278 Other studies suggest they may directly cross the membrane via transient pores.279 Recently, arginine-rich CPPs were shown to go through vesicles and live cells via vesicular fusion induced by calcium influx, similar to the mechanism of membrane multilinearity and fusion.
Peptides with both polar and nonpolar regions are known as amphipathic CPPs, typically classified as primary, secondary, or proline-rich peptides.280 Primary amphipathic CPPs are chimeric peptides formed by covalently attaching a hydrophobic domain to a nuclear localization signal (NLS), enhancing their ability to target cell membranes effectively. The peptide carrier MPG, derived from the fusion peptide domain of the HIV-1 gp41 protein combined with the nuclear localization sequence of the SV40 large T antigen, efficiently delivers siRNAs into mammalian cells.281 Furthermore, a refined and shorter variant of the amphipathic peptide carrier MPG, called MPG-8 (AFLGWLGAWGTMGWSPKKKRK), enhances the efficiency of siRNA delivery both in vitro and in vivo, without triggering an immune response.282 In a mouse xenograft tumor model, stable and noncovalent nanoparticles were generated with a cyclin B1 siRNA to block tumor cell proliferation and tumor growth.282 Additionally, peptide transduction domain–dsRNA binding domain (PTD-DRBD) fusion proteins efficiently deliver siRNAs.283 In vivo delivery of EGFR and Akt2 siRNAs via PTD-DRBD triggered apoptosis specifically in tumor cells and prolonged survival in mice bearing glioblastoma.284 Moreover, secondary amphipathic α-helical CPPs feature a highly hydrophobic patch on one side, whereas the other side can be cationic, anionic, or polar.285,286 In an interesting study, the siRNA delivery efficiencies of cationic and amphipathic CPPs were compared, and the results revealed that the amphipathic CPPs are more suitable as carrier moieties for delivering siRNA polyplexes.287 In addition to MPG and Pep-1, other known primary amphipathic CPPs are derived from natural proteins. These CPPs include an 18-amino acid-long peptide originating from vascular endothelial cadherin (pVEC),288 a 22-amino acid-long peptide derived from the N-terminal region of p14ARF, named ARF (1–22),289 and a peptide sourced from the N-terminus of the unprocessed bovine prion protein (BPrPr).290 Secondary amphipathic CPPs typically display a unique structure, adopting an α-helical conformation wherein hydrophilic and hydrophobic amino acids are segregated onto separate faces of the helix.291 The use of secondary amphipathic CPP-based delivery systems provides therapeutic carriers with high delivery efficiency and no cytotoxicity or immunogenicity. Model amphipathic peptide (MAP) is a well-studied CPP featuring an α-helical structure with hydrophilic and hydrophobic residues arranged on opposite sides of the helix.292 Comprising a sequence of alanines, leucines, and lysines (KLALKLALKALKAALKLA),292 MAP has been shown to be an effective carrier for delivering siRNA into cells.293,294 CADY is a 20-residue peptide that adopts a helical conformation within cell membranes and forms stable complexes with siRNAs.291,295 Another type of amphipathic CPPs contains proline-rich CPPs. Although these CPPs vary in sequence and structure across different families, they all feature a proline pyrrolidine template.296,297,298 Apidaecin and oncocin belong to this group of peptides and can permeate the BBB in mice.299,300 PR39 is a proline- and arginine-rich peptide implicated in wound healing and protection against myocardial ischemia.301 PR39 has been utilized as an innovative carrier for delivering siRNAs into the cell cytoplasm, and loading a Stat3 siRNA has shown synergistic effects in suppressing the invasion and migration of 4T1 cells.302
Hydrophobic CPPs with a low positive or negative net charge tend to have poor solubility and easily aggregate.303 Hydrophobic CPPs have recently received increasing amounts of attention due to the extensive presence of positive charges that cause cationic CPPs to be toxic.304 For example, natural C105Y, its C-terminal portion (PFVYLI), and Pep-7 are part of this group, and the affinity of their nonpolar amino acids for the hydrophobic domain of cell membranes aids in their translocation.305,306,307 Several modified peptides can also be generated using various approaches.308 The chemically modified hydrophobic CPP TP10 has four cationic amino acid (Lys) residues among the many hydrophobic amino acid residues, enabling efficient delivery of a splice-correcting 2’-OMe RNA oligonucleotide.309 Therefore, hydrophobic CPPs hold significant potential for the noncovalent delivery of negatively charged oligonucleotides.
Although the groundbreaking and versatile peptide tools have received heightened interest in their application within gene therapy, peptide nanocarriers must be tested in clinical trials before they can be applied in clinical settings. Therefore, additional basic science and preclinical studies are needed to accelerate peptide-based vector development.
Polymers
Since the first human trial approval (NCT01644890) was obtained in the early 1990s, polymeric drugs have entered the market as potent therapeutics.310,311 Innovative polymer-based drug delivery systems have gained significant interest in gene therapy. The polymers employed for gene delivery differ greatly in terms of molecular weight, structure, and composition.312 With linear, branched or dendritic structures, these polymers electrostatically bind negatively charged nucleic acids through their inherent proton sponge behavior, offering protection and promoting cellular uptake.312,313,314 Further chemical modifications of polymers optimize the transfection efficiency, biocompatibility, cell selectivity, and in vivo distribution and reduce cytotoxicity.313,315 In general, synthetic and natural polymers are completely distinct. Polymers derived from natural sources, such as plants, animals, and microorganisms, have the advantages of biocompatibility, mechanical properties, biodegradability and renewability, whereas synthetic polymers are easier to generate and modify and have been widely utilized in all applications due to their structural and mechanical properties; however, they are unable to perform certain biological functions.316,317,318 One of the first widely utilized polymers in gene delivery was polyethyleneimine (PEI), has shown great potential for transfecting dividing cells.319 Regrettably, its cytotoxicity has restricted its application in vivo and in clinical trials, as it has been found to cause membrane damage and initiate apoptosis in clinically relevant human cell lines.320 Currently, a range of polymers, such as poly(b-amino esters), poly(amido ethylenimines), and dendrimers, have emerged,321 demonstrating high transfection efficiency and low cytotoxicity (Table 3).322,323,324,325,326,327,328
Poly(L-lysine) (PLL)
Poly-L-lysine (PLL), synthesized from L-lysine found in high-protein foods like meat and eggs, has inherent properties of non-toxicity, non-antigenicity, biocompatibility, and biodegradability.329,330 As a cationic polymer, PLL can be protonated in physiological conditions, allowing it to electrostatically bind with negatively charged nucleic acids and form complexes.331 Although PLLs are biodegradable, their high degree of cationic toxicity limits their use in nucleic acid drug delivery.322,332 Chemical modifications, such as the introduction of polyethylene glycol (PEG), have been implemented to reduce the toxicity and increase the transfection efficiency of PLLs and thus improve their gene delivery performance. For instance, Kazunori Kataoka’s group developed a PEG-block-poly(L-lysine) (PEG-b-PLL) that features lysine amines modified with 2-iminothiolane (2IT) and the cyclo-Arg-Gly-Asp (cRGD) peptide at the PEG terminus.333 This modification of PEG-b-PLL resulted in better control over micelle formation and enhanced siRNA stability in the bloodstream; subsequently, the incorporation of siRNA into these micelle structures increased cellular uptake, improved the gene silencing efficacy of the siRNAs and increased drug accumulation in both the tumor tissue and tumor-associated blood vessels following intravenous injection.333 This successful application of the PLL modification potentially expands the utility of polymer-based siRNA therapies for cancer treatments that require intravenous injection.333 Dendritic PLL, which allows for the control of molecular size and shape, can also be utilized for gene delivery.334 The 6th generation of dendritic poly(L-lysine) (KG6), with 128 amine groups on its surface, was demonstrated to be an efficient siRNA carrier with minimal cytotoxicity.335 Another star-shaped copolymer consisting of a β-cyclodextrin core and PLL dendron arms was used for docetaxel and MMP-9 siRNA codelivery.336 A biomimetic nanocomplex consisting of a cationic nanocore formed by a membrane-penetrating helical polypeptide (P-Ben) and Sav1 siRNA, a charge-reversal intermediate layer of PLL-cis-aconitic acid (PC), and an outer shell of a hybrid membrane, was administered intravenously. This nanodrug effectively accumulated in the ischemia‒reperfusion-injured myocardium.337 Although polymeric materials are increasingly used in the development of second-generation polymer therapeutics, the applications of PLLs are still relatively restricted compared to other polycations like chitosan (CS), poly(ethyleneimine) (PEI), and poly(amido amine) (PAMAM) and further investigations should explore the development of an appropriate regulatory framework that can be universally applied in research.
PEI
PEI is a synthetic polymer introduced in 1995, featuring an amine group and two CH2 spacers making it a cationic polymer suitable for nucleic acid delivery..338,339 PEI has two distinct chemical structures, namely, branched PEI (BPEI) and linear PEI (LPEI), with molecular weights ranging from 1 to 1000 kDa.340,341 BPEIs contain all types of various primary, secondary and tertiary amines, whereas LPEIs contain only secondary and primary amino groups.341 Interestingly, the capacity of PEI for gene delivery and cytotoxicity are greatly affected by its molecular weight. Therefore, it is important to carefully adjust the PEI structure to balance transfection efficiency and toxicity.342 Qiao et al. developed a successful PEI-siRNA delivery system, the dual siRNA-PEI (siRP) complex locally knocked down the target gene.343 Additionally, SNS01-T is a PEI-based formulation containing two nucleic acids: an siRNA and a plasmid. It has been shown to inhibit tumor growth in various animal models of B-cell cancers and demonstrates acceptable tolerability.344,345 In a Phase I/II clinical trial (NCT01435720), SNS01-T was administered intravenously in multiple doses and dose escalation stages to evaluate the safety and tolerability of the vector in patients with recurrent and refractory B-cell lymphoma. Although the trial was activated in 2011 and was completed in 2014, the results have not yet been published. Only one PEI-based small nucleic acid drug has been tested in clinical trials, highlighting the significant potential of novel PEI-based formulations to overcome current challenges related to drug stability.
Poly(β-amino esters) (PBAEs)
Poly(β-amino esters) (PBAEs) are a promising group of cationic polymers made from acrylates and amines, extensively developed for drug delivery.346,347 PBAEs are pH sensitive and protonated in acidic environments; they can be soluble at acidic pH levels but insoluble at physiological pH levels.348 PBAE-based drug delivery systems primarily enter cells by energy-dependent endocytosis and are split into separate vesicles by lipid bilayers.349 These separate vesicles are internalized into endosomes (pH<5.5), which subsequently transform into lysosomes (pH<4.5).350,351 Various PBAEs, synthesized from different diacrylate and amine combinations, offer diverse physicochemical and mechanical properties for drug delivery.346,352 In recent years, PBAEs have been extensively studied for convenient synthesis, low cost and good biocompatibility, and they can effective at activating antigens and boosting immune responses.353,354,355,356 Bioreducible PBAEs self-assemble with siRNAs in aqueous conditions to form nanoparticles and the ability of simple polymeric nanoparticles to efficiently knock down genes in primary human glioblastoma cells was greater than that of Lipo2000.357 Additionally, synthetic end-modified poly(beta-amino ester) (PBAE)-based nanoparticles can improve siRNA delivery into human mesenchymal stem cells (hMSCs).358 Although PBAEs were not approved for clinical use following initial human studies, their significant benefits make them promising candidates for medical applications.
Dendrimers
Dendrimers are highly branched, monodisperse, tree-like macromolecules.359 Unlike hyperbranched polymers, dendrimers exhibit random branching and have well-defined and regular nanoarchitectures with spherical shapes.360,361 Moreover, the surface of dendrimers can be chemically modified in multiple ways to alter the functionality of the macromolecules, and the branched nature of dendrimers results in a very high surface-to-volume ratio.362 A relatively empty dendrimer matrix is amenable to host molecule entrapment, facilitating precise, controlled payload release. Dendrimers are prepared via divergent or convergent synthesis.363 The divergent synthesis method has the advantage of modifying the dendrimer molecules starting from the core, whereas the convergent method allows greater control of the modification of molecules at specific positions responsible for specific functions.364,365 Commercially available poly(amidoamine) (PAMAM; Starburstk) dendrimers and poly(propylenemine) (also called PPI, DAB; AstramolR) dendrimers have been most widely studied and applied in drug delivery.366,367 Therapeutic drugs can be encapsulated within the core of PAMAM or conjugated to their surface, facilitating delivery to target cells. The development of covalently bonded hydroxyl-terminated PAMAM dendrimer–siRNA conjugates allows for precise nucleic acid loading, which has been observed using a GFP-targeted siRNA (siGFP) conjugate (D-siGFP).368 Additionally, the conjugation of PAMAM to the thermosome acts as an anchor for siRNAs, and further modification of the protein cage with a CPP increases the delivery efficiency of the siRNA transfection reagents.369 However, the synthesis of dendritic architectures through convergent or divergent methods may disrupt the design and construction, resulting in increased difficulty in mass production. A novel PEG modification strategy that preserves the surface amino groups of polymers has been proposed.370 Catechol-PEG polymers were used to modify the surface of PBA-modified generation 5 (G5) PAMAM dendrimers (G5PBA) via reversible boronate esters.370 This approach maintains the free amines of G5PBA, aiding in siRNA loading, stable complex formation, and increased transfection efficacy in serum.370 PEG-modified dendrimer/siRNA polyplexes show a similar gene silencing efficacy to non-PEG-modified polyplexes under serum-free conditions but superior performance in serum due to PEG shielding.370 In vivo, PEG- and RGD-modified dendrimer/siRNA nanoassemblies target tumors effectively, with PEG dissociating in acidic environments, allowing efficient gene silencing by G5PBA/siRNA polyplexes.370 Generally, the activation of functional groups on both PAMAM and the thermosome facilitates conjugation, typically using a crosslinking agent such as EDC/NHS (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide) to form stable amide bonds.371 Once the PAMAM dendrimers are conjugated to the thermosome, the positively charged surface amine groups of PAMAM can electrostatically interact with the negatively charged phosphate backbone of the siRNAs. This strong electrostatic interaction allows siRNA molecules to bind efficiently to the surface of the modified thermosome.369,368 One of the most common methods for attaching CPPs to the protein cage involves covalent bonding372 This process is typically achieved through reactive side chains on the amino acids of the protein cage. For example, lysine residues, which contain primary amine groups, can be targeted for conjugation with CPPs that have been preactivated with functional groups such as NHS esters or maleimides.373
Click chemistry is an efficient method for dendronizing β-cyclodextrin macrocycles, and the emergence of click chemistry has led to the generation of a dendritic delivery system with good yield and increased uniformity.374,375 This approach facilitates the rapid and reliable formation of covalent bonds between molecular components, resulting in the synthesis of dendritic systems with excellent yields. Moreover, click chemistry reduces the likelihood of side reactions, leading to increased uniformity of the final product.376 Copper-assisted azide‒alkyne cycloadditions (CuAAC), thiol‒ene click (TEC) reactions and Diels–Alder (DA) reactions are commonly applied to dendrimers. Luis José López-Méndez et al. introduced a novel dendritic material created by combining β-cyclodextrin (βCD) with second-generation poly(ester) dendrons.374 The dendrons were selectively attached to the seven positions on the primary face of βCD via a CuAAC click reaction. This method, along with a straightforward work-up process, enables the production of monodisperse materials with exceptionally high yields.374 Several hundred articles were surveyed and analyzed in this field. Due to the length of this review, we have not given special attention to the click chemistry applied to dendrimer synthesis.
Exosomes
Exosomes (also called extracellular vesicles) are nanosized vesicles, ranging from 30 nm to 150 nm, composed of various proteins and RNAs surrounded by a lipid bilayer membrane, similar to liposomes in structure.377 The concept of exosomes was first proposed in 1981; Trams et al. reported that exfoliated membrane vesicles with 5’-nucleotidase activity are present in the culture supernatants of some cell lines and these vesicles may have a physiological function.378 Natural exosomes are derived from the endocytosis of plasma membrane.379,380 First, invagination of the cytoplasmic membrane is processed to form the early endosomes (ESEs). Then, by exchanging materials with other organelles, the early endosomes form late endosomes (LSEs) and further sprout into multivesicular bodies (MVBs). After MVBs fusing with cell membrane, exosomes are finally bud.381 Since exosomes are derived from natural cells, they not only are highly biocompatible but also have low immunogenicity, thus realizing high transfection (in vitro) and delivery (in vivo) efficiencies without inducing serious adverse effects.382 Once considered a trivial biological phenomenon, exosomes have recently garnered much attention for use as nucleic acid delivery systems.383 Rosas, L. E. et al. added of HEK293T-derived exosomes into human monocytic cell lines, results showed that no potential cytotoxic effects were observed.384 Furthermore, a series of studies revealed that no severe immune reactions were observed in mice or humans following repeated administration of exosomes either from mice or humans.385,386,387 In addition, exosomes possess a unique homing effect in which they preferentially target the parent cell from which the exosomes are produced.388
There are two major types for nucleic acids loading, including active loading, which includes mechanical extrusion,389 sonication,390 electroporation391,392,393,394,395,396 and repeated freeze‒thaw cycles,397 and passive loading, which includes direct incubation398 and exosome transfection.399,400 Shan et al. used exosome membranes obtained from repeated freeze‒thaw cycles to encapsulate DEP-siRNAs by coextrusion with a liposome extruder (220 µm, 12 times), thus generating a stable EM@DEP-siRNA complex.401 The mixture consisting of siRNAs and exosomes was ultrasonicated at an ultrasonic power of 25 W for 6 cycles. After incubated for 30 min at 4 °C, Angiopep-2 was added for a 24 h incubation. Finally, the unconjugated An2 and unloaded siRNA were removed to obtain Exo-An2-siRNA.390 Electroporation is the most established method used for this purpose; however, the conditions may vary among protocols, and numerous methods can achieve efficient loading to modulate target gene expression.402,403,404,405,406 The direct incubation of exosomes and siRNAs is time consuming with low efficiency. Tian et al. modified the siRNAs with 2′Ome through the conjugation of cholesterol at the 3′-terminus, thus increased the siRNAs loading efficiency. The loading efficiency of cholesterol-modified siRNAs in exosomes was highly increased and nearly 3-fold greater than that resulting from electroporation.398 Moreover, Exo-Fect is commonly used as a cell transfection reagent. After the exosomes were mixed with Exo-Fect and the desired nucleic acid cargo and incubated for an hour, the complex was added to the recipient cells. The Exo-Fect-induced loading procedure is time-saving and easy and increases the cellular uptake of the nucleic acid cargo.407 Nucleic acids loaded exosomes can be endocytosed by cells through different uptake pathways, including clathrin-coated pit-mediated internalization and micropinocytosis.408 The exosomal uptake efficiency of phagocytic cells is much greater than that of nonphagocytic cells.409 These internalization pathways can be divided into two main types: direct membrane fusion, which is the most likely route, and receptor-mediated endocytosis.399
However, the development of exosome delivery systems faces many difficulties. First, natural exosomes can migrate everywhere in vivo and do not have specific organ-targeting abilities, as verified by multiple experiments.410 Therefore, engineered exosomes are conducted to enhance their specific targeting of cells and organs. The most commonly used strategy involves modifying specific ligands or receptors on the membrane of exosomes. Through genetic engineering technology, homing proteins or polypeptides that effectively target recipient cells can be expressed on the membrane of exosomes. Generally, the plasmids are inserted with targeting peptide-fused exosomal membrane proteins, such as CD63, CD81, and Lamp2.411 For example, Liang et al. fused Her2, involved in tumor progression, to the exosomal surface to generate exosomes with the ability to target tumor cells, and the results revealed efficient accumulation and therapeutic effects both in vitro and in vivo.412 Additionally, Li et al. constructed peptide-fused Exos that contained CP05 and titanium-binding peptide (TBP), thereby achieving an exosome-targeted titanium implant.413 On the other hand, chemical modifications can directly conjugate target proteins or peptides to the membrane of exosomes. For example, Gunassekaran et al. produced IL4R-Exos (si/mi), the surface of which was modified with IL4RPep-1, and the accumulation of these exosomes was greater in tumors than in the liver.414 Ran et al. inserted neuron-targeting RVG peptides into the membrane with His-CP05 to form AP-ExoRs, and ex vivo imaging revealed a significantly increased fluorescence intensity.415 Chemical modification after exosome extraction is easier and more convenient than chemical modification prior to extraction, and biological engineering can protect exosomes without introducing other unwanted chemicals. In addition, selective organ targeting can also be achieved through physical engineering. For example, vesicle shuttles, consisted of an Fe3O4 core, a silica shell and anti-CD63 antibodies (exosome capture), can lead to a local release of the captured exosomes via magnetic fields.416 However, large-scale exosome production for clinical application is still difficult and expensive.417 In addition to stimulating exosome release through processes such as phototherapy-based light induction,418 the upregulation of certain genes (STEAP3, syndecan-4 and a fragment of L-aspartate oxidase),419 the introduction of electrical pulses provided by a cellular nanoporation biochip,420 and the production of bioinspired exosome-mimetic nanovesicles (NVs) have greatly increased the yield. NVs are produced by the breakdown of cells through serial extrusion using several filters of different decreasing sizes (10, 5, and 1 μm), similar to the natural production of exosomes.421 Furthermore, exosomes derived from tumor cells, especially tumor cells with an amplified oncogene, may transfer this oncogene along with nucleic acid drugs to target cells, further stimulating tumor growth since additional tumor-specific genetic properties, such as oncogenic DNA (c-Myc) and retrotransposon elements (HERV, L1 and Alu sequences), are present in these exosomes.422 However, in addition to immortalized cells, all nucleated cells present some level of risk for horizontal gene transfer (HGT). Usman et al. proposed generating exosomes from mature human red blood cells, which lack both nuclear and mitochondrial DNA, to resolve this issue, thus completely abrogating the risk of gene transfer.423 More detailed information about the exosome systems mentioned in this section is listed in Table 4.
Conjugates
A drug conjugate is an integrated drug delivery system comprising therapeutic agents and one or more carriers through a biodegradable linker.424,425 The conjugate that has received the most clinical attention is a trivalent carbohydrate ligand based on N-acetylgalactosamine (GalNAc), which has been approved by the FDA.426,427,428 The strategy of GalNAc-based conjugation relies on the specific recognition and combination of GalNAc with the asialoglycoprotein receptor (ASGP-R), located on the basolateral membrane of hepatocytes with a very high density.429 ASGP-R was first isolated in 1974. Both Hudgin et al. and Stockert et al. reported that ASGP-R is a galactose-binding protein,430,431 whereas Baenziger et al. reported ASGP-R prefers GalNAc, compared with galactose.432 ASGP-R contains four functional domains, namely, the cytosolic domain, transmembrane domain, stalk, and carbohydrate recognition domain (CRD), which can bind to the GalNAc ligand.433 Moreover, in a previous study, the sialic acid residue of ASGP-R was removed to expose the sugar residues and improve the binding affinity to GalNAc.434 Classical GalNAc-based nucleic acid drugs comprise three components: a triantennary GalNAc, a linker and a nucleic acid molecule.435 GalNAc is a sugar derivative obtained by acetylation after the C-2 hydroxyl group on galactose is replaced by an amino group. Lee et al. demonstrated that the inhibitory potency of the tetraantennary molecule was greater than that of the triantennary molecule, whereas that of the triantennary molecule was much greater than that of both the biantennary and monoantennary molecules (tetraantennary > triantennary >> biantennary >> monoantennary).436 Furthermore, the spacers between the GalNAc moieties and the branching point of the dendrite can also change the affinity for ASGP-R. The most potent sugar spacing was 20 Å (20 Å >10 Å >4 Å).437 Another investigation suggested that the cargo size of GalNAc-based drugs should be less than 70 nm for proper receptor recognition and efficient endocytosis.438
After GalNAc-conjugated drugs bind to ASGP-Rs, the complex is endocytosed through clathrin-mediated pathway.439 The GalNAc linker is then quickly removed before the nucleic acid escapes into the cytoplasm.440 Moreover, ASGP-R is recycled to the cell surface quickly (less than 15 min) for another internalization of nucleic acid cargo.441 Many studies have verified that attachment of the GalNAc moiety can greatly increase the efficiency of hepatocyte-targeted delivery by approximately 6–7-fold.442,443 Prakash et al. reported that unconjugated ASOs are predominantly ( > 70%) taken up by nonparenchymal cells in the liver, while GalNAc-conjugated ASOs prefer hepatocyte fraction in the liver ( > 80%).444 Sirnaomics designed an upgraded GalNAc platform named peptide docking vehicle-GalNAc (PDoV-GalNAc) which provides two binding sites for siRNAs and exhibits excellent property in endosomal escape. Thus, the PDoV-GalNAc can both accelerate the speed at which the drug cargo is delivered and increase the efficiency of target gene knockdown.
Other types of conjugates include cholesterol, tocopherol, vitamin E and fatty acids. Wolfrum et al. constructed cholesterol-conjugated siRNAs and performed in vivo experiments showing that they can silence gene expression, whose endocytosis is associated with some lipoprotein particles.445 Moreover, through covalent binding of alpha-tocopherol (vitamin E) to the 5’ end of antisense strand, the endogenous mRNA target in the liver can be effectively knocked down by conjugated siRNAs.446
Since ASGP-R is highly expressed on parenchymal hepatocytes and minimally expressed on other cells, GalNAc conjugates can be constructed to target liver lesions. Scharner et al. ectopically expressed ASGP-R in nonhepatic cancer cell lines to achieve extrahepatic delivery, results showed increased internalization efficiency of GalNAc-conjugated ASOs in vitro and greater splicing modulation than that mediated by unconjugated ASOs in vivo.447 In addition, lipid conjugation can cause small nucleic acids to accumulate in extrahepatic tissues and subsequently leads to gene silencing, providing an opportunity to realize extrahepatic delivery of therapeutic small nucleic acids. By systematically comparing the body distributions of a set of fatty acid-conjugated siRNAs, Biscans et al. reported that trivalent lipid-conjugated siRNAs mainly resided at the injection site, whereas monovalent lipid-conjugated siRNAs were rapidly released into the circulation and subsequently accumulated mainly in the kidney, and divalent lipid-conjugated siRNAs exhibited intermediate behavior and preferentially accumulates in the liver.448
Inorganic nanoparticles
Inorganic nanoparticles mainly include metal, semiconductor, and metal oxide nanoparticles and exhibit various physicochemical properties.449,450,451 Inorganic nanoscaffolds offer access to distinctive magnetic and optical properties based on their unique physiochemical characteristics and structural capabilities.452,453 The inorganic nanoparticles employed for nucleic acid delivery have become a critical component in the targeted treatment of diseases. We focus on four classes of inorganic materials in this section.454
Gold
Gold is a versatile nanoparticle core for vector delivery and can form monodisperse nanostructures with high specificity.455,456 Gold nanoparticles (AuNPs) are tiny particles ranging from 1 to 100 nm in diameter, and their flexible sizes and shapes facilitate the loading of proteins, peptides, oligonucleotides, or small drug molecules.457,458 The in vivo characteristics of various AuNPs are determined by their sizes, shapes, surface charges, and surface coatings.459,460,461,462 For example, gold nanorods (AuNRs) were created, conjugated with CLPFFD (a brain-targeting peptide) and associated with siRNA-PARP-1, and the results revealed that this complex can cross the BBB of neonatal rats.463 Among the different sizes of AuNPs, medium- to large-sized AuNPs ( ≥ 10 nm) have been extensively studied due to their advantageous physical properties.464 A variety of coating methods, such as ligand substitution, chemical moieties or embedding in a carrier matrix, can also be applied to better employ AuNPs in pharmacology.465 The most widely utilized coating polymer is PEG, and PEG-coated AuNPs have a nearly neutral surface and are highly hydrophilic. Therefore, the PEG modification prevents nonspecific protein adsorption on the AuNP surface, increasing the uptake efficiency of the EPR and increasing the retention time in the circulation.466,467,468 Nucleic acid strands can be easily modified and attached to AuNP cores in a selective and cooperative manner, usually via thiol moieties.469,470 In early years, the synthesis and characterization of polyvalent RNA‒gold nanoparticle conjugates (RNA‒AuNPs) were reported.471 The particles were stable and protected from degrading enzymes, with a high efficiency in loading siRNA duplexes onto their surface, and produced robust gene knockdown.471 For the past few years, extensive delivery of small nucleic acids via AuNPs has been performed in animal experiments. Wan et al. developed a PEG-SH-GNP-SAPNS@miR-29a delivery system aimed at repairing injured spinal cords by creating a regenerative microenvironment that encourages the recruitment of endogenous neural stem cells.472 Chen et al. created aptamer-siRNA chimeras combined with PEI/PEG/AuNP/collagen membranes that can sequentially activate T cells through a layer-by-layer assembly approach.473 PLL is also used to decorate gold nanoparticles and efficiently deliver a Sema3A siRNA.474 Moreover, gold cores are inert, highly biocompatible, and safe.459,460 However, many aspects can be improved for an ideal AuNP drug delivery process. Prolonging the plasma circulation time, enhancing the target accumulation, improving the cellular uptake and controlling the intracellular release of AuNPs are necessary to optimize their pharmacology.
Silica nanoparticles (SiNPs)
Due to their advantages in terms of drug solubility and an extended release rate, SiNPs, especially porous nanoparticles, have attracted increasing interest.475,476 SiNPs possess tunable porous structures, excellent stability, and ease of functionalization, which make them promising platforms for nucleic acid delivery.477,478 SiNPs commonly offer alternative routes for medical treatment, such as inhalation, ingestion, transdermal absorption, and intravenous injection.479 Significant advancements have been achieved in the use of SiNPs for nucleic acid delivery and application to improve intentional modifications as well as change the surface chemistry, size, shape, and charge.480,481 The repulsive negative charges on the surfaces of nucleic acids and mesoporous silica nanoparticles (MSNPs) prevent them from interacting directly; thus, nucleic acids can be incorporated into MSNP pores via hydrogen bonding.482 Kumar et al. developed an MSNP-based multifunctional nanocarrier for the codelivery of drugs that kill drug-resistant triple-negative breast cancer cells and validated its therapeutic effects on a Shahin et al. used hyaluronic acid (HA)-modified mesoporous silica nanoparticles (MSNPs) to target CD44 on cancer cells, delivering TWIST-siRNA to inhibit TWIST protein expression in ovarian cancer cells..483,484 Moreover, the oral administration of only one MSNP system has been approved by the FDA.485 Additionally, the unique properties of MSNPs, such as their negative charge and large surface area, can be covalently modified to generate cationic substances and greatly improve drug encapsulation and release rates; PEI, dendrimers and lipids have been utilized to modify the surfaces of MSNPs for the delivery of nucleic acids.486,487
Moreover, prior studies have shown that exposure to SiNPs can cause adverse biological effects in various organs.488,489 The dose, exposure, route of administration, particle size, shape and composition have combined effects on nanoparticle toxicity.490 Acute toxicity is commonly observed with SiNPs, but there is a lack of information regarding their long-term toxicity. The toxicity of SiNPs depends on their physicochemical properties and is mediated by distinct mechanisms. Some researchers have confirmed that pristine SiNPs with a diameter of 50 nm caused targeted lung injury, while silica particles with a diameter of 3 μm significantly reduced the severity of lung damage. Additionally, recent evaluations have assessed the biocompatibility of SiNPs in the liver.488 This research group also revealed that SiNPs promoted lipid accumulation and aggravated metabolic-associated fatty liver disease (MAFLD) progression.488 Additionally, the cardiovascular effects of SiNP exposure have been gradually confirmed.491,492,493 A long-term study compared the acute (10-day) and subchronic (60-day and 180-day) toxicity of nonporous SiNPs with diameters of approximately 50 nm and 500 nm, as well as mesoporous SiNPs with a diameter of about 500 nm, following a single-dose intravenous injection into male and female BALB/c mice.494 Pathological lesions were predominantly observed with the administration of large nonporous SiNPs, while small nonporous or mesoporous SiNPs showed no significant toxicity.495
Iron oxide nanoparticles (IONPs)
IONPs contain maghemite (γ-Fe2O3) and magnetite (Fe3O4) at certain sizes, which allows them to be used as delivery vehicles.496,497 In recent years, various researchers have investigated the structure, magnetic properties, and biological effects of IONPs, especially surface-engineered cationic IONPs, on electrostatic interactions with nucleic acids.498,499 In the late 1970s, Widder and Senyi were the first to document the capability of magnetic fields to control nanoparticles for drug delivery purposes.500,501 This application delivers drugs directly to the brain, which is difficult to achieve using traditional methods. Small ( < 1 μm) magnetic nanoparticles can reside in glioma tissues.502 Recently, IONPs functionalized with peptides were used to deliver si-MGMT to glioma cells, which exhibited significantly increased sensitivity to temozolomide treatment.503 With the assistance of RVG (a brain-targeting peptide), biomimetic iron oxide nanorods loaded with an NF-κB siRNA targeting neurons have been developed and shown to improve memory and cognitive abilities in Alzheimer’s disease model mice.504 In addition, IONPs combined with engineered exosomes can penetrate the BBB and encapsulate siRNAs for GBM therapy.505 IONPs offer several benefits, including the ability to be magnetically directed to disease sites, tracked using contrast imaging, and precisely release drugs upon heat stimulation (Table 5).506
Vector-free delivery
Although delivery systems can increase uptake efficiency in vitro and alter accumulation in target organs in vivo, delicately designed small nucleic acid drugs, which can be directly used without any vectors, exhibit high biosafety, few side effects and low immunogenicity in the human body.507 ALN-RSV01, which targets the human respiratory syncytial virus (RSV) nucleocapsid gene, can reduce the viral titer to background levels after the intranasal instillation of the molecule with 2’-methoxylation of certain nucleotides and the addition of hydroxyproline in RSV-infected mice.508 SYL040012, an siRNA that targets ADRB2, can be rapidly distributed among structures of the anterior segment of the eye after direct administration in eye drops.509 Zheng et al. constructed an siRNA-based vehicle (siRNAsome) composed of self-assembled siRNA-disulfide-poly(N-isopropylacrylamide) diblock copolymers with a hydrophilic interior. This siRNAsome can be directly endocytosed by MDR MCF-7 cells without a transfection agent.510 Through the grafting of a cationic photosensitizer (NB-Br) onto siRNAs, an amphiphilic conjugate can be obtained for further self-assembly into nanoparticles. In vitro experiments revealed rapid and efficient cell endocytosis and endosomal escape; in vivo experiments revealed the inhibition of tumor growth.511 However, several hurdles must be overcome before the wide use of vector-free small nucleic acids in the clinic, including their easy degradation by nucleases, lack of specific delivery, poor stability and unpredictable in vivo fate.512
Commercial platforms for smart small nucleic acid drug design
The landscape of small nucleic acid therapeutics has rapidly advanced, driven by innovative design platforms that enable the precise targeting of genetic material. These platforms represent the forefront of next-generation medicine.
Dicerna possesses delivery platforms capable of targeting the (GalXC™) and extrahepatic tissues (GalXC-Plus™), both of which can greatly complement Novo Nordisk’s existing therapeutic pipeline. The GalXC™ platform is a cutting-edge technology designed to precisely target the liver in drug delivery. By leveraging the high liver selectivity of GalNAc, GalXC™ constructs structurally specific siRNAs. The Dicer enzyme is the natural starting point for RNAi within human cells, and Dicerna directly links the GalNAc to the extended region of proprietary Dicer-substrate short interfering RNA (DsiRNA) molecules. This strategy allows the creation of various conjugated delivery structures that can flexibly and efficiently bind to target ligands while stabilizing the RNAi duplex, achieving precise targeting. Unlike other GalNAc delivery systems, GalXC™ introduces a unique tetraloop structure in the passenger strand, with monovalent GalNAc conjugation that enhances conjugate stability. Additionally, this design accurately positions multiple GalNAc ligands, effectively delivering siRNAs to liver cells. Furthermore, GalXC RNAi technology has exceptional pharmacological properties that significantly reduce the risks associated with drug development. GalXC-Plus builds upon the strong preclinical and clinical characteristics of the original GalXC platform, offering customizable nucleic acid structures and a variety of ligands. This platform enables RNAi drugs to be delivered to a wide range of tissues, including the central nervous system, muscles, adipose tissue, and tumors. The platform provides additional flexibility for drug optimization and expansion into new therapeutic areas. Dicerna’s Dicer-substrate RNA and tetraloop conjugation technology theoretically provide certain advantages over Alnylam’s corresponding technology. The successful completion of phase 3 clinical trials and subsequent approval of nedosiran demonstrated that GalXC™ technology is not only theoretically sound but also effective in practice.
The Arrowhead TRiM™ platform is a powerful and universal tool that employs a comprehensive array of RNAi delivery technologies, chemical modifications and structural designs. The platform utilizes specialized targeting ligands combined with unique RNA modifications to achieve the highly effective and efficient suppression of AGER gene expression. ARO-RAGE is an investigational RNAi-based drug designed using the TRiM™ platform that is intended for the treatment of inflammatory lung diseases, such as asthma. ARORAGE-1001 is an ongoing phase 1/2a randomized, double-blind, placebo-controlled study designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of ARO-RAGE in normal healthy volunteers and asthma patients (NCT05276570).
Nano-systems play a crucial role in the delivery of small nucleic acids, as they enhance the stability, targeting, and efficacy of these therapies. Each type of carrier system offers unique benefits that make it suitable for specific applications. In conclusion, leveraging the unique properties of various carrier systems can significantly enhance the effectiveness of small nucleic acid therapies, facilitating their translation from bench to clinic and back again (Table 6).
Clinical translational studies of nucleic acid therapy
Small nucleic acids represent promising therapeutic options for various diseases, and their clinical translation is advancing with the approval of multiple ASOs and siRNA drugs for clinical use (Table 7). This section reviews the clinical trial outcomes and the current status of each drug that has been approved or is in the clinical trial phase (Fig. 6).
Summary of the characteristics of small nucleic acid drugs used in clinical trials. a Various administration routes for small nucleic acid drugs, including subcutaneous, intravitreal, intravenous, inhalation, and oral administration. Blue box: in a clinical trial; green box: on the market; red box: delisted. b Administration routes of small nucleic acid drugs on the market. c Administration routes of small nucleic acid drugs in clinical trials. d Different phases of clinical trials for small nucleic acid drugs. e Different target organs of small nucleic acid drugs in clinical trials
siRNA- and ASO-based therapeutics
Infectious diseases
Hundreds of millions of people suffer from acute or chronic infectious diseases for which no satisfactory treatments or effective vaccinations are available. In fact, in contrast to patients with monogenic disorders or cancer, patients with infectious diseases are rarely treated with nucleic acid drugs.513 Moreover, among these infectious diseases, hepatitis, including HBV, HCV and HDV, is the most intensively researched disease in the context of treatment with small nucleic acid delivery systems.514
Among the 6 ASO drugs tested, GSK3228836, also called bepirovirsen, has entered phase III clinical trials (B-Well 1 and B-Well 2), the last step before submission for approval by the FDA, and the aims of the trial are to evaluate its safety and efficacy in treating chronic HBV (NCT05630820 and NCT05630807, respectively). This ASO drug is modified with 2ʹ-MOE to target all HBV RNAs, and this trial is expected to enroll 900 patients and be completed in 2026. Moreover, GSK3389404, also developed by GlaxoSmithKline; RO7062931, developed by Roche; and ALG-020572, developed by Aligos Therapeutics, are all GalNAc conjugates with the same target as bepirovirsen. Unfortunately, ALG-020572 was terminated because of multiple reports of a sudden increase in alanine transaminase (ALT) levels in patients enrolled in a phase 1 clinical trial, which suggested the occurrence of severe adverse events resulting from hepatotoxicity (NCT05001022). In addition to hepatitis virus RNAs, host microRNAs can also be optimal antiviral targets for treating hepatitis infection. Early studies documented that miR-122, specifically located in liver, is an essential host factor for HCV infection that can stabilize HCV RNA genomes.515,516,517 For example, RG-101 is a kind of ASO drug that target miR-122.
Most siRNA drugs target the RNA of all hepatitis viruses, whereas VIR-2218 (BRII-835 or ALN-HBV-02) and RBD-1016 target the X gene of HBV; however, RG6346 (DCR-HBVS), ARC-520 and ALG-125755 target the S gene of HBV. As a next-generation RNAi therapy from Arbutus Biopharma, AB-729 showed a significant improvement in efficacy compared with ARB-001467 and ARE-1740 in clinical trials. However, the AB-729 study was terminated early by the study sponsor for strategic reasons (NCT04820686). Moreover, although both animal and clinical trials have shown an efficient decrease in HBsAg levels after ARC-520 and ARC-521 treatment, both drugs were still terminated because of the death of nonhuman primates (chimpanzees) after administration, which might be associated with the excipient-related toxicity of the ARC-EX1 delivery system.518,519,520
Additionally, siRNA drugs have been used to treat other infectious diseases. ALN-RSV01 is a naked siRNA that was developed to be intranasally administered to silence the nucleocapsid protein transcripts of respiratory syncytial virus (RSV). In clinical trials, ALN-RSV01 was shown to have protective effects and reduce the infection rate in healthy individuals (NCT00658086), potentially leading to long-term benefits in lung transplant patients infected with RSV (NCT01065935). Additionally, MIR 19®, a siRNA-based drug, target SARS-CoV-2 RNA-dependent RNA polymerase (RdRp). The results of a phase 2 clinical trial revealed that MIR 19® decreased the recovery time of patients hospitalized with moderate COVID-19 (NCT05184127). Moreover, siRNA drugs for the treatment of Ebola virus infection include TKM-130803, TKM-100201 (TKM-EBOV-001) and TKM-100802, all of which are composed of LNPs and target L polymerase (Lpol) and Viral Protein 35 (VP35), and TKM-100201 and TKM-100802 also target VP24. However, clinical trials have shown that an intravenous infusion of 0.3 mg/kg/d TKM-130803 into adult patients with severe EVD does not improve survival compared with that of historic controls (PACTR201501000997429).521 Due to the corporate decision to reformulate the test product, both the TKM-100201 (NCT01518881) and TKM-100802 (NCT02041715) clinical trials were terminated.
Unexpected toxicity and pathogen escape from mutation pressure are still extraordinary challenges for small nucleic acid therapeutic in infectious diseases. Further improvement is essential to create well-tolerated and effective nucleic acid drugs.
Liver disease
The liver is a major organ of the reticuloendothelial system (RES) that actively participates in many physiological processes.522 Since liver is a central hub for most metabolic pathways, liver dysfunction can lead to systemic and liver-specific diseases.523,524 Owing to the development of GalNAc conjugation and the discovery of the first-pass effect, which refers to the phenomenon in which drugs greater than 300 Da in size accumulate in the liver following administration, several small ASOs and siRNAs for liver-related disorders have been approved.524 In this section, we discuss small nucleic acid drugs developed to treat liver disorders and focus on therapeutics for metabolic disorders such as hyperlipidemia, hemophilia, porphyria and primary hyperoxaluria.
A total of 8 RNA-based drugs, including 3 ASOs and 5 siRNAs, have been approved by the FDA or European Medicines Agency (EMA) for the treatment of liver disorders. As a trailblazer in oligonucleotide therapy, Carlsbad developed Kynamro (mipomersen), approved by the FDA in 2013 for the treatment of heterozygous familial hypercholesterolemia (HoFH) by reducing the expression of apolipoprotein B-100 (ApoB-100).525 Clinical trials showed that long-time mipomersen treatment can sustainedly reduce the expression of all atherosclerotic lipoproteins without serious toxicity observed (NCT00694109).526 In addition, inotersen (TegsediTM), which is the first RNA-targeting hATTR therapeutic, is an ASO drug launched by the Ionis Company for the treatment of hATTR-mediated amyloidosis.527 Inotersen can inhibit the production of transthyretin by binding to the TTR mRNA in the liver, the main TTR synthesis site, to reduce the circulating levels of TTR avoiding TTR deposition in tissues, which can further induce polyneuropathy.528 Phase 3 clinical trials have shown that long-term treatment with inotersen (for >3 years) slows the progression of polyneuropathy and improves the quality of life of hATTR patients without causing new safety issues (NCT01737398, NCT02175004).529,530 Moreover, volanesorsen is a 2ʹ-O-methoxyethyl-modified ASO drug, targeting hepatic apolipoprotein C-III (APOC3) synthesis to further reduce the plasma triglyceride concentration for the treatment of familial chylomicronaemia syndrome (FCS).531 Four key phase III clinical trials supporting the commercial application of volanesorsen (NCT02658175, NCT02300233, NCT02211209, and NCT02527343) have been conducted. These results showed that the administration of volanesorsen works in 77% of patients with FCS, where the triglyceride level was reduced to less than 750 mg per deciliter avoiding acute pancreatitis events; however, thrombocytopenia and injection site reactions are also common adverse events.532,533 As a result, the FDA rejected its marketing application on August 27, 2018, because of the risk of thrombocytopenia. Fortunately, on May 07, 2019, the EMA approved volanesorsen serve as an adjunct for FCS.
The first siRNA drug approved for marketing by the FDA in 2018 was the hATTR-mediated amyloidosis therapeutic drug patisiran (Onpattro™), which is also the only approved siRNA drug that does not rely on GalNAc but rather LNPs to target the liver.15 Since then, interest in siRNA therapies has increased, even surpassing the interest in ASOs. Patisiran can specifically bind to TTR mRNA at the 3’ untranslated region to further block its production in the liver.534 Givosiran (Givlaari™), lumasiran (Oxlumo™), vutrisiran (Amvuttra™) and inclisiran (Leqvio®) are conjugated with GalNAc. Vutrisiran is another siRNA drug used to treat hATTR whose chemical stability and liver targeting are both largely enhanced through GalNAc conjugation, which decreases the subcutaneous injection frequency to once every 3 months.535 In addition, givosiran was approved in 2019 for the treatment of acute hepatic porphyria (AHP), targeting the aminolevulinate synthase 1 (ALAS1) mRNA.16 ALAS is associated with accumulation of neurotoxic δ-aminolevulinic acid and porphobilinogen, which may trigger acute porphyria attacks.536,537 Lumasiran (Oxlumo™) is a siRNA drug targeting the hydroxyacid oxidase 1 gene (HAO1), for primary hyperoxaluria type 1 (PH1) by subcutaneous administration. The HAO1 gene encodes glycolate oxidase, which is involved in the synthesis of oxalate.538 Inclisiran (Leqvio®) targets the proprotein convertase subtilisin kexin type 9 (PCSK9), an enzyme secreted primarily by the liver that can modulate the degradation of the low-density lipoprotein receptor (LDLR), further influencing the concentration of LDL-cholesterol (LDL-C), a factor contributing to atherosclerotic cardiovascular disease (ASCVD).539 Inclisiran exhibits excellent efficacy in lowering LDL-C levels, which can lead to a half reduction in LDL-C levels in ASCVD through subcutaneous administration every 6 months.540
Ocular diseases
Since the 1990s, ASOs have been developed for effective ocular therapeutic use. The initial application of ASO therapy for ocular conditions involved injecting the IGF-I ASO during conditioned eye-blink learning in rats, which resulted in the normalization of cerebellar IGF-I levels following treatment.541 The delivery of ASOs to the retina has also been explored.542 Fomivirsen is a 21-mer ASO modified with a PS backbone, designed to target and inhibit the viral IE-2 protein.543 As the first approved ASO-based drug, fomivirsen treats cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome (AIDS) through intravitreal (IVT) administration. Fomivirsen inhibits human cytomegalovirus replication by binding to complementary sequences on mRNAs derived from the major immediate-early transcriptional unit of virus. Treatment with fomivirsen notably slowed disease progression in patients with advanced, refractory, sight-threatening cytomegalovirus retinitis or newly diagnosed unilateral peripheral cytomegalovirus retinitis.543 The most commonly reported side effects of fomivirsen in clinical trials were elevated intraocular pressure and certain eye inflammation.544 Regrettably, fomivirsen was withdrawn from the European market in 2002 and from the United States in 2006, as more effective antiretroviral therapies for the same indication had been developed. Moreover, vascular endothelial growth factor (VEGF) is also a well-established target for treating neovascular diseases. In preclinical studies of ocular ASO therapeutics, a VEGF ASO drug was applied in vivo to inhibit choroidal neovascularization (CNV) in the eye. ASO therapy also targets inflammatory factors, and the therapeutic effects of these ASOs on keratitis, chorioretinitis and inflammation after glaucoma surgery have been assessed.
ASO therapy for patients with inherited retinal degeneration (IRD) is an effective therapeutic approach for treating CEP290-associated Leber’s congenital amaurosis (LCA). Two studies addressing the c.2991 + 1655 A > G mutation in CEP290 demonstrated that ASO therapy could correct the abnormal splicing of pre-mRNAs and restore ciliation in the patients’ fibroblasts.545,546 Furthermore, ASO therapy has been studied for other genetic conditions, such as retinitis pigmentosa (RP) and Stargardt disease.547 Recently, the USH2A gene was targeted for RP treatment, and experimental studies showed that protein domain-specific ASO-induced dual-exon skipping restored usherin expression in the zebrafish retina, correcting photopigment mislocalization in zebrafish with USH2A mutations.548
Currently, a total of seven ASO drugs that target the eye have been progressed in human trials. QR-421a was administered to patients with RP due to mutations in exon 13 of the USH2A gene; however, no updates on these three trials are available. QR-110 (a target of CEP290) has been explored for the LCA administration and has shown vision improvement at 3 months and sustained visual gain at 15 months in one patient. Additionally, aganirsen, an ASO that targets IRS1, for ischemic central retinal vein occlusion that reduces the patient burden by topical treatment.549
With respect to siRNA therapies being investigated in clinical trials for eye disease, most drugs are administered via IVT injection. AGN211745 (sirna-027) is the first siRNA drug aimed at eye conditions that began clinical trials, and this siRNA drug targeted the mRNA of VEGF receptor 1 (VEGFR-1). A notable decrease in the area of neovascularization was detected in a murine model of laser-induced CNV after the IVT injection of AGN211745 in mice.550 Further clinical trials have investigated its value in ocular disease. A phase I/II trial (NCT00363714) administered different doses of AGN211745 to 26 neovascular age-related macular degeneration (nAMD) participants via IVT, and the participants were observed for more than 24 months. Regrettably, no study results were found. A Phase II trial, which ended in 2007, involved administering various doses of AGN211745 to 138 participants over a 2-month period, with follow-up observations extending for 24 months. However, the company decided to terminate this study early; since then, no clinical studies have focused on AGN211745. In addition to targeting VEGFR, bevasiranib directly inhibits VEGF for the treatment of nAMD and has shown clinical promise in phase III trials. However, the occurrence of serious adverse effects, such as diminished visual acuity and endophthalmitis, led to the discontinuation of one clinical trial (NCT00499590). A Phase III trial of codosiran, which was designed to target CASP2 for glaucoma treatment, was also halted due to unexplained reasons (NCT02341560).
Three trials investigated the topical administration of siRNA drugs for ocular disease treatment. Tivanisiran, which targets TRPV1, finished phase III trials in 2020 for the treatment of dry eye disease (NCT03108664). SYL040012 is thought to exert its therapeutic effect by downregulating ADRB2 production. For patients with both open-angle glaucoma (NCT02250612) and ocular hypertension (NCT00990743), SYL040012 led to a substantial decrease in intraocular pressure. SYL1801 inhibited NRARP, and the safety and tolerability of different SYL1801 doses, along with the pharmacokinetic profile of the drug, were evaluated in healthy volunteers (NCT04782271). Moreover, the safety and effects of three different doses of SYL1801 eye drops on visual acuity were evaluated in patients with wet AMD (NCT05637255). Additionally, RXI-109 (NCT02599064) and PF-0423655 (NCT01445899) may reduce the progression of subretinal fibrosis in subjects with neovascular age-related macular degeneration and diabetic macular edema, respectively; however, neither of these studies has published results. Vectors loaded with different small nucleic acids are promising for treating eye diseases in the future.
Lung disorders
Pulmonary diseases are prominent public health and medical problems and are among the primary causes of death globally, as anatomical barriers hinder the effective delivery of medications to the lungs.551 Nanomedicine has emerged as a promising solution to address the limitations of existing treatments for pulmonary diseases.552 Inhalation of a nanocarrier-based drug delivery system (DDS) is currently being widely investigated.553 A total of 8 RNA-based drugs, comprising 6 ASOs and 2 siRNAs, have been evaluated in clinical trials. For the treatment of asthma, three ASO products, TPI ASM8 (NCT01158898), SB010 (NCT01743768) and AiR645 (NCT00941577), are presently being tested in clinical trials. Another drug candidate, TPI 1100 (NCT00914433), is an unmodified ASO that inhibits phosphodiesterases 4 and 7, which are crucial in the progression of chronic obstructive pulmonary disorder (COPD).
Eluforsen, a full PS and full 2ʹ-OMe ASO developed to target the mRNA region surrounding the F508 deletion, the most common mutation leading to cystic fibrosis (CF). Its goal is to restore the function of the cystic fibrosis transmembrane conductance regulator (CFTR) protein in the airway epithelium.554 Preclinical data have shown that eluteforsen enhanced CFTR function in F508del-CFTR cells and animal models.555 This ASO has exhibited potential improvements in lung function and advantages for homozygous del-508 CF patients in two clinical trials (NCT02564354 and NCT02532764).554,556 The nebulization of IONIS-ENAC-2.5Rx, a PS 2ʹ-cEt ASO that aimed at reducing epithelial sodium channel (ENaC) expression in the lungs and promoting RNase H1 activity, is ongoing in a Phase II clinical trial involving CF patients (NCT04441788). According to preclinical studies, ENaC mRNA expression is obviously reduced in mouse models of CF-related lung disease, resulting in significant functional improvements.557 In a phase I trial, IONIS-ENAC-2.5Rx also demonstrated a significant reduction in ENaC mRNA expression in healthy volunteers who received the drug via nebulization (NCT03647228).
Central nervous system disorders
Most neurological diseases result from gain‑of‑function mutations; thus, ASOs are extensively utilized to suppress the expression of mutant proteins in various diseases. An intrathecal infusion of 2ʹ‑MOE‑PS ASOs aimed at the human HTT transgene in a Huntington’s disease (HD) mouse model suppressed huntingtin accumulation and delayed disease progression.558 Furthermore, allele-specific ASOs that are both potent and selective can correct single nucleotide polymorphisms (SNPs) that are prevalent in the HD allele.127,559 Unlike tominersen, WVE-120101 and WVE-120102 are experimental stereopure ASOs designed to selectively suppress mHTT by targeting SNPs associated with the CAG repeat expansion in the haplotype phase.560 The two ASOs currently undergoing evaluation in patients with HD in two phase Ib/IIa studies. According to Wave Life Sciences, the results of the mHTT from the PRECISION-HD trials do not justify the continued development of WVE-120102 and WVE-120101, as the occurrence of serious adverse events was higher in the 32 mg group compared to the low-dose group. Additionally, the company is currently enrolling Huntington’s disease patients for its phase 1b/2a trial of the WVE-003 (SNP3) program.
Moreover, atherosclerosis (ALS) is a progressive neuromuscular disease caused by the degeneration and impairment of neurons in motor pathways, resulting in muscle function loss and ultimately leading to death within 3 to 5 years.561 Tofersen received its initial approval in the USA for treating ALS in adults with mutations in SOD1 gene. In a clinical study of the intrathecal administration of tofacersen, a PS 2′-MOE ASO was developed to decrease the levels of both wild-type and mutant SOD1 mRNA through RNase H1-mediated degradation.562 In a phase 1/2 ascending-dose trial (NCT02623699) that assessed cerebrospinal fluid (CSF), SOD1 concentrations showed a decline following the intrathecal administration of the highest tofersen dose over 12 weeks, with a manageable incidence of adverse events related to lumbar punctures.563 In a phase III trial (NCT02623699), tofersen lowered SOD1 concentrations in the cerebrospinal fluid and reduced neurofilament light chain levels in the plasma over 28 weeks. However, it did not demonstrate improvements in clinical endpoints and was associated with adverse events.562 ALS can also result from mutations in the C9orf72 gene. A phase I/II study was conducted to evaluate BIIB078 (IONIS-C9Rx), a PS 2′-MOE ASO aimed at selectively degrading the mutant C9orf72 mRNA. Regrettably, BIIB078 did not achieve any of the secondary efficacy endpoints and failed to provide a clinical benefit. No consistent differences were observed between the 60 mg BIIB078 group and the placebo group. However, participants in the 90 mg BIIB078 cohort showed a tendency for greater reductions in secondary endpoints compared to those in the 60 mg BIIB078 group and the placebo group. Based on these disappointing results, the clinical development program for BIIB078 will be discontinued, including the ongoing open-label extension study.
Spinal muscular atrophy (SMA) is among the most common and severe genetic disorders affecting children,564 with the loss of function of SMN2 being the primary genetic cause of this condition. Nusinersen is a fully modified 2ʹ-MOE polysaccharide ASO that modifies the splicing of the SMN2 pre-mRNA.565 It promotes the production of functional full-length SMN protein throughout the spinal column and central nervous system (including motor neurons) by inducing the retention of exon 7 in SMN2.566 A few clinical trials have demonstrated that the intrathecal administration of nusinersen to SMA patients is well tolerated and results in improved motor function. Nusinersen significantly improved event-free survival and overall survival in infants with type 1 SMA, showing comparable results in patients with type 2 SMA and later-onset type 3 SMA.567,568 These promising outcomes from clinical trials led to the FDA’s approval of nusinersen for the treatment of SMA in both pediatric and adult patients in the United States.
siRNAs are often highly modified or encapsulated in LNPs to increase their stability. Patisiran was the first siRNA therapeutic to obtain FDA approval in 2018 for treating polyneuropathy with hATTR.15 However, this drug is administered intravenously and does not directly target the nervous system. Patisiran inhibits TTR production in the liver, significantly decreasing neuropathy and stopping the advancement of the disease in patients with hATTR.569 Patisiran is known for its immense potential as an siRNA for treating brain diseases, and many siRNA therapies were later evaluated in late clinical trials for the treatment of Alzheimer’s disease (AD), Parkinson’s disease (PD), and spinocerebellar ataxia. Revusiran is the first-generation GalNAc–siRNA with ‘Standard Template Chemistry’ (STC) that contains two terminal phosphorothioate (PS) linkages and is fully modified with 2′-F,570 which targets TTR. In a phase 1 study, a mean reduction in TTR levels of approximately 90% was observed with multiple revusiran doses, indicating the potential clinical use of subcutaneously administered GalNAc-siRNAs for liver-related diseases. ALN-APP was designed to treat early-onset Alzheimer’s disease (EOAD) by attaching siRNAs to a lipophilic C16 fatty acid chain, which significantly enhanced the distribution of the siRNA in the brains of rats and monkeys after intrathecal injection. In 2022, a clinical trial for ALN-APP, which could specifically target the CNS for the treatment of AD (NCT05231785), was announced.
Muscle diseases
Duchenne muscular dystrophy (DMD) is a severe muscle disorder caused by in DMD mutations, leading to progressive muscle degeneration and, in many cases, premature death.571 A single-exon or multiexon skipping strategy to restore dystrophin expression would be applicable for 90% of DMD mutations. A strategy involving single-exon or multiexon skipping to restore dystrophin expression could potentially address 90% of DMD mutations.572 Seven ASOs with different chemical modifications targeting DMD have progressed to clinical trials. To date, the clinical therapies available for patients with confirmed DMD mutations that are amenable to exon 51, 45 and 53 skipping include the approved DMD therapies casimersen, eteplirsen, golodirsen, and viltolarsen, respectively. However, only viltolarsen has provided evidence supporting a treatment-related clinical improvement, as assessed by the 6-minute walk test.573 The rapid development of ASO-mediated therapies for DMD opens the door for the application of nucleic acid drugs in treating a variety of diseases with well-understood pathogenesis.
Cancer
Over the decades, the therapeutic delivery of nucleic acids into cancer cells through gene therapy has been acknowledged as a hopeful new strategy for meeting the unmet needs in cancer care. The initial clinical trial of ASOs in cancer was a phase 2 study that began in 1993 (NCT00002592). This ASO specifically targeted G4460 in chronic myelogenous leukemia (CML), binding to the mRNA of the proto-oncogene CMYB and triggering RNAse H-dependent degradation.574 Previous experiments demonstrated that ASOs markedly reduced the proliferation and differentiation of human leukemia cells.574,575 Furthermore, findings from trial NCT00780052 revealed that nearly half of the CML patients who were ineligible for allografts experienced substantial decreases in CMYB mRNA levels following treatment with the G4460 ASO.576 Danvatirsen (AZD9150) is a chimeric generation 2.5 ASO (16-mer ASO) modified with phosphorothioate, aimed at degrading human STAT3 through RNase H1 activity. In the phase 1b trial (NCT01563302), Danvatirsen demonstrated a favorable safety profile and was well-tolerated at both dosing levels. Additionally, it exhibited efficacy in a subset of pretreated patients diagnosed with diffuse large B-cell lymphoma (DLBCL).577 Moreover, further trials of danvatirsen have been conducted in patients with different types of cancer, but the results have not been published to date.
IONIS-AR-2.5Rx (AZD5312) is a second PS 2ʹ-cEt ASO (constrained ethyl bicyclic nucleic acid) specifically designed to target full-length, splice variant and mutated forms of AR. A phase 1 study revealed that IONIS-AR-2.5Rx is tolerable in heavily pretreated mCRPC patients, showing lasting reductions in PSA and CTC levels in some individuals (NCT02144051).578 IONIS-AR-2.5Rx has limited efficacy, possibly due to multiple tumor pathways and mutations. Further investigations may focus on the effectiveness of these agents in combination with other anti-androgen drugs. EZN-4176 is a 16-mer third-generation locked ASO that effectively downregulates AR expression at both the transcriptional and translational levels in androgen-sensitive and castration-resistant PCa, as demonstrated in vitro and in animal models.579 A phase I study of EZN-4176 in CRPC patients (NCT01337518) reported dose-limiting toxicity (DLT) with elevated ALT at 10 mg/kg on a weekly schedule, preventing dose escalation. Minimal antitumor activity was observed, leading to trial termination.
The number of siRNA drugs approved for cancer immunotherapy is steadily increasing. CALAA-01, the first targeted siRNA drug, was developed in 2008 for the treatment of advanced solid tumors. It is composed of a cyclodextrin-containing polymer (CDP), a PEG stabilization agent, human transferrin, and an RRM2 siRNA. These components can self-assemble into nanoparticles suitable for pharmaceutical applications and can be delivered intravenously.580 In the case of CALAA-01, the delivery system allows for the targeted delivery of functional siRNA; however, its full potential was not evaluated in the initial phase I clinical trial (NCT00689065).581 Multiple patients encountered dose-limiting toxicities (DLTs) such as diarrhea, fever, and fatigue, leading to the termination of the clinical trial.581 The probable reason is NP instability and the degradation of various components in the bloodstream.582
A local drug eluter (LODER) was developed for treating pancreatic ductal adenocarcinoma (PDA). The local prolonged siRNA delivery system (Local Drug EluteR, LODER) was employed to silence mutated KRAS, and the siG12D LODER was583developed based on the slow release of the biodegradable polymer matrix that encompasses the siRNA.583 A phase 1/2a study (NCT01188785) was launched for first-line treatment of non-operable locally advanced pancreatic cancer (LAPC). Patients received a single dose of siG12D-LODER with chemotherapy, showing a reported therapeutic effect.584 Phase II trials are currently underway for siG12D-LODER in combination with gemcitabine+nab-paclitaxel, FOLFIRINOX, or modified FOLFIRINOX (NCT01676259).
Atu027 is a lipoplex siRNA that targets PKN3 and is formulated using positively charged liposomes composed of cationic and fusogenic lipids complexed with negatively charged PKN3 siRNA, leading to RNAi induction in the vasculature of mice following systemic administration.585,586 Furthermore, intravenous administration of Atu027 is considered an effective drug for preventing lung metastasis in experimental lung metastasis models and mouse models of spontaneous metastasis mouse models.587 The first-in-human study (NCT00938574) revealed that Atu027 was safe in patients with advanced solid tumors.588 The first-in-human trial (NCT00938574) confirmed the safety of Atu027 in patients with advanced solid tumors. Moreover, in an orthotopic pancreatic cancer model, Atu027 was shown to boost the antitumor effects of gemcitabine. As a result, a phase I/II combination trial for advanced pancreatic cancer (NCT01808638) is currently in progress.588
Tyrosine kinase EphA2 is overexpressed in tumor tissues while maintaining relatively low levels in most normal tissues, making it a promising target for cancer therapy.589 An EphA2-targeting DOPC-encapsulated siRNA (siRNA EphA2-DOPC) was developed for efficient siRNA delivery.590 In an orthotopic mouse model of ovarian cancer, the injection of siRNA EphA2-DOPC led to a significant reduction in tumor growth.590 Moreover, the combination of siRNA EphA2-DOPC with paclitaxel demonstrated greater tumor inhibition than treatment with paclitaxel alone.590 Meanwhile, no observed AEs resulting from the EphA2-DOPC siRNA were considered to have occurred at >225 μg/kg when the siRNA was given as a single intravenous injection to CD-1 mice, there was an indication of a mild to moderate inflammatory response after two weekly injections.591 Combined with previously reported in vivo validation data, these findings indicate that the EphA2-DOPC siRNA is effective and well tolerated. The ongoing first-in-human phase I clinical trial enrolled patients with advanced solid tumors to intravenously inject the siRNA EphA2-DOPC (NCT01591356).
MYC proteins are major drivers of human tumorigenesis.592 For targeting MYC, Dicer-substrate siRNA (DsiRNA) formulated in EnCore lipid nanoparticles (DCR-MYC) has demonstrated efficacy in various tumor models in vivo.593,594 In one patient, DCR-MYC anticancer therapy was linked to thrombotic microangiopathy in the kidney.595 The patient ultimately died from cancer progression one year after DCR-MYC treatment was halted.595 Moreover, DCR-MYC is the first MYC-targeting siRNA to undergo human trials (NCT02110563). Anthony W. Tolcher reported that DCR-MYC is well-tolerated and shows encouraging clinical and metabolic effects across a range of doses.596 In a phase Ib/II clinical trial (NCT02314052), DCR-MYC was evaluated in patients with advanced HCC to assess its safety and tolerability. Despite the encouraging outcomes of the phase I study supporting MYC as a therapeutic target, both studies were halted due to sponsor decisions.597
ALN-VSP02, consisting of an LNP loaded with two distinct siRNAs (siVEGF and siKSP), marks the first dual-targeted siRNA therapy employed in clinical trials aimed at treating solid tumors.598,599 Multiple doses of ALN-VSP notably extend the survival of mice with advanced orthotopic liver tumors.600 A phase 1 trial in patients with liver-involved solid tumors assessed the safety, tolerability, pharmacokinetics, and pharmacodynamics of ALN-VSP02, indicating that ALN-VSP02 was well tolerated at the highest dose (1.25 mg/kg) with biweekly intravenous injections.601 The pharmacodynamics suggest robust antitumor activity, including a complete regression of liver metastases in patients with endometrial cancer.601 An extension study was also performed for patients who continued ALN-VSP02 to collect long-range safety response (NCT01158079).
Arbutus Biopharma Corporation developed TKM-080301, a LNP formulation of a PLK1 siRNA targeting human PLK1.602 TKM-080301 demonstrated strong antiproliferative effects and gene-specific silencing in cancer cell lines, showing antitumor activity in PDX models of tumors implanted intrahepatically or subcutaneously.603 Its toxicity was mainly limited to the liver and spleen in HCC patients, reflecting the distribution of the LNPs.603 An open‐label, multicenter, dose escalation study revealed that the antitumor effect of TKM 080301 as a single agent was limited (NCT02191878).
In recent years, extensive efforts have been dedicated to identifying critical oncogenes through genomic databases, with small nucleic acids emerging as a promising method for regulating target gene expression in precision medicine.604 However, challenges remain in improving delivery efficiency, targeting control, release, and reducing side effects.605 Nanotechnology has earned attention for enhancing precision cancer therapy, with NP-mediated delivery systems (passive, active, and endogenous targeting) effectively increasing drug accumulation in tumors.604,606 Thus, nucleic acid drugs are employed in the early stages of cancer treatment.
More detailed information about the LNP systems referenced in this section is cataloged in Table 8 (ASO-based therapeutics) and Table 9 (siRNA-based therapeutics).
Aptamer-based therapies
Due to their unique three-dimensional structure, aptamers can recognize target molecules through their three-dimensional conformation and exhibit high binding affinity. Aptamer-based drugs include a variety of technologies, featuring novel shapes, chemistries, and delivery methods, and are utilized for both therapeutic and diagnostic purposes.607
Aptamers are chemically synthesized in vitro, resulting in shorter synthesis times, lower costs, greater stability, and greater specificity. The small and flexible structure of aptamers allows them to bind to smaller targets or hidden epitopes that some antibodies cannot access. Over the last few years, aptamer-based drugs that have garnered significant interest in clinical applications as alternatives to traditional monoclonal antibody-based therapies have been developed. Pegaptanib, developed by Valeant, was the first nucleic acid aptamer drug approved for the treatment of wet age-related macular degeneration and received FDA approval in 2004. It was subsequently approved by the EMA in January 2006 and by the PMDA in July 2008.
Structurally, pegaptanib is a 28-base RNA oligonucleotide conjugated to a 20 kDa branched PEG.608 Mechanistically, pegaptanib selectively binds to the VEGF 165 isoform, thereby slowing the development of choroidal neovascularization and reducing leakage from abnormal blood vessels.609 Preclinical data indicate that the drug is metabolized in vivo by endonucleases and exonucleases and is excreted in the urine in both its original form and as a metabolite. Pharmacokinetic studies of IVT in rhesus monkeys have shown that drug concentrations in the vitreous and blood are dose dependent and that the drug’s half-life is also dose dependent, following first-order kinetic processes.610 In a phase II clinical trial, a multicenter, open-label, repeated-dose study involving 21 patients with choroidal neovascularization secondary to AMD was conducted over 3 months. The results revealed that 87.5% of patients who received pegaptanib monotherapy experienced stable or improved vision, with 25% of treated eyes gaining 3 or more lines of visual acuity. Additionally, 60% of patients treated with a combination of pegaptanib and photodynamic therapy (PDT) had visual acuity improvements of 3 lines or more, suggesting a potential synergistic effect between the two treatments.611 These findings confirmed the safety of the drug, with no clear drug-related complications observed.
Avacincaptad pegol is also an RNA aptamer. Structurally, it is composed of 39 bases, with a PEG modification at the 5’ end, a capped structure at the 3’ end, and a molecular weight of nearly 56 kDa.612 It can efficiently and specifically bind to complement C5 in patients with AMD and received FDA approval for geographic atrophy in 2023.612 In a phase II AMD study (NCT03362190), 43 subjects received monthly doses of 0.3, 1, or 2 mg of avacincaptad pegol along with 0.5 mg of Lucentis (OphthoTech Corporation). OphthoTech announced results from a phase 2a safety trial of Zimura® in combination with Lucentis® for wet age-related macular degeneration (media release, 16 Nov 2018). After 6 months, the mean improvement in visual activity was 13.6, 11.7, and 15.3 letters for patients receiving low, medium, and high doses, respectively, with 46%, 47%, and 60% of patients improving by more than 3 lines. Compared with the control group receiving Lucentis alone, a significant portion of patients in the combination therapy group experienced improved vision.
To date, most therapeutic aptamers are still in preclinical or early clinical development stages. Aptamer drugs that target ophthalmic diseases, cardiovascular conditions, tumors, and inflammation have already entered clinical trials. AS1411 is the first aptamer to enter clinical trials for cancer treatment. Its nucleic acid sequence is rich in guanine, which easily forms a quadruplex structure.613 This unique structure not only resists nuclease degradation but also inhibits the proliferation of cancer cells by specifically targeting nucleolin.614,615 In a phase I clinical trial involving patients with advanced cancer (NCT00881244), AS1411 was well tolerated after six months of treatment, with no adverse reactions observed, except in patients with renal cell carcinoma. However, in the phase II clinical trial, only limited efficacy was observed, leading to the suspension of the trial (NCT00740441).
NOX-A12 binds to and neutralizes CXC chemokine ligand (CXCL12), disrupting the CXCL12 gradient established by bone marrow stromal cells and ultimately sensitizing CLL cells to cytotoxic drugs.616 The FDA granted NOX-A12 the orphan drug designation for use in combination with radiation therapy for patients with glioblastoma in 2014. This aptamer is currently being developed under the trade name olaptesed pegol for the treatment of various malignancies. In a recent phase I/II clinical trial (NCT01486797), 28 patients with relapsed/refractory chronic lymphocytic leukemia received olaptesed pegol in combination with bendamustine and rituximab.617 The monotherapy was well tolerated, with no dose-limiting toxicity observed, and the overall response rate for the combination therapy was 86%, with 11% of patients achieving complete remission and 75% achieving partial remission.617 In animal experiments, the PEGylated L-RNA aptamer olaptesed pegol was highly effective in a rat brain tumor model of highly refractory glioblastoma (GBM). An open-label, multicenter phase I/II trial known as the GLORIA trial was conducted to evaluate the clinical safety and efficacy of combining radiotherapy with NOX-A12 (NCT04121455). This study reported the safety of RT and NOX-A12 in patients with newly diagnosed chemotherapy-resistant GBM, achieving the primary endpoint of the trial.618
DTRI-031 is a dose-dependent platelet aggregation-inhibiting aptamer capable of reopening vessels occluded by platelet-rich thrombi.619 A randomized, double-blind, single-center, placebo-controlled phase I study is underway in healthy volunteers to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single intravenous injection of DTRI-031 (NCT05005520).
Several coagulation aptamers are being investigated in different stages of clinical trials. REG1 is an aptamer-based factor IXa inhibitor developed for percutaneous coronary intervention (PCI) in patients with acute coronary syndrome.620 It consists of the RNA aptamer pegnivacogin (RB006, with a 40 kDa polyethylene glycol carrier attached to its tail) and the antidote anivamersen (RB007). A phase I clinical trial (NCT00113997) of REG1 for anticoagulation showed positive results, with lower toxicity during PCI, but the phase II trial was prematurely terminated due to severe allergic reactions (NCT01848106), and the system is currently being optimized.621
ARC1779 is the first DNA aptamer that targets the A1 domain of von Willebrand factor (vWF), with indications primarily for thrombotic thrombocytopenic purpura, von Willebrand disease, cerebrovascular embolism, and thrombotic microangiopathy.622 Preliminary evidence suggests that low doses of ARC1779 can be used to correct vWF and/or FVIII deficiencies in patients with hereditary bleeding disorders. A clinical trial (NCT00432770) evaluated the safety, pharmacokinetics, and pharmacodynamics of ARC1779 in patients with VWF-related platelet function disorders. These results indicate that ARC1779 can inhibit platelet aggregation without significantly increasing the risk of bleeding.622 A phase II clinical trial (NCT00742612) investigated the effect of ARC1779 on cerebral microemboli in patients immediately after carotid endarterectomy; however, the study had to be paused because of insufficient patient enrollment.
Due to their high binding specificities and affinities, as well as several advantages over antibodies, aptamers have become excellent alternatives to antibodies in the treatment of various diseases. Currently, attention to therapeutic aptamers is increasing significantly annually, with several aptamer-based drugs undergoing proof-of-concept studies and various stages of clinical trials, and they have already shown great potential in the treatment of serious diseases (Table 10).
MicroRNA therapeutics
To date, miRNAs have been demonstrated to play a role in the pathogenesis of human diseases, especially viral infection, metabolic disorders and cancer.623,624 The main function of miRNAs is to suppress gene expression by binding to the 3’-UTRs (untranslated regions) of mRNAs in a post-transcriptional manner.625 Advancements in miRNA research concerning human diseases has allowed miRNAs to hold great prognostic value and to become therapeutic agents.626,627 Here, we present a summary of miRNA-related preclinical development and clinical trials, and miRNA mimics and miRNA inhibitors currently show promise as novel therapeutic drugs (Table 11).
The first miRNA-targeted drug to advance into clinical development was miravirsen, a locked nucleic acid-modified oligonucleotide that designed to antagonize miR-122 for treating hepatitis C virus (HCV) infection. Patients with chronic HCV infection who received miravirsen demonstrated sustained, dose-dependent decreases in HCV levels, with no signs of viral resistance or long-term safety issues.628,629,630 This treatment, which makes the reality of miRNA therapy undeniable, facilitated the progression of miravirsen into further studies involving long-term follow-up, a larger patient population, and multi-drug combinations. RG-101 is a modified phosphorothioate oligonucleotide that inhibits miR-122 and is conjugated to a multivalent N-GalNAc structure, specifically engineered to improve oligonucleotide absorption by hepatocytes. This drug has undergone phase I trials in patients infected with HCV.444,631,632 While RG-101 led to a significant decrease in viral load among all treated patients, the trial was terminated by the FDA due to reports of jaundice.
The most advanced cancer-targeting miRNA drug is MRX34, a synthetic miR-34a mimic, double-stranded and encapsulated within the NOV40 lipid carrier.633 A preclinical study revealed that systematic injection of MRX34 markedly inhibited tumor growth and significantly increased the survival of mouse models with orthotopic hepatocellular carcinoma, and no immunostimulatory effects or dose-limiting toxicities were observed.634,635 The first clinical trial of miRNA therapy conducted on humans was the Phase 1 clinical trial (NCT01829971) of MRX34, which enrolled 155 participants diagnosed with seven distinct cancer types, such as primary liver cancer, SCLC, lymphoma, melanoma, multiple myeloma, renal cell carcinoma, and NSCLC.636,637 Another clinical trial (NCT02862145) evaluated the therapeutic effects of MRX34 in combination with dexamethasone on melanoma patients.
Many preclinical and clinical trials focused on miRNAs have since been conducted, describing the potential of miRNAs as therapeutic agents. The disparity between foundational research on miRNAs and their practical use in clinical settings continues to be considerable. A phase 1 clinical trial of the miR-29 mimic MRG-201 for the treatment of scleroderma was conducted in 2021 (NCT02603224). In addition, MRG-229 is a modified miR-29 mimic that features enhanced stability due to conjugation to the internalization moiety BiPP, was found to be a potent candidate therapeutic for fibrotic conditions in the preclinical stage.638 The drug MRG-110 is a locked ASO of miR-92a, which focuses on angiogenesis during wound healing and demonstrates potential therapeutic benefits for both chronic and acute wounds.639 A phase 1 clinical trial (NCT03603431) showed that a systemic infusion of MRG-110 effectively inhibited miR-92a in human blood.640 Activation of cardiac miR-132 results in adverse remodeling and pathological hypertrophy. A preclinical study assessed the safety and effectiveness of CDR132L in a clinically relevant pig model of chronic heart failure.641 CDR132L treatment markedly reduced the expression of fibrosis markers and reversed cardiac remodeling in a phase Ib trial (NCT04045405). The encouraging efficacy and suitable tolerance of these regimens provide a basis for further clinical research to further confirm the beneficial effects of CDR132L treatment.642 MRG-106, a miR-155 inhibitor, is synthesized as an LNA-modified oligonucleotide and can be administered via subcutaneous injection, intravenous infusion, or directly into cancerous skin lesions.643,644 Patients diagnosed with specific lymphomas and leukemias were recruited, and this trial was the first to utilize miRNA inhibitors instead of the previously tested miRNA mimics. Although the phase 1 results (NCT02580552) were encouraging, the phase 2 clinical trials (NCT03713320 and NCT03837457) were halted early by the sponsoring company for commercial reasons. LNA-i-Mir-221, MesomiR-1, and INT-1B3 were all systemically administered and completed phase 1 trials. However, the cellular effects of miRNAs are so extensive that off-target effects are inevitable. Therefore, the primary challenges for effectively tackling these issues require further development of synthetic RNA technologies and specific delivery systems to ensure the safe and targeted administration of miRNA therapeutics.
Small activating RNA therapeutics
Unlike other gene overexpression techniques, such as plasmids, viruses or mRNAs, saRNAs are small, versatile and safe, with lower overall research costs.645 Thus, saRNAs constitute an alternative category of therapeutics that can restore the expression of essencial genes in disease conditions. A growing number of animal and clinical studies have investigated saRNA-based therapies for multiple diseases (Table 11).
Several in vivo studies have validated the therapeutic effectiveness of saRNAs in animal models. To induce endogenous p21 expression, Li et al. targeted a 19-nucleotide saRNA at the –322 position of the p21 promoter relative to the transcription start site and synthesized it into a novel saRNA, p21-saRNA-322, according to design guidelines.105 p21-saRNA-322 successfully hindered the growth of several cancer models, including PCa, HCC, NSCLC, pancreatic cancer and bladder cancer.646,647,648,649,650,651 However, the major obstacle to successful saRNA therapy is the absence of efficient drug delivery systems. A 2’-fluoro-modified derivative (dsP21-322-2’F) of LNPs was formulated for intravesical drug delivery, which led to increased urothelial uptake and prolonged survival in mice with established orthotopic human bladder cancer.652 Moreover, a rectal delivery system, TSLPP-p21-saRNA-322, consisting of a PEI/p21-saRNA-322 polyplex core and a hyaluronan-modulated lipid shell, was developed to treat colorectal cancer.653 Although p21-saRNA-322 induced substantial tumor shrinkage in animal models, intratumor delivery of the encapsulated saRNAs may delay the application of the findings into a clinical practice. Although most saRNA-based drugs are still in the preclinical stage, MTL-EBPA is the cutting-edge therapeutic saRNA developed by the National University Cancer Institute (NCIS) and MiNA Therapeutics, entered a phase 2 trial in March 2016.654 MTL-EBPA is intended for targeting solid tumors and is composed of SMARTICLE liposomal nanoparticles and 2′-O-Me saRNAs, which can increase the expression of the C/EBPα gene. C/EBPα is a transcription factor strongly implicated in myelopoiesis and the proliferation and differentiation of cancer cells.655,656,657,658 In preclinical studies, evidence has shown that injectable saRNAs that successfully effectively enhance C/EBPα expression, decrease tumor burden, and ameliorate liver function in a cirrhotic rat model of multifocal liver tumors.659 Another targeted delivery system for C/EBPα-saRNAs, employing RNA aptamers specific to pancreatic ductal adenocarcinoma (PDAC), has shown antiproliferative effects in vivo660; further studies have shown that linking C/EBPα-saRNAs to TR14 could offer promising therapeutic effects on advanced PDAC.661 These comprehensive and exciting preclinical studies prompted the development of clinical trials. The initial phase 1a/b clinical trial of MTL-CEBPA in humans revealed a favorable safety and tolerability profile for patients with hepatocellular carcinoma linked to hepatitis B and/or C infections (NCT02716012).662 Moreover, this trial demonstrated that pretreatment with MTL-CEBPA ameliorates the immunosuppressive HCC microenvironment to enhance the treatment benefits of tyrosine kinase inhibitors (TKIs).663 Thus, many clinical trials of the combination of MTL-CEBPA with other small-molecule drugs, such as PD-1 inhibitors (NCT04105335), atezolizumab and bevacizumab (NCT05097911), have been conducted.612 Moreover, the combination of MTL-CEBPA and sorafenib has entered a phase 2 clinical trial (NCT04710641).
Clinical feedback and drug tailoring in development
To date, small nucleic acid drugs have achieved remarkable success in clinical trials. Additionally, an increasing number of pharmaceutical companies are now engaged in researching and developing small nucleic acid-based therapies for the treatment of various diseases. The integration of clinical feedback significantly enhances the drug development process.664,665 By analyzing data from clinical trials, researchers can refine drug formulations to better meet patient needs. This iterative process ensures that therapies are not only effective but also safe and well-tolerated.
During the initial phases of development, preclinical studies offer essential insights into the potential efficacy and safety of drugs. However, the data collected during clinical trials truly inform the refinement process. Patient responses, side effects, and therapeutic outcomes all contribute valuable insights.666 These insights allow researchers to adjust dosages, modify delivery mechanisms, and even alter the chemical structure of the drug to optimize drug performance. Furthermore, tailoring drugs based on the clinical data involves a deep understanding of patient demographics and genetic profiles. Personalized medicine has become a key focus, as variations in the genetic makeup can significantly impact how patients respond to treatments.667 By incorporating these data, researchers can develop more targeted therapies that are effective for specific patient groups, reducing the trial-and-error approach traditionally associated with drug development.668
The incorporation of clinical feedback not only improves the efficacy of drugs but also accelerates the approval process. Patient clinical data provide information for laboratory research to realize the rapid transformation of the results of the study. Regulatory agencies are more likely to approve drugs that demonstrate clear benefits and minimal risks and are supported by comprehensive clinical data. This streamlined process helps bring new therapies to market faster, benefiting patients who need them the most. Ultimately, the continuous loop of feedback and adjustment creates a robust framework for developing small nucleic acid therapies. By prioritizing clinical data, researchers ensure that each iteration of a drug is better suited to meet the clinical needs, laying the foundation for more effective and personalized treatments.
The transition from Onpattro® to Vutrisiran® represents an advancement in small nucleic acid drug development that benefitted from clinical feedback. Building on the success of Onpattro®, researchers have applied the lessons learned from the clinical limitations identified in Onpattro® to enhance the next generation of therapies.669,670 Vutrisiran® exemplifies how integrating patient data can lead to improved drug formulations with greater efficacy and safety.569 This continuous cycle of feedback and improvement underscores the importance of patient-centric approaches in drug development. Onpattro® requires intravenous infusion every three weeks, meaning that patients need to visit the hospital frequently for treatment, increasing both time and financial burdens. In contrast, Vutrisiran® has a relatively lower dosing frequency, requiring subcutaneous injection every three months.535,670 This schedule not only reduces the treatment frequency for patients but also decreases the occurrence of adverse reactions, thereby improving patients’ quality of life.535,670 Moreover, drawing from the experiences with inotersen, researchers have improved their methods to boost the efficacy and safety of eplontersen. The design of eplontersen reflects a more patient-centric approach, addressing the limitations observed with inotersen. Eplontersen shares a similar design and identical nucleobase sequence with inotersen, an ASO previously approved for the treatment of polyneuropathy of ATTR, but uses unique chemistry and GalNAc conjugation to specifically target hepatocytes, the main source of TTR in the body.671,672 This targeted approach allows eplontersen to be administered at lower doses and less frequently while still achieving efficacy.671,672
In recent years, the clinical use of small nucleic acid drugs has decreased, whereas laboratory research on these drugs has increased, which is marked by a resurgence in fundamental investigations. This shift reflects a strategic pivot toward refining the foundational aspects of small nucleic acid therapeutics. By conducting more basic science experiments, researchers aim to deepen their understanding of molecular mechanisms, optimize drug designs, and innovate delivery strategies.673 This iterative process promises to fortify the clinical pipeline, ensuring that future advancements are grounded in robust scientific insights and paving the way for transformative treatments in diverse therapeutic landscapes. Insights gained from patient outcomes and clinical data can be used to tailor drug development to meet real-world needs.674 This feedback loop allows researchers to make necessary adjustments, increasing the efficacy and safety of small nucleic acid therapeutics. The integration of clinical experiences with laboratory research fosters a dynamic environment where theoretical knowledge and practical application inform each other.
Future directions for small nucleic acid therapeutics
Small nucleic acid therapeutics represent an innovative approach, leveraging the precise gene expression modulation abilities of these molecules through their binding affinity to specific RNA sequences. With a deeper understanding of genes and RNA structures, along with ongoing advancements in biotechnology, the prospects for small nucleic acid therapeutics are highly promising. Over the past years, preclinical and clinical data have confirmed the potential of small nucleic acid therapies to treat various diseases. However, several advances are needed to fully reach their potential (Fig. 7).
The challenges and future directions of small nucleic acid drugs. Various challenges in the development of small nucleic acid drugs persist (red box). First, the development of new drugs is expensive and time-consuming. In addition, NPs may cause adverse immune effects, such as an inflammatory cytokine storm. Moreover, cost and regulatory challenges exist. In addition, insufficient organ and cell selectivity reduce the effectiveness of small nucleic acid drugs. Many ideas have been proposed to solve these barriers as future directions for small nucleic acid drugs (green boxes). The first is the application of artificial intelligence (AI) in drug discovery. The second is improving the safety profile, which should focus on both the delivery vector and the sequence of small nucleic acids. The third is surface modifications of nucleic acid drugs for delivery to specific cell types. Finally, vectors are screened to deliver nucleic acid drugs more efficiently
Current limitations and shortcomings
Despite the significant progress in the development of small nucleic acid therapies, several limitations and shortcomings persist. These issues hinder the full realization of their potential as next-generation medicines.
Financial and time constraints
The process of translating these drugs from the laboratory to the clinic is notably time intensive.675,676 Extensive research, rigorous testing, and multiple phases of clinical trials are needed to ensure safety and efficacy. In addition to the time involved, the cost of developing small nucleic acid drugs is a major barrier.677 The complexity of drug design and production, coupled with stringent regulatory requirements, contributes to the high financial burden. These expenses can limit the ability of researchers and companies to sustain long-term development efforts and restrict access to these therapies once they are approved. Moreover, the financial risks associated with this lengthy development process can deter investment in these innovative drugs. The high cost and uncertainty of success may discourage potential investors, further slowing progress.
Unintended immune reactions
Unlike small-molecule drugs, small nucleic acid drugs primarily exert their effects by binding to target mRNAs once inside the body. Oligonucleotides generally share certain molecular characteristics with naturally occurring oligonucleotides, which may result in immunotoxicity.678 However, during the development process, these compounds are often chemically modified to increase their stability, safety, cellular uptake, and efficacy.167 According to the literature, the mechanism of immunogenic reactions is due primarily to the binding of oligonucleotide drugs to pattern recognition receptors (e.g., TLRs), which activate the innate immune system, leading to an immune response, and the immunomodulatory effect of oligonucleotides is influenced mainly by their sequences, with guanine- and uridine-rich sequences being more prone to binding to TLR7/8.679,680,681 Additionally, some modifications, such as PS modifications, promote inflammatory responses, which are known to induce immunogenic reactions.682 Thrombocytopenia has occasionally been observed in preclinical models, particularly for PS-ASOs such as volanesorsen and inotersen.533,683 PS backbone modifications have been proven to be one of the major causes of reduced platelet counts. Studies have reported that PS-ASOs specifically bind to platelet glycoprotein VI, which in turn activates human platelets, triggering the formation of platelet‒leukocyte aggregates.684
Moreover, nanoparticle toxicity remains a significant concern in the development of small nucleic acid drugs. For example, SiO2 NPs have been shown to cause toxicity in various human organs, including lung epithelial cells, liver cells, and intestinal cells, as well as in the lungs and kidneys.685 Although research on nanomaterial toxicity has yielded mixed results regarding its extent and mechanisms, some materials previously deemed biocompatible due to the safety of their bulk forms can clearly be toxic. Factors such as the nanomaterial size, shape, surface chemistry, and aggregation affect the generation of free radicals and the resulting oxidative stress. Nanoparticle toxicology is still an emerging field, with most studies focusing on acute toxicity. Understanding the long-term effects of and chronic exposure to these materials is essential for a comprehensive assessment of their in vivo toxicology.
Vector screening to improve the efficiency of nucleic acid delivery
The advancement of delivery systems and techniques continues to be the primary challenge in unlocking the vast potential of nucleic acid drugs for gene therapy. Many NPs are composed of diverse chemical materials, and drug delivery mediated by each of these NPs has been evaluated in vitro. Moreover, the quantification of potent vector data in vitro was used to select a minority of NPs for in vivo research. Driven by the need for the systematic design and testing of delivery vehicles, establishing a reliable and efficient in vivo screening method to identify chemical properties that enhance drug delivery. In-depth research has been conducted on the design of LNPs aimed at the efficient delivery of therapeutic RNAs to the lungs via nebulization.686 Through the evaluation of the composition, molar ratios, and structure optimization of LNPs, An excellent LNP was found suitable for the nebulized delivery of low-dose mRNA.686 This systematic method for LNP design can be modified to optimize LNPs for various administration routes and therapeutic applications. In a related study, researchers synthesized and evaluated a library of 720 biodegradable ionizable lipids to develop inhalable delivery vehicles for mRNAs and CRISPR‒Cas9 gene editors through a high-throughput platform.687 Further multistep screening of DNA/LNPs and codelivery with siRNAs provides opportunities for nucleic acid-based gene therapy applications.688 This multistep screening platform integrates both in vitro and in vivo strategies to efficiently identify effective LNP candidates from a library of more than 1000 formulations.688 Another related advance is the construction of an in vivo library selection platform to optimize the therapeutic performance of protein NPs and protein linkers in living mammals.689 This approach may enable the identification of effective delivery platforms for peptide-based gene therapy.
Unlike in monoclonal antibody discovery and engineering, high-throughput sequencing has been utilized to gather extensive data on the diversity of antibody repertoires, and mature materials have been developed.690 The underlying mechanism through which NP properties engage with biological systems is unclear, however, it is broadly recognized that these properties are crucial in inducing biological responses..691 Moreover, the wide range of different types of NPs and the many unknown factors influencing their formation and biological behavior complicate the design process significantly. It is necessary to screen various combinations of oligonucleotide sequences, component ratios, and target organs to identify the principles that regulate the desired characteristics and therapeutic effectiveness of these agents.692,693 These experimental screens involve high workloads and costs, limiting the number of combinations that can be evaluated.692,694 Recently, computational models and artificial intelligence techniques have emerged for the design of NP candidates and nucleic acid sequences, accelerating the discovery of new functional drugs.695,696 Nevertheless, machine learning algorithms can provide predictive benefits to the speed of screening; aid in understanding physicochemical properties, encapsulation efficiencies, biodistribution and toxicity; and provide great value before synthesizing materials.697,698,699 Using machine learning algorithms, researchers can construct and identify NP-specific biomarkers via genomic NP trafficking networks,700 which can be further applied for a quick and high-throughput analysis of multicomponent NP mixtures and solutions.701 To effectively implement computational selection methods, there is a need for more reliable data collection tools and better algorithms to enhance formulation scanning throughput.
Artificial intelligence in small nucleic acid drug discovery
The drug discovery process is complex and includes identifying and validating drug targets, designing and synthesizing compounds, and assessing their efficacy and safety for clinical development. It is estimated that the median cost for discovering and developing a new drug is approximately $985 million, which includes expenses incurred from unsuccessful trials.702 The emergence of artificial intelligence (AI)- and machine learning-based methods has led to the introduction of new and rapid algorithms for drug exploration, predicting drug responses and potent drug combinations.703 Here, we focus on AI in small nucleic acid drug discovery.
TREAT is an all-in-one platform for target selection, drug design, and optimization. It combines coding and noncoding genes from 81 biological networks under different physiological conditions and employs three advanced algorithms for target ranking and identification. For RNA sequence optimization and siRNA design, TREAT uses user-defined criteria to produce deterministic outputs within a specified search space.704 Another model, ‘MysiRNA,’ was trained on 2431 siRNA records and evaluated using three additional datasets. When compared to 11 other scoring tools, MysiRNA demonstrated superior performance based on the correlation coefficient and receiver operating characteristic (ROC) curve, increasing prediction accuracy by as much as 18% compared to the sensitivity and specificity of the best existing tools.705 In addition to siRNAs, the sequence and efficiency of ASOs have also received attention. eSkip-Finder (https://eskip-finder.org) is the first online resource that aids researchers in locating available exon-skipping ASOs. This tool facilitates rapid analysis of chosen exon/intron sequences and ASO lengths, leveraging a machine learning model trained on experimental data to identify effective ASOs for exon skipping.706
Chemical modifications of the siRNA itself could increase serum stability and target delivery efficiency.707 Most recently, a machine learning approach to predicting the efficacy of chemically modified siRNA efficacy has the potential to greatly enhance the siRNA design process for chemical modifications, thereby reducing the time and costs involved in siRNA drug development.708 Liu et al. created the Cm-siRPred algorithm utilizing a multiview learning strategy to aid in the design of chemically modified siRNA drugs.708 The algorithm utilizes a multiview strategy for predicting the efficacy of chemical modifications to siRNAs and assisting in designing chemical modifications of siRNAs, comprising a module for designing chemical modifications and a module for predicting the efficiency of chemically modified siRNAs.708 Furthermore, a model designed to predict the silencing activity of siRNAs featuring different chemical modification patterns has been documented, illustrating the application of machine learning to reveal the correlation between the siRNA sequence, chemical modification pattern, and silencing efficacy.709 More importantly, AI and ML integrated with recent advancements in the biomedical or chemical field can significantly accelerate technological progress in healthcare. By exploring the search space of ionizable lipid molecules using the synergy of deep learning and combinatorial chemistry, Xu et al. developed an AI-guided ionizable lipid engineering (AGILE) platform.710 The AGILE platform learns structural information for many small molecules through pretrained deep learning neural networks and uses self-supervised methods to discriminate and differentiate lipid structures. After fine-tuning and high-throughput screening, AGILE can accurately identify novel lipid structures with high mRNA transfection efficacy.710 Methods that combine systems and synthetic biology with ML models, including graph neural networks, sequence-to-function and sequence-to-structure frameworks, as well as generative models, provide new opportunities for drug candidates and drug discovery approaches.711,712 Sebastian M Castillo-Hair and Georg Seelig integrated high-throughput assays and deep-learning techniques to develop predictive models and build quantitative models related to ribosome loading that optimize protein expression for mRNA therapeutic applications.713 This strategy is not only quicker but also less likely to become trapped in local sequence optima.713
AI depends on the merging of various technologies with basic science methods to leverage extensive multimodal data through predictive modeling to support decision-making.714 Through the automated processing and analysis of large-scale data from laboratory and clinical trials, AI not only improves research efficiency but also reduces the likelihood of human error. With the assistance of AI, scientists can identify key data patterns and trends more quickly, thus advancing research more effectively.
Surface modifications of nucleic acids for delivery to specific cell types
A primary challenge in using NPs for nucleic acid delivery is ensuring selectivity for specific organs or cells, primarily because of the many unpredictable factors that affect how nanoparticles distribute in vivo.38 Currently, the clinical efficacy of tissue-specific delivery methods for RNAi therapeutics is mainly limited to hepatocyte targeting, achieved through LNPs (passive targeting), GalNAc conjugates (active targeting), or local administration.715,716 For instance, the clinically approved siRNA drug Onpattro can efficiently target the liver when administered through the intravenous route. The GalNAc-siRNA conjugate not only can mediate cellular uptake as a ligand without relying on cationic particles, but also can specifically target hepatocytes.717 Thus, approaches to improve the systemic drug delivery efficiency of NPs are highly desirable. A promising strategy involves using targeting ligands and chemical probes, molecules that can specifically bind to surface markers on affected cell populations. This approach is particularly advantageous for single-component delivery systems with well-defined compositions. The RGD peptide can specifically target cancer cells and the tumor vasculature by binding to these integrins. By integrating RGD into lipid‒protamine nanoparticles, Rengaswamy et al. reported a statistically significant tumor growth delay, as well as the inhibition of tumor initiation.718 The RVG peptide conjugate, which is specific to the central nervous system, binds to the acetylcholine receptor and can modify various types and forms of nanoparticles for effective delivery to the brain.719,720 The addition of RVG to nontoxic SSPEI nanovectors increased microRNA accumulation in the brain by approximately 1.5-fold.721 In addition, Tang et al. employed T7, a brain-targeting peptide with a high affinity for the transferrin receptor, to construct lipid-based nanoparticles with DP7 and cholesterol, and the results revealed enhanced brain targeting and extended survival compared with those of T7 unmodified LBNPs.722 In addition, novel passive NPs (such as lipid-based, polymer-based, and biomimetic NPs) for organ-selective systemic nucleic acid delivery have made breakthroughs in extrahepatic targeted therapy.723 Cheng et al. conceived a strategy to add a fifth molecule to LNPs, which is a selective organ-targeting (SORT) molecule, to establish new compositions without destroying the core 4-component ratios. The results showed that SORT enabled existing liver-targeting LNPs to be tuned to deliver nucleic acids to the spleen or lungs.724 Among them, linear PEIs are widely investigated for their capacity to enable lung-selective targeted delivery.725 In a novel dry siRNA powder for inhalation, PEI is the delivery vector, and the results showed that a low dose of 3 μg of siRNA resulted in strong and specific gene silencing activity in the lungs without severe lung injury.726 A “passive” strategy can be integrated with other methods, such as stimuli-responsive delivery, to attain precise organ selectivity. In addition to NPs, cellular organelles such as extracellular vesicles, mitochondria, lysosomes, and lipid droplets are being actively explored as viable drug delivery systems for therapeutic intervention.727 Organelle carriers possess biocompatibility and can be tailored through their specific biological structures or surface modifications to facilitate targeted drug delivery. They predominantly transport drugs to target cells through processes like membrane fusion, receptor-mediated endocytosis, and macropinocytosis.728,729,730 Wei et al. constructed a smart Janus-like surface-coated mitochondrial system that can not only target tumors but also penetrate the depth of tumor tissues and be retained for a long period.731 Through the introduction of tumor cell nuclei into activated macrophages, Wang et al. obtained chimeric exosomes with excellent accumulation in both lymph nodes and tumors, which constitute a powerful delivery system for tumor immunotherapy.732 Among the different kinds of organelle-based drug delivery systems, exosomes are currently a research hotspot and have entered clinical trials.733 The large-scale production of exosomes is challenging and not economically feasible, which also limits the translation of exosomes from the bench to the clinic. Besides the development and screening of NP formulations and drug delivery systems, a fundamental understanding of NP-mediated tissue tropism has also been obtained. Further research is necessary to clarify how these factors contribute to achieving targeted delivery to specific tissues and to refine the design of next-generation delivery systems for clinical use.
Improved safety profiles
The Oligonucleotide Safety Working Group has issued comprehensive guidelines for evaluating oligonucleotide safety. Unfortunately, trial results have been mixed, with some studies showing strong effects while others report significant adverse reactions. Tolerability issues primarily result from pathogen-associated molecular pattern (PAMP) receptors, like Toll-like receptors (TLRs), recognizing RNA structures or nanoparticles, which trigger adverse immune effects.69 An example is the miR-34 mimic MRX34, which resulted in serious treatment-related AEs in five patients with advanced solid tumors in a multicenter clinical trial (NCT01829971).637 In preclinical studies, the application of cationic liposomes has been constrained by their toxicity at the administration site.734 Therefore, a primary obstacle in bringing nucleic acid therapies to clinical practice and the market is ensuring their absolute safety. By understanding the potential toxicity mechanisms, studies have paid attention to mitigating the adverse effects of NPs.735,736 The most commonly reported strategies involve altering the surface chemistry and properties of nanoparticles.737,738,739 Adding biocompatible PEG polymers to SiO2 synthesis systems can attenuate chemical toxicity.740,741 On the other hand, an innovative strategy to overcome this barrier is to encapsulate NPs in cell plasma membranes, such as membranes obtained from erythrocytes.742
For optimal safety, nucleic acid drugs need to ensure that only the antisense strand is selected, which can be achieved by optimizing the thermodynamic stability of the double-stranded RNA.743 Sequence selection, including the target site and oligonucleotide length, has decisive effects on both false off-target activities and on-target functions.744 Numerous researchers have worked to establish consistent and practical algorithms to increase the chance of oligonucleotide binding success.745 In recent decades, certain online design software programs have aimed to improve the quality of siRNA design based on the most highly cited algorithms.745,746
Rational selection of the appropriate target gene
Identifying the correct target gene is essential for ensuring the efficacy of therapy, as it determines the specific genetic pathway to be modulated. Mutations in the superoxide dismutase 1 (SOD1) gene account for approximately 15% of familial ALS cases.564 Designing ASO drugs that specifically target these SOD1 gene mutations and reduce the levels of the harmful SOD1 protein represents a promising therapeutic approach.561 ASO drugs targeting SOD1 have been used to treat familial ALS in human clinical trials and slow disease progression.561,747 This targeted gene selection makes the treatment more focused, significantly improving patient outcomes. In Alzheimer’s disease research, mutations in the amyloid precursor protein (APP) gene are closely linked to the onset of the disease.748,749 ALN-APP is a novel RNAi therapeutic developed by Alnylam using proprietary C16-siRNA conjugation technology that targets APP for AD treatment. The conjugation of the siRNA with C16 increases cellular uptake in the central nervous system, and the C16-siRNA binds to the APP mRNA, which is then cleaved and degraded by the RISC, leading to reduced APP protein expression, a subsequent decrease in Aβ protein levels, a further reduction in the level of the substrate for brain amyloid deposition, and an improvement in neurological function. This approach demonstrates how targeting the APP gene with RNAi can intervene at the root cause of the disease, thereby increasing treatment efficacy.
As we move forward, the integration of advanced genomic tools and technologies will further refine this selection process, enabling researchers to identify the most promising genetic targets with greater accuracy. These advancements will be crucial in expanding the range of treatable conditions and in developing personalized therapies tailored to individual genetic profiles. Next-generation sequencing (NGS) and whole-genome sequencing (WGS) have transformed how we identify target genes. NGS provides an extensive view of genetic variations, revealing genes that may be linked to diseases. Similarly, WES focuses on the coding regions of the genome, which are often related to genetic disorders because mutations affect protein function. Bioinformatics tools are essential for interpreting genomic data. Gene Ontology and pathway analyses help researchers associate genes with specific biological functions and processes. Predictive modeling uses computational algorithms to forecast how genetic variations impact protein functions and disease outcomes, assisting in target gene identification. Single-cell genomics adds another layer of detail, providing insights into gene expression at the individual cell level, revealing cellular diversity and identifying specific cell types or states relevant to disease. Furthermore, functional genomics approaches, such as gene knockdown and overexpression studies, are valuable for validating target genes. These methods use RNAi or gene overexpression techniques to evaluate the roles of specific genes in disease models, confirming their potential as therapeutic targets. By integrating these advanced genomic tools and technologies, researchers can more accurately select and validate target genes, paving the way for more precise and personalized treatment strategies.
Optimizing nucleic acid sequences to reduce off-target effects
For optimal safety, nucleic acid drugs need to ensure the exclusive selection of the antisense strand, which can be accomplished by tuning the thermodynamic stability of dsRNA.743 Sequence selection, including the target site and oligonucleotide length, has decisive effects on both false off-target activities and on-target functions.744 Clinically utilized siRNAs undergo chemical modifications to increase their potency, minimize immune reactions, and reduce off-target effects (OTEs).750,751 A notable example involves the development of 2’-deoxy-2’-α-F-2’-β-C-methyl (2’-F/Me) modifications incorporated into siRNAs, which reduce the thermal stability of double-stranded structures due to steric hindrance. At the ends of oligonucleotides, 2’-F/Me modifications provide greater resistance to nuclease degradation than do 2’-F modifications. Compared with unmodified siRNAs, siRNAs with 2’-F/Me modifications delivered via LNPs or GalNAc showed equal or improved silencing activity in cells and mice. The 2’-F/Me modification at the 7th position of the siRNA antisense strand also reduced the OTEs. When combined with 5’-vinylphosphonate modifications, both the E and Z isomers had similar silencing activities to unmodified siRNA. These findings indicate that the 2’-F/Me modification is a promising tool for increasing the potency, duration, and safety of nucleic acid therapeutics.752 The patent CN118202046A by Zhongtian Biotech discloses a strategy for modifying siRNA molecules aimed at reducing OTEs. By introducing PS nucleotide linkages at positions 5–8 in the seed region of the antisense strand, the specificity and silencing efficiency of the siRNA are improved. This modified siRNA can more precisely target and silence HIF1α, thereby reducing OTEs and increasing the safety and efficacy of treatment.
Numerous researchers have attempted to introduce uniform and practical algorithms to increase the chance of oligonucleotide binding success.745 In recent decades, certain design software programs have aimed to improve the quality of design based on the most frequently cited algorithms745,746; by carefully refining their length, composition, and binding affinity, researchers can increase specificity, ensuring that the nucleic acid sequences bind only to the desired targets. This process involves rigorous testing and computational modeling to predict and eliminate potential off-target interactions. Furthermore, ongoing advancements in predictive algorithms, especially machine learning and artificial intelligence, are increasingly being integrated into bioinformatics platforms, providing powerful tools to identify and assess OTEs before clinical application. These tools allow the simulation of interactions across the entire genome, enabling the selection of sequences with the highest precision and the lowest risk of unintended consequences. OligoWalk, a partition function calculation that considers all possible secondary structures, is used to predict target site accessibility, improving upon methods that consider only the structures with the lowest free energy.753 These thermodynamic features, along with siRNA sequence features, are input into a support vector machine to select functional siRNAs.753 The method effectively predicts efficient siRNAs (70% efficacy) in a large Novartis dataset, with a positive predictive value of 87.6%.753 Very recently, a novel approach called AttSiOff was developed for the prediction of siRNA inhibition and off-target effects. It integrates a self-attention-based siRNA inhibition predictor, an mRNA search package, and an off-target filter. The predictor analyzes siRNA and local mRNA sequences embedded from the pretrained RNA-FM model, capturing key features that influence siRNA inhibition. Tests on five siRNA drugs and a new target gene (AGT) confirmed the practicality and effectiveness of AttSiOff.754
Another area of exploration is the development of more comprehensive databases that include a broader range of genomic data, encompassing diverse populations and rare genetic variations. These databases would enable more accurate modeling of potential OTEs, ensuring that therapies are effective and safe across different genetic backgrounds. Novel methods, such as AI-based techniques, may increase the predictive power of algorithms in complex situations.755 In the future, collaboration among computational scientists, biologists, and clinicians will be crucial in overcoming these challenges. Together, they can develop next-generation tools that not only predict off-target effects with greater accuracy but also provide strategies to mitigate them.
Finding new types of small nucleic acid systems
To date, numerous well-studied nucleic acid systems have been selected for clinical therapies, shedding light on the treatment of intractable diseases. In terms of fundamental studies, the discovery of new types of small nucleic acid systems is essential. Several novel systems have been developed, with laboratory data showing encouraging results, positioning them as potential candidates for clinical trials. For example, preclinical experiments have shown that thiourea-based nucleic acid (TNA)-based modifications of ASOs and siRNA, which offer an alternative to natural nucleic acids, exhibit enhanced nuclease resistance compared to conventional 2’-O-methyl or 2’-fluororibose modifications.756,757 Additionally, Depmeier et al. pioneered the synthesis of TNA with an expanded genetic alphabet (exTNA), further broadening the scope of TNA-based systems.758 Single-stranded oligonucleotides can also be folded into a tetrahedral framework nucleic acid (tFNA). Natural tFNA has inherent reactive oxygen species (ROS) scavenging capabilities and exhibits enhanced structural programmability and efficient endocytosis, thanks to its optimal size and geometry.759 Thus, tFNA shows promise as a nanodelivery system for small nucleic acids. For instance, Zhang et al. developed a novel transdermal RNAi drug utilizing tFNA to deliver siRNAs, which demonstrated increased resistance to enzymatic, serum, and lysosomal degradation. In vivo results indicated that this system effectively and specifically silenced the target gene, nuclear factor kappa-B (NF-κB) p65, thereby maintaining the stability of the skin’s microenvironment and restoring normal immune defense.760 Moreover, Moreno et al. successfully locked multiple functional siRNAs into a bimolecular “caged-siRNA” structure. This structure, formed from single-stranded siRNA and a DNA dendron, self-assembles through the identification and pairing of sense and antisense strands. Their findings revealed that this caged-siRNA system, with multiple RNAi triggers, holds significant potential for therapeutic applications.761
References
Schambach, A. et al. A new age of precision gene therapy. Lancet 403, 568–582 (2024).
Crooke, S. T., Baker, B. F., Crooke, R. M. & Liang, X. H. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discov. 20, 427–453 (2021).
Wang, Z. et al. Development and applications of mRNA treatment based on lipid nanoparticles. Biotechnol. Adv. 65, 108130 (2023).
Xu, L. et al. Exploring precision treatments in immune-mediated inflammatory diseases: Harnessing the infinite potential of nucleic acid delivery. Exploration 4, 20230165 (2024).
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020).
Ramasamy, T. et al. Nano drug delivery systems for antisense oligonucleotides (ASO) therapeutics. J. Control Release 352, 861–878 (2022).
Stephenson, M. L. & Zamecnik, P. C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl. Acad. Sci. USA 75, 285–288, (1978).
Bennett, C. F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu Rev. Med. 70, 307–321 (2019).
Whangbo, J. S. & Hunter, C. P. Environmental RNA interference. Trends Genet 24, 297–305 (2008).
Agrawal, N. et al. RNA interference: biology, mechanism, and applications. Microbiol Mol. Biol. Rev. 67, 657–685 (2003).
Downward, J. RNA interference. Bmj 328, 1245–1248 (2004).
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Song, E. et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 9, 347–351 (2003).
Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067–1070 (2010).
Hoy, S. M. Patisiran: First Global Approval. Drugs 78, 1625–1631 (2018).
Scott, L. J. Givosiran: First Approval. Drugs 80, 335–339 (2020).
Rinaldi, C. & Wood, M. J. A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 14, 9–21 (2018).
Monia, B. P. et al. Evaluation of 2’-modified oligonucleotides containing 2’-deoxy gaps as antisense inhibitors of gene expression. J. Biol. Chem. 268, 14514–14522 (1993).
Vickers, T. A. & Crooke, S. T. The rates of the major steps in the molecular mechanism of RNase H1-dependent antisense oligonucleotide induced degradation of RNA. Nucleic Acids Res. 43, 8955–8963, (2015).
Liang, X. H., Sun, H., Nichols, J. G. & Crooke, S. T. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol. Ther. 25, 2075–2092 (2017).
Vickers, T. A. et al. Fully modified 2’ MOE oligonucleotides redirect polyadenylation. Nucleic Acids Res. 29, 1293–1299 (2001).
Boiziau, C. et al. Inhibition of translation initiation by antisense oligonucleotides via an RNase-H independent mechanism. Nucleic Acids Res. 19, 1113–1119 (1991).
Popp, M. W. & Maquat, L. E. Organizing principles of mammalian nonsense-mediated mRNA decay. Annu Rev. Genet. 47, 139–165 (2013).
Nomakuchi, T. T., Rigo, F., Aznarez, I. & Krainer, A. R. Antisense oligonucleotide-directed inhibition of nonsense-mediated mRNA decay. Nat. Biotechnol. 34, 164–166 (2016).
Bennett, C. F. & Swayze, E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev. Pharm. Toxicol. 50, 259–293 (2010).
Dominski, Z. & Kole, R. Restoration of correct splicing in thalassemic pre-mRNA by antisense oligonucleotides. Proc. Natl. Acad. Sci. USA 90, 8673–8677, (1993).
Shen, X. & Corey, D. R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 46, 1584–1600 (2018).
Kole, R., Krainer, A. R. & Altman, S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 11, 125–140, (2012).
Ji, C. et al. Aptamer-Protein Interactions: From Regulation to Biomolecular Detection. Chem. Rev. 123, 12471–12506 (2023).
Di Mauro, V. et al. Diagnostic and Therapeutic Aptamers: A Promising Pathway to Improved Cardiovascular Disease Management. JACC Basic Transl. Sci. 9, 260–277 (2024).
Lao, Y. H., Phua, K. K. & Leong, K. W. Aptamer nanomedicine for cancer therapeutics: barriers and potential for translation. ACS Nano 9, 2235–2254, (2015).
Oelkrug, C. et al. Antibody- and aptamer-strategies for GvHD prevention. J. Cell Mol. Med. 19, 11–20 (2015).
Šmuc, T., Ahn, I. Y. & Ulrich, H. Nucleic acid aptamers as high affinity ligands in biotechnology and biosensorics. J. Pharm. Biomed. Anal. 81-82, 210–217 (2013).
Hoinka, J. et al. Large scale analysis of the mutational landscape in HT-SELEX improves aptamer discovery. Nucleic Acids Res. 43, 5699–5707 (2015).
Gotrik, M. R. et al. Advancements in Aptamer Discovery Technologies. Acc. Chem. Res. 49, 1903–1910 (2016).
Li, Q., Zhao, X., Liu, H. & Qu, F. Low pH capillary electrophoresis application to improve capillary electrophoresis-systematic evolution of ligands by exponential enrichment. J. Chromatogr. A 1364, 289–294 (2014).
Ishida, R. et al. RaptRanker: in silico RNA aptamer selection from HT-SELEX experiment based on local sequence and structure information. Nucleic Acids Res. 48, e82 (2020).
Kanasty, R., Dorkin, J. R., Vegas, A. & Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 12, 967–977, (2013).
Ahn, I., Kang, C. S. & Han, J. Where should siRNAs go: applicable organs for siRNA drugs. Exp. Mol. Med. 55, 1283–1292 (2023).
Schwarz, D. S. et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115, 199–208 (2003).
Hu, B. et al. Therapeutic siRNA: state of the art. Signal Transduct. Target Ther. 5, 101 (2020).
Liu, J. et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441 (2004).
Schürmann, N. et al. Molecular dissection of human Argonaute proteins by DNA shuffling. Nat. Struct. Mol. Biol. 20, 818–826 (2013).
Yamaguchi, S. et al. Structure of the Dicer-2-R2D2 heterodimer bound to a small RNA duplex. Nature 607, 393–398 (2022).
Noland, C. L., Ma, E. & Doudna, J. A. siRNA repositioning for guide strand selection by human Dicer complexes. Mol. Cell 43, 110–121, (2011).
Masliah, G. et al. Structural basis of siRNA recognition by TRBP double-stranded RNA binding domains. Embo j. 37, (2018).
Elbashir, S. M. et al. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. Embo j. 20, 6877–6888 (2001).
Wittrup, A. & Lieberman, J. Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet. 16, 543–552, (2015).
Humphreys, S. C. et al. Emerging siRNA Design Principles and Consequences for Biotransformation and Disposition in Drug Development. J. Med Chem. 63, 6407–6422 (2020).
Schwarz, D. S., Hutvágner, G., Haley, B. & Zamore, P. D. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol. Cell 10, 537–548 (2002).
Martinez, J. et al. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 110, 563–574 (2002).
Kim, D. H. et al. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 23, 222–226 (2005).
Manche, L., Green, S. R., Schmedt, C. & Mathews, M. B. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol. Cell Biol. 12, 5238–5248 (1992).
Gantier, M. P. & Williams, B. R. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 18, 363–371 (2007).
Patzel, V. et al. Design of siRNAs producing unstructured guide-RNAs results in improved RNA interference efficiency. Nat. Biotechnol. 23, 1440–1444 (2005).
Pei, Y. & Tuschl, T. On the art of identifying effective and specific siRNAs. Nat. Methods 3, 670–676 (2006).
Tomari, Y. et al. A protein sensor for siRNA asymmetry. Science 306, 1377–1380 (2004).
Petri, S. & Meister, G. siRNA design principles and off-target effects. Methods Mol. Biol. 986, 59–71 (2013).
Ranjbar, S., Zhong, X. B., Manautou, J. & Lu, X. A holistic analysis of the intrinsic and delivery-mediated toxicity of siRNA therapeutics. Adv. Drug Deliv. Rev. 201, 115052 (2023).
Hornung, V. et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med 11, 263–270 (2005).
Judge, A. D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 23, 457–462 (2005).
Fedorov, Y. et al. Off-target effects by siRNA can induce toxic phenotype. Rna 12, 1188–1196 (2006).
Pelletier, D., Rivera, B., Fabian, M. R. & Foulkes, W. D. miRNA biogenesis and inherited disorders: clinico-molecular insights. Trends Genet 39, 401–414 (2023).
Han, J. et al. Posttranscriptional crossregulation between Drosha and DGCR8. Cell 136, 75–84 (2009).
Lund, E. et al. Nuclear export of microRNA precursors. Science 303, 95–98 (2004).
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Lee, Y. Y. et al. Structure of the human DICER-pre-miRNA complex in a dicing state. Nature 615, 331–338 (2023).
Treiber, T., Treiber, N. & Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 20, 5–20 (2019).
Winkle, M., El-Daly, S. M., Fabbri, M. & Calin, G. A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 20, 629–651 (2021).
Lu, Z. G. et al. Nucleic acid drug vectors for diagnosis and treatment of brain diseases. Signal Transduct. Target Ther. 8, 39 (2023).
Majid, S. et al. MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer 116, 5637–5649 (2010).
Matsui, M. et al. Promoter RNA links transcriptional regulation of inflammatory pathway genes. Nucleic Acids Res 41, 10086–10109 (2013).
Turner, M. J., Jiao, A. L. & Slack, F. J. Autoregulation of lin-4 microRNA transcription by RNA activation (RNAa) in C. elegans. Cell Cycle 13, 772–781 (2014).
Xiao, M. et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 14, 1326–1334 (2017).
Bader, A. G., Brown, D., Stoudemire, J. & Lammers, P. Developing therapeutic microRNAs for cancer. Gene Ther. 18, 1121–1126, (2011).
Yates, L. A., Norbury, C. J. & Gilbert, R. J. The long and short of microRNA. Cell 153, 516–519 (2013).
Helwak, A., Kudla, G., Dudnakova, T. & Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153, 654–665, (2013).
Gu, S. et al. Weak base pairing in both seed and 3’ regions reduces RNAi off-targets and enhances si/shRNA designs. Nucleic Acids Res. 42, 12169–12176 (2014).
Peters, L. & Meister, G. Argonaute proteins: mediators of RNA silencing. Mol. Cell 26, 611–623, (2007).
Aravin, A. et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207 (2006).
Girard, A., Sachidanandam, R., Hannon, G. J. & Carmell, M. A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202 (2006).
Lau, N. C. et al. Characterization of the piRNA complex from rat testes. Science 313, 363–367 (2006).
Saito, K. et al. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 20, 2214–2222 (2006).
Grivna, S. T., Beyret, E., Wang, Z. & Lin, H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 20, 1709–1714 (2006).
Nishimasu, H. et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature 491, 284–287 (2012).
Chen, S. et al. The biogenesis and biological function of PIWI-interacting RNA in cancer. J. Hematol. Oncol. 14, 93 (2021).
Tushir, J. S., Zamore, P. D. & Zhang, Z. SnapShot: mouse piRNAs, PIWI proteins, and the ping-pong cycle. Cell 139, 830–830.e831 (2009).
Houwing, S. et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 129, 69–82 (2007).
Ortogero, N. et al. A novel class of somatic small RNAs similar to germ cell pachytene PIWI-interacting small RNAs. J. Biol. Chem. 289, 32824–32834 (2014).
Nishida, K. M. et al. Respective functions of two distinct Siwi complexes assembled during PIWI-interacting RNA biogenesis in Bombyx germ cells. Cell Rep. 10, 193–203 (2015).
Honda, S. et al. Increasing cell density globally enhances the biogenesis of Piwi-interacting RNAs in Bombyx mori germ cells. Sci. Rep. 7, 4110 (2017).
Grivna, S. T., Pyhtila, B. & Lin, H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc. Natl. Acad. Sci. USA 103, 13415–13420 (2006).
Charlesworth, A. G., Nitschko, V., Renaud, M. S. & Claycomb, J. M. PIWI puts spermatogenesis in its place. Dev. Cell 57, 149–151 (2022).
Siomi, M. C., Sato, K., Pezic, D. & Aravin, A. A. PIWI-interacting small RNAs: the vanguard of genome defence. Nat. Rev. Mol. Cell Biol. 12, 246–258, (2011).
Gainetdinov, I. et al. Relaxed targeting rules help PIWI proteins silence transposons. Nature 619, 394–402 (2023).
Yin, J. et al. piR-823 contributes to colorectal tumorigenesis by enhancing the transcriptional activity of HSF1. Cancer Sci. 108, 1746–1756 (2017).
Xiao, L. et al. Disorders and roles of tsRNA, snoRNA, snRNA and piRNA in cancer. J. Med Genet. 59, 623–631 (2022).
Liu, T. et al. Piwi-interacting RNA-651 promotes cell proliferation and migration and inhibits apoptosis in breast cancer by facilitating DNMT1-mediated PTEN promoter methylation. Cell Cycle 20, 1603–1616 (2021).
Cheng, J. et al. piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells. Cancer Lett. 315, 12–17 (2012).
Wang, S. et al. piR-823 inhibits cell apoptosis via modulating mitophagy by binding to PINK1 in colorectal cancer. Cell Death Dis. 13, 465 (2022).
Shen, S. et al. PIWIL1/piRNA-DQ593109 Regulates the Permeability of the Blood-Tumor Barrier via the MEG3/miR-330-5p/RUNX3 Axis. Mol. Ther. Nucleic Acids 10, 412–425 (2018).
Tan, L. et al. PIWI-interacting RNA-36712 restrains breast cancer progression and chemoresistance by interaction with SEPW1 pseudogene SEPW1P RNA. Mol. Cancer 18, 9 (2019).
Visel, A. et al. Ultraconservation identifies a small subset of extremely constrained developmental enhancers. Nat. Genet 40, 158–160 (2008).
Pennacchio, L. A. et al. In vivo enhancer analysis of human conserved non-coding sequences. Nature 444, 499–502 (2006).
Li, L. C. et al. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl. Acad. Sci. USA 103, 17337–17342 (2006).
Levin, A. A. Treating Disease at the RNA Level with Oligonucleotides. N. Engl. J. Med. 380, 57–70 (2019).
Hartman, T. R. et al. RNA helicase A is necessary for translation of selected messenger RNAs. Nat. Struct. Mol. Biol. 13, 509–516 (2006).
Weinmann, L. et al. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell 136, 496–507 (2009).
Wei, Y. et al. Importin 8 regulates the transport of mature microRNAs into the cell nucleus. J. Biol. Chem. 289, 10270–10275 (2014).
Portnoy, V. et al. saRNA-guided Ago2 targets the RITA complex to promoters to stimulate transcription. Cell Res. 26, 320–335 (2016).
Kim, J., Guermah, M. & Roeder, R. G. The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell 140, 491–503 (2010).
Yue, X. et al. Transcriptional regulation by small RNAs at sequences downstream from 3’ gene termini. Nat. Chem. Biol. 6, 621–629 (2010).
Zheng, L., Wang, L., Gan, J. & Zhang, H. RNA activation: promise as a new weapon against cancer. Cancer Lett. 355, 18–24 (2014).
Janowski, B. A. et al. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat. Chem. Biol. 3, 166–173 (2007).
Watts, J. K. et al. Effect of chemical modifications on modulation of gene expression by duplex antigene RNAs that are complementary to non-coding transcripts at gene promoters. Nucleic Acids Res 38, 5242–5259 (2010).
Setten, R. L., Lightfoot, H. L., Habib, N. A. & Rossi, J. J. Development of MTL-CEBPA: Small Activating RNA Drug for Hepatocellular Carcinoma. Curr. Pharm. Biotechnol. 19, 611–621 (2018).
Kingwell, K. Small activating RNAs lead the charge to turn up gene expression. Nat. Rev. Drug Discov. 20, 573–574 (2021).
Egli, M. & Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 51, 2529–2573 (2023).
Opalinska, J. B. & Gewirtz, A. M. Nucleic-acid therapeutics: basic principles and recent applications. Nat. Rev. Drug Discov. 1, 503–514, (2002).
Wan, W. B. & Seth, P. P. The Medicinal Chemistry of Therapeutic Oligonucleotides. J. Med Chem. 59, 9645–9667 (2016).
Geary, R. S., Norris, D., Yu, R. & Bennett, C. F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 87, 46–51 (2015).
Bramsen, J. B. et al. A large-scale chemical modification screen identifies design rules to generate siRNAs with high activity, high stability and low toxicity. Nucleic Acids Res. 37, 2867–2881 (2009).
Morrissey, D. V. et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology 41, 1349–1356 (2005).
Sato, Y. et al. Highly specific delivery of siRNA to hepatocytes circumvents endothelial cell-mediated lipid nanoparticle-associated toxicity leading to the safe and efficacious decrease in the hepatitis B virus. J. Control Release 266, 216–225 (2017).
Hatakeyama, H. et al. The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice. J. Control Release 173, 43–50 (2014).
Voutila, J. et al. Development and Mechanism of Small Activating RNA Targeting CEBPA, a Novel Therapeutic in Clinical Trials for Liver Cancer. Mol. Ther. 25, 2705–2714 (2017).
Carroll, J. B. et al. Potent and selective antisense oligonucleotides targeting single-nucleotide polymorphisms in the Huntington disease gene / allele-specific silencing of mutant huntingtin. Mol. Ther. 19, 2178–2185 (2011).
Schlegel, M. K. et al. Chirality Dependent Potency Enhancement and Structural Impact of Glycol Nucleic Acid Modification on siRNA. J. Am. Chem. Soc. 139, 8537–8546 (2017).
Gao, S. et al. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol. Ther. 17, 1225–1233 (2009).
Snead, N. M., Escamilla-Powers, J. R., Rossi, J. J. & McCaffrey, A. P. 5’ Unlocked Nucleic Acid Modification Improves siRNA Targeting. Mol. Ther. Nucleic Acids 2, e103 (2013).
Carlile, T. M. et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515, 143–146 (2014).
Bernard, M. C. et al. The impact of nucleoside base modification in mRNA vaccine is influenced by the chemistry of its lipid nanoparticle delivery system. Mol. Ther. Nucleic Acids 32, 794–806 (2023).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175, (2005).
Vaidyanathan, S. et al. Uridine Depletion and Chemical Modification Increase Cas9 mRNA Activity and Reduce Immunogenicity without HPLC Purification. Mol. Ther. Nucleic Acids 12, 530–542 (2018).
Heuberger, B. D. et al. Replacing uridine with 2-thiouridine enhances the rate and fidelity of nonenzymatic RNA primer extension. J. Am. Chem. Soc. 137, 2769–2775 (2015).
Zhang, C. et al. The landscape of m(1)A modification and its posttranscriptional regulatory functions in primary neurons. Elife 12, e85324 (2023).
Zaidi, Ali et al. Engineering siRNA therapeutics: challenges and strategies. J. Nanobiotechnol. 21, 381 (2023).
Lockhart, J. et al. Nucleotide Modification Alters MicroRNA-Dependent Silencing of MicroRNA Switches. Mol. Ther. Nucleic Acids 14, 339–350 (2019).
Zhang, J. et al. Modification of the siRNA passenger strand by 5-nitroindole dramatically reduces its off-target effects. Chembiochem 13, 1940–1945 (2012).
Renneberg, D. et al. Antisense properties of tricyclo-DNA. Nucleic Acids Res. 30, 2751–2757 (2002).
Sheng, L. et al. Comparison of the efficacy of MOE and PMO modifications of systemic antisense oligonucleotides in a severe SMA mouse model. Nucleic Acids Res. 48, 2853–2865 (2020).
De Mesmaeker, A., Altmann, K. H., Waldner, A. & Wendeborn, S. Backbone modifications in oligonucleotides and peptide nucleic acid systems. Curr. Opin. Struct. Biol. 5, 343–355, (1995).
Barnaby, S. N., Lee, A. & Mirkin, C. A. Probing the inherent stability of siRNA immobilized on nanoparticle constructs. Proc. Natl. Acad. Sci. USA 111, 9739–9744, (2014).
Jahns, H. et al. Stereochemical bias introduced during RNA synthesis modulates the activity of phosphorothioate siRNAs. Nat. Commun. 6, 6317 (2015).
Hogrefe, R. I. et al. Deprotection of methylphosphonate oligonucleotides using a novel one-pot procedure. Nucleic Acids Res. 21, 2031–2038 (1993).
Vasquez, G. et al. Evaluation of Phosphorus and Non-Phosphorus Neutral Oligonucleotide Backbones for Enhancing Therapeutic Index of Gapmer Antisense Oligonucleotides. Nucleic Acid Ther. 32, 40–50 (2022).
Yang, X., Dinuka Abeydeera, N., Liu, F. W. & Egli, M. Origins of the enhanced affinity of RNA-protein interactions triggered by RNA phosphorodithioate backbone modification. Chem. Commun. (Camb.) 53, 10508–10511 (2017).
Torigoe, H. & Maruyama, A. Synergistic stabilization of nucleic acid assembly by oligo-N3’->P5’ phosphoramidate modification and additions of comb-type cationic copolymers. J. Am. Chem. Soc. 127, 1705–1710 (2005).
Laurent, A. et al. Chiral and steric effects in the efficient binding of alpha-anomeric deoxyoligonucleoside N-alkylphosphoramidates to ssDNA and RNA. Nucleic Acids Res. 27, 4151–4159 (1999).
Pallan, P. S. & Egli, M. Selenium modification of nucleic acids: preparation of phosphoroselenoate derivatives for crystallographic phasing of nucleic acid structures. Nat. Protoc. 2, 640–646, (2007).
Meade, B. R. et al. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat. Biotechnol. 32, 1256–1261 (2014).
Hall, A. H. et al. RNA interference using boranophosphate siRNAs: structure-activity relationships. Nucleic Acids Res. 32, 5991–6000 (2004).
An, H. et al. Synthesis of novel 3’-C-methylene thymidine and 5-methyluridine/cytidine H-phosphonates and phosphonamidites for new backbone modification of oligonucleotides. J. Org. Chem. 66, 2789–2801 (2001).
Gryaznov, S. M. et al. Oligonucleotide N3’->P5’ phosphoramidates. Proc. Natl. Acad. Sci. USA 92, 5798–5802 (1995).
Mutisya, D. et al. Amides are excellent mimics of phosphate internucleoside linkages and are well tolerated in short interfering RNAs. Nucleic Acids Res. 42, 6542–6551 (2014).
Cowsert, L. M. et al. MMI linkage modification increases potency and stability of H-ras antisense oligonucleotides. Nucleosides Nucleotides 18, 1383–1384 (1999).
Kolarovic, A. et al. Interplay of structure, hydration and thermal stability in formacetal modified oligonucleotides: RNA may tolerate nonionic modifications better than DNA. J. Am. Chem. Soc. 131, 14932–14937 (2009).
Rice, J. S. & Gao, X. Conformation of formacetal and 3’-thioformacetal nucleotide linkers and stability of their antisense RNA.DNA hybrid duplexes. Biochemistry 36, 399–411 (1997).
Freier, S. M. & Altmann, K. H. The ups and downs of nucleic acid duplex stability: structure-stability studies on chemically-modified DNA:RNA duplexes. Nucleic Acids Res. 25, 4429–4443 (1997).
Jahns, H. et al. Chirality matters: stereo-defined phosphorothioate linkages at the termini of small interfering RNAs improve pharmacology in vivo. Nucleic Acids Res. 50, 1221–1240 (2022).
Iwamoto, N. et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 35, 845–851 (2017).
Østergaard, M. E. et al. Understanding the effect of controlling phosphorothioate chirality in the DNA gap on the potency and safety of gapmer antisense oligonucleotides. Nucleic Acids Res. 48, 1691–1700 (2020).
Wan, W. B. et al. Synthesis, biophysical properties and biological activity of second generation antisense oligonucleotides containing chiral phosphorothioate linkages. Nucleic Acids Res. 42, 13456–13468 (2014).
Byrne, M. et al. Stereochemistry Enhances Potency, Efficacy, and Durability of Malat1 Antisense Oligonucleotides In Vitro and In Vivo in Multiple Species. Transl. Vis. Sci. Technol. 10, 23 (2021).
Wu, S. Y. et al. 2’-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity. Nat. Commun. 5, 3459 (2014).
Baker, Y. R. et al. An LNA-amide modification that enhances the cell uptake and activity of phosphorothioate exon-skipping oligonucleotides. Nat. Commun. 13, 4036 (2022).
Shen, W. et al. Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index. Nat. Biotechnol. 37, 640–650 (2019).
Flierl, U. et al. Phosphorothioate backbone modifications of nucleotide-based drugs are potent platelet activators. J. Exp. Med. 212, 129–137 (2015).
Swayze, E. E. et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 35, 687–700 (2007).
Burdick, A. D. et al. Sequence motifs associated with hepatotoxicity of locked nucleic acid-modified antisense oligonucleotides. Nucleic Acids Res. 42, 4882–4891 (2014).
Kuntsche, J., Horst, J. C. & Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int J. Pharm. 417, 120–137 (2011).
Hu, M. et al. Physiological Barriers and Strategies of Lipid-Based Nanoparticles for Nucleic Acid Drug Delivery. Adv. Mater. 36, e2303266 (2024).
Kansız, S. & Elçin, Y. M. Advanced liposome and polymersome-based drug delivery systems: Considerations for physicochemical properties, targeting strategies and stimuli-sensitive approaches. Adv. Colloid Interface Sci. 317, 102930 (2023).
Bangham, A. D., Standish, M. M. & Watkins, J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 13, 238–252, (1965).
Gregoriadis, G. & Ryman, B. E. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem J. 124, 58p (1971).
Felgner, P. L. & Ringold, G. M. Cationic liposome-mediated transfection. Nature 337, 387–388, (1989).
Harding, C. V. et al. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell 64, 393–401 (1991).
Malone, R. W., Felgner, P. L. & Verma, I. M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 86, 6077–6081, (1989).
Yano, J. et al. Antitumor activity of small interfering RNA/cationic liposome complex in mouse models of cancer. Clin. Cancer Res. 10, 7721–7726 (2004).
Lonez, C., Vandenbranden, M. & Ruysschaert, J. M. Cationic liposomal lipids: from gene carriers to cell signaling. Prog. Lipid Res. 47, 340–347, (2008).
Qian, Y. et al. Hyaluronan Reduces Cationic Liposome-Induced Toxicity and Enhances the Antitumor Effect of Targeted Gene Delivery in Mice. ACS Appl Mater. Interf. 10, 32006–32016 (2018).
Villares, G. J. et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 68, 9078–9086 (2008).
Zhang, Y. et al. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 121, 12181–12277 (2021).
Jeong, M. et al. Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications. Adv. Drug Deliv. Rev. 200, 114990 (2023).
Yang, L. et al. Efficient Delivery of Antisense Oligonucleotides Using Bioreducible Lipid Nanoparticles In Vitro and In Vivo. Mol. Ther. Nucleic Acids 19, 1357–1367 (2020).
Ma, Y., Li, S., Lin, X. & Chen, Y. A perspective of lipid nanoparticles for RNA delivery. Exploration 4, 20230147 (2024).
Akinc, A. et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14, 1084–1087 (2019).
Chonn, A., Semple, S. C. & Cullis, P. R. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J. Biol. Chem. 267, 18759–18765, (1992).
Hald Albertsen, C. et al. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 188, 114416 (2022).
Nakamura, Y., Mochida, A., Choyke, P. L. & Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 27, 2225–2238 (2016).
Cullis, P. R. & Hope, M. J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 25, 1467–1475 (2017).
Schlich, M. et al. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 6, e10213 (2021).
Jayaraman, M. et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int Ed. Engl. 51, 8529–8533 (2012).
Kedmi, R., Ben-Arie, N. & Peer, D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials 31, 6867–6875, (2010).
Samaridou, E., Heyes, J. & Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 154-155, 37–63 (2020).
Eygeris, Y., Gupta, M., Kim, J. & Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res 55, 2–12 (2022).
Gjetting, T. et al. In vitro and in vivo effects of polyethylene glycol (PEG)-modified lipid in DOTAP/cholesterol-mediated gene transfection. Int J. Nanomed. 5, 371–383, (2010).
Issa, H. et al. Nanoparticle-mediated targeting of the fusion gene RUNX1/ETO in t(8;21)-positive acute myeloid leukaemia. Leukemia 37, 820–834 (2023).
Suzuki, T. et al. PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: Faster PEG shedding attenuates anti-PEG IgM production. Int J. Pharm. 588, 119792 (2020).
Belliveau, N. M. et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 1, e37 (2012).
Lam, K. et al. Optimizing Lipid Nanoparticles for Delivery in Primates. Adv. Mater. 35, e2211420 (2023).
Krause, M. R. & Regen, S. L. The structural role of cholesterol in cell membranes: from condensed bilayers to lipid rafts. Acc. Chem. Res 47, 3512–3521, (2014).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).
Patel, S. et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 11, 983 (2020).
Cheng, X. & Lee, R. J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 99, 129–137 (2016).
Chen, D. et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 134, 6948–6951 (2012).
Maeki, M. et al. Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J. Control Release 344, 80–96 (2022).
Kim, K. H. et al. Optimization of HPLCCAD method for simultaneous analysis of different lipids in lipid nanoparticles with analytical QbD. J. Chromatogr. A 1709, 464375 (2023).
Bost, J. P. et al. Delivery of Oligonucleotide Therapeutics: Chemical Modifications, Lipid Nanoparticles, and Extracellular Vesicles. ACS Nano 15, 13993–14021 (2021).
Gruenberg, J. & Maxfield, F. R. Membrane transport in the endocytic pathway. Curr. Opin. Cell Biol. 7, 552–563, (1995).
Troelnikov, A. et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J. Allergy Clin. Immunol. 148, 91–95 (2021).
Ibrahim, M. et al. Polyethylene glycol (PEG): The nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Control Release 351, 215–230 (2022).
Knop, K., Hoogenboom, R., Fischer, D. & Schubert, U. S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int Ed. Engl. 49, 6288–6308, (2010).
Ganson, N. J. et al. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther. 8, R12 (2006).
Dams, E. T. et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharm. Exp. Ther. 292, 1071–1079 (2000).
Ishida, T. et al. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J. Control Release 88, 35–42 (2003).
Wang, H. et al. Polyethylene glycol (PEG)-associated immune responses triggered by clinically relevant lipid nanoparticles in rats. NPJ Vaccines 8, 169 (2023).
Abu Lila, A. S., Kiwada, H. & Ishida, T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J. Control Release 172, 38–47 (2013).
Veronese, F. M. & Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 10, 1451–1458, (2005).
Torchilin, V. P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 58, 1532–1555 (2006).
Herold, D. A., Keil, K. & Bruns, D. E. Oxidation of polyethylene glycols by alcohol dehydrogenase. Biochem Pharm. 38, 73–76, (1989).
Judge, A., McClintock, K., Phelps, J. R. & Maclachlan, I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol. Ther. 13, 328–337 (2006).
Chen, S. et al. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control Release 235, 236–244 (2016).
Mui, B. L. et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of siRNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids 2, e139 (2013).
Hatakeyama, H. et al. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG-lipid. Biomaterials 32, 4306–4316 (2011).
Juang, V. et al. pH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and microRNA to Enhance Tumor-Specific Therapy. Small 15, e1903296 (2019).
El Moukhtari, S. H. et al. Lipid nanoparticles for siRNA delivery in cancer treatment. J. Control Release 361, 130–146 (2023).
Walters, A. A. et al. Nanoparticle-Mediated In Situ Molecular Reprogramming of Immune Checkpoint Interactions for Cancer Immunotherapy. ACS Nano 15, 17549–17564 (2021).
Lin, C. et al. Targeting Ligand Independent Tropism of siRNA-LNP by Small Molecules for Directed Therapy of Liver or Myeloid Immune Cells. Adv. Healthc Mater. e2202670, (2023).
Zhang, R. et al. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater. Sci. 9, 1449–1463 (2021).
Lee, J. B. et al. A Glu-urea-Lys Ligand-conjugated Lipid Nanoparticle/siRNA System Inhibits Androgen Receptor Expression In Vivo. Mol. Ther. Nucleic Acids 5, e348 (2016).
Ramishetti, S. et al. Systemic Gene Silencing in Primary T Lymphocytes Using Targeted Lipid Nanoparticles. ACS Nano 9, 6706–6716 (2015).
Younis, M. A. et al. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J. Control Release 331, 335–349 (2021).
Passos Gibson, V. et al. Hyaluronan decorated layer-by-layer assembled lipid nanoparticles for miR-181a delivery in glioblastoma treatment. Biomaterials 302, 122341 (2023).
Bai, X. et al. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci. Adv. 8, eabn7162 (2022).
Wang, Z. et al. Inhibition of retinal neovascularization by VEGF siRNA delivered via bioreducible lipid-like nanoparticles. Graefes Arch. Clin. Exp. Ophthalmol. 258, 2407–2418 (2020).
Hörmann, K. & Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions - A review. J. Control Release 223, 85–98 (2016).
Xu, Y. et al. Surface Modification of Lipid-Based Nanoparticles. ACS Nano 16, 7168–7196 (2022).
Kretzer, I. F. et al. Simvastatin increases the antineoplastic actions of paclitaxel carried in lipid nanoemulsions in melanoma-bearing mice. Int J. Nanomed. 11, 885–904, (2016).
Jiang, S. P. et al. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int J. Nanomed. 8, 3141–3150 (2013).
Kaneda, M. M. et al. Mechanisms of nucleotide trafficking during siRNA delivery to endothelial cells using perfluorocarbon nanoemulsions. Biomaterials 31, 3079–3086 (2010).
Gehrmann, S. & Bunjes, H. Preparation of lipid nanoemulsions by premix membrane emulsification with disposable materials. Int J. Pharm. 511, 741–744 (2016).
Baumann, N., Baumgarten, J., Kunick, C. & Bunjes, H. Prolonged release from lipid nanoemulsions by modification of drug lipophilicity. J Control Release 374, 478–488 (2024).
Naseri, N., Valizadeh, H. & Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 5, 305–313 (2015).
Tapeinos, C., Battaglini, M. & Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control Release 264, 306–332 (2017).
Liu, M. et al. Nanoencapsulation of lutein within lipid-based delivery systems: Characterization and comparison of zein peptide stabilized nano-emulsion, solid lipid nanoparticle, and nano-structured lipid carrier. Food Chem. 358, 129840 (2021).
Shirvani, A. et al. Fabrication of edible solid lipid nanoparticle from beeswax/propolis wax by spontaneous emulsification: Optimization, characterization and stability. Food Chem. 387, 132934 (2022).
del Pozo-Rodríguez, A., Solinís, M. A., Gascón, A. R. & Pedraz, J. L. Short- and long-term stability study of lyophilized solid lipid nanoparticles for gene therapy. Eur. J. Pharm. Biopharm. 71, 181–189 (2009).
Wang, T. et al. Solvent injection-lyophilization of tert-butyl alcohol/water cosolvent systems for the preparation of drug-loaded solid lipid nanoparticles. Colloids Surf. B Biointerf. 79, 254–261 (2010).
Sandri, G. et al. Chitosan-coupled solid lipid nanoparticles: Tuning nanostructure and mucoadhesion. Eur. J. Pharm. Biopharm. 110, 13–18 (2017).
Huang, Z. et al. Relationship between particle size and lung retention time of intact solid lipid nanoparticle suspensions after pulmonary delivery. J. Control Release 325, 206–222 (2020).
Mohamed, J. M. et al. Pectin co-functionalized dual layered solid lipid nanoparticle made by soluble curcumin for the targeted potential treatment of colorectal cancer. Carbohydr. Polym. 252, 117180 (2021).
Rosiaux, Y., Jannin, V., Hughes, S. & Marchaud, D. Solid lipid excipients - matrix agents for sustained drug delivery. J. Control Release 188, 18–30 (2014).
Mehrad, B. et al. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res Int. 105, 962–969 (2018).
Costa, C. P., Moreira, J. N., Sousa Lobo, J. M. & Silva, A. C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 11, 925–940 (2021).
da Silva, M. G. et al. Developed and characterization of nanostructured lipid carriers containing food-grade interesterified lipid phase for food application. Food Res. Int.155, 111119 (2022).
Sharma, G. et al. Nanostructured Lipid Carriers: A New Paradigm in Topical Delivery for Dermal and Transdermal Applications. Crit. Rev. Ther. Drug Carr. Syst. 34, 355–386 (2017).
Dima, C., Assadpour, E., Dima, S. & Jafari, S. M. Nutraceutical nanodelivery; an insight into the bioaccessibility/bioavailability of different bioactive compounds loaded within nanocarriers. Crit. Rev. Food Sci. Nutr. 61, 3031–3065 (2021).
Müller, R. H., Radtke, M. & Wissing, S. A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 54, S131–S155 (2002).
Oner, E., Kotmakci, M. & Kantarci, A. G. A promising approach to develop nanostructured lipid carriers from solid lipid nanoparticles: preparation, characterization, cytotoxicity and nucleic acid binding ability. Pharm. Dev. Technol. 25, 936–948 (2020).
Rajala, A. et al. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 14, 5257–5263 (2014).
Urandur, S. & Sullivan, M. O. Peptide-Based Vectors: A Biomolecular Engineering Strategy for Gene Delivery. Annu Rev. Chem. Biomol. Eng. 14, 243–264 (2023).
Frankel, A. D. & Pabo, C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55, 1189–1193, (1988).
Guidotti, G., Brambilla, L. & Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharm. Sci. 38, 406–424 (2017).
Lehto, T., Ezzat, K., Wood, M. J. A. & El Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 106, 172–182 (2016).
Yang, Y., Liu, Z., Ma, H. & Cao, M. Application of Peptides in Construction of Nonviral Vectors for Gene Delivery. Nanomaterials (Basel) 12, 4076 (2022).
Kwak, M. & Herrmann, A. Nucleic acid/organic polymer hybrid materials: synthesis, superstructures, and applications. Angew. Chem. Int Ed. Engl. 49, 8574–8587 (2010).
Dhasaiyan, P. & Prasad, B. L. V. Self-Assembly of Bolaamphiphilic Molecules. Chem. Rec. 17, 597–610 (2017).
Khan, M. M., Filipczak, N. & Torchilin, V. P. Cell penetrating peptides: A versatile vector for co-delivery of drug and genes in cancer. J. Control Release 330, 1220–1228 (2021).
Rothbard, J. B. et al. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J. Am. Chem. Soc. 126, 9506–9507 (2004).
Morris, M. C., Deshayes, S., Heitz, F. & Divita, G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol. Cell 100, 201–217, (2008).
De Coupade, C. et al. Novel human-derived cell-penetrating peptides for specific subcellular delivery of therapeutic biomolecules. Biochem J. 390, 407–418 (2005).
Yang, Y. et al. Enhanced nose-to-brain delivery of siRNA using hyaluronan-enveloped nanomicelles for glioma therapy. J. Control Release 342, 66–80 (2022).
Zhang, R. et al. Cholesterol modified DP7 and pantothenic acid induce dendritic cell homing to enhance the efficacy of dendritic cell vaccines. Mol. Biomed. 2, 37 (2021).
Zhao, B. et al. Sustained and targeted delivery of siRNA/DP7-C nanoparticles from injectable thermosensitive hydrogel for hepatocellular carcinoma therapy. Cancer Sci. 112, 2481–2492 (2021).
Zhang, R. et al. A Peptide-Based Small RNA Delivery System to Suppress Tumor Growth by Remodeling the Tumor Microenvironment. Mol. Pharm. 18, 1431–1443 (2021).
Liu, M. et al. In Situ Cocktail Nanovaccine for Cancer Immunotherapy. Adv. Sci. (Weinh.) 10, e2207697 (2023).
Wadia, J. S., Stan, R. V. & Dowdy, S. F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med 10, 310–315, (2004).
Sun, D., Forsman, J., Lund, M. & Woodward, C. E. Effect of arginine-rich cell penetrating peptides on membrane pore formation and life-times: a molecular simulation study. Phys. Chem. Chem. Phys. 16, 20785–20795, (2014).
Pärnaste, L. et al. The Formation of Nanoparticles between Small Interfering RNA and Amphipathic Cell-Penetrating Peptides. Mol. Ther. Nucleic Acids 7, 1–10 (2017).
Simeoni, F., Morris, M. C., Heitz, F. & Divita, G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. 31, 2717–2724, (2003).
Crombez, L. et al. Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth. Nucleic Acids Res. 37, 4559–4569 (2009).
Eguchi, A. et al. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat. Biotechnol. 27, 567–571 (2009).
Michiue, H., Eguchi, A., Scadeng, M. & Dowdy, S. F. Induction of in vivo synthetic lethal RNAi responses to treat glioblastoma. Cancer Biol. Ther. 8, 2306–2313, (2009).
Pooga, M. et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat. Biotechnol. 16, 857–861 (1998).
Taylor, B. N. et al. Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res. 69, 537–546 (2009).
Mo, R. H., Zaro, J. L. & Shen, W. C. Comparison of cationic and amphipathic cell penetrating peptides for siRNA delivery and efficacy. Mol. Pharm. 9, 299–309 (2012).
Elmquist, A., Lindgren, M., Bartfai, T. & Langel, U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp. Cell Res 269, 237–244 (2001).
Johansson, H. J. et al. Characterization of a novel cytotoxic cell-penetrating peptide derived from p14ARF protein. Mol. Ther. 16, 115–123 (2008).
Magzoub, M. et al. N-terminal peptides from unprocessed prion proteins enter cells by macropinocytosis. Biochem Biophys. Res Commun. 348, 379–385 (2006).
Crombez, L. et al. A new potent secondary amphipathic cell-penetrating peptide for siRNA delivery into mammalian cells. Mol. Ther. 17, 95–103 (2009).
Fernández-Carneado, J., Kogan, M. J., Pujals, S. & Giralt, E. Amphipathic peptides and drug delivery. Biopolymers 76, 196–203 (2004).
Wada, S. I. et al. Influence of lysine residue in amphipathic helical peptides on targeted delivery of RNA into cancer cells. Bioorg. Med Chem. Lett. 29, 1934–1937 (2019).
Wada, S. I., Shibaike, A., Hayashi, J. & Urata, H. Influence of Aib-Containing Amphipathic Helical Chain Length in MAP(Aib)-cRGD as Carrier for siRNA Delivery. Chem. Biodivers. 19, e202100728 (2022).
Konate, K. et al. Optimisation of vectorisation property: A comparative study for a secondary amphipathic peptide. Int J. Pharm. 509, 71–84 (2016).
Kragol, G. et al. Identification of crucial residues for the antibacterial activity of the proline-rich peptide, pyrrhocoricin. Eur. J. Biochem 269, 4226–4237 (2002).
Otvos, L. Jr The short proline-rich antibacterial peptide family. Cell Mol. Life Sci. 59, 1138–1150 (2002).
Sadler, K. et al. Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7. Biochemistry 41, 14150–14157 (2002).
Stalmans, S. et al. Blood-brain barrier transport of short proline-rich antimicrobial peptides. Protein Pept. Lett. 21, 399–406 (2014).
Frøslev, P. et al. Highly cationic cell-penetrating peptides affect the barrier integrity and facilitates mannitol permeation in a human stem cell-based blood-brain barrier model. Eur. J. Pharm. Sci. 168, 106054 (2022).
Wu, J. et al. PR39 inhibits apoptosis in hypoxic endothelial cells: role of inhibitor apoptosis protein-2. Circulation 109, 1660–1667 (2004).
Tian, W. et al. Suppression of tumor invasion and migration in breast cancer cells following delivery of siRNA against Stat3 with the antimicrobial peptide PR39. Oncol. Rep. 28, 1362–1368 (2012).
Mueller, L. K., Baumruck, A. C., Zhdanova, H. & Tietze, A. A. Challenges and Perspectives in Chemical Synthesis of Highly Hydrophobic Peptides. Front Bioeng. Biotechnol. 8, 162 (2020).
Schmidt, S. et al. Identification of Short Hydrophobic Cell-Penetrating Peptides for Cytosolic Peptide Delivery by Rational Design. Bioconjug Chem. 28, 382–389 (2017).
Gao, C. et al. A cell-penetrating peptide from a novel pVII-pIX phage-displayed random peptide library. Bioorg. Med Chem. 10, 4057–4065 (2002).
Desale, K., Kuche, K. & Jain, S. Cell-penetrating peptides (CPPs): an overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 9, 1153–1188 (2021).
Rhee, M. & Davis, P. Mechanism of uptake of C105Y, a novel cell-penetrating peptide. J. Biol. Chem. 281, 1233–1240 (2006).
Wang, F. et al. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control Release 174, 126–136 (2014).
Mäe, M. et al. A stearylated CPP for delivery of splice correcting oligonucleotides using a non-covalent co-incubation strategy. J. Control Release 134, 221–227 (2009).
Haag, R. & Kratz, F. Polymer therapeutics: concepts and applications. Angew. Chem. Int Ed. Engl. 45, 1198–1215 (2006).
Hamaguchi, T. et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 92, 1240–1246 (2005).
Aied, A., Greiser, U., Pandit, A. & Wang, W. Polymer gene delivery: overcoming the obstacles. Drug Discov. Today 18, 1090–1098, (2013).
Jäger, M. et al. Branched and linear poly(ethylene imine)-based conjugates: synthetic modification, characterization, and application. Chem. Soc. Rev. 41, 4755–4767 (2012).
Dey, D. et al. Efficient gene delivery of primary human cells using peptide linked polyethylenimine polymer hybrid. Biomaterials 32, 4647–4658 (2011).
Gueta, O. & Amiram, M. Expanding the chemical repertoire of protein-based polymers for drug-delivery applications. Adv. Drug Deliv. Rev. 190, 114460 (2022).
Sbordone, F. et al. Embedding Peptides into Synthetic Polymers: Radical Ring-Opening Copolymerization of Cyclic Peptides. J. Am. Chem. Soc. 145, 6221–6229 (2023).
Jung, K., Corrigan, N., Wong, E. H. H. & Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 34, e2105063 (2022).
Zhu, S. et al. Recent Advances in Patterning Natural Polymers: From Nanofabrication Techniques to Applications. Small Methods 5, e2001060 (2021).
Li, Y. et al. Synthesis of Polymer-Lipid Nanoparticles by Microfluidic Focusing for siRNA Delivery. Molecules. 21, (2016).
Moghimi, S. M. et al. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol. Ther. 11, 990–995 (2005).
Mastrobattista, E. & Hennink, W. E. Polymers for gene delivery: Charged for success. Nat. Mater. 11, 10–12, (2011).
Min, H. S. et al. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew. Chem. Int Ed. Engl. 59, 8173–8180 (2020).
Araújo, D. et al. Polyamide Microsized Particulate Polyplex Carriers for the 2’-OMethylRNA EFG1 Antisense Oligonucleotide. ACS Appl. Bio. Mater. 4, 4607–4617 (2021).
Guo, S. et al. pH-Responsive polymer boosts cytosolic siRNA release for retinal neovascularization therapy. Acta Pharm. Sin. B 14, 781–794 (2024).
Ansari, A. S. et al. Lipopolymer mediated siRNA delivery targeting aberrant oncogenes for effective therapy of myeloid leukemia in preclinical animal models. J. Control Release 367, 821–836 (2024).
Guo, S. et al. Spatiotemporal-Controlled NIR-II Immune Agonist Sensitizes Cancer Immunotherapy. Adv. Mater. 36, e2400228 (2024).
Zhang, Y. et al. Hydrophobization of Ribonucleic Acids for Facile Systemic Delivery and Multifaceted Cancer Immunotherapy. Nano Lett. 24, 1376–1384 (2024).
Ma, Y. et al. A miRNA-based gene therapy nanodrug synergistically enhances pro-inflammatory antitumor immunity against melanoma. Acta Biomater. 155, 538–553 (2023).
Rutherfurd, S. M., Rutherfurd-Markwick, K. J. & Moughan, P. J. Available (ileal digestible reactive) lysine in selected pet foods. J. Agric Food Chem. 55, 3517–3522, (2007).
Khmara, I. et al. Preparation of poly-L-lysine functionalized magnetic nanoparticles and their influence on viability of cancer cells. J. Magn. Magn. Mater. 427, 114–121 (2017).
Estrada-Osorio, D. V. et al. Poly-L-lysine-modified with ferrocene to obtain a redox polymer for mediated glucose biosensor application. Bioelectrochemistry 146, 108147 (2022).
Yang, S. et al. Drug-free neutrally charged polypeptide nanoparticles as anticancer agents. J. Control Release 345, 464–474 (2022).
Christie, R. J. et al. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano 6, 5174–5189 (2012).
Yamagata, M. et al. Structural advantage of dendritic poly(L-lysine) for gene delivery into cells. Bioorg. Med Chem. 15, 526–532 (2007).
Inoue, Y. et al. Efficient delivery of siRNA using dendritic poly(L-lysine) for loss-of-function analysis. J. Control Release 126, 59–66 (2008).
Liu, T. et al. Star-shaped cyclodextrin-poly(l-lysine) derivative co-delivering docetaxel and MMP-9 siRNA plasmid in cancer therapy. Biomaterials 35, 3865–3872 (2014).
Hatakeyama, S. & Yasuno, M. Chronic effects of Cd on the reproduction of the guppy (Poecilia reticulata) through Cd-accumulated midge larvae (Chironomus yoshimatsui). Ecotoxicol. Environ. Saf. 14, 191–207 (1987).
Molinaro, R. et al. Polyethylenimine and chitosan carriers for the delivery of RNA interference effectors. Expert Opin. Drug Deliv. 10, 1653–1668 (2013).
Casper, J. et al. Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. J. Control Release 362, 667–691 (2023).
Boussif, O. et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA 92, 7297–7301 (1995).
Lungu, C. N., Diudea, M. V., Putz, M. V. & Grudziński, I. P. Linear and Branched PEIs (Polyethylenimines) and Their Property Space. Int J. Mol. Sci. 17, 555 (2016).
Dai, J. et al. Polyethylenimine-grafted copolymer of poly(l-lysine) and poly(ethylene glycol) for gene delivery. Biomaterials 32, 1694–1705 (2011).
Qiao, F., Zou, Y., Bie, B. & Lv, Y. Dual siRNA-Loaded Cell Membrane Functionalized Matrix Facilitates Bone Regeneration with Angiogenesis and Neurogenesis. Small 20, e2307062 (2024).
Francis, S. M. et al. SNS01-T modulation of eIF5A inhibits B-cell cancer progression and synergizes with bortezomib and lenalidomide. Mol. Ther. 22, 1643–1652 (2014).
Taylor, C. A. et al. Modulation of eIF5A expression using SNS01 nanoparticles inhibits NF-κB activity and tumor growth in murine models of multiple myeloma. Mol. Ther. 20, 1305–1314 (2012).
Liu, Y., Li, Y., Keskin, D. & Shi, L. Poly(β-Amino Esters): Synthesis, Formulations, and Their Biomedical Applications. Adv. Health Mater. 8, e1801359 (2019).
Wei, J. et al. Recent progress and applications of poly(beta amino esters)-based biomaterials. J. Control Release 354, 337–353 (2023).
Zhang, J. et al. Poly(β-amino ester)s-based nanovehicles: Structural regulation and gene delivery. Mol. Ther. Nucleic Acids 32, 568–581 (2023).
Hou, D. Y. et al. A Lysosome-Targeting Self-Condensation Prodrug-Nanoplatform System for Addressing Drug Resistance of Cancer. Nano Lett. 22, 3983–3992 (2022).
Routkevitch, D. et al. Efficiency of Cytosolic Delivery with Poly(β-amino ester) Nanoparticles is Dependent on the Effective pK(a) of the Polymer. ACS Biomater. Sci. Eng. 6, 3411–3421 (2020).
Sugie, Y. et al. [A case of systemic lupus erythematosus associated with lateral medullary syndrome and unilateral internuclear ophthalmoplegia]. Rinsho Shinkeigaku 29, 75–79 (1989).
Green, J. J., Langer, R. & Anderson, D. G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 41, 749–759, (2008).
Chen, Q. et al. Poly(beta-amino ester)-Based Nanoparticles Enable Nonviral Delivery of Base Editors for Targeted Tumor Gene Editing. Biomacromolecules 23, 2116–2125 (2022).
Qiao, Z. Y. et al. Self-assembly of cytotoxic peptide conjugated poly(β-amino ester)s for synergistic cancer chemotherapy. J. Mater. Chem. B 3, 2943–2953 (2015).
Vaughan, H. J. et al. Polymeric nanoparticles for dual-targeted theranostic gene delivery to hepatocellular carcinoma. Sci. Adv. 8, eabo6406 (2022).
Rhodes, K. R. et al. Biodegradable Cationic Polymer Blends for Fabrication of Enhanced Artificial Antigen Presenting Cells to Treat Melanoma. ACS Appl. Mater. Interf. 13, 7913–7923 (2021).
Kozielski, K. L., Tzeng, S. Y., De Mendoza, B. A. & Green, J. J. Bioreducible cationic polymer-based nanoparticles for efficient and environmentally triggered cytoplasmic siRNA delivery to primary human brain cancer cells. ACS Nano 8, 3232–3241, (2014).
Tzeng, S. Y., Hung, B. P., Grayson, W. L. & Green, J. J. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials 33, 8142–8151, (2012).
Klajnert, B. & Bryszewska, M. Dendrimers: properties and applications. Acta Biochim Pol. 48, 199–208 (2001).
Duncan, R. & Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 57, 2215–2237, (2005).
Kim, Y., Park, E. J. & Na, D. H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 41, 571–582 (2018).
Deriu, M. A. et al. Elucidating the role of surface chemistry on cationic phosphorus dendrimer-siRNA complexation. Nanoscale 10, 10952–10962 (2018).
Abbasi, E. et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 9, 247 (2014).
Chen, J. et al. Synthesis and use of an amphiphilic dendrimer for siRNA delivery into primary immune cells. Nat. Protoc. 16, 327–351 (2021).
Patil, N. G. et al. Synthesis of Stimuli-Responsive Heterofunctional Dendrimer by Passerini Multicomponent Reaction. ACS Omega 4, 6660–6668 (2019).
Fox, L. J., Richardson, R. M. & Briscoe, W. H. PAMAM dendrimer - cell membrane interactions. Adv. Colloid Interf. Sci. 257, 1–18 (2018).
Shao, N. et al. Comparison of generation 3 polyamidoamine dendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, release behavior, and cytotoxicity. Int J. Nanomed. 6, 3361–3372, (2011).
Liyanage, W., Wu, T., Kannan, S. & Kannan, R. M. Dendrimer-siRNA Conjugates for Targeted Intracellular Delivery in Glioblastoma Animal Models. ACS Appl. Mater. Interf. 14, 46290–46303 (2022).
Nussbaumer, M. G. et al. Chaperonin-Dendrimer Conjugates for siRNA Delivery. Adv. Sci. (Weinh.) 3, 1600046 (2016).
Liu, H. et al. Nanoassemblies with Effective Serum Tolerance Capability Achieving Robust Gene Silencing Efficacy for Breast Cancer Gene Therapy. Adv. Mater. 33, e2003523 (2021).
Figueroa, E. R. et al. Optimization of PAMAM-gold nanoparticle conjugation for gene therapy. Biomaterials 35, 1725–1734 (2014).
Lee, W. et al. Dual peptide-dendrimer conjugate inhibits acetylation of transforming growth factor β-induced protein and improves survival in sepsis. Biomaterials 246, 120000 (2020).
Harada, A. et al. Intracellular environment-responsive stabilization of polymer vesicles formed from head-tail type polycations composed of a polyamidoamine dendron and poly(L-lysine). Molecules 18, 12168–12179 (2013).
López-Méndez, L. J. et al. Synthesis of a poly(ester) dendritic β-cyclodextrin derivative by “click” chemistry: Combining the best of two worlds for complexation enhancement. Carbohydr. Polym. 184, 20–29 (2018).
Franc, G. & Kakkar, A. K. Click” methodologies: efficient, simple and greener routes to design dendrimers. Chem. Soc. Rev. 39, 1536–1544, (2010).
Paioti, P. H. S. et al. Click processes orthogonal to CuAAC and SuFEx forge selectively modifiable fluorescent linkers. Nat. Chem. 16, 426–436 (2024).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, 640–655 (2020).
Trams, E. G., Lauter, C. J., Salem, N. Jr & Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys. Acta 645, 63–70 (1981).
Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Invest 126, 1208–1215 (2016).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Smyth, T. et al. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control Release 199, 145–155 (2015).
Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 20, 6–7 (2021).
Graham, F. L., Smiley, J., Russell, W. C. & Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36, 59–74 (1977).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Mendt, M. et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3, e99263 (2018).
Zhu, X. et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 6, 1324730 (2017).
Park, E. J. et al. Exosomal regulation of lymphocyte homing to the gut. Blood Adv. 3, 1–11 (2019).
Yang, Y. et al. Exosome/antimicrobial peptide laden hydrogel wound dressings promote scarless wound healing through miR-21-5p-mediated multiple functions. Biomaterials 308, 122558 (2024).
Liang, S. F., Zuo, F. F., Yin, B. C. & Ye, B. C. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT3. Biomater. Sci. 10, 1582–1590 (2022).
Kim, G. et al. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control Release 317, 273–281 (2020).
Yang, J. et al. Exosome-mediated delivery of antisense oligonucleotides targeting α-synuclein ameliorates the pathology in a mouse model of Parkinson’s disease. Neurobiol. Dis. 148, 105218 (2021).
Zhang, R. et al. sEVs(RVG) selectively delivers antiviral siRNA to fetus brain, inhibits ZIKV infection and mitigates ZIKV-induced microcephaly in mouse model. Mol. Ther. 30, 2078–2091 (2022).
Izco, M. et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 27, 2111–2122 (2019).
Han, G. et al. Oral TNF-α siRNA delivery via milk-derived exosomes for effective treatment of inflammatory bowel disease. Bioact. Mater. 34, 138–149 (2024).
Lai, N. et al. Systemic exosomal miR-193b-3p delivery attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage in mice. J. Neuroinflamm. 17, 74 (2020).
Zhao, L. et al. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control Release 318, 1–15 (2020).
Tian, T. et al. Immune Checkpoint Inhibition in GBM Primed with Radiation by Engineered Extracellular Vesicles. ACS Nano 16, 1940–1953 (2022).
Uddin, N. et al. Targeted delivery of RNAi to cancer cells using RNA-ligand displaying exosome. Acta Pharm. Sin. B 13, 1383–1399 (2023).
Wang, Y. et al. G-MDSC-derived exosomes mediate the differentiation of M-MDSC into M2 macrophages promoting colitis-to-cancer transition. J. Immunother Cancer 11, e006166 (2023).
Shan, S. et al. Functionalized Macrophage Exosomes with Panobinostat and PPM1D-siRNA for Diffuse Intrinsic Pontine Gliomas Therapy. Adv. Sci. (Weinh.) 9, e2200353 (2022).
Aqil, F. et al. Milk exosomes - Natural nanoparticles for siRNA delivery. Cancer Lett. 449, 186–195 (2019).
Zhou, W. et al. Exosomes derived from immunogenically dying tumor cells as a versatile tool for vaccination against pancreatic cancer. Biomaterials 280, 121306 (2022).
Xu, L. et al. Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials 264, 120369 (2021).
Cui, Y. et al. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact. Mater. 10, 207–221 (2022).
El-Andaloussi, S. et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 7, 2112–2126 (2012).
Zheng, Z. et al. Folate-displaying exosome mediated cytosolic delivery of siRNA avoiding endosome trapping. J. Control Release 311-312, 43–49 (2019).
Yuan, D. et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142, 1–12 (2017).
Feng, D. et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic 11, 675–687 (2010).
Tian, T. et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150, 137–149 (2018).
Xu, M. et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 11, 8926–8944 (2021).
Liang, G. et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 18, 10 (2020).
Li, X. et al. A drug delivery system constructed by a fusion peptide capturing exosomes targets to titanium implants accurately resulting the enhancement of osseointegration peri-implant. Biomater. Res. 26, 89 (2022).
Gunassekaran, G. R., Poongkavithai Vadevoo, S. M., Baek, M. C. & Lee, B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 278, 121137 (2021).
Ran, N. et al. Autologous exosome facilitates load and target delivery of bioactive peptides to repair spinal cord injury. Bioact. Mater. 25, 766–782 (2023).
Liu, S. et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat. Biomed. Eng. 4, 1063–1075 (2020).
Ha, D., Yang, N. & Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B 6, 287–296, (2016).
Ruan, S., Erwin, N. & He, M. Light-induced high-efficient cellular production of immune functional extracellular vesicles. J. Extracell. Vesicles 11, e12194 (2022).
Kojima, R. et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 9, 1305 (2018).
Yang, Z. et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 4, 69–83 (2020).
Jang, S. C. et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 7, 7698–7710 (2013).
Balaj, L. et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180 (2011).
Usman, W. M. et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 9, 2359 (2018).
Zhu, Y. S., Tang, K. & Lv, J. Peptide-drug conjugate-based novel molecular drug delivery system in cancer. Trends Pharm. Sci. 42, 857–869 (2021).
Li, W. Q. et al. The promising role of antibody drug conjugate in cancer therapy: Combining targeting ability with cytotoxicity effectively. Cancer Med. 10, 4677–4696 (2021).
Ray, K. K. et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 382, 1507–1519 (2020).
Garrelfs, S. F. et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 384, 1216–1226 (2021).
Balwani, M. et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 382, 2289–2301 (2020).
Kumar, V. & Turnbull, W. B. Targeted delivery of oligonucleotides using multivalent protein-carbohydrate interactions. Chem. Soc. Rev. 52, 1273–1287 (2023).
Stockert, R. J., Morell, A. G. & Scheinberg, I. H. Mammalian hepatic lectin. Science 186, 365–366, (1974).
Hudgin, R. L. et al. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J. Biol. Chem. 249, 5536–5543 (1974).
Baenziger, J. U. & Maynard, Y. Human hepatic lectin. Physiochemical properties and specificity. J. Biol. Chem. 255, 4607–4613, (1980).
Meier, M. et al. Crystal structure of the carbohydrate recognition domain of the H1 subunit of the asialoglycoprotein receptor. J. Mol. Biol. 300, 857–865 (2000).
Steirer, L. M., Park, E. I., Townsend, R. R. & Baenziger, J. U. The asialoglycoprotein receptor regulates levels of plasma glycoproteins terminating with sialic acid alpha2,6-galactose. J. Biol. Chem. 284, 3777–3783 (2009).
Zhou, Y. et al. Development of Triantennary N-Acetylgalactosamine Conjugates as Degraders for Extracellular Proteins. ACS Cent. Sci. 7, 499–506 (2021).
Lee, Y. C. et al. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. Dependence on fine structural features. J. Biol. Chem. 258, 199–202 (1983).
Biessen, E. A. et al. Synthesis of cluster galactosides with high affinity for the hepatic asialoglycoprotein receptor. J. Med Chem. 38, 1538–1546 (1995).
Rensen, P. C. et al. Determination of the upper size limit for uptake and processing of ligands by the asialoglycoprotein receptor on hepatocytes in vitro and in vivo. J. Biol. Chem. 276, 37577–37584 (2001).
Huang, Y. Preclinical and Clinical Advances of GalNAc-Decorated Nucleic Acid Therapeutics. Mol. Ther. Nucleic Acids 6, 116–132 (2017).
Debacker, A. J. et al. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther. 28, 1759–1771 (2020).
Schwartz, A. L., Fridovich, S. E. & Lodish, H. F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J. Biol. Chem. 257, 4230–4237 (1982).
Crooke, S. T. et al. Integrated Assessment of the Clinical Performance of GalNAc(3)-Conjugated 2’-O-Methoxyethyl Chimeric Antisense Oligonucleotides: I. Human Volunteer Experience. Nucleic Acid Ther. 29, 16–32 (2019).
Watanabe, A. et al. Comparative Characterization of Hepatic Distribution and mRNA Reduction of Antisense Oligonucleotides Conjugated with Triantennary N-Acetyl Galactosamine and Lipophilic Ligands Targeting Apolipoprotein B. J. Pharm. Exp. Ther. 357, 320–330 (2016).
Prakash, T. P. et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 42, 8796–8807 (2014).
Wolfrum, C. et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 25, 1149–1157 (2007).
Nishina, K. et al. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol. Ther. 16, 734–740 (2008).
Scharner, J. et al. Delivery of GalNAc-Conjugated Splice-Switching ASOs to Non-hepatic Cells through Ectopic Expression of Asialoglycoprotein Receptor. Mol. Ther. Nucleic Acids 16, 313–325 (2019).
Biscans, A., Coles, A., Echeverria, D. & Khvorova, A. The valency of fatty acid conjugates impacts siRNA pharmacokinetics, distribution, and efficacy in vivo. J. Control Release 302, 116–125 (2019).
Anikeeva, P. O., Halpert, J. E., Bawendi, M. G. & Bulović, V. Quantum dot light-emitting devices with electroluminescence tunable over the entire visible spectrum. Nano Lett. 9, 2532–2536, (2009).
Gao, Y. & Tang, Z. Design and application of inorganic nanoparticle superstructures: current status and future challenges. Small 7, 2133–2146 (2011).
Ferdows, B. E. et al. RNA cancer nanomedicine: nanotechnology-mediated RNA therapy. Nanoscale 14, 4448–4455 (2022).
Hirschbiegel, C. M. et al. Inorganic nanoparticles as scaffolds for bioorthogonal catalysts. Adv. Drug Deliv. Rev. 195, 114730 (2023).
Karakoti, A. S., Das, S., Thevuthasan, S. & Seal, S. PEGylated inorganic nanoparticles. Angew. Chem. Int Ed. Engl. 50, 1980–1994, (2011).
He, X. et al. AIE Featured Inorganic-Organic Core@Shell Nanoparticles for High-Efficiency siRNA Delivery and Real-Time Monitoring. Nano Lett. 19, 2272–2279 (2019).
Luo, Z. et al. Evolution of the Ligand Shell Morphology during Ligand Exchange Reactions on Gold Nanoparticles. Angew. Chem. Int. Ed. Engl. 56, 13521–13525 (2017).
Gupta, A. et al. Interfacing Nanomaterials with Biology through Ligand Engineering. Acc. Chem. Res. 56, 2151–2169 (2023).
Love, J. C. et al. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105, 1103–1169, (2005).
Daniel, M. C. & Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 104, 293–346 (2004).
Hauck, T. S., Ghazani, A. A. & Chan, W. C. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small 4, 153–159 (2008).
Pan, Y. et al. Size-dependent cytotoxicity of gold nanoparticles. Small 3, 1941–1949 (2007).
Groysbeck, N. et al. Bioactivated and PEG-Protected Circa 2 nm Gold Nanoparticles for in Cell Labelling and Cryo-Electron Microscopy. Small Methods 7, e2300098 (2023).
Wang, J. et al. Size- and surface chemistry-dependent pharmacokinetics and tumor accumulation of engineered gold nanoparticles after intravenous administration. Metallomics 14, 516–524 (2022).
Vio, V. et al. Gold nanorods/siRNA complex administration for knockdown of PARP-1: a potential treatment for perinatal asphyxia. Int J. Nanomed. 13, 6839–6854 (2018).
Gupta, A. et al. Ultrastable and Biofunctionalizable Gold Nanoparticles. ACS Appl Mater. Interf. 8, 14096–14101 (2016).
Lee, S. et al. Controlled drug release with surface-capped mesoporous silica nanoparticles and its label-free in situ Raman monitoring. Eur. J. Pharm. Biopharm. 131, 232–239 (2018).
Niidome, T. et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Control Release 114, 343–347 (2006).
Akiyama, Y., Mori, T., Katayama, Y. & Niidome, T. The effects of PEG grafting level and injection dose on gold nanorod biodistribution in the tumor-bearing mice. J. Control Release 139, 81–84 (2009).
Mieszawska, A. J., Mulder, W. J., Fayad, Z. A. & Cormode, D. P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 10, 831–847, (2013).
Lee, S. E. et al. Biologically functional cationic phospholipid-gold nanoplasmonic carriers of RNA. J. Am. Chem. Soc. 131, 14066–14074 (2009).
Ding, Y. et al. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 22, 1075–1083 (2014).
Giljohann, D. A. et al. Gene regulation with polyvalent siRNA-nanoparticle conjugates. J. Am. Chem. Soc. 131, 2072–2073 (2009).
Wan, J. et al. PEG-SH-GNPs-SAPNS@miR-29a delivery system promotes neural regeneration and recovery of motor function after spinal cord injury. J. Biomater. Sci. Polym. Ed. 34, 2107–2123 (2023).
Chen, W. et al. Aptamer-siRNA chimera and gold nanoparticle modified collagen membrane for the treatment of malignant pleural effusion. Front Bioeng. Biotechnol. 10, 973892 (2022).
Kim, S. J. et al. Axon guidance gene-targeted siRNA delivery system improves neural stem cell transplantation therapy after spinal cord injury. Biomater. Res. 27, 101 (2023).
Slowing, I. I., Vivero-Escoto, J. L., Wu, C. W. & Lin, V. S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 60, 1278–1288 (2008).
Fu, J. et al. Silica Nanoparticles with Virus-Mimetic Spikes Enable Efficient siRNA Delivery In Vitro and In Vivo. Res. (Wash. D. C.) 2022, 0014 (2022).
Niu, D. et al. Monodispersed and ordered large-pore mesoporous silica nanospheres with tunable pore structure for magnetic functionalization and gene delivery. Adv. Mater. 26, 4947–4953 (2014).
Borchert, K. B. L. et al. Tuning the pore structure of templated mesoporous poly(melamine-co-formaldehyde) particles toward diclofenac removal. J. Environ. Manag. 324, 116221 (2022).
Krug, H. F. & Wick, P. Nanotoxicology: an interdisciplinary challenge. Angew. Chem. Int Ed. Engl. 50, 1260–1278, (2011).
Inoue, M. et al. Size and surface modification of silica nanoparticles affect the severity of lung toxicity by modulating endosomal ROS generation in macrophages. Part Fibre Toxicol. 18, 21 (2021).
Khazieva, A. et al. Surface modification of silica nanoparticles by hexarhenium anionic cluster complexes for pH-sensing and staining of cell nuclei. J. Colloid Interf. Sci. 594, 759–769 (2021).
Kamegawa, R., Naito, M. & Miyata, K. Functionalization of silica nanoparticles for nucleic acid delivery. Nano Res. 11, 5219–5239 (2018).
Kumar, P., Salve, R., Paknikar, K. M. & Gajbhiye, V. Nucleolin aptamer conjugated MSNPs-PLR-PEG multifunctional nanoconstructs for targeted co-delivery of anticancer drug and siRNA to counter drug resistance in TNBC. Int J. Biol. Macromol. 229, 600–614 (2023).
Shahin, S. A. et al. Hyaluronic acid conjugated nanoparticle delivery of siRNA against TWIST reduces tumor burden and enhances sensitivity to cisplatin in ovarian cancer. Nanomedicine 14, 1381–1394 (2018).
Noureddine, A. et al. Future of Mesoporous Silica Nanoparticles in Nanomedicine: Protocol for Reproducible Synthesis, Characterization, Lipid Coating, and Loading of Therapeutics (Chemotherapeutic, Proteins, siRNA and mRNA). ACS Nano 17, 16308–16325 (2023).
Ahmadi, E. et al. Synthesis and surface modification of mesoporous silica nanoparticles and its application as carriers for sustained drug delivery. Drug Deliv. 21, 164–172 (2014).
Kulkarni, J. A. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643 (2021).
Abulikemu, A. et al. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox Biol. 59, 102569 (2023).
Li, X. et al. Long-term respiratory exposure to amorphous silica nanoparticles promoted systemic inflammation and progression of fibrosis in a susceptible mouse model. Chemosphere 300, 134633 (2022).
Lérida-Viso, A. et al. Biosafety of mesoporous silica nanoparticles; towards clinical translation. Adv. Drug Deliv. Rev. 201, 115049 (2023).
Duan, J. et al. Cardiovascular toxicity evaluation of silica nanoparticles in endothelial cells and zebrafish model. Biomaterials 34, 5853–5862 (2013).
Wang, F. et al. Silica nanoparticles induce pyroptosis and cardiac hypertrophy via ROS/NLRP3/Caspase-1 pathway. Free Radic. Biol. Med. 182, 171–181 (2022).
Guo, C., Liu, Y. & Li, Y. Adverse effects of amorphous silica nanoparticles: Focus on human cardiovascular health. J. Hazard Mater. 406, 124626 (2021).
Mohammadpour, R. et al. Subchronic toxicity of silica nanoparticles as a function of size and porosity. J. Control Release 304, 216–232 (2019).
Pinese, C. et al. Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing. Acta Biomater. 76, 164–177 (2018).
Hufschmid, R. et al. Observing the colloidal stability of iron oxide nanoparticles in situ. Nanoscale 11, 13098–13107 (2019).
Insin, N. et al. Incorporation of iron oxide nanoparticles and quantum dots into silica microspheres. ACS Nano 2, 197–202 (2008).
Mao, Z., Li, X., Wang, P. & Yan, H. Iron oxide nanoparticles for biomedical applications: an updated patent review (2015-2021). Expert Opin. Ther. Pat. 32, 939–952 (2022).
Li, J., Xue, S. & Mao, Z. W. Nanoparticle delivery systems for siRNA-based therapeutics. J. Mater. Chem. B 4, 6620–6639 (2016).
Widder, K. J., Senyel, A. E. & Scarpelli, G. D. Magnetic microspheres: a model system of site specific drug delivery in vivo. Proc. Soc. Exp. Biol. Med. 158, 141–146 (1978).
Senyei, A., Widder, K. & Czerlinski, G. Magnetic guidance of drug‐carrying microspheres. J. Appl. Phys. 49, 3578–3583 (1978).
Pulfer, S. K., Ciccotto, S. L. & Gallo, J. M. Distribution of small magnetic particles in brain tumor-bearing rats. J. Neurooncol. 41, 99–105 (1999).
Chung, S., Sugimoto, Y., Huang, J. & Zhang, M. Iron Oxide Nanoparticles Decorated with Functional Peptides for a Targeted siRNA Delivery to Glioma Cells. ACS Appl Mater. Interf. 15, 106–119 (2023).
Wu, Y. et al. A Biomimic Nanobullet with Ameliorative Inflammatory Microenvironment for Alzheimer’s Disease Treatments. Adv. Health Mater. 13, e2302851 (2024).
Li, B. et al. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv. Sci. (Weinh.) 9, e2105451 (2022).
Luther, D. C. et al. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 156, 188–213 (2020).
Liu, L.-H. & Zhang, X.-Z. Carrier-free nanomedicines for cancer treatment. Prog. Mater Sci. 125, 100919 (2022).
Alvarez, R. et al. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrob. Agents Chemother. 53, 3952–3962 (2009).
Martínez, T. et al. In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol. Ther. 22, 81–91 (2014).
Zheng, M. et al. The siRNAsome: A Cation-Free and Versatile Nanostructure for siRNA and Drug Co-delivery. Angew. Chem. Int Ed. Engl. 58, 4938–4942 (2019).
Yang, Y. et al. Electrostatic Attractive Self-Delivery of siRNA and Light-Induced Self-Escape for Synergistic Gene Therapy. Adv. Mater. 35, e2301409 (2023).
Fang, F. & Chen, X. Carrier-Free Nanodrugs: From Bench to Bedside. ACS Nano. 18, 23827–23841 (2024).
Galy, A. et al. Recent Advances Using Genetic Therapies Against Infectious Diseases and for Vaccination. Hum. Gene Ther. 34, 896–904 (2023).
Forgham, H., Kakinen, A., Qiao, R. & Davis, T. P. Keeping up with the COVID’s—Could siRNA-based antivirals be a part of the answer? Exploration 2, 20220012 (2022).
Luna, J. M. et al. Hepatitis C virus RNA functionally sequesters miR-122. Cell 160, 1099–1110 (2015).
Bandiera, S., Pfeffer, S., Baumert, T. F. & Zeisel, M. B. miR-122-a key factor and therapeutic target in liver disease. J. Hepatol. 62, 448–457, (2015).
Sedano, C. D. & Sarnow, P. Hepatitis C virus subverts liver-specific miR-122 to protect the viral genome from exoribonuclease Xrn2. Cell Host Microbe 16, 257–264 (2014).
Yuen, M. F. et al. RNA Interference Therapy With ARC-520 Results in Prolonged Hepatitis B Surface Antigen Response in Patients With Chronic Hepatitis B Infection. Hepatology 72, 19–31 (2020).
Yuen, M. F. et al. Long-term serological, virological and histological responses to RNA inhibition by ARC-520 in Chinese chronic hepatitis B patients on entecavir treatment. Gut 71, 789–797 (2022).
Schluep, T. et al. Safety, Tolerability, and Pharmacokinetics of ARC-520 Injection, an RNA Interference-Based Therapeutic for the Treatment of Chronic Hepatitis B Virus Infection, in Healthy Volunteers. Clin. Pharm. Drug Dev. 6, 350–362 (2017).
Dunning, J. et al. Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial. PLoS Med. 13, e1001997 (2016).
Maestro, S. et al. Novel vectors and approaches for gene therapy in liver diseases. JHEP Rep. 3, 100300 (2021).
D’Souza, A. A. & Devarajan, P. V. Asialoglycoprotein receptor mediated hepatocyte targeting - strategies and applications. J. Control Release 203, 126–139 (2015).
Gardin, A. et al. Modern therapeutic approaches to liver-related disorders. J. Hepatol. 76, 1392–1409 (2022).
Kling, J. Safety signal dampens reception for mipomersen antisense. Nat. Biotechnol. 28, 295–297, (2010).
Santos, R. D. et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur. Heart J. 36, 566–575 (2015).
Keam, S. J. Inotersen: First Global Approval. Drugs 78, 1371–1376 (2018).
Marques, J. H., Coelho, J., Menéres, M. J. & Beirão, J. M. Progressive vitreous deposits during treatment with inotersen for hereditary ATTR amyloidosis. Amyloid 28, 275–276 (2021).
Benson, M. D. et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 379, 22–31 (2018).
Brannagan, T. H. et al. Long-term efficacy and safety of inotersen for hereditary transthyretin amyloidosis: NEURO-TTR open-label extension 3-year update. J. Neurol. 269, 6416–6427 (2022).
Khetarpal, S. A., Wang, M. & Khera, A. V. Volanesorsen, Familial Chylomicronemia Syndrome, and Thrombocytopenia. N. Engl. J. Med. 381, 2582–2584 (2019).
Gouni-Berthold, I. et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diab. Endocrinol. 9, 264–275 (2021).
Witztum, J. L. et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med 381, 531–542 (2019).
Fernández-Ruiz, I. Patisiran is beneficial for the treatment of TTR cardiac amyloidosis. Nat. Rev. Cardiol 21, 8–8 (2023).
Keam, S. J. Vutrisiran: First Approval. Drugs 82, 1419–1425 (2022).
Gonzalez-Aseguinolaza, G. Givosiran - Running RNA Interference to Fight Porphyria Attacks. N. Engl. J. Med. 382, 2366–2367 (2020).
Syed, Y. Y. Givosiran: A Review in Acute Hepatic Porphyria. Drugs 81, 841–848 (2021).
Scott, L. J. & Keam, S. J. Lumasiran: First Approval. Drugs 81, 277–282 (2021).
Santos, R. D. & Rocha, V. Z. Cholesterol lowering with inclisiran: a new chapter in the PCSK9 story book. Eur. Heart J. 44, 139–141 (2023).
Fernández-Ruiz, I. Twice-yearly inclisiran injections halve LDL-cholesterol levels. Nat. Rev. Cardiol. 17, 321 (2020).
Castro-Alamancos, M. A. & Torres-Aleman, I. Learning of the conditioned eye-blink response is impaired by an antisense insulin-like growth factor I oligonucleotide. Proc. Natl Acad. Sci. USA 91, 10203–10207 (1994).
Rakoczy, P. E. et al. Initiation of impaired outer segment degradation in vivo using an antisense oligonucleotide. Curr. Eye Res. 15, 119–123 (1996).
Perry, C. M. & Balfour, J. A. Fomivirsen. Drugs 57, 375–380 (1999). discussion 381.
A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDS. Am. J. Ophthalmol. 133, 467–474, (2002).
Gerard, X. et al. AON-mediated Exon Skipping Restores Ciliation in Fibroblasts Harboring the Common Leber Congenital Amaurosis CEP290 Mutation. Mol. Ther. Nucleic Acids 1, e29 (2012).
Collin, R. W. et al. Antisense Oligonucleotide (AON)-based Therapy for Leber Congenital Amaurosis Caused by a Frequent Mutation in CEP290. Mol. Ther. Nucleic Acids 1, e14 (2012).
Xue, K. & MacLaren, R. E. Antisense oligonucleotide therapeutics in clinical trials for the treatment of inherited retinal diseases. Expert Opin. Investig. Drugs 29, 1163–1170 (2020).
Schellens, R. T. W. et al. A protein domain-oriented approach to expand the opportunities of therapeutic exon skipping for USH2A-associated retinitis pigmentosa. Mol. Ther. Nucleic Acids 32, 980–994 (2023).
Lorenz, K. et al. A prospective, randomised, placebo-controlled, double-masked, three-armed, multicentre phase II/III trial for the Study of a Topical Treatment of Ischaemic Central Retinal Vein Occlusion to Prevent Neovascular Glaucoma - the STRONG study: study protocol for a randomised controlled trial. Trials 18, 128 (2017).
Shen, J. et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 13, 225–234 (2006).
He, S. B. et al. Advances and perspectives of nanozymes in respiratory diseases. J. Mater. Chem. B 11, 7041–7054 (2023).
García-Fernández, A., Sancenón, F. & Martínez-Máñez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 177, 113953 (2021).
Patil, T. S. & Deshpande, A. S. Nanostructured lipid carriers-based drug delivery for treating various lung diseases: A State-of-the-Art Review. Int J. Pharm. 547, 209–225 (2018).
Sermet-Gaudelus, I. et al. Antisense oligonucleotide eluforsen improves CFTR function in F508del cystic fibrosis. J. Cyst. Fibros. 18, 536–542 (2019).
Beumer, W. et al. Evaluation of eluforsen, a novel RNA oligonucleotide for restoration of CFTR function in in vitro and murine models of p.Phe508del cystic fibrosis. PLoS One 14, e0219182 (2019).
Drevinek, P. et al. Antisense oligonucleotide eluforsen is safe and improves respiratory symptoms in F508DEL cystic fibrosis. J. Cyst. Fibros. 19, 99–107 (2020).
Girón Moreno, R. M. et al. Treatment of Pulmonary Disease of Cystic Fibrosis: A Comprehensive Review. Antibiotics (Basel) 10, 486 (2021).
Kordasiewicz, H. B. et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron 74, 1031–1044 (2012).
Lombardi, M. S. et al. A majority of Huntington’s disease patients may be treatable by individualized allele-specific RNA interference. Exp. Neurol. 217, 312–319 (2009).
Svrzikapa, N. et al. Investigational Assay for Haplotype Phasing of the Huntingtin Gene. Mol. Ther. Methods Clin. Dev. 19, 162–173 (2020).
McCampbell, A. et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J. Clin. Invest. 128, 3558–3567 (2018).
Miller, T. M. et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 387, 1099–1110 (2022).
Miller, T. et al. Phase 1-2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 383, 109–119 (2020).
Abati, E., Bresolin, N., Comi, G. & Corti, S. Silence superoxide dismutase 1 (SOD1): a promising therapeutic target for amyotrophic lateral sclerosis (ALS). Expert Opin. Ther. Targets 24, 295–310 (2020).
Pechmann, A. et al. Effect of nusinersen on motor, respiratory and bulbar function in early-onset spinal muscular atrophy. Brain 146, 668–677 (2023).
Finkel, R. S. et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Finkel, R. S. et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388, 3017–3026 (2016).
Montes, J. et al. Nusinersen improves walking distance and reduces fatigue in later-onset spinal muscular atrophy. Muscle Nerve 60, 409–414 (2019).
Aimo, A. et al. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat. Rev. Cardiol. 19, 655–667 (2022).
Janas, M. M. et al. Safety evaluation of 2’-deoxy-2’-fluoro nucleotides in GalNAc-siRNA conjugates. Nucleic Acids Res. 47, 3306–3320 (2019).
Duan, D. et al. Duchenne muscular dystrophy. Nat. Rev. Dis. Prim. 7, 13 (2021).
Echigoya, Y. et al. Quantitative Antisense Screening and Optimization for Exon 51 Skipping in Duchenne Muscular Dystrophy. Mol. Ther. 25, 2561–2572 (2017).
Clemens, P. R. et al. Safety, Tolerability, and Efficacy of Viltolarsen in Boys With Duchenne Muscular Dystrophy Amenable to Exon 53 Skipping: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 77, 982–991 (2020).
Anfossi, G., Gewirtz, A. M. & Calabretta, B. An oligomer complementary to c-myb-encoded mRNA inhibits proliferation of human myeloid leukemia cell lines. Proc. Natl Acad. Sci. USA 86, 3379–3383, (1989).
Tondelli, L. et al. Highly efficient cellular uptake of c-myb antisense oligonucleotides through specifically designed polymeric nanospheres. Nucleic Acids Res. 26, 5425–5431 (1998).
Luger, S. M. et al. Oligodeoxynucleotide-mediated inhibition of c-myb gene expression in autografted bone marrow: a pilot study. Blood 99, 1150–1158 (2002).
Reilley, M. J. et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J. Immunother. Cancer 6, 119 (2018).
Chowdhury, S. et al. A phase I dose escalation, safety and pharmacokinetic (PK) study of AZD5312 (IONIS-ARRx), a first-in-class Generation 2.5 antisense oligonucleotide targeting the androgen receptor (AR). Eur. J. Cancer. 69, S145 (2016).
Zhang, Y. et al. Reduced expression of the androgen receptor by third generation of antisense shows antitumor activity in models of prostate cancer. Mol. Cancer Ther. 10, 2309–2319 (2011).
Davis, M. E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 6, 659–668 (2009).
Zuckerman, J. E. et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl Acad. Sci. USA 111, 11449–11454 (2014).
Zuckerman, J. E. & Davis, M. E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 14, 843–856, (2015).
Zorde Khvalevsky, E. et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20723–20728 (2013).
Golan, T. et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 6, 24560–24570 (2015).
Aleku, M. et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 68, 9788–9798 (2008).
Santel, A. et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 13, 1222–1234 (2006).
Santel, A. et al. Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin. Cancer Res 16, 5469–5480 (2010).
Schultheis, B. et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 32, 4141–4148 (2014).
Xiao, T. et al. Targeting EphA2 in cancer. J. Hematol. Oncol. 13, 114 (2020).
Landen, C. N. Jr. et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 65, 6910–6918 (2005).
Wagner, M. J. et al. Preclinical Mammalian Safety Studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol. Cancer Ther. 16, 1114–1123 (2017).
Baluapuri, A., Wolf, E. & Eilers, M. Target gene-independent functions of MYC oncoproteins. Nat. Rev. Mol. Cell Biol. 21, 255–267 (2020).
Doré-Savard, L. et al. Central delivery of Dicer-substrate siRNA: a direct application for pain research. Mol. Ther. 16, 1331–1339 (2008).
Jovanović, K. K. et al. Targeting MYC in multiple myeloma. Leukemia 32, 1295–1306 (2018).
Miller, A. J., Chang, A. & Cunningham, P. N. Chronic Microangiopathy Due to DCR-MYC, a Myc-Targeted Short Interfering RNA. Am. J. Kidney Dis. 75, 513–516 (2020).
Tolcher, A. W. et al. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J. Clin. Oncol. 33, 11006–11006 (2015).
Cuciniello, R., Filosa, S. & Crispi, S. Novel approaches in cancer treatment: preclinical and clinical development of small non-coding RNA therapeutics. J. Exp. Clin. Cancer Res. 40, 383 (2021).
Zhang, Y. & Xu, W. Progress on kinesin spindle protein inhibitors as anti-cancer agents. Anticancer Agents Med Chem. 8, 698–704 (2008).
Doan, C. C. et al. Simultaneous silencing of VEGF and KSP by siRNA cocktail inhibits proliferation and induces apoptosis of hepatocellular carcinoma Hep3B cells. Biol. Res 47, 70 (2014).
Toudjarska, I. et al. Abstract 4064: Development of ALN-VSP: An RNAi therapeutic for solid tumors. Cancer Res. 70, 4064–4064 (2010).
Tabernero, J. et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 3, 406–417 (2013).
Mok, W. C., Wasser, S., Tan, T. & Lim, S. G. Polo-like kinase 1, a new therapeutic target in hepatocellular carcinoma. World J. Gastroenterol. 18, 3527–3536 (2012).
Semple, S. C. et al. Abstract 2829: Preclinical characterization of TKM-080301, a lipid nanoparticle formulation of a small interfering RNA directed against polo-like kinase 1. Cancer Res. 71, 2829–2829 (2011).
Kara, G., Calin, G. A. & Ozpolat, B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv. Drug Deliv. Rev. 182, 114113 (2022).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019).
Wang, X. et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023).
Ding, D. et al. The First-in-Human Whole-Body Dynamic Pharmacokinetics Study of Aptamer. Res.(Wash. D. C.) 6, 0126 (2023).
Siddiqui, M. A. & Keating, G. M. Pegaptanib: in exudative age-related macular 39 degeneration. Drugs. 65, 1571–1577; discussion 1578–1579, (2005).
Drolet, D. W., Green, L. S., Gold, L. & Janjic, N. Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther. 26, 127–146, (2016).
Drolet, D. W. et al. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (NX1838) following injection into the vitreous humor of rhesus monkeys. Pharm. Res 17, 1503–1510 (2000).
Schmidt-Erfurth, U. et al. Photodynamic therapy of subfoveal choroidal neovascularization: clinical and angiographic examples. Graefes Arch. Clin. Exp. Ophthalmol. 236, 365–374 (1998).
Kang, C. Avacincaptad Pegol: First Approval. Drugs 83, 1447–1453 (2023).
Fu, X. et al. Repurposing AS1411 for constructing ANM-PROTACs. Cell Chem. Biol. 31, 1290–1304.e1297 (2024).
Zhao, H. et al. Energy-storing DNA-based hydrogel remodels tumor microenvironments for laser-free photodynamic immunotherapy. Biomaterials 309, 122620 (2024).
Sun, X. et al. AS1411 binds to nucleolin via its parallel structure and disrupts the exos-miRNA-27a-mediated reciprocal activation loop between glioma and astrocytes. Biochim. Biophys. Acta Mol. Basis Dis. 1870, 167211 (2024).
Hoellenriegel, J. et al. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 123, 1032–1039 (2014).
Ludwig, H. et al. Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib-dexamethasone in relapsed/refractory multiple myeloma: a Phase IIa Study. Leukemia 31, 997–1000 (2017).
Giordano, F. A. et al. L-RNA aptamer-based CXCL12 inhibition combined with radiotherapy in newly-diagnosed glioblastoma: dose escalation of the phase I/II GLORIA trial. Nat. Commun. 15, 4210 (2024).
Nimjee, S. M. et al. Preclinical Development of a vWF Aptamer to Limit Thrombosis and Engender Arterial Recanalization of Occluded Vessels. Mol. Ther. 27, 1228–1241 (2019).
Dyke, C. K. et al. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation 114, 2490–2497 (2006).
Povsic, T. J. et al. A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial. Eur. Heart J. 34, 2481–2489 (2013).
Gilbert, J. C. et al. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation 116, 2678–2686 (2007).
Lu, T. X. & Rothenberg, M. E. MicroRNA. J. Allergy Clin. Immunol. 141, 1202–1207 (2018).
Lee, Y. S. & Dutta, A. MicroRNAs in cancer. Annu Rev. Pathol. 4, 199–227 (2009).
Fabian, M. R., Sonenberg, N. & Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu Rev. Biochem. 79, 351–379, (2010).
Li, Y. & Kowdley, K. V. MicroRNAs in common human diseases. Genom. Proteom. Bioinf. 10, 246–253 (2012).
Srinivasan, S. et al. MicroRNAs -the next generation therapeutic targets in human diseases. Theranostics 3, 930–942 (2013).
Janssen, H. L. et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 368, 1685–1694 (2013).
van der Ree, M. H. et al. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antivir. Res. 111, 53–59 (2014).
Ottosen, S. et al. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob. Agents Chemother. 59, 599–608 (2015).
van der Ree, M. H. et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet 389, 709–717 (2017).
Stelma, F. et al. Immune phenotype and function of natural killer and T cells in chronic hepatitis C patients who received a single dose of anti-MicroRNA-122, RG-101. Hepatology 66, 57–68 (2017).
Zhang, L., Liao, Y. & Tang, L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 38, 53 (2019).
Daige, C. L. et al. Systemic delivery of a miR34a mimic as a potential therapeutic for liver cancer. Mol. Cancer Ther. 13, 2352–2360 (2014).
Bader, A. G. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 3, 120 (2012).
Ling, H., Fabbri, M. & Calin, G. A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 12, 847–865, (2013).
Hong, D. S. et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 122, 1630–1637 (2020).
Chioccioli, M. et al. A lung targeted miR-29 mimic as a therapy for pulmonary fibrosis. EBioMedicine 85, 104304 (2022).
Gallant-Behm, C. L. et al. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen. 26, 311–323 (2018).
Abplanalp, W. T. et al. Efficiency and Target Derepression of Anti-miR-92a: Results of a First in Human Study. Nucleic Acid Ther. 30, 335–345 (2020).
Batkai, S. et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur. Heart J. 42, 192–201 (2021).
Täubel, J. et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 42, 178–188 (2021).
Seto, A. G. et al. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 183, 428–444 (2018).
Anastasiadou, E. et al. Cobomarsen, an Oligonucleotide Inhibitor of miR-155, Slows DLBCL Tumor Cell Growth In Vitro and In Vivo. Clin. Cancer Res. 27, 1139–1149 (2021).
Tan, C. P. et al. RNA Activation-A Novel Approach to Therapeutically Upregulate Gene Transcription. Molecules 26, 6530 (2021).
Kosaka, M., Kang, M. R., Yang, G. & Li, L. C. Targeted p21WAF1/CIP1 activation by RNAa inhibits hepatocellular carcinoma cells. Nucleic Acid Ther. 22, 335–343 (2012).
Place, R. F. et al. Formulation of Small Activating RNA Into Lipidoid Nanoparticles Inhibits Xenograft Prostate Tumor Growth by Inducing p21 Expression. Mol. Ther. Nucleic Acids 1, e15 (2012).
Wei, J. et al. p21WAF1/CIP1 gene transcriptional activation exerts cell growth inhibition and enhances chemosensitivity to cisplatin in lung carcinoma cell. BMC Cancer 10, 632 (2010).
Wu, Z. M. et al. Anti-cancer effects of p21WAF1/CIP1 transcriptional activation induced by dsRNAs in human hepatocellular carcinoma cell lines. Acta Pharm. Sin. 32, 939–946 (2011).
Zhang, Z. et al. Up-regulation of p21WAF1/CIP1 by small activating RNA inhibits the in vitro and in vivo growth of pancreatic cancer cells. Tumori 98, 804–811 (2012).
Yang, K. et al. Up-regulation of p21WAF1/Cip1 by saRNA induces G1-phase arrest and apoptosis in T24 human bladder cancer cells. Cancer Lett. 265, 206–214 (2008).
Kang, M. R. et al. Intravesical delivery of small activating RNA formulated into lipid nanoparticles inhibits orthotopic bladder tumor growth. Cancer Res. 72, 5069–5079 (2012).
Gosney, J. R., Sissons, M. C. & O’Malley, J. A. Quantitative study of endocrine cells immunoreactive for calcitonin in the normal adult human lung. Thorax 40, 866–869, (1985).
Zhang, C. & Zhang, B. RNA therapeutics: updates and future potential. Sci. China Life Sci. 66, 12–30 (2023).
Huang, K. W. et al. MTL-CEBPA Combined with Immunotherapy or RFA Enhances Immunological Anti-Tumor Response in Preclinical Models. Int. J. Mol Sci. 22, 9168 (2021).
Zhou, J. et al. Anti-inflammatory Activity of MTL-CEBPA, a Small Activating RNA Drug, in LPS-Stimulated Monocytes and Humanized Mice. Mol. Ther. 27, 999–1016 (2019).
Leroy, H. et al. CEBPA point mutations in hematological malignancies. Leukemia 19, 329–334 (2005).
Lourenço, A. R. & Coffer, P. J. A tumor suppressor role for C/EBPα in solid tumors: more than fat and blood. Oncogene 36, 5221–5230 (2017).
Reebye, V. et al. Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo. Hepatology 59, 216–227 (2014).
Li, Y., Fung, J. & Lin, F. Local Inhibition of Complement Improves Mesenchymal Stem Cell Viability and Function After Administration. Mol. Ther. 24, 1665–1674 (2016).
Yoon, S. et al. Targeted Delivery of C/EBPα-saRNA by RNA Aptamers Shows Anti-tumor Effects in a Mouse Model of Advanced PDAC. Mol. Ther. Nucleic Acids 18, 142–154 (2019).
Reebye, V. et al. Gene activation of CEBPA using saRNA: preclinical studies of the first in human saRNA drug candidate for liver cancer. Oncogene 37, 3216–3228 (2018).
Sarker, D. et al. MTL-CEBPA, a Small Activating RNA Therapeutic Upregulating C/EBP-α, in Patients with Advanced Liver Cancer: A First-in-Human, Multicenter, Open-Label, Phase I Trial. Clin. Cancer Res. 26, 3936–3946 (2020).
Bian, S., Zhu, B., Rong, G. & Sawan, M. Towards wearable and implantable continuous drug monitoring: A review. J. Pharm. Anal. 11, 1–14 (2021).
Hahn, J. O., Dumont, G. A. & Ansermino, J. M. Closed-loop anesthetic drug concentration estimation using clinical-effect feedback. IEEE Trans. Biomed. Eng. 58, 3–6 (2011).
Zaragoza Domingo, S. et al. Methods for Neuroscience Drug Development: Guidance on Standardization of the Process for Defining Clinical Outcome Strategies in Clinical Trials. Eur. Neuropsychopharmacol. 83, 32–42 (2024).
Amin, M. B. et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99 (2017).
Feng, F. et al. Large-scale pharmacogenomic studies and drug response prediction for personalized cancer medicine. J. Genet Genomics 48, 540–551 (2021).
Adams, D. et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. 20, 49–59 (2021).
Garcia-Pavia, P. et al. Impact of vutrisiran on exploratory cardiac parameters in hereditary transthyretin-mediated amyloidosis with polyneuropathy. Eur. J. Heart Fail 26, 397–410 (2024).
Viney, N. J. et al. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: preclinical and phase 1 data. ESC Heart Fail 8, 652–661 (2021).
Coelho, T. et al. Eplontersen for Hereditary Transthyretin Amyloidosis With Polyneuropathy. Jama 330, 1448–1458 (2023).
Foster, D. J. Muscarinic receptors: from clinic to bench to clinic. Trends Pharm. Sci. 43, 461–463 (2022).
Liang, X., Li, D., Leng, S. & Zhu, X. RNA-based pharmacotherapy for tumors: From bench to clinic and back. Biomed. Pharmacother. 125, 109997 (2020).
Garattini, L. & Padula, A. Pharmaceutical pricing conundrum: time to get rid of it? Eur. J. Health Econ. 19, 1035–1038 (2018).
Gorgulla, C. et al. An open-source drug discovery platform enables ultra-large virtual screens. Nature 580, 663–668 (2020).
Rawlins, M. D. Cutting the cost of drug development? Nat. Rev. Drug Discov. 3, 360–364 (2004).
Hagedorn, P. H. et al. Acute Neurotoxicity of Antisense Oligonucleotides After Intracerebroventricular Injection Into Mouse Brain Can Be Predicted from Sequence Features. Nucleic Acid Ther. 32, 151–162 (2022).
Janssens, S. & Beyaert, R. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol Rev. 16, 637–646, (2003).
Lee, J. et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl Acad. Sci. USA 100, 6646–6651 (2003).
Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Pollak, A. J. et al. Insights into innate immune activation via PS-ASO-protein-TLR9 interactions. Nucleic Acids Res. 50, 8107–8126 (2022).
Brannagan, T. H. et al. Early data on long-term efficacy and safety of inotersen in patients with hereditary transthyretin amyloidosis: a 2-year update from the open-label extension of the NEURO-TTR trial. Eur. J. Neurol. 27, 1374–1381 (2020).
Slingsby, M. H. L. et al. Sequence-specific 2’-O-methoxyethyl antisense oligonucleotides activate human platelets through glycoprotein VI, triggering formation of platelet-leukocyte aggregates. Haematologica 107, 519–531 (2022).
Zhang, Z. et al. Mechanistic study of silica nanoparticles on the size-dependent retinal toxicity in vitro and in vivo. J. Nanobiotechnol. 20, 146 (2022).
Lokugamage, M. P. et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 5, 1059–1068 (2021).
Li, B. et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat. Biotechnol. 41, 1410–1415 (2023).
Zhu, Y. et al. Multi-step screening of DNA/lipid nanoparticles and co-delivery with siRNA to enhance and prolong gene expression. Nat. Commun. 13, 4282 (2022).
Olshefsky, A. et al. In vivo selection of synthetic nucleocapsids for tissue targeting. Proc. Natl Acad. Sci. USA 120, e2306129120 (2023).
Parola, C., Neumeier, D. & Reddy, S. T. Integrating high-throughput screening and sequencing for monoclonal antibody discovery and engineering. Immunology 153, 31–41 (2018).
Kon, E., Elia, U. & Peer, D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 73, 329–336 (2022).
Sago, C. D. et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc. Natl Acad. Sci. USA 115, E9944–e9952 (2018).
Ramishetti, S. et al. A Combinatorial Library of Lipid Nanoparticles for RNA Delivery to Leukocytes. Adv. Mater. 32, e1906128 (2020).
Billingsley, M. M. et al. Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering. Nano Lett. 20, 1578–1589 (2020).
Yamankurt, G. et al. Exploration of the nanomedicine-design space with high-throughput screening and machine learning. Nat. Biomed. Eng. 3, 318–327 (2019).
Shamay, Y. et al. Quantitative self-assembly prediction yields targeted nanomedicines. Nat. Mater. 17, 361–368 (2018).
Zhu, M. et al. Machine-learning-assisted single-vessel analysis of nanoparticle permeability in tumour vasculatures. Nat. Nanotechnol. 18, 657–666 (2023).
Gong, D. et al. Machine learning guided structure function predictions enable in silico nanoparticle screening for polymeric gene delivery. Acta Biomater. 154, 349–358 (2022).
Huang, J. et al. Identification of potent antimicrobial peptides via a machine-learning pipeline that mines the entire space of peptide sequences. Nat. Biomed. Eng. 7, 797–810 (2023).
Boehnke, N. et al. Massively parallel pooled screening reveals genomic determinants of nanoparticle delivery. Science 377, eabm5551 (2022).
Heil, C. M., Patil, A., Dhinojwala, A. & Jayaraman, A. Computational Reverse-Engineering Analysis for Scattering Experiments (CREASE) with Machine Learning Enhancement to Determine Structure of Nanoparticle Mixtures and Solutions. ACS Cent. Sci. 8, 996–1007 (2022).
Wouters, O. J., McKee, M. & Luyten, J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. Jama 323, 844–853 (2020).
Lv, Q., Zhou, F., Liu, X. & Zhi, L. Artificial intelligence in small molecule drug discovery from 2018 to 2023: Does it really work? Bioorg. Chem. 141, 106894 (2023).
Fitzgibbons, R. J. Jr et al. Recognition and treatment of patients with hereditary nonpolyposis colon cancer (Lynch syndromes I and II). Ann. Surg. 206, 289–295 (1987).
Mysara, M., Elhefnawi, M. & Garibaldi, J. M. MysiRNA: improving siRNA efficacy prediction using a machine-learning model combining multi-tools and whole stacking energy (ΔG). J. Biomed. Inf. 45, 528–534, (2012).
Chiba, S. et al. eSkip-Finder: a machine learning-based web application and database to identify the optimal sequences of antisense oligonucleotides for exon skipping. Nucleic Acids Res. 49, W193–w198 (2021).
Joo, M. K., Yhee, J. Y., Kim, S. H. & Kim, K. The potential and advances in RNAi therapy: chemical and structural modifications of siRNA molecules and use of biocompatible nanocarriers. J. Control Release 193, 113–121, (2014).
Liu, T. et al. Cm-siRPred: Predicting chemically modified siRNA efficiency based on multi-view learning strategy. Int J. Biol. Macromol. 264, 130638 (2024).
Martinelli, D. D. From sequences to therapeutics: Using machine learning to predict chemically modified siRNA activity. Genomics 116, 110815 (2024).
Xu, Y. et al. AGILE platform: a deep learning powered approach to accelerate LNP development for mRNA delivery. Nat. Commun. 15, 6305 (2024).
Wong, F., de la Fuente-Nunez, C. & Collins, J. J. Leveraging artificial intelligence in the fight against infectious diseases. Science 381, 164–170 (2023).
He, S., Leanse, L. G. & Feng, Y. Artificial intelligence and machine learning assisted drug delivery for effective treatment of infectious diseases. Adv. Drug Deliv. Rev. 178, 113922 (2021).
Castillo-Hair, S. M. & Seelig, G. Machine Learning for Designing Next-Generation mRNA Therapeutics. Acc. Chem. Res 55, 24–34 (2022).
Moingeon, P., Kuenemann, M. & Guedj, M. Artificial intelligence-enhanced drug design and development: Toward a computational precision medicine. Drug Discov. Today 27, 215–222 (2022).
Won Lee, J. et al. RNAi therapies: Expanding applications for extrahepatic diseases and overcoming delivery challenges. Adv. Drug Deliv. Rev. 201, 115073 (2023).
Mendes, B. B. et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers. 2, 24 (2022).
Dong, Y., Siegwart, D. J. & Anderson, D. G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 144, 133–147 (2019).
Rengaswamy, V., Zimmer, D., Süss, R. & Rössler, J. RGD liposome-protamine-siRNA (LPR) nanoparticles targeting PAX3-FOXO1 for alveolar rhabdomyosarcoma therapy. J. Control Release 235, 319–327 (2016).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Kim, J. Y., Choi, W. I., Kim, Y. H. & Tae, G. Brain-targeted delivery of protein using chitosan- and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials 34, 1170–1178, (2013).
Hwang, D. W. et al. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials 32, 4968–4975 (2011).
Tang, L. et al. A blood-brain barrier- and blood-brain tumor barrier-penetrating siRNA delivery system targeting gliomas for brain tumor immunotherapy. J. Control Release 369, 642–657 (2024).
Fu, L. et al. Passive’ nanoparticles for organ-selective systemic delivery: design, mechanism and perspective. Chem. Soc. Rev. 52, 7579–7601 (2023).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Bonnet, M. E. et al. Systemic delivery of sticky siRNAs targeting the cell cycle for lung tumor metastasis inhibition. J. Control Release 170, 183–190 (2013).
Okuda, T. et al. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J. Control Release 279, 99–113 (2018).
Hu, J. et al. Cellular organelles as drug carriers for disease treatment. J. Control Release 363, 114–135 (2023).
Liang, Y., Duan, L., Lu, J. & Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 11, 3183–3195 (2021).
Giacomello, M., Pyakurel, A., Glytsou, C. & Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 21, 204–224 (2020).
Joshi, B. S., de Beer, M. A., Giepmans, B. N. G. & Zuhorn, I. S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 14, 4444–4455 (2020).
Chen, W., Huang, T., Shi, K., Chu, B., & Qian, Z. Chemotaxis-based self-accumulation of surface-engineered mitochondria for cancer therapeutic improvement. Nano Today. Nano Today. 35, (2020).
Wang, S. et al. Macrophage-tumor chimeric exosomes accumulate in lymph node and tumor to activate the immune response and the tumor microenvironment. Sci. Transl. Med. 13, eabb6981 (2021).
Raguraman, R. et al. Tumor-targeted exosomes for delivery of anticancer drugs. Cancer Lett. 558, 216093 (2023).
Wahane, A. et al. Role of Lipid-Based and Polymer-Based Non-Viral Vectors in Nucleic Acid Deliveryfor Next-Generation Gene Therapy. Molecules 25, 2866 (2020).
Yang, W. et al. Nanoparticle Toxicology. Annu Rev. Pharm. Toxicol. 61, 269–289 (2021).
Shatkin, J. A. The Future in Nanosafety. Nano Lett. 20, 1479–1480 (2020).
Wilhelm, S. Perspectives for Upconverting Nanoparticles. ACS Nano 11, 10644–10653 (2017).
Buchman, J. T., Hudson-Smith, N. V., Landy, K. M. & Haynes, C. L. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Acc. Chem. Res 52, 1632–1642 (2019).
Eftekhari, A. et al. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed. Pharmacother. 103, 1018–1027 (2018).
Jing, X. et al. Surface engineering of colloidal nanoparticles. Mater. Horiz. 10, 1185–1209 (2023).
Lin, Y. S. & Haynes, C. L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 132, 4834–4842, (2010).
Rao, L. et al. Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging. ACS Appl. Mater. Interf. 9, 2159–2168 (2017).
Weng, Y. et al. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol. Adv. 37, 801–825 (2019).
Reynolds, A. et al. Rational siRNA design for RNA interference. Nat. Biotechnol. 22, 326–330 (2004).
Fakhr, E., Zare, F. & Teimoori-Toolabi, L. Precise and efficient siRNA design: a key point in competent gene silencing. Cancer Gene Ther. 23, 73–82 (2016).
Ui-Tei, K. et al. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 32, 936–948 (2004).
Boros, B. D., Schoch, K. M., Kreple, C. J. & Miller, T. M. Antisense Oligonucleotides for the Study and Treatment of ALS. Neurotherapeutics 19, 1145–1158 (2022).
Saito, T. et al. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 17, 661–663 (2014).
Xia, Q. et al. The Protective A673T Mutation of Amyloid Precursor Protein (APP) in Alzheimer’s Disease. Mol. Neurobiol. 58, 4038–4050 (2021).
Egli, M. & Manoharan, M. Re-Engineering RNA Molecules into Therapeutic Agents. Acc. Chem. Res. 52, 1036–1047 (2019).
Khvorova, A. & Watts, J. K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 35, 238–248 (2017).
Guenther, D. C. et al. Role of a “Magic” Methyl: 2’-Deoxy-2’-α-F-2’-β-C-methyl Pyrimidine Nucleotides Modulate RNA Interference Activity through Synergy with 5’-Phosphate Mimics and Mitigation of Off-Target Effects. J. Am. Chem. Soc. 144, 14517–14534 (2022).
Lu, Z. J. & Mathews, D. H. OligoWalk: an online siRNA design tool utilizing hybridization thermodynamics. Nucleic Acids Res. 36, W104–W108 (2008).
Liu, B. et al. AttSiOff: a self-attention-based approach on siRNA design with inhibition and off-target effect prediction. Med-X 2, 5 (2024).
Tang, Q. & Khvorova, A. RNAi-based drug design: considerations and future directions. Nat. Rev. Drug Discov. 23, 341–364 (2024).
Wang, Y., Nguyen, K., Spitale, R. C. & Chaput, J. C. A biologically stable DNAzyme that efficiently silences gene expression in cells. Nat. Chem. 13, 319–326 (2021).
Matsuda, S. et al. Shorter Is Better: The α-(l)-Threofuranosyl Nucleic Acid Modification Improves Stability, Potency, Safety, and Ago2 Binding and Mitigates Off-Target Effects of Small Interfering RNAs. J. Am. Chem. Soc. 145, 19691–19706 (2023).
Depmeier, H. & Kath-Schorr, S. Expanding the Horizon of the Xeno Nucleic Acid Space: Threose Nucleic Acids with Increased Information Storage. J. Am. Chem. Soc. 146, 7743–7751 (2024).
Zhang, T., Tian, T. & Lin, Y. Functionalizing Framework Nucleic-Acid-Based Nanostructures for Biomedical Application. Adv. Mater. 34, e2107820 (2022).
Zhang, M. et al. Transcutaneous Immunotherapy for RNAi: A Cascade-Responsive Decomposable Nanocomplex Based on Polyphenol-Mediated Framework Nucleic Acid in Psoriasis. Adv. Sci. (Weinh.) 10, e2303706 (2023).
Moreno, P. M. D., Cortinhas, J., Martins, A. S. & Pêgo, A. P. Engineering a Novel Self-Assembled Multi-siRNA Nanocaged Architecture with Controlled Enzyme-Mediated siRNA Release. ACS Appl Mater. Interfaces 14, 56483–56497 (2022).
Wu, X. et al. In vitro and in vivo activities of antimicrobial peptides developed using an amino acid-based activity prediction method. Antimicrob. Agents Chemother. 58, 5342–5349 (2014).
Zhang, R. et al. Novel Self-Assembled Micelles Based on Cholesterol-Modified Antimicrobial Peptide (DP7) for Safe and Effective Systemic Administration in Animal Models of Bacterial Infection. Antimicrob. Agents Chemother. 62, e00368–18 (2018).
Oehlke, J. et al. Extensive cellular uptake into endothelial cells of an amphipathic beta-sheet forming peptide. FEBS Lett. 415, 196–199 (1997).
Acknowledgements
All images, including the graphical abstract image and the figures, were created with BioRender (https://www.biorender.com/). This work was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYGD23008); by the Frontiers Medical Center, Tianfu Jincheng Laboratory Foundation (No. TFJC202310005); and by the Scientific Research and Innovation Team Program of Sichuan University of Science and Technology (No. SUSE652B003).
Author information
Authors and Affiliations
Contributions
L.Y. contributed to the design of the study. M.H.L. and Y.S.W. drafted the manuscript and created the illustrations. M.H.L., Y.S.W., Y.B.Z., D.H., L.T. and B.L.Z reviewed and prepared the tables. All authors have read and approved the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, M., Wang, Y., Zhang, Y. et al. Landscape of small nucleic acid therapeutics: moving from the bench to the clinic as next-generation medicines. Sig Transduct Target Ther 10, 73 (2025). https://doi.org/10.1038/s41392-024-02112-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-02112-8
This article is cited by
-
Exosomes in cancer nanomedicine: biotechnological advancements and innovations
Molecular Cancer (2025)
-
The concurrent silencing of Tim-3 and STAT-3 promotes tumor regression both in vitro and in ovo
BMC Cancer (2025)
-
Precision engineering of macrophage reprogramming with RNA interference-loaded lipid nanoparticles: a game-changer in cancer immunotherapy
Drug Delivery and Translational Research (2025)