Abstract
The progression of malignant tumors leads to the development of secondary tumors in various organs, including bones, the brain, liver, and lungs. This metastatic process severely impacts the prognosis of patients, significantly affecting their quality of life and survival rates. Research efforts have consistently focused on the intricate mechanisms underlying this process and the corresponding clinical management strategies. Consequently, a comprehensive understanding of the biological foundations of tumor metastasis, identification of pivotal signaling pathways, and systematic evaluation of existing and emerging therapeutic strategies are paramount to enhancing the overall diagnostic and treatment capabilities for metastatic tumors. However, current research is primarily focused on metastasis within specific cancer types, leaving significant gaps in our understanding of the complex metastatic cascade, organ-specific tropism mechanisms, and the development of targeted treatments. In this study, we examine the sequential processes of tumor metastasis, elucidate the underlying mechanisms driving organ-tropic metastasis, and systematically analyze therapeutic strategies for metastatic tumors, including those tailored to specific organ involvement. Subsequently, we synthesize the most recent advances in emerging therapeutic technologies for tumor metastasis and analyze the challenges and opportunities encountered in clinical research pertaining to bone metastasis. Our objective is to offer insights that can inform future research and clinical practice in this crucial field.
Similar content being viewed by others
Introduction
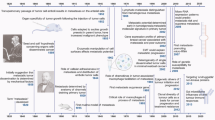
Tumor metastasis represents a pivotal event in the progression of malignancy, accounting for over 90% of cancer-related deaths and posing a formidable challenge to the clinical management of the vast majority of advanced patients with cancer.1,2,3 This intricate process encompasses the uncontrolled proliferation of primary tumor foci and the transmigration of cancerous cells across tissue barriers, which contributes to new lesions in distant organs. This substantially compromises patients’ survival rates and quality of life.4,5,6 The polymorphism and complexity of tumor metastasis are evident in its impact on virtually all vital organs throughout the body, including the lungs, liver, brain, and bones. The intricate interplay between cancer cells and the microenvironment of the target organ represents the core of this metastatic cascade. This interplay involves dynamic changes in numerous cytokines, growth factors, and signaling pathways, collectively creating a microenvironment conducive to tumor growth and dissemination7,8,9 (Fig. 1).
While significant advancements have been made in fundamental research on tumor metastasis, effectively translating these findings into clinical practice remains a considerable challenge. Current clinical studies usually prioritize the development and evaluation of pharmacological treatments, with a relative lack of emphasis on the comprehensive understanding of metastasis mechanisms, the specific mechanisms underlying organ-specific metastasis, and the exploration of targeted therapies. Therefore, this review aims to examine the multidimensional nature of tumor metastasis, mainly focusing on bone, brain, liver, and lung metastasis as archetypal representatives. By integrating the “seed and soil” theory with the “multiclonal metastasis” theory, we aim to analyze the interactions between tumor cells and the microenvironments of various organs, thereby uncovering the pivotal signaling pathways and regulatory mechanisms underlying metastasis. Moreover, an exhaustive review of existing clinical research and trials will be conducted to evaluate the efficacy of pharmacological, non-pharmacological, and comprehensive management strategies in treating tumor metastasis. The objective of this endeavor is to provide a more comprehensive and scientific basis for the clinical management of tumor metastasis. We aim to identify the key challenges within the field and propose forward-thinking solutions, with the ultimate goal of fostering the continuous optimization and advancement of diagnostic and therapeutic strategies for tumor metastasis.
Clinical significance of cancer metastasis
Metastasis represents a defining characteristic of malignancy, with a documented causal role in over 90% of cancer-related deaths.10 The brain, lungs, liver, and bones are the most common sites for metastasis, with various cancer types exhibiting distinct patterns of dissemination to specific organs or tissues11 (Table 1). This organ affinity indicates that metastasis is driven by intricate biological mechanisms rather than mere statistical correlation.12 A comprehensive understanding of the epidemiology of cancer metastasis is essential for identifying high-risk populations and the development of targeted screening programs. Recognizing organ-specific tendencies in different cancers facilitates more effective monitoring and management of patients by clinicians. This knowledge is crucial for improving patient outcomes and reducing the global burden of cancer-related mortality (Fig. 2).
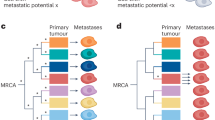
Metastasis of cancer cells. Tumor cells with inherent genomic instability accumulate mutations leading to significant heterogeneity. Metastasis involves the colonization of distant sites by various clones from the primary tumor, resulting in polyclonal metastasis. Studies on various solid cancer metastasis patterns support this concept by revealing polyclonal seeding and heterogeneity within metastatic lesions. The bidirectional flow of cancer cells, as proposed by tumor self-/cross- seeding (indicated by green and orange arrows) or secondary metastasis from metastatic site (blue arrows), adds metastasis complexity, indicating potential intra- and interpatient heterogeneity in treatment response and resistance
Approximately half of all intracranial tumors are brain metastasis. The most common site of intracranial metastasis is the brain parenchyma. In addition, cancer can metastasize to the skull, dura mater, and meninges, with metastasis occurring simultaneously, which can result in severe neurological complications.13 As evidenced by published studies, the incidence of brain metastasis ranges from 8.3 to 14.3 per 100,000 individuals,14,15,16 with a prevalence of 1.9% to 9.6% among patients with cancer.17,18,19 Previously, the diagnosis of brain metastasis primarily depended on the pathological verification of surgically removed specimens and autopsies of deceased patients. However, considering that neurosurgeons usually treat patients with localized brain metastasis and those with longer expected survival rates,20 and not all autopsies include central nervous system examinations, the incidence of brain metastasis has probably been underestimated.13,17 The efficacy of chemotherapy in extending survival periods21,22,23 has increased the likelihood of cancer cells spreading to the brain. Furthermore, the ongoing advancement in imaging technologies has improved detection, contributing to the increased incidence of brain metastasis.13,21
Statistical data indicates that over 19 million new cancer cases are registered worldwide annually, with over 60% of these cases ultimately developing into metastatic disease.24,25 Bone metastasis represents a substantial proportion of these cases. It is noteworthy that the incidence of bone metastasis in patients with breast, prostate, and lung cancers is as high as 75, 70–85, and 40%, respectively.26,27,28,29 Bone metastasis affects bone health and often results in severe complications, including skeletal-related events (SREs), such as fractures and increased pain. These complications have a markedly deleterious impact on patients’ quality of life and a considerable increase in the overall medical burden.30,31 In patients with prostate cancer, the three-year and five-year survival rates are 50 and 65%, respectively, in patients with bone metastasis compared to those without, which demonstrates the adverse impact of bone metastasis on the survival of patients with cancer.32 Furthermore, in patients with lung cancer and bone metastasis, the incidence of SREs within one year of diagnosis is as high as 55%, resulting in a notable reduction in survival rates.33
Liver metastasis is a prevalent complication in the advanced stages of various cancers, affecting approximately 5% of patients with cancer. It is notably prevalent in young women with breast cancer and young men with colorectal cancer.34 However, with increasing age, the types of primary cancers causing liver metastasis to diversify, extending beyond the lung, pancreatic, and colorectal cancers to include esophageal, gastric, and small-intestine cancers.35,36,37,38 The liver’s distinctive physiological structure and function render it a “haven” for numerous tumor cells,10,39 contributing to elevated liver metastasis rates in countries like the United States compared to those of primary liver cancer.40,41 Notably, the survival rate of patients with liver metastasis is markedly inferior, with a one-year survival rate of only 15.1%, which is considerably lower than the 24.0% observed in patients without liver metastasis.34 Moreover, the consumption of medical resources is exacerbated by liver metastasis, thereby imposing a significant economic and psychological burden on the patients’ families and society.
The incidence of lung metastasis is as high as 17.92 per 100,000 individuals42 and commonly occurs in cancers such as lung and colorectal cancers.43,44,45 Approximately 4% of patients with cancer present with synchronous lung metastasis at the time of diagnosis.42 Among patients with primary lung cancer, the proportion of patients with lung metastasis was as high as 13%. In contrast, it was the lowest in patients with prostate cancer, at only 0.5%, with this rate continuously increasing.42 This phenomenon may be closely related to the widespread use of advanced imaging technologies, such as CT and PET, which facilitate more precise detection of lung metastasis.46 However, the prognosis for patients with lung metastasis is generally poor, with overall survival rates significantly lower than those of patients without lung metastasis. This cohort predominantly comprised elderly males with late-stage cancers42]. Therefore, improving early screening, accurate diagnosis, and comprehensive treatment of this high-risk population is imperative.
Mechanisms of cancer metastasis
Organ tropism and metastasis theories
Metastasis is a defining characteristic of malignancy that presents significant challenges in oncology, owing to the spread of cancer cells from the primary sites to distant organs. Metastatic cells often exhibit organ-specific preferences, known as “organ tropism”. Determining this predilection is vital for advancing preventive and therapeutic measures. Two pivotal theories, the “seed and soil” hypothesis, and the “multiclonal metastasis” theory, enhance our understanding of bone tropism. Introduced by Paget in 1889, the “seed and soil” hypothesis posits that metastasis is not random.47 It proposes that the “seed” (cancer cells) requires a conducive “soil” (metastatic site) for successful growth, with specific tissue niches providing factors that facilitate their development. Furthermore, the origin of metastatic cells is not limited to the action of a singular dominant “seed.” Instead, it is the collective contribution of various cancer cell subpopulations within the primary tumor, known as “multiclonal metastasis,” orchestrating this metastatic process.48 This underscores the inherent heterogeneity within primary tumors, which is crucial for their metastatic capabilities.
Seed and soil theory
The “seed and soil” theory offers a framework for understanding the intricate process of cancer metastasis. The successful spread of cancer cells (the “seed”) to distant organs or tissues depends on both their intrinsic properties and the distal colonized microenvironment (the “soil”). Metastasis occurs when circulating tumor cells (CTCs) interact with the microenvironment of a distant organ, creating conditions conducive to their survival, proliferation, and colonization.49 After detachment from the primary tumor, CTCs enter the bloodstream and must survive a hostile environment, evade immune surveillance, adhere to the narrow capillaries of distant organs, and extravasate into the surrounding tissue. This extravasation step is particularly significant for organ tropism, as it determines whether cancer cells can establish a niche within specific target organs (Fig. 2). We summarized key signaling molecules and pathways reported for organ tropism in Table 2.
Equally important is the “soil,” or the microenvironment at the metastatic site. This environment is composed of a complex array of growth factors, cytokines, and extracellular matrix components, and diverse cell types (Table 2). Cancer cells, tissue-specific niches, and immune cells engage in intensive cell-cell communication to shape a tumor-favoring ecosystem. Tissue structure also influences metastasis patterns; for example, the lymphatic system often serves as a primary route for dissemination, with lymph nodes providing initial sites for cancer cell trapping and proliferation before further spreading via lymphatic and circulatory systems.50 Likewise, the liver and lungs are common metastasis sites due to their distinctive blood flow patterns.51,52 These insights underscore the complex interplay between the genetic makeup of cancer cells and permissive distant microenvironments. Recognizing these “seed and soil” dynamics may guide the development of more effective therapeutic strategies that disrupt supportive niches and impede the colonization and growth of metastatic cancer cells. Continued research will refine our understanding of bone metastasis and inform improved management of various cancers.
The blood and lymphatic circulation patterns play a crucial role in determining metastatic sites. Anatomical factors greatly influence the site at which cancer cells disseminate, with the liver and lungs being common metastasis sites owing to their distinctive blood flow patterns.51,52 For example, gastrointestinal cancers often metastasize to the liver owing to the direct blood flow from the intestines via the portal vein system.53 Additionally, adhesion molecules such as integrins and selectins expressed on cancer cell surfaces enable these cells to adhere to and invade the target organs by binding to endothelial cells.54 Integrin-mediated organ tropism has been illustrated in various model systems. For instance, studies using melanoma and patient-derived MDA-MB-231 breast cancer cells55 have shown that exosomes carrying α6β1 and α6β4 preferentially direct metastases to the lungs, whereas αvβ5-bearing exosomes facilitate liver colonization. In breast cancer, exosomes carry β3 integrin and sialylated N-glycans/integrins have been implicated in promoting brain metastasis.56 Furthermore, αv integrin has been shown to promote bone colonization by interacting with and dysregulating osteoclast functions.57,58,59,60 It has been selected for potential targets in treating bone metastasis.61
The influence of chemotactic factors and their receptors on organ tropism is important in this process. Specific organs secrete chemokines and growth factors that attract cancer cells to express their corresponding receptors. For instance, the CXCL12/CXCR4 axis plays a crucial role in the metastasis of breast cancer to the lungs and bones by directing cancer cells to these sites.62
The composition of the ECM in different organs can influence the process of metastatic colonization. Specific ECM components provide a supportive niche for metastatic cancer cells, facilitating their growth and survival. ECM proteins, such as fibronectin and laminin, enhance the adhesion and invasion capabilities of cancer cells.63
The formation of a pre-metastatic niche (PMN) is initiated by tumor-secreted factors, preparing distant organs for cancer cells even before their arrival. Exosomes, cytokines, and other molecular components secreted by the primary tumor can modify the microenvironment of the target organs to support metastasis. Another critical factor in cancer metastasis is immune evasion, whereby cancer cells avoid detection and destruction by the immune system to colonize new sites.64 For instance, some organs, such as the brain, provide a distinctive immune milieu that safeguards metastatic cells from immune surveillance.65 Finally, organ-specific growth factors support the growth of metastatic cells. Specific organs produce growth factors that favor the proliferation of specific types of cancer. For example, IGF-1 in bone marrow supports prostate cancer metastasis.66
Multiclonal metastasis
The “multiclonal metastasis” theory emphasizes that metastasis arises through a complex, dynamic evolutionary process (Fig. 2). Due to inherent genomic instability, tumor cells accumulate numerous mutations, resulting in substantial heterogeneity and enabling different tissues to be colonized by multiple, genetically distinct tumor clones (i.e., polyclonal metastasis).67 Whole-genome sequencing studies have shown that metastases can originate from intermingling multiple tumor clones across metastatic sites, highlighting the multifaceted nature of metastatic dissemination.68 This concept aligns with the evolutionary dynamics described by Turajlic and Swanton,69 who demonstrated that emerging metastatic subclones contribute significantly to genetic diversity within metastatic tumors.
Further evidence for polyclonal metastasis includes polyclonal lymph node metastases in colorectal cancer arising from disparate regions of the primary tumor,70 as well as breast cancer metastases driven by collective dissemination of keratin 14-expressing tumor cell clusters.71 In the TRACERx study, both polyclonal and monoclonal metastases were observed in non-small-cell lung cancer (NSCLC), illustrating that metastatic clones often represent expansions of subclones from the primary tumor.72,73 Colorectal cancer (CRC) patients, in particular, frequently exhibit polyclonal metastasis in lymph nodes compared to other organs,74,75,76 and triple-negative breast cancer (TNBC) metastasis often displays heterogeneous subclone populations characteristic of polyclonal seeding.77 Similar patterns have been noted in colorectal and pancreatic cancers, where distinct subclones give rise to metastatic lesions, further reinforcing the polyclonal nature of metastasis and its implications for treatment heterogeneity.78,79,80 The concept of tumor self-/cross-seeding introduces the possibility that circulating tumor cells can repopulate both primary and metastatic lesions, augmenting tumor heterogeneity even further,81 a phenomenon also supported by recent liver cancer studies employing novel labeling systems (Fig. 2).82
Collectively, these insights underscore that multiclonal metastasis has profound implications for bone metastasis and beyond. Distinct subclones may respond differently to therapeutic interventions based on their unique genetic makeup, contributing to variable treatment responses and resistance within a single patient. Understanding this complex clonal architecture is critical for developing targeted, practical strategies to manage bone metastases and improve patient outcomes.
Metastatic cascade
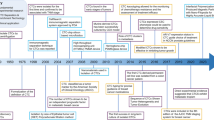
Metastasis is a biological process involving complex interactions between colonized cancer cells and metastatic microenvironment. At the primary tumor site, cancer-associated fibroblasts (CAFs), stromal cells, and other cells establish a “niche” conducive to tumor cell metastasis through remote regulatory mechanisms, providing the necessary microenvironment for tumor cell migration and facilitating their detachment from the primary site and embarking on their invasive journey. As tumor cells migrate, they evade immune surveillance and interact with circulating CAFs and myeloid cells to enhance survival and invasiveness. Upon reaching the metastatic site, tumor cells extravasate through frequent interactions with the local microenvironment (Fig. 3).
Mechanisms of the cancer metastasis cascade. At primary tumor sites, cancer-associated fibroblasts (CAFs) and stromal cells create a metastasis-conducive niche to support cancer cell EMT process, dissemination, and migration. As migrating cancer cells interact with circulating CAFs and myeloid cells to enhance survival and invasiveness while evading immune detection, they eventually reach the metastatic sites where they transition from dormancy and interact with the microenvironment to initiate active proliferation. The metastatic cascade initiated by primary tumor cells invading adjacent tissues via EMT is facilitated by CAFs that promote motility and ECM degradation. Moreover, macrophages and tumor-associated neutrophils (TANs) significantly contribute to ECM breakdown, facilitating cancer cell intravasation and survival in circulation by forming aggregates with platelets and myeloid cells to evade immune surveillance. Key interactions between cancer cells and the endothelium facilitate adhesion and extravasation into bone marrow, supported by the metabolic reprogramming of osteoblasts and osteoclasts. In addition, myeloid cells enhance cancer cell survival and metastasis through immune suppression, metabolic support, and ECM remodeling, including the crucial activities of neutrophils and macrophages in facilitating tumor cell adhesion, invasion, and metastatic proliferation at secondary sites
Pre-metastatic niche
The formation of PMN in the bone results from interactions between primary tumor cells and various distal niche cells. These interactions facilitate molecular and cellular changes in distant organs, setting the stage for metastatic seeding before clinically detectable collective and massive metastasis. The establishment of the PMN sets the basis for organ tropism. For instance, fibroblasts are crucial for establishing an environment conducive to metastatic colonization. The significance of fibroblasts in forming the metastatic niche is underscored by the essential role of periostin expression in the proliferation of early disseminated cancer stem cells at secondary sites, highlighting the critical influence of stromal niche signals.83 Moreover, the tumor-associated stroma, comprising fibroblasts and myofibroblasts, plays an active role in supporting tumor expansion by promoting neo-angiogenesis and the proliferation and invasion of cancer cells, thus aiding distal seeding, such as bone colonization.84 Studies on highly metastatic HCC cells have indicated that the secretion of exosomal miR-1247-3p activates fibroblasts and promotes lung metastasis in liver cancer.85 Secreted extracellular vesicles (sEVs) play critical roles in forming PMN in the lungs and in the preparation of lung and brain metastasis from various cancers.86 These sEVs contain a variety of molecules, including nucleic acids, signaling proteins, enzymes, lipids, and metabolites, that can influence cellular functions and communication.87
In the bone, the initiation of PMN has been attributed to VEGFR1-positive hematopoietic progenitor cells, which migrate to specific pre-metastatic sites and form clusters in anticipation of tumor cell arrival, suggesting the role of bone marrow in PMN initiation.88 The interaction between cancer cells and hematopoietic and mesenchymal stem/progenitor cells residing within the bone metastatic niche facilitates reciprocal communication between tumor cells and the bone metastatic stroma.89 In myelomas, osteoblasts undergo metabolic reprogramming in response to the primary tumor, characterized by increased glucose uptake and enhanced glycolysis. This metabolic shift facilitates the production of lactate and other metabolites that are utilized by cancer cells for energy production. Osteoclasts adopt a high-energy state, which increases bone resorption.90
Myeloid cells and their progenitors within PMN help establish chronic inflammation in secondary organs, which may be an immune response to infection.91 This inflammation, in turn, compromises the immune system’s ability to initiate an effective response, thereby facilitating the successful establishment of metastatic lesions.92 Primary breast tumors can induce the mobilization of CD11b+ myeloid cells to the lungs, creating an immunosuppressive microenvironment that dampens the cytotoxic activities of NK93 and T cells,94 thus promoting metastatic colonization in the lung. Furthermore, lipid metabolites in lung-resident neutrophils have been identified as significant energy sources influencing lung metastasis in breast cancer (BC).95 Moreover, primary lung and BC tumor growth can remotely disrupt myelopoiesis through sEVs, leading to abnormal myeloid lineage differentiation in the bone marrow, which accumulates myeloid cells in the bone marrow and supports tumor progression96,97 (Fig. 3). In colorectal cancer (CRC), Kupffer cells can phagocytose exosomes carrying highly expressed miR-135a-5p from the bloodstream into the liver, thereby establishing liver tropism.98 In addition, secreted molecules such as tissue inhibitors of metalloproteinases (TIMP-1),99 VEGFA,100,101,102, and CCL15103 can accumulate various myeloid cells in the liver and form PMN in the liver.
Cancer cell dissemination and intravasation
The metastatic cascade begins with the invasion of primary tumor cells into adjacent tissues. This invasive process often involves epithelial-mesenchymal transition (EMT), which enables cancer cells to acquire migratory and invasive properties. Simultaneously, primary tumor cells break the ECM and create pathways for dissemination. The local tumor microenvironment (TME) supports EMT and ECM breakdown, enabling intravasation into the bloodstream or lymphatic system (Fig. 3).
CAFs play multiple roles in cancer metastasis. One crucial mechanism involves the induction of EMT in tumor cells. CAFs secrete factors such as TGF-β, downregulating E-cadherin and upregulating N-cadherin and vimentin, signifying a mesenchymal phenotype.104,105,106 This transition promotes tumor cell motility and invasiveness, facilitating their escape from the primary tumor. In addition, CAFs drive metastasis by remodeling the ECM. Furthermore, they secrete matrix metalloproteinases (MMPs), which degrade ECM components and decrease cell-cell adhesion, aiding tumor cell invasion and migration.107 Moreover, direct interactions between CAFs and carcinoma cells influence invasion, with CAFs reorganizing collagen fibrils within the ECM, creating pathways for tumor cell progression.108 Additionally, primed CAFs support tumor cell invasion through metabolic crosstalk by secreting metabolites such as lactate and glutamine, which cancer cells readily utilize, fueling pathways that enhance their invasive potential.109,110 Moreover, these metabolic alterationsv not only promote primary tumor growth and metastasis but also foster immune evasion by increasing glycolysis (Warburg effect111), suppressing anti-tumor responses of NK cells,112 impairing macrophage pro-inflammatory stimulation,113 dysregulating myeloid cell function,114,115 limiting dendritic cell antigen presentation,116 and promoting regulatory T cell infiltration.117
In addition to CAFs, tumor-associated neutrophils (TANs) and tumor-associated macrophages (TAMs) support cancer invasion through ECM degradation via the secretion of MMPs.118 In addition, they secrete osteonectin, promoting tumor cells and ECM interaction.119 Further ECM remodeling is driven by TAM- and TAN-derived factors such as elastases, cathepsins, and proteinases-3.120,121 Changes in the bone microenvironment fuel the invasion process. Metabolic reprogramming, accompanied by metabolite release during osteoclast-mediated bone breakdown, generates a highly acidic environment. This acidosis activates proteases, such as cathepsin K, promoting ECM degradation, and facilitating the early steps of tumor cell dissociation and invasion.122
The tumor microenvironment for metastasis (TMEM) is a strong predictor of metastasis in human BC. Invadopodia formation, driven by interactions between macrophages and tumor cells, is key to tumor cell intravasation within TMEM.123,124,125 A paracrine loop involving macrophage-derived growth factors and tumor cell-produced colony-stimulating factor 1 fuels these interactions. In addition, transient vascular permeability within the TMEM facilitates tumor cell escape into circulation. Cancer stem cells (CSCs) accumulate at TMEM sites near TAMs. Their lower inherent migration ability suggests that CSCs may efficiently intravasate by exploiting macrophage-endothelium connections.126,127
In a polyomavirus middle T antigen-overexpressing BC model, TAMs promoted cancer cell intravasation by partly inducing angiogenesis via VEGFA secretion, thereby increasing blood vessel density.128 In addition, a subset of Tie2+ TAMs transdifferentiate into perivascular macrophages that promote vascular leakage and directly facilitate the intravasation of tumor cells.129,130,131 TANs also promote tumor cell intravasation but through different processes. One hypothesis suggests that migrating neutrophils create tunnels in the ECM, allowing tumor cells to disseminate into the vasculature. Furthermore, tumor cells may adhere directly to neutrophils, using them to facilitate transport through the endothelium132,133 (Fig. 3).
Cancer cell circulation
The circulatory system presents a harsh environment, presenting numerous obstacles to tumor cell dissemination. However, most of the tumor cells were Ki67+, suggesting they are in a state of active proliferation. CTCs have evolved strategies to avoid immune surveillance to survive and ultimately metastasize. A growing body of research elucidates these mechanisms, highlighting how CTCs evade detection and persist as they migrate to distant bone regions (Fig. 3).
One key mechanism involves physical cloaking within the platelet aggregates, obscuring them from immune surveillance.134 Both selectins and integrins facilitate this interaction. In addition to physical shielding, platelets release signaling factors that induce EMT in CTCs, promoting invasiveness, stemness, motility, and resistance to anoikis.135 CAFs can also accompany CTCs into circulation, aiding them in several ways. CAF-secreted MMPs degrade physical barriers, whereas growth factors, such as vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), support CTC survival. Moreover, CAFs facilitate the metabolic adaptability required for CTCs to withstand stress during circulation.136,137
Myeloid cells also support CTCs, often forming cellular aggregates with disseminated tumor cells. Initial in vitro studies have indicated that TANs promote aggregation of both breast and CRC cells. Subsequently, neutrophil extracellular traps (NETs) were linked to the emergence of venous thrombi in the lungs.138 Further investigation revealed that neutrophil-associated CTCs, both in the 4T1 mammary tumor model and in patients with BC, displayed a pro-tumoral gene expression profile characterized by the enrichment of positive regulators of cell cycle progression and DNA replication. This pro-tumor phenotype contributes to enhanced metastatic capabilities. Consistent with these observations, using antibodies to block neutrophils reduced the incidence of bone metastasis. Conversely, upregulation of the granulocyte-colony-stimulating factor intensifies the clustering of TANs and CTCs, thereby exacerbating metastasis.139
Neutrophils play a dual role in shielding CTCs during immune surveillance in patients with BC. They inhibit the responsiveness of natural killer (NK) cells by attenuating signaling through cell surface receptors.140 Furthermore, neutrophils protect tumor cells from antitumor T-cell responses. An increase in T-cell-suppressive neutrophils has been systemically observed in mammary tumor models.141 Neutrophils derived from patients with melanoma and renal cell carcinoma exhibit elevated levels of ARG1, an enzyme that inhibits T-cell-mediated cytotoxic responses. Neutrophil depletion restores cytotoxic T-cell proliferation.142,143 Moreover, recent findings suggest a collaborative mechanism wherein CTCs activate platelets and neutrophils through direct cell-cell interactions, creating a protective network within the vasculature.144 This mutual activation facilitates the clustering of disseminated tumor cells with neutrophils and platelets and likely shields CTCs from mechanical and immune-mediated destruction during the metastatic process (Fig. 3).
Cancer cell extravasation and seeding in the distal tissue
Besides surviving in circulation, CTCs must navigate several steps to establish colonies in distant organs. These include adhesion to and transendothelial migration through the endothelium, ECM degradation, parenchyma invasion. Initial interactions between CTCs and the endothelium are crucial in the metastatic niche. Endothelial selectins, such as E-selectin, facilitate CTC tethering and rolling through ligand-receptor interactions.145 Adhesion is further stabilized by specific integrin pairings, notably αvβ3 integrin, on CTCs and their endothelial ligands.146,147,148
Adhesion is a critical preliminary step when CTCs invade tissues. Given that different tissues possess distinct homeostatic environments, CTCs utilize various strategies, each adapted to the specific conditions of the tissue in question, to ensure successful extravasation and seeding. This process entails distinctive molecular interactions and adaptations to the distinctive microenvironments of target tissues, thereby ensuring effective colonization and growth. Because the bone represents a primary organ frequently targeted by a multitude of cancers, this discussion will focus on elaborating on the phenomenon of bone metastasis in various tissues.
Chemokine signaling via the CXCL12-CXCR4 axis directs CTCs to CXCL12-rich bone sites.149,150 After attaching to the endothelium, CTCs must penetrate the vascular barrier to reach the bone marrow, secrete VEGF to increase vascular permeability, and aid in their transendothelial journey.151 Interactions with vascular lining cells, including pericytes, can either facilitate or impede CTC extravasation152 (Fig. 3).
In the bone microenvironment, stromal cell networks significantly affect CTC metastatic potential. Osteoblasts emit chemoattractants, such as receptor activators of nuclear factor-κB ligand (RANKL), attracting CTCs that express the RANK receptor to the bone niche.153 CTCs manipulate bone remodeling by inducing osteoclasts via parathyroid hormone-related protein (PTHrP) secretion and releasing cytokines and growth factors from the bone matrix to create a nurturing environment for CTC proliferation.154 Some CTCs may enter dormancy within the bone and be reactivated under favorable conditions.155,156,157,158
Enhanced oxidative phosphorylation in macrophages is linked to the secretion of pro-tumor factors, aiding tumor cell survival and EMT via pathways such as Wnt/β-catenin signaling at metastatic sites.159,160,161 Neutrophils colocalize with tumor cells at metastatic sites, facilitating their adhesion and arrest, especially in the lung and liver, thus promoting the retention of tumor cells.162,163 Studies on aggressive mammary tumors and TNBC specimens have shown elevated neutrophil-derived NETs in the lungs.164 NETosis enhances tumor cell adhesion to neutrophil monolayers, a process mitigated by inhibiting NET formation. NETs encapsulate adherent tumor cells, trapping them at distant sites and correlating with increased metastatic burden, whereas NET inhibition reduces metastasis in vivo.164,165,166 Moreover, transendothelial migration of CTCs is mediated by neutrophil MMP8/9, with inhibition or genetic ablation of these enzymes, thus reducing the metastatic burden in murine models140 (Fig. 3).
In tumor immunology, monocytes are categorized as pro-tumoral, classical, anti-tumoral, and non-classical. Classical monocytes enhance cancer cell invasiveness, as evidenced by their co-culture with human BC cells, resulting in elevated MMP9, TNF, and growth factor production.167 Dormant tumor cells actively recruit circulating monocytes, which facilitates extravasation. Ly6C+ monocyte recruitment is driven by CCL2 secretion, promoting BC cell extravasation into the lung tissue via VEGFA and MMP9.168 Similarly, Gr-1 + CD11b+ myeloid lineages contribute by releasing MMP9, disrupting endothelial monolayers, and enhancing vascular permeability.169 Collectively, these studies highlight the role of neutrophils and classical monocytes in regulating endothelial permeability, facilitating cancer cell extravasation, and bone colonization (Fig. 3).
Metastatic cancer dormancy, reactivation, and outgrowth
Metastatic cancer dormancy and reactivation are complex processes within a metastatic microenvironment. Disseminated tumor cells dynamically interact with local stromal cells, pivotal for transitioning tumor cells from a dormant state to active metastatic proliferation.
The maintenance of dormancy in metastatic cells depends on several mechanisms. Interactions with the ECM, including fibronectin,170 tenascin C, and periostin,171 are crucial for survival, with adhesion molecules, such as integrins, playing a pivotal role. Maintenance of the dormant state is facilitated by stress signaling pathways, including p38 MAPK and the unfolded protein response.172 Hypoxic conditions and their associated signaling pathways also contribute to the maintenance of cellular quiescence. Furthermore, dormant cells can evade immune detection, enabling them to survive in a non-proliferative state. These integrated mechanisms facilitate the persistence of dormant cells in a stable state until the conditions are conducive to reactivation and growth173,174,175,176 (Fig. 3).
In breast and prostate cancer models, prolonged systemic inflammation induces neutrophil infiltration and NET formation at metastatic sites. NETs, by remodeling the ECM component laminin, activate a cascade involving WNT signaling, integrin signaling, and the FAK/ERK/MLCK/YAP pathways, which awaken dormant tumor cells, thereby promoting their proliferation.177,178,179,180,181 In addition, chronic inflammation increases reactive oxygen species and promotes angiogenesis, which breaks the dormancy.178,179,180 Neutrophil accumulation in the lungs precedes significant tumor cell invasion, and systemic perturbation in myelopoiesis is evident in both mouse models and human BC.96 In melanoma, factors that inhibit macrophage migration stimulate Kupffer cells to secrete TGF-β, attract bone marrow-derived macrophages, and elevate fibronectin levels in the liver, underscoring the vital role of resident myeloid cells in metastasis.182 In mammary tumors, the myeloid lineage of the primary tumor influences distant pre-metastatic niches, where tumor-derived CCL2 fosters TAM accumulation and elevated IL1β secretion, thereby facilitating immunosuppression at metastatic sites.183,184
Metastasis-associated macrophages (MAMs) have recently been identified as distinct macrophage subpopulations and critical players in metastatic processes that facilitate the proliferation of metastatic cells. The reduction in metastatic outgrowth following macrophage depletion highlights the critical role of macrophages in metastasis.185 MAMs promote metastatic cell survival by activating the Akt pathway, which provides resistance to pro-apoptotic cytokines. Furthermore, MAMs interact with CTCs via integrins such as vascular cell adhesion molecule-1 (VCAM-1) to form protective clusters that improve cancer cell survival during migration.186 Gr-1 + CD11b+ monocytes promote the establishment of metastatic tumor cells in the lungs, particularly in breast tumor-bearing mice, through mechanisms such as PDGF-BB-induced angiogenesis and CCL9 production,187 which support tumor cell survival188 (Fig. 3).
Mechanisms of organ tropism
The distinct genomic and epigenomic variation patterns observed across diverse tumor types and their subtypes, the specific molecules expressed by tumor cells, and the intricate interactions between these cells and the metastatic organ microenvironment collectively constitute a fundamental framework for understanding organ tropism mechanisms189,190 (Table 2). This intricate and sophisticated network offers several potential targets for developing targeted therapeutic strategies.191
Bone metastasis
Bone is the preferred site for metastasis in several types of cancer, which is closely related to the unique microenvironment within bones, including high vascularization, hypoxic conditions, and a high local calcium concentration.24,192,193 The propensity for bone metastasis to predominantly affect the axial bones, such as the spine, pelvis, and ribs, rather than the distal bones, such as those found in the extremities, is significantly associated with the distribution of red bone marrow.194,195 The distinctive sinusoidal configuration of the skeletal vasculature endows bones with enhanced accessibility to CTC, thereby establishing them as primary targets for metastatic colonization.11 Furthermore, the bone exhibits a markedly hypoxic microenvironment, with prevailing oxygen tension frequently declining below 2%.196 This hypoxic microenvironment induces the activation of hypoxia-inducible factor (HIF) signaling in tumors, thereby triggering a cascade of events, including EMT, cell invasion, and angiogenesis. These processes facilitate the infiltration, metastasis, and colonization of tumor cells within the bone.197 Analysis of primary breast cancer specimens from bone metastasis has consistently demonstrated an elevation in the expression of HIFs, highlighting the critical role of hypoxia in driving organ tropism.198 In bone tissue, calcium levels typically range from 2 to 4 mmol/L, whereas in zones of active remodeling, they can reach concentrations of 8–40 mmol/L.199 Elevated local calcium concentrations can activate calcium-sensing receptors (CaSRs) in cancer cells, potentially amplifying proliferation, enhancing migratory capabilities, and blunting apoptotic signals.200 A distinctive attribute of CaSR in malignant cells is its inclination toward Gαs proteins, a deviation that results in the production of cAMP and PTHrP, further promoting tumor progression and dissemination.201,202,203
The concept of the “PMN” is important for understanding how specific secondary sites become the preferred locations for cancer metastasis. In the context of TNBC, the bone microenvironment is notably enriched with CXCL12 (also known as SDF-1) and insulin-like growth factor 1 (IGF-1), which are secreted by CAFs. These cytokines selectively drive the bone-tropic metastasis of cancer cells exhibiting elevated Src activity via stimulation of the PI3K-Akt pathway, which is pivotal in regulating cellular survival and motility.204 SCUBE2, a tumor-secreted glycoprotein, is a crucial facilitator of bone metastasis in luminal breast cancer, particularly during the initial stages of niche formation.205 SCUBE2 indirectly inhibits leukocyte-associated Ig-like receptor 1 (LAIR1) signaling, impairing NK cell function and promoting tumor cell persistence and growth within the bone. Exosomes are nanoscale vesicles secreted by tumor cells that serve as vital communicators between neoplastic cells and pre-metastatic niches, demonstrating a predilection to home organs expressing cognate ligands.55 Upon engagement, exosomal microRNAs (miRNAs) can modulate gene expression within target cells, thereby engineering a hospitable microenvironment conducive to the anchorage and proliferation of tumor cells.206,207 Furthermore, growth differentiation factor 15 (GDF15), secreted by prostate cancer cells, has also been identified as a factor contributing to the increased propensity for bone metastasis, as demonstrated in preclinical xenograft models.208
The proclivity for bone metastasis is inextricably linked to a vicious cycle that involves tumor cells and osteoclasts.209 Tumor cells secrete osteolytic substances, including PTHrP, IL-11, and Jagged 1, which induce bone resorption. These secretions activate the RANK/RANKL and Notch pathways, stimulating osteoclastogenesis and activation, exacerbating bone destruction, and providing a conducive environment for metastatic growth.154,210,211 Osteolysis in metastatic bone releases of key biological factors, including transforming growth factor beta (TGF-β), IGF-1, and calcium.200,212,213 These substances profoundly influence cancer cell growth, proliferation, and propensity for bone metastasis, thereby creating an environment conducive to the establishment and progression of skeletal lesions.
Brain metastasis
Brain metastasis, a regrettable common occurrence among patients diagnosed with lung, breast, and melanoma cancers, is associated with unfavorable prognoses and reduced survival rates.214 Transmigration of tumor cells across the blood-brain barrier (BBB) through diverse mechanisms represents a critical step in the inception of brain metastasis. It is a determinant factor in the organotropism observed in cancer dissemination.215 A compromised BBB integrity, frequently associated with the upregulation of specific genes, plays a pivotal role in this process.216,217 For instance, the proteolytic action of cathepsin S, mediated by its interaction with the adhesion molecule JAM-B, can induce BBB leakage. Inhibition of cathepsin S expression markedly reduces the likelihood of brain metastasis.218 Brain metastases from triple negative or basal-type breast cancers frequently disrupt the BBB, in contrast to those from HER2/neu-positive breast cancer, which are inclined to maintain the BBB’s integrity. This phenomenon is closely associated with the differential expression of glucose transporter 1 (GLUT1) and breast cancer resistance protein (BCRP).219 Furthermore, elevated expression of adhesion molecules, including MUC1, VCAM1, and VLA-4, in breast cancer cells has been identified as a contributing factor in the facilitation of brain metastasis, thereby enhancing tumor cell adherence.220 Notably, primary tumor cells can also facilitate brain metastasis by exchanging exosomes, which are envelopes carrying miRNAs that communicate with pre-metastatic niches.221 Once cancer cells successfully colonize the brain, the BBB may transform its role, shifting from a protective barrier to an impediment against therapeutic interventions, thereby complicating treatment efficacy.21
The metabolic mechanisms of tumor cells are important for cancer invasion and metastasis. Different tumor types can result in significant variations in the metabolic characteristics of brain metastasis.222,223 Some tumors demonstrate a proclivity for anaerobic glycolysis, whereas others rely on oxidative phosphorylation (OXPHOS) for energy production.224 Given the distinctive energy storage and consumption mechanisms of the brain, tumor cells are compelled to adapt to their metabolic microenvironment.225 Genomic analysis of brain metastasis in melanoma has revealed that tumor cells can express genes related to the OXPHOS pathway at high levels.226 Additionally, inhibition of OXPHOS activity has been demonstrated to prevent melanoma brain metastasis in a mouse model.226 Furthermore, elevated expression of fatty acid-binding protein 7 (FABP7) in breast cancer is closely associated with a high incidence of brain metastasis. FABP7 facilitates a glycolytic phenotype and storage of lipid droplets, thereby enabling HER2-positive breast cancer cells to adapt more effectively to the relatively hypoxic and nutrient-restricted microenvironment of the brain.227
Neurons and glial cells work together to construct the PMN of the brain, and their interactions with cancer cells are crucial for promoting brain metastasis.228 Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter that plays a critical role in the central nervous system.229 In the clinical analysis of HER2+ and TNBC brain metastasis, cancer cells have been observed to overexpress GABA-related proteins (such as GABAA receptors, GABA transporters, GABA transaminases, and glutamate decarboxylase). This overexpression influences organotropism of tumor metastasis.230 Glutamatergic neurons can form pseudo-tripartite synapses with breast cancer cells, a distinctive intercellular structure that directs glutamate signaling from neurons to cancer cells.231 This cross-cell interaction supports the survival and proliferation of breast cancer cells in the brain, thereby explaining the specific preference of these cells for this organ. Furthermore, brain metastatic tumor cells can deliver the second messenger cGAMP (cyclic GMP-AMP) to astrocytes via gap junctions, thereby activating the STAT1 (signal transducer and activator of transcription 1) and nuclear factor kappa B (NF-κB) signaling pathways within tumor cells through a series of cascades.232 These two signaling pathways are intimately associated with the growth, proliferation, and survival of tumor cells.225,233
Liver metastasis
The liver is a highly vascularized organ that serves as a distal metastatic target for a multitude of solid tumors, including those of breast, pancreas, and colorectal origin.234,235 Tumor cells are disseminated to potential niches via the bloodstream, and the liver receives blood from both the hepatic artery (~25%) and portal vein (~75%), providing a direct pathway for tumor cells to reach the liver.45,236 Conversely, the low flow rate in the hepatic sinusoids allows for the retention and deposition of tumor cells in the liver, prolonging their retention time. Conversely, the high permeability of hepatic sinusoidal endothelial cells and the incomplete basement membrane facilitate tumor cell penetration of the vascular wall and subsequent entry into the liver parenchyma.225,237 CRC cells are more likely to be “captured” in the liver during the metastatic process than to remain in the peripheral blood. This increased propensity for liver metastasis is characteristic of CRC.235,238 Furthermore, the liver exhibits distinctive immune tolerance, particularly toward NK cells, which can impede the immune system’s capacity to eradicate tumor cells.239,240
Exosomes play a pivotal role in intercellular communication and are instrumental in PMN formation in the liver.235 In pancreatic cancer liver metastasis, macrophage migration inhibitory factor (MIF)-containing exosomes can specifically activate Kupffer cells in the liver under the mediation of αvβ5 integrin, thereby inducing the secretion of TGF-β and promoting tumor-specific liver metastasis.182 In addition, hepatocytes can respond to cytokines secreted by tumors, such as IL-6, by producing myeloid cell chemoattractants, such as serum amyloid A (SAA). They participate in formating an inflammatory and fibrotic microenvironment that favors the growth and dissemination of metastatic tumor cells in the liver.234 In addition, exosomes can carry CD39 and CD73, which inhibit T-cell function and aid tumor escape from immune surveillance.241 Neutrophils can release NETs, which contain DNA components that promote the proliferation and migration of tumor cells by activating CCDC25 (a cell cycle regulatory protein), thus facilitating liver metastasis.242
Tumor cells adapt to changes in the metastatic microenvironment of the liver through specific gene mutations or aberrant expression. DAMTS10, NELL1, and RXFP3 are regarded as liver metastasis-specific genes, exhibiting mutations that are exclusive to liver metastatic regions but are absent in CRC lacking liver metastasis.243 Liver metastatic CRC cells upregulate the GATA6 transcription factor, increaseing the expression of aldolase B (ALDOB). This confers the ability to metabolize fructose and enhances the proliferative potential of tumor cells following metastasis.244 In the context of liver metastasis, elevated SERPINE2 expression has been observed to enhance epidermal growth factor receptor (EGFR) signaling, which is conducive to the proliferation of tumor cells.245 The expression of SERPINE2 is influenced by the status of DNA methylation, thereby indicating that epigenetic alterations may also affect the process of liver metastasis.246
Lung metastasis
The lung has the highest incidence of metastasis, particularly in patients with breast cancer, melanoma, and thyroid cancer.225,247 The extensive vascular network of the lungs provides a conducive environment for tumor cells to adhere, extravasate, and establish micrometastasis. For example, thyroid cancer cells usually metastasize via the bloodstream, and because the lung is the terminal point of the superior vena cava system, this facilitates their metastasis to the lung.45,248 The typically high oxygen levels in the lung starkly contrast with the hypoxic environments of the bone and liver. Tumor cells that metastasize to the lungs must adapt their metabolic pathways to accommodate the microenvironment and mitigate oxidative damage.249 Examination of single-cell transcriptomes of breast cancer micrometastasis in the lung demonstrated elevated levels of OXPHOS activity, which contrasts with the energy production methods observed in primary breast cancer cells.250 Activation of the Notch signaling pathway promotes the expression of EMT-related genes, enabling breast cancer cells to acquire migratory and invasive capabilities that facilitate metastasis.251 For example, when breast cancer cells invade the lung, they produce tenascin C, which enhances Notch signaling.252 Secreted protein acidic and rich in cysteine (SPARC) derived from melanoma promotes lung metastasis by increasing vascular permeability and promoting the adhesion of tumor cells to vascular endothelial cells, mainly through VCAM-1-dependent mechanisms.253
Both breast and melanoma cancer cells express high levels of CXCR4 and CCR7 receptors, which enables them to migrate to the lungs in response to CXCL12 and CCL21 ligands.254 Exosomes released from CD103+ CSCs are enriched for miR-15b-3p, promoting the colonization and growth of clear cell renal cell carcinoma (CCRCC) cells in the lung and accelerating metastasis.255 EVs released by hepatocellular carcinoma (HCC) cells contain nidogen 1, which enhances angiogenesis and activates fibroblasts in the lungs, thereby contributing to the survival and proliferation of tumor cells.256
Osteosarcoma, a highly representative primary malignancy within bone and soft tissue sarcomas, primarily disseminates through the vascular system without involving the lymphatic system, with the lung often serving as its primary metastatic destination.257 The microenvironment plays a pivotal role in regulating the behavior of osteosarcoma cells, influencing not only their proliferation, quiescence, invasion, migration, and drug resistance characteristics but also contributing to the development of intrinsic tumor heterogeneity.257 By releasing EVs, osteosarcoma cells can remotely manipulate the lung environment, thereby predisposing a pre-metastatic niche for migrating tumor cells.258
Moreover, mesenchymal stem cells (MSCs) exhibit a profound connection with both the metastatic progression and therapeutic resistance of osteosarcoma.259 Specifically, EVs secreted by osteosarcoma cells, containing TGF-β, are capable of inducing MSCs to release IL-6, which in turn activates STAT3-mediated tumor progression pathways. This activation drives the formation of metastatic foci in the lungs and fosters the maturation of these MSCs.260 Furthermore, EVs originating from both osteosarcoma cells and metastatic niches possess the remarkable ability to reprogram myofibroblasts and osteosarcoma stem cells into fibrotic phenotypes, a process that is pivotal for metastatic colonization.261,262 In the osteosarcoma metastatic cascade, the cytoskeletal linker protein Ezrin provides essential scaffolding, thereby enhancing the survival of disseminated tumor cells under initial stress conditions in the lungs.263 Furthermore, the RANK-RANKL-OPG system has been identified as significant factors influencing the formation of the pre-metastatic niche in the lungs.264
Other metastasis
A more profound understanding of tumor cell metastasis in the lymphatic system and peritoneum could facilitate the development of more efficacious treatment strategies and enhance our understanding of cancer staging.191 The structure of lymphatic vessels permits the passage of larger cells, and the slow flow rate of lymph provides opportunities for cancer cells to adhere to the walls of the lymphatic vessels.265 Lymph nodes serve not only as the initial destination for tumor cells following their departure from the primary tumor266 but also as pre-tumor niches, providing a protected and supportive environment for the proliferation of disseminated tumor cells.267 Furthermore, lymph nodes are typically located in key areas of the body, such as the neck, armpit, and groin, near many vital organs. This configuration facilitates the dissemination of cancer cells through the lymphatic system. Lymph nodes serve as sentinels of the immune system and exhibit rich nutrient and growth factor contents.268 However, cancer cells use immune evasion mechanisms, such as the upregulation of MHC-I and PD-L1 expression, to evade immune surveillance and proliferate within lymph nodes.269
Peritoneal metastasis is a common occurrence in ovarian cancer, whereby cancer cells that have detached from the primary tumor surface are carried by the peritoneal fluid and adhere in large numbers to the peritoneal surface.270 The presence of a substantial number of adipocytes in the peritoneum has been associated with the promotion of tumor cell growth through the release of lipids, cytokines (such as IL-8), and upregulation of fatty acid-binding protein 4 expression.271
Targeting cancer metastasis
In oncology, the prevention or reversal of tumor metastasis is one of the most challenging and urgent clinical objectives. To address this challenge, scientists and clinicians have investigated the molecular mechanisms of tumor metastasis from a multitude of perspectives by directly targeting metastatic cancer cells. Crucially, cancer cells do not function in isolation but engage in a complex “symbiotic relationship” with their surrounding microenvironment. This underscores the need for targeting this environment as a pivotal therapeutic strategy. Treatment strategies for tumor metastasis are progressing toward greater precision, efficiency, and multi-target capabilities, offering promising avenues for advancing cancer care (Fig. 4) (Table 3).
Therapeutic strategies targeting cancer metastasis. These strategies range from directly and precisely attacking metastatic cancer cells to delicately modulating the intricate TME, and further encompass personalized treatment plans for specific organ metastases. Specifically, these strategies integrate chemotherapy, targeted therapy, immunotherapy, local therapy, and combined therapy to achieve precise elimination of cancer cells. Additionally, anti-angiogenic therapy, targeting the ECM and tumor-associated cells, and regulating tumor metabolic mechanisms indirectly impact the TME, ultimately providing a more precise therapeutic approach for cancer metastasis
Targeting metastatic cancer cells
Chemotherapy
Chemotherapy is the conventional approach to cancer treatment. This entails the utilization of drugs that disrupt the growth and division of cancer cells, ultimately leading to their eradication or proliferation inhibition. Among the many metastatic cancers, metastatic lung cancer is one of the solid tumors most responsive to chemotherapy.272 Platinum-based drugs combined with gemcitabine, paclitaxel, docetaxel, or vinorelbine represent primary therapeutic options for patients with metastatic lung cancer. Other agents, including docetaxel, erlotinib, and pemetrexed, have received clinical approval as second-line treatments.273 Oxaliplatin exhibits a broad spectrum of anticancer activity and was approved for the treatment of colorectal cancer. It enters cells through passive diffusion and active transport mechanisms. It undergoes non-enzymatic biotransformation with nucleophilic reagents such as glutathione (GSH), converting it into more reactive species. These reactive forms of oxaliplatin subsequently form covalent DNA adducts with DNA, which inhibit DNA synthesis and transcription, ultimately leading to cellular apoptosis.274
In cancer brain metastasis, the BBB presents a significant challenge for the diffusion of chemotherapeutic drugs into the brain. In addition to direct injection into the cerebral circulation, the use of drugs capable of traversing the BBB, such as the alkylating agent temozolomide, has emerged as the preferred strategy.275,276 Notably, the expression level of P-glycoprotein in blood vessels at metastatic brain sites is lower than that in normal vessels and primary brain tumors.277 This influences drug efflux and results in increased pharmacological concentrations of paclitaxel in metastatic brain tumors. This observation indicates that metastatic brain tumors may be more susceptible to chemotherapy than primary brain tumors.278
Despite the efficacy of chemotherapeutic agents against rapidly proliferating cancer cells, they confront numerous challenges in suppressing metastatic cancer cells, including the emergence of drug resistance. During the process of chemotherapy resistance, CSCs can persist in a dormant state for decades after initial treatment, evading elimination by chemotherapy and retaining their self-renewal and differentiation properties, which are critical factors underlying recurrence and therapeutic resistance.279 Recent studies have indicated that CSCs become enriched following chemotherapy or radiotherapy, suggesting that treatment may induce reprogramming or dedifferentiation of normal cancer cells into those with enhanced CSC characteristics.280,281 During chemotherapy, CSCs in various cancers express abundant ATP-binding cassette transporters, which efflux chemotherapeutic drugs, leading to drug resistance.282,283,284 Additionally, the tumor microenvironment fosters the growth and proliferation of CSCs, contributing to metastasis and drug resistance.285 In colorectal cancer cells, exosomes secreted by CAFs trigger CSC activation, resulting in resistance to 5-fluorouracil.286 Trastuzumab resistance has been demonstrated to be mediated by an IL-6 inflammatory loop in HER2+ breast cancer CSCs.287
Targeted therapy
Targeted therapy is of paramount significance in the management of metastatic cancer cells, given that these cells often escape the confines of the primary tumor and disseminate through the bloodstream or lymphatic system to distant sites within the body. Although traditional chemotherapeutics can inhibit cancer cell growth to a certain extent, they often lack specificity and inadvertently damage healthy cells. Targeted therapies are designed to interfere with specific molecular pathways associated with cancer cell growth, division, metastasis, and viability. Targeted therapies can enhance treatment efficacy while minimizing adverse effects by recognizing and acting on these unique molecular targets exclusive to cancer cells.
These agents typically target receptors on cancer cell surfaces, signaling pathways, or intracellular enzymes. In patients with breast cancer and brain metastasis, HER2-targeted therapies, including trastuzumab, pertuzumab, neratinib, tucatinib, and pyrotinib, have demonstrated efficacy in the treatment of brain cancer.288,289,290,291,292,293 In patients with HER2-positive breast cancer who have undergone treatment with anthracyclines or taxanes and have developed brain metastasis, the combination of pyrotinib with capecitabine has been demonstrated to yield a superior median progression-free survival (PFS) of 11.1 months compared with 4.1 months in the placebo arm.294 Moreover, the combination of tucatinib with trastuzumab and capecitabine demonstrated enhanced therapeutic efficacy, with augmented rates of central nervous system responses and prolonged PFS.295 In patients with HER2-negative, hormone receptor-positive breast cancer and brain metastasis, the CDK4/6 inhibitor abemaciclib demonstrated an intracranial clinical benefit rate of 24%, indicating its potential for further investigation.296 Inhibition of EGFR represents a promising therapeutic strategy for metastatic lung cancer.297
EGFR-specific tyrosine kinase inhibitors, including erlotinib, cetuximab, and gefitinib, reversibly inhibit EGFR by blocking its intracellular ATP-binding domain, thereby effectively treating metastatic lung cancer with EGFR mutations.298,299,300 Furthermore, ALK inhibitors such as crizotinib, ceritinib, and alectinib have demonstrated efficacy in treating brain metastasis in non-small cell lung cancer (NSCLC).301 Dasatinib, a Src kinase inhibitor that impedes cancer cell growth, is another promising antagonist.302
Inhibition of the PI3K-Akt-mTOR pathway may be a promising therapeutic option for approximately 70% of breast cancer patients with bone metastasis.303 The ability of these agents to effectively cross the BBB remains a significant challenge. However, GDC-0084 and GDC-0068 have demonstrated the capacity to overcome this hurdle by inhibiting the PI3K-Akt-mTOR pathway and exhibiting potential to treat breast cancer brain metastasis.303,304
Additionally, dovitinib, an oral FGFR inhibitor, demonstrated moderate antitumor activity in patients with mCRPC, with controllable toxicity. Patients who do not undergo chemotherapy may benefit more from dovitinib than from docetaxel.305 Tanezumab, a NGF inhibitor, is commonly used to treat bone and joint arthritis and to alleviate chronic lower back pain. In phase III clinical trial for severe bone metastasis cancer pain, tanezumab demonstrated a more significant improvement in pain site intensity than opioid drugs at 8 weeks.306 Enfortumab vedotin, an antibody-drug conjugate targeting nectin-4, has been demonstrated to be safe and effective in treating metastatic urothelial carcinoma, with an objective response rate of 44%.307
Immunotherapy
Immunotherapy, which is directed toward metastatic cancer cells, represents a revolutionary advancement in cancer treatment. This is achieved by leveraging and enhancing the capacity of a patient’s immune system to accurately identify and eradicate cancer cells that have disseminated to other regions of the body. This approach overcomes the limitations of conventional therapies and offers a promising avenue for patients with metastatic cancer who are unresponsive or have developed resistance to traditional chemotherapy, radiotherapy, and similar treatments.
Immunotherapy exerts its effects through a multitude of mechanisms, with immune checkpoint inhibitors garnering particular attention. These agents remove the immunosuppressive “brakes” imposed by cancer cells on the immune system, such as the PD-1/PD-L1 pathway, thereby unleashing the full potential of immune cells like T cells to recognize and attack cancer cells more efficiently.308,309 Notably, the PD-1/PD-L1 pathway serves as a pivotal immune regulatory axis, with drugs such as pembrolizumab and nivolumab targeting PD-1. These drugs effectively manage brain metastasis in patients with melanoma and NSCLC and alleviate central nervous system symptoms.310,311 Socazolimab (ZKAB001), a PD-L1-specific monoclonal antibody, has been demonstrated to be safe in nonprogressive localized high-grade osteosarcoma and beneficial for PD-L1-positive and microsatellite instability-high subgroups of patients.312 However, not all anti-PD-1 monoclonal antibodies (mAbs) exhibit therapeutic effects. Pembrolizumab has been shown to improve distant metastasis-free survival in patients with stage IIB and IIC melanoma after surgical resection.313 However, the same effect has not been observed in patients with advanced osteosarcoma.314 Following anti-angiogenic therapy for metastatic clear cell RCC, patients with bone metastasis treated with nivolumab exhibited a poorer prognosis with lower PFS and objective response rates.315 Moreover, ipilimumab, which targets the CTLA-4 checkpoint, when combined with nivolumab in the treatment of melanoma brain metastasis, significantly enhances both the response rates within the central nervous system and objective response rates within the intracranial region.316
Cellular therapies such as CAR-T cell therapy exhibit considerable promise. For example, Priceman et al. optimized the 4-1BB co-stimulatory domain in HER2-CAR-T cells. Compared to CD28 co-stimulation, this modification demonstrated a reduction in T-cell exhaustion phenotypes, enhanced proliferation, and potent antitumor activity in breast cancer brain metastasis models.317
Local therapy
In addition to the specific targeting of tumor cells, therapeutic interventions for metastatic tumors include surgical resection, and minimally invasive and noninvasive modalities.
In the context of oligometastatic disease, surgical procedures, such as lung resection,318 hepatectomy for liver metastasis,319 and craniotomy for brain metastasis,273 have demonstrated that current surgical techniques remain viable options for treating metastatic disease. However, given the complexity of metastatic behavior and the potential involvement of multiple organ systems, minimally invasive or noninvasive approaches have emerged as the primary choice among healthcare professionals.320
External beam radiation therapy (EBRT) uses radiation beams that traverse normal tissues and adjacent organs to target specific pathological sites. It encompasses a variety of techniques, including radiofrequency ablation (RFA), stereotactic body radiation therapy (SBRT), three-dimensional conformal radiation therapy (3D-CRT), and hypofractionated stereotactic ablative radiotherapy (HSRT).321 The advent of image-guided technology has led to a surge in the popularity of RFA for hepatic metastasis. This approach has demonstrated efficacy in controlling tumors with lesion sizes < 3 cm and ablation margins > 5 mm, with long-term local control rates > 90%.322 Similarly, in a study of over 1,000 patients with lung metastasis treated with RFA, the four-year local control rate was 89%, with superior outcomes observed in smaller tumors.323 Stereotactic ablative body radiation therapy (SABR) is an effective treatment for a wide range of lesions, including lung, liver, and bone metastasis, as evidenced by robust data from various clinical settings.324,325 HSRT improves local control through fractionated high-dose regimens, resulting in enhanced 5- and 10-year overall survival (OS) rates in breast cancer patients with oligometastasis. The number of lesions may influence the risk of recurrence, necessitating further research to identify patients with breast cancer who would potentially benefit from metastasis-directed radiotherapy.326 Notably, radiation therapy alters the blood cytokine profile, thereby mediating analgesia in bone metastasis through the modulation of cytokine production. Several factors, including MIP-1δ, MCP-2, TIMP-1, RANTES, IGFBP3, and TNF-α, have been observed to undergo significant changes both before and after radiotherapy. These changes may play a role in the mechanisms underlying pain related to cancer metastasis.327
Magnetic resonance-guided focused ultrasound (MRgFUS, also known as MRgHIFU) uses focused ultrasound beams to generate thermal, mechanical, and cavitation effects within soft tissues, thereby rapidly heating the target area to achieve tissue coagulation and necrosis.328,329 Extensive research has been conducted on MRgFUS to treat a range of conditions, including uterine fibroids, osteoid osteoma, essential tremors, and cancers of the breast, prostate, liver, pancreas, and bone metastasis.330,331 The therapeutic mechanism of MRgHIFU in cancerous bone metastasis is primarily periosteal nerve ablation.332,333 A prospective, open-label, non-randomized Phase II study comparing MRgHIFU and EBRT for bone metastasis revealed comparable overall response rates and quality of life scores at one month, accompanied by a diminished incidence of adverse events in the MRgHIFU cohort.331
Combined therapy
Comprehensive therapy incorporates the advantages of various treatment modalities, addressing the processes of cancer cell proliferation and invasion holistically and synergistically. This approach emphasizes not only the direct eradication of cancer cells but also the restoration and enhancement of the patient’s immune system function and modulation of the TME, with the ultimate goal of achieving comprehensive control over metastatic cancer.
Comprehensive therapy typically encompasses a combination of targeted therapy, immunotherapy, chemotherapy, and radiotherapy, among other modalities. A clinical trial (RTOG 0320) observed an increased risk of cytotoxicity when whole-brain radiation therapy (WBRT) was combined with EGFR tyrosine kinase inhibitors (TKIs), specifically erlotinib or temozolomide. Nevertheless, other studies have demonstrated the efficacy and safety of EGFR-TKIs with WBRT for the treatment of brain metastasis from advanced lung cancer.334 Compared with erlotinib monotherapy, patients who received stereotactic radiosurgery (SRS) or WBRT demonstrated similar OS rates but a longer time to intracranial progression.273,335 Androgen deprivation therapy (ADT) is frequently used to improve patient survival in the management of metastatic prostate cancer.336 In a comparative study, Kyriakopoulos et al. examined the outcomes of chemotherapy with hormonal therapy versus ADT in patients with extensive-disease prostate cancer. The researchers defined high-volume disease as the presence of visceral metastasis and/or ≥4 bone metastasis with at least one outside the spine and pelvis. The findings revealed that docetaxel prolonged OS in patients with high-volume disease, but not in those with low-volume disease.336 In a study of patients with metastatic castration-resistant prostate cancer (mCRPC), Heery et al. investigated the efficacy of a therapeutic vaccine, PSA-TRICOM, combined with the radiopharmaceutical, samarium-153-ethylenediamine tetramethylenephosphonate (Sm-153-EDTMP). The study demonstrated that combination therapy resulted in improved PFS, as well as a trend toward enhanced PSA decline and PSA-specific T-cell responses, compared to Sm-153-EDTMP alone.337
Targeting metastatic tumor microenvironment
Antiangiogenic therapy
Antiangiogenic therapy represents a precise therapeutic strategy that targets the vital processes of angiogenesis during tumor growth and metastasis. Angiogenesis is a pivotal factor in tumor progression in the metastatic TME. Cancer cells secrete a multitude of angiogenic factors (such as VEGF and FGF), which prompt the proliferation and migration of adjacent vascular endothelial cells. These cells form novel vascular networks, thereby supplying crucial nutrients and oxygen to tumors.338 By inhibiting the activity of angiogenic factors within the TME, this therapy impedes the formation and maturation of new blood vessels, thereby depriving tumor cells of essential nutrients and oxygen, which ultimately inhibit their growth, invasion, and metastasis.339
Antiangiogenic therapy uses specific inhibitors, such as bevacizumab and anlotinib, to interrupt the actions of angiogenic factors or to directly act on vascular endothelial cells, disrupting their proliferation and migration and suppressing tumor angiogenesis. Early studies in metastatic CRC have demonstrated improved median OS (20.3 months with bevacizumab vs. 15.6 months without; P < 0.001) and PFS; 10.6 vs. 6.2 months; P < 0.001) for the bevacizumab arm compared to traditional triplet regimens including 5-fluorouracil (5-FU), irinotecan, and leucovorin calcium (5-FU/LV/Irinotecan). In contrast, randomized clinical trials and long-term follow-up have demonstrated that bevacizumab does not confer a survival benefit in patients with metastatic breast cancer and is associated with a significant increase in severe adverse effects. Consequently, the FDA withdrew its approval for this indication, although Medicare and Medicaid Services continue to support the use of bevacizumab as a first-line treatment for metastatic breast cancer.340
Aflibercept, an engineered VEGF receptor, has been approved for Phase III clinical trials of metastatic NSCLC and pancreatic CRC.273 TAS-115 specifically targets VEGFR2 and completely inhibits both MET and tumor progression by blocking angiogenesis.341 Ramucirumab, a human IgG1 antibody that binds to both HER2 and VEGFR, offers limited benefits when combined with docetaxel in the treatment of certain advanced digestive system malignancies.342 However, it also provides a promising avenue for antibody-based therapies.343 Additionally, Taquimmod specifically targets S100A9, influencing the tumor-infiltrating myeloid cells in such a way that they undergo a phenotypic transformation. This change converts them from pro-angiogenic and immunosuppressive M2-like TAMs into pro-inflammatory M1-like macrophages, which have immunomodulatory, anti-angiogenic and metastatic inhibitory effects.344
Targeting the ECM
The ECM, a critical element of the TME, provides physical support to tumor cells and regulates their biological behavior through an array of growth factors, proteolytic enzymes, cytokines, and other mediators.345 During the process of tumor metastasis, remodeling and degradation of the ECM facilitate the invasion and migration of tumor cells, thereby promoting disease progression.345 Consequently, therapeutic strategies that target the ECM have been designed to impede tumor metastasis by intervening in this complex process.
Extracellular heat shock proteins (HSPs) play a pivotal role in ECM remodeling and augmentation of MMP activity, which is crucial for tumor metastasis.346 AUY922, an inhibitor of HSP90, exemplifies this approach by reducing fibronectin secretion into the ECM and impeding prostate cancer invasion.347
Lysyl oxidase (LOX) is another crucial enzyme involved in ECM remodeling. LOX inhibitors have been the subject of extensive preclinical research.348 Among these, β-aminopropionitrile (BAPN) and the aminomethyl thiophene-based inhibitor CCT365623 effectively suppress the migration and invasion of breast cancer cells.349,350 Furthermore, PAT-1251/GB2064, a highly selective LOXL2 inhibitor, has shown promise in reducing collagen accumulation and inhibiting tumor growth in preclinical settings.351 CCT365623 disrupts HTRA1 multimerization, activates TGF-β1 signaling, suppresses MATN2 expression, inhibits EGFR surface retention, and attenuates EGFR signaling. Ultimately, akin to BAPN or LOX gene ablation, CCT365623 downregulates MATN2, impeding EGFR plasma membrane localization in tumors and inhibiting tumor growth and metastasis in mice.352
Integrins, which are highly expressed in solid tumors, serve as signaling hubs that transmit messages from the interior to the exterior of the cell. This process modulates cell-ECM interactions, which alter cell adhesion, migration, and ECM properties.63 Notably, integrin αvβ3 is more prevalent in metastatic tumors than in primary pancreatic and breast cancers.353 This enhances tumor migration and metastasis by recruiting Src kinase. Selective expression of integrin αvβ3 at metastatic sites offers a promising avenue for enhancing the delivery of chemotherapeutic agents to bone-resident breast cancer cells with greater precision.354
Given the pervasive role of the ECM across various tumor types, therapies targeting the ECM have broad application prospects. However, it is essential to recognize that the ECM is a highly complex and dynamic system, and its composition and structure vary significantly among tumor types, stages, and even individuals. Therefore, precision medicine requires careful consideration of these differences when developing ECM-targeted therapeutics.
Targeting cancer-associated cells
Targeting cancer-associated cells within the TME, which encompasses CAFs, represents a promising approach for impeding tumor metastasis and progression. This can be achieved by modulating the activity, function, and interactions of these cells with tumor cells.
CCL5, produced by CD4 and CD8 T lymphocytes in liver metastasis of colorectal cancer, elicits protumorigenic effects via CCR5 signaling. This fosters monocyte recruitment and M2 polarization, enhances CAF expansion, and potentiates the TGF-β-mediated killing of CD8 T cells by Tregs. Notably, the CCR5 antagonist maraviroc, which inhibits CCR5, has been linked to the repolarization of tumor-associated macrophages, emerging as a promising avenue for further scientific and clinical exploration.355
Given the established correlation between the crosstalk of metastatic cancer cells and their microenvironment in the progression of cancer metastasis, there is growing emphasis on developing therapeutic strategies that target this microenvironment or the crosstalk itself. For example, silibinin impedes the growth of brain metastasis by targeting STAT3 in tumor-associated astrocytes, thereby reducing their crosstalk with cancer cells and microglia.356,357
Therapeutic strategies targeting CAFs offer notable advantages, as they not only directly impact crucial cellular components of the TME but also indirectly inhibit tumor growth and metastasis by influencing the entire microenvironment. Furthermore, given the critical roles that CAFs play in a multitude of tumor types, this approach offers significant potential for broad-spectrum applications.
Targeting metabolic reprogramming
Metabolic reprogramming of tumor cells is characterized by aberrant uptake and utilization of nutrients, such as glucose and glutamine, as well as abnormal accumulation and excretion of metabolic byproducts. This phenomenon is the hallmark of malignant transformation.358 This reprogramming satisfies the energetic demands of rapid tumor cell proliferation and influences their ability to invade, migrate, and evade immune surveillance by altering metabolite composition within the microenvironment.359 Consequently, therapeutic strategies targeting metabolic reprogramming seek to impede tumor metastasis and progression by modulating glycolysis, glutaminolysis, one-carbon metabolism, and lipid metabolism.
The proliferation and metastasis of tumor cells are closely associated with aberrant glycolysis.360 The glycolytic inhibitor 2-deoxy-D-glucose (2-DG) has been demonstrated to reduce the invasiveness of 5-FU-resistant CRC cells by downregulating glycolytic enzyme expression, while impairing the secretion of EMT-related cytokines and inactivating integrins, MMP-10, and MMP-17.361
Tumor cells utilize glutamine as a fuel source for the tricarboxylic acid (TCA) cycle, and targeting intermediates of the TCA cycle involved in glutaminolysis has proven to be an effective anticancer approach.362 New specific glutaminase (GLS) inhibitors, including CB-839 selenadiazole-derivatives CPD-20 and CPD-23, have been observed to demonstrate enhanced uptake in tumor cells and exhibit higher anticancer activity against CRC cells. CPD-20 and CPD-23 exhibit improved cellular and tumor accumulation, enhanced GLS inhibition, elevated ROS induction, and a better effect on eliminating cancer cells.363 Additionally, when CB-839 was combined with erlotinib in a dual therapy for mouse NSCLC xenografts, rapid tumor regression was observed in vivo. This combination simultaneously hindered cancer cells’ utilization of Gln and Glc, disrupted redox homeostasis, and induced autophagy to combat cancer.364 Likewise, CB-839 demonstrated notable antitumor activity in two xenograft models of TNBC cell lines, both as a monotherapy and in conjunction with paclitaxel.365
Abnormalities in lipid metabolism have been linked to several tumorigenic processes, including cancer progression and metastasis.366 Luteolin, functioning as a fatty acid synthase inhibitor, obstructs the de novo biosynthesis of long-chain fatty acids and exerts its anticancer effects in CRC by modulating a multitude of tumor signaling pathways, including IGF-1, Keap1-Nrf2-ARE, and Wnt-β-catenin.367
One-carbon metabolism is a pathway that generates one-carbon units that are essential for nucleotide synthesis, methylation, and NADH/NADPH production. These processes sustain a high proliferation rate of tumor cells.368 Methotrexate and chemotherapeutic agents that target key enzymes involved in one-carbon metabolism, such as dihydrofolate reductase and thymidylate synthase, are clinically utilized to treat a range of cancers, including CRC.358
Targeting organ-specific metastasis
Targeting bone metastasis
A multifaceted approach is essential for the effective management of bone metastasis, encompassing the use of monoclonal antibodies, TKIs, hormone-related medications, bisphosphonates, small molecules, and radiopharmaceuticals.
In the context of targeted therapy for bone metastasis, denosumab, a monoclonal antibody, has demonstrated particular efficacy by specifically binding to and inhibiting RANKL, thereby impeding osteoclast-mediated bone resorption. It is the standard treatment for cancer patients with bone metastasis.369,370,371,372 Furthermore, biosimilars of denosumab, such as HS-20090 and QL1206, provide novel therapeutic options for patients with bone metastasis.373,374 Abituzumab, a pan-αv integrin inhibitor, has demonstrated promising PFS and a low cumulative incidence of bone lesion progression in asymptomatic or patients with mildly symptomatic metastatic castration-resistant prostate cancer (mCRPC). These findings highlight the specific activity of abituzumab in prostate cancer-related bone diseases and warrant further investigation.375
Cabozantinib, an oral TKI, has been demonstrated to reduce osteoblast proliferation and enhance osteoblast activation. This is achieved by inhibiting the MET and VEGFR-2 rearrangements that occur during the transduction of osteoblasts.376,377 In addition, it affects tumor cells and the TME.378 Nevertheless, the clinical efficacy of other TKIs remains to be elucidated.379,380
ADT is a fundamental component of prostate cancer management, particularly in metastatic and advanced stages. Enzalutamide, an oral androgen receptor antagonist, effectively halts receptor nuclear translocation upon binding, thereby disrupting androgen signaling.381 It markedly enhances outcomes for bone disease in both low- and high-volume settings382 and displays a favorable efficacy and safety profile in patients aged 75 years and above.383 However, it should be noted that patients with prostate cancer undergoing ADT may experience early bone loss (within three months),384 thereby increasing the risk of osteoporotic fractures.385
Bisphosphonates represent another essential class of bone-modifying agents (BMAs). These agents are analogs of pyrophosphate that bind to hydroxyapatite binding sites on bone, thereby inhibiting osteoclast-mediated bone resorption. In addition, they regulate osteoclast function by reducing the development, recruitment, and promotion of osteoclast progenitor cells as well as promoting osteoclast apoptosis.386,387 Bisphosphonates have been approved for the prevention and treatment of SREs associated with solid tumor bone metastasis.386 Nevertheless, long-term use of zoledronic acid in patients with bone metastasis requires careful consideration.388
Advances in our understanding of the mechanisms underlying bone metastasis have led to the identification of several key molecules that may represent promising therapeutic targets. Elevated expression of phosphatase of regenerating liver-3 (PRL-3) in tumor tissues has been demonstrated to contribute to metastasis. A Phase 1 clinical trial demonstrated the safety and tolerability of PRL3-zumab in advanced tumors, thereby encouraging further advancement of PRL-3-targeted therapies.389 Inhibition of the poly(ADP-ribose) polymerase (PARP) isoforms (PARP-1, -2, -3) has been demonstrated to reduce tumor growth in vitro and in vivo in human cancer models. In Phase III trials, olaparib, a PARP inhibitor, demonstrated significantly prolonged radiographic PFS in patients with mCRPC compared to enzalutamide or abiraterone.390 Tumor vascular receptors represent attractive targets for ligand-directed drug discovery and development. BMTP-11, developed by Pasqualini et al., targets bone marrow tumors by binding to the ligand-binding motif of interleukin-11 receptor α (IL-11Rα) in the human tumor vasculature, thereby inducing apoptosis.391
Radioisotopes release α, β, γ, and other rays, which cause ionization, generation of free radicals, DNA strand breaks, and ultimately, cancer cell apoptosis. Clinical trials on bone metastasis have utilized radium-223, Sn-117, rhenium-188, lutetium-177, and samarium-153 to demonstrate antitumor effects and pain relief. Radium-223 is primarily used in clinical trials to treat bone metastasis and exhibits antitumor and analgesic effects.392,393 In clinical practice, radium-223 reduces the length of hospital stay, improves survival without symptomatic skeletal events (SSEs), and enhances health-related quality of life (QOL) in patients with mCRPC.394 Analyses of the EuroQoL 5D and Functional Assessment of Cancer Therapy-Prostate instruments revealed that the survival benefits associated with radium-223 administration resulted in notable improvements in QOL. These benefits encompass an increased proportion of patients with castration-resistant prostate cancer experiencing enhanced QOL and a slower rate of decline in QOL.395 Sn-117 meta-diethylenetriaminepentaacetic acid (DTPA) (Sn-117m-DTPA) is a radioactive isotope of tin developed as a radioactive drug.396 Compared to radium-223, Sn-117m-DTPA appears to have lower bone marrow toxicity. A phase II clinical trial is underway to validate the efficacy of Sn-117m-DTPA for pain relief and antitumor activity in patients with mCRPC.397 Rhenium-188 was obtained from the 188W/188Re generator and involved fewer radiation protection issues than 131I did. 188Re-HEDP is safe and well tolerated, is effective in relieving pain in patients with painful bone metastasis from lung cancer, and improves the overall quality of life.398 Samarium-153 is a highly bone-seeking radioactive drug, similar to bisphosphonates, linked to ethylenediamine tetramethylene phosphonic acid (EDTMP).399 In advanced stages, requiring palliative treatment for bone metastasis, intravenous injection of 153Sm-EDTMP represents a potential option for managing cancer pain in patients who are intolerant or resistant to drug therapy. Patients receiving samarium therapy require fewer or no analgesics and are more cost-effective.400 In bone pain caused by multifocal myeloma or mixed lytic metastatic lesions, 153Sm-EDTMP and 177Lu-EDTMP demonstrated high affinity for lesions. As measured using the Edmonton Symptom Assessment System, ECOG performance status, and a numeric rating scale, 153Sm-EDTMP and 177Lu-EDTMP have been demonstrated to be safe and effective radioactive drugs for the relief of cancer pain caused by metastatic lesions with myeloma or mixed lytic characteristics.401
EBRT enhances prognosis and reduces SREs in patients with asymptomatic bone metastasis. The Phase II trial conducted by Gillespie et al. demonstrated the efficacy of EBRT in reducing the risk of SREs, and hospitalizations, and improving OS in patients with high-risk, asymptomatic bone metastasis.321 EBRT is recognized as the standard treatment for alleviating pain associated with symptomatic bone metastasis, offering an effective and commonly adopted approach.321 When formulating treatment plans, it is essential to consider the unique circumstances and characteristics of each patient. Further research and trials must validate the therapeutic outcomes and provide more guidance for clinical practice.
Targeting brain metastasis
Whole-brain radiotherapy (WBRT), surgical intervention, and stereotactic radiosurgery are the preferred options for the localized treatment of brain metastasis.402 A review of past studies has shown that surgical intervention can effectively delay recurrence in certain patients with brain metastasis and thereby improve their median survival rates.403,404 The combination of surgery and WBRT has been demonstrated to significantly prolong the time to recurrence and markedly improve median survival, thereby building upon the benefits of WBRT alone.405,406
In addition to localized therapies, systemic treatments such as chemotherapy and targeted therapy are also important in the management of brain metastasis. However, traditional cytotoxic chemotherapy has exhibited limited efficacy in the treatment of brain metastasis owing to the BBB.407 Targeted therapies designed to target specific mutations in lung cancer, breast cancer, and melanoma have emerged as important tools in the treatment of brain metastasis.
TKIs, such as erlotinib, gefitinib, and osimertinib, which target EGFR mutations in NSCLC brain metastasis, have demonstrated response rates exceeding 50%, effectively extending median survival.408,409,410 In cases of brain metastasis associated with ALK rearrangement in NSCLC, alectinib, ceritinib, and brigatinib, which demonstrate effective penetration of the BBB, show promise and warrant further investigation.411,412,413
Among patients with breast cancer and brain metastasis, those with HER2-positive status are more likely to have a higher incidence of brain involvement. Targeted therapies tailored to the molecular subtypes of primary breast cancers have been developed. In HER2-positive brain metastasis, a range of anti-HER2 agents has been the subject of clinical investigation, including monoclonal antibodies (e.g., trastuzumab and pertuzumab), antibody-drug conjugates (e.g., T-DM1), and small-molecule TKIs (e.g., lapatinib, neratinib, tucatinib, and pyrotinib).414 Preclinical trials demonstrated the limited activity of lapatinib as a single agent but exhibited good performance in combination with capecitabine, achieving over 65% response rates in radiotherapy-naive patients and 20% in radioresistant patients.415,416,417 In a phase 3 study involving 991 patients who had undergone treatment for HER2-positive brain metastasis, trastuzumab demonstrated superior efficacy and safety outcomes compared to capecitabine plus lapatinib.288 The combination of HER2-targeted inhibitors with cytotoxic chemotherapy demonstrated some efficacy in clinical trials; however, concerns have been raised regarding the response instability and frequency of adverse events. Thus, further efforts must be made to optimize efficacy and reduce toxicity.418
In patients with melanoma and brain metastasis, mutations in BRAF and NRAS indicate potential for targeted therapeutic approaches.419 Early clinical studies have demonstrated the efficacy and tolerability of dabrafenib and trametinib in patients with BRAF-mutated brain metastasis, with approximately one-third of the patients with unresectable or metastatic melanoma experiencing long-term benefits.420,421 Moreover, MEK inhibitors have been combined with BRAF inhibitors, including encorafenib plus binimetinib and vemurafenib plus cobimetinib, and have demonstrated effective clinical responses.422,423
Targeting liver metastasis
Despite advancements in chemotherapy that have led to improved survival rates, only 10–20% of patients with liver metastasis are eligible for resection. Radical surgery remains the standard treatment for this patient population.424 The feasibility of liver resection is clinically assessed based on patient tolerance, extent of the tumor, and residual liver function, often with systemic chemotherapy. This enables some patients to develop resectable tumors following the initial drug therapy.425,426 Surgical resection has been demonstrated to confer substantial survival benefits, with 5-year OS rates ranging from 20 to 50%.427,428 Conversely, the role of radiotherapy in the management of liver tumors is limited by concerns regarding local control and toxicity.429 SBRT is a precise and effective means of delivering high-dose radiation to liver metastasis. This approach effectively induces DNA damage and fragmentation, resulting in excellent local control with minimal side effects.429
It is estimated that over 50% of patients diagnosed with CRC will eventually develop metastasis, with the liver being the most common site of recurrence.430 In addition to downstaging tumors and converting unresectable to resectable cases,431 the use of 5-FU and leucovorin significantly enhances the median survival of patients with liver metastasis.432 The combination of irinotecan, fluorouracil, and leucovorin significantly prolongs PFS, improve response rates and extend OS.433 Moreover, the incorporation of oxaliplatin into fluoropyrimidine regimens markedly enhanced pathological complete response rates and reduced perioperative metastasis.434
Antiangiogenic agents are frequently used in targeted therapy for liver metastasis, often in combination with chemotherapy, to enhance efficacy. The incorporation of bevacizumab into first-line chemotherapy for metastatic CRC significantly increased the median PFS from 8.0 to 9.4 months.435 Furthermore, cetuximab, when combined with other agents, is well tolerated, reduces the risk of disease progression, and significantly increases the overall response rate (61% vs. 37%) compared to fluorouracil/leucovorin plus oxaliplatin.436
In addition to antiangiogenic therapies, immunotherapy has emerged as a promising treatment option. In patients with CRC and liver metastasis, responses to immune checkpoint blockade depend on the status of DNA microsatellite instability (MSI) or mismatch repair.437,438 In patients with high MSI (MSI-H) or deficient mismatch repair, nivolumab, a PD-1 inhibitor, achieved an objective response rate of 31.1%. This demonstrates durable responses and disease control and may represent a new treatment paradigm for these patients.439 The PD-L1 inhibitor atezolizumab, when used in combination with fluorouracil, leucovorin, oxaliplatin, irinotecan, and bevacizumab, has been demonstrated to be safe and effective in improving PFS.440
Targeting lung metastasis
The management of lung metastasis requires a multifaceted approach encompassing surgical, chemotherapeutic, radiotherapeutic, immunotherapeutic, and targeted therapeutic modalities, which are tailored to the stage and characteristics of the tumor.189 Specifically, surgical and radiotherapy procedures are primarily utilized to treat limited metastatic lesions, whereas chemotherapy, immunotherapy, and targeted therapy are more effective in addressing widespread systemic metastasis. However, chemotherapy is associated with considerable adverse effects, and certain agents, including paclitaxel441 and gemcitabine,442 may unintentionally contribute to the development of lung metastasis, necessitating caution among clinicians. Consequently, targeted therapy and immunotherapy, which offer substantial precision and reduced toxicity profiles, have emerged as promising avenues of research for the management of lung metastasis.
Aberrant gene expression is a key driver of tumor progression, treatment resistance, and lung metastasis.443 Notably, these dysregulated genes also represent novel therapeutic targets. Lung metastasis is a prevalent phenomenon in TNBC, and the response gene to complement 32 protein (RGCC) has been identified as a key driver of TNBC lung metastasis. This is achieved through the enhancement of PLK1 kinase activity, which drives AMPKα2 phosphorylation and subsequent downstream signaling. Notably, the combination of RGCC targeting with paclitaxel/carboplatin effectively inhibits TNBC lung metastasis in mice, indicating the potential of RGCC as a target for treatment strategies.444
Within the ECM, aberrant expression of collagen type X (COL10A1) in TNBC may activate the PI3K/AKT pathway, stimulating tumor-associated angiogenesis, and promoting TNBC growth and lung metastasis.445,446 Therefore, the downregulation of COL10A1 represents a potential therapeutic strategy. Tirapazamine, a pan-PI3K inhibitor, inhibits the development of lung metastasis in TNBC by targeting PI3K/Akt/mTOR complexes 1 and 2 (mTORC1/2), which reduces the expression of proteins associated with EMT.447 Similarly, plant-derived isoflavone ononin has been demonstrated to inhibit the expression of EMT markers and matrix metalloproteinases, thereby reversing the EMT process and attenuating the growth of TNBC tumors and the formation of lung metastasis.448
Targeting primary tumor cells can indirectly mitigate the progression of lung metastasis. To suppress nasopharyngeal carcinoma (NPC) lung metastasis, strategies include knockdown of aryl hydrocarbon receptor nuclear translocator-like 2 (ARNTL2) or targeting the PLUNC-NLRP3 inflammasome axis to promote NLRP3 ubiquitination and degradation, as well as inhibition of the AMOTL2/LATS/YAP pathway, which reduces NPC cell migration and invasion.449,450 In chondrosarcoma lung metastasis, nicotinamide phosphoribosyltransferase promotes lysyl oxidase (LOX) production via c-Src and Akt signaling, thereby enhancing LOX-dependent chondrosarcoma cell migration and invasion.451 In a study by Wang et al., inhibition of the c-MYC/Nrf2 pathway hindered the migration and invasion of CRC cells, thereby suppressing lung metastasis.452 In the context of osteosarcoma lung metastasis, the SDC4-TGF-β signaling feedback loop and Wnt/β-catenin pathway represent promising avenues for therapeutic intervention.453,454 Moreover, nerve growth factor (NGF) upregulates matrix metalloproteinase-2 (MMP-2) via the MEK/ERK pathway, thereby enhancing osteosarcoma cell migration and invasion.455 Larorectinib, a tropomyosin receptor kinase inhibitor, has been demonstrated to potently inhibit the effect of NGF on lung metastasis, thereby emerging as a potential therapeutic candidate for osteosarcoma lung metastasis.455
Targeting lymphatic metastasis
Lymph node metastasis (LNM) is a common feature of most solid malignancies and serves as a crucial basis for disease staging and prognosis. Furthermore, colonization of tumor cells within lymph nodes results in tumor immune tolerance, which facilitates distant metastasis and disease progression.269 Consequently, addressing LNM is of paramount significance for enhancing the prognosis of patients with cancer.
In the context of LNM, lymph node dissection has two principal functions. First, it prevents recurrence by eradicating tumor lesions in tumor-draining lymph nodes (tdlns). Second, it provides precise information for the staging, diagnosis, treatment, and prognostic prediction of patients with cancer.265,456 These effects positively influence tumor recurrence, metastasis, and survival. Radiotherapy, a crucial treatment modality for low-metastatic tumors, has markedly benefited patients with LNM. A large cohort study by Cavano et al. demonstrated that 90% of patients with LNM underwent SBRT in a safe and well-tolerated manner, resulting in high local control and survival rates.457 Among patients with nasopharyngeal carcinoma and parotid LNM, those who received intensity-modulated radiotherapy to the parotid lymph nodes exhibited superior survival outcomes compared to those with preserved parotid lymph nodes.458 Nevertheless, it is advisable that patients with cancer undergoing immunotherapy exercise caution when contemplating surgical or radiotherapeutic intervention, as lymph node resection has the potential to disrupt host immune structures and functions, thereby attenuating antitumor immune responses and potentially rendering immunotherapy ineffective.459,460 Furthermore, local treatments such as surgery and radiotherapy are frequently inadequate for addressing occult LNM. Therefore, it is imperative to investigate the potential of immunotherapeutic or targeted therapies for lymph nodes to manage LNM more effectively.
Lymphangiogenesis plays a pivotal role in facilitating tumor cell dissemination to the lymph nodes,265 rendering it a promising therapeutic strategy for LNM. The activated C kinase 1 receptor has been demonstrated to promote lymphangiogenesis in vivo through galectin-1-dependent mechanisms461 and activation of the glycolytic AKT/mTOR signaling pathway.462 This consequently facilitates the development of cervical cancer LNM. In addition, targeting enzymes involved in specific lipid metabolic pathways represents a promising avenue for the development of novel therapeutic strategies to treat cervical cancer LNM.463 In a recent study, Mei et al. demonstrated that 7-dehydrocholesterol reductase (DHCR7) plays a crucial role in promoting LNM by activating the KANK4/PI3K/AKT signaling pathway and enhancing VEGF-C secretion. These findings further validated that DHCR7 is a novel potential therapeutic target.464 Sterol O-acyltransferase 1 (SOAT1), which is overexpressed in gastric cancer, promotes lipid synthesis, induces lymphangiogenesis, and promotes gastric cancer LNM by upregulating VEGF-C expression. Therefore, SOAT1 could serve as a potential therapeutic target.465 Specific non-coding RNAs may emerge as potential targets to inhibit lymphangiogenesis, which could prove to be an effective strategy for the prevention and treatment of LNM. MicroRNAs such as miR-182-5p, miR-431-5p, and circular RNA NFIB1 and VESTAR, can inhibit tumor lymphangiogenesis by regulating VEGF expression.466,467,468,469
The inhibition of lymphatic metastasis can be achieved by targeting cancer cells directly. In head and neck squamous cell carcinoma, silencing of RING1 and YY1 binding protein expression470 and activation of the Wnt/β-catenin/Slug signaling axis471 contribute to cancer cell invasion and migration. In laryngeal cancer, silencing of HOXC6 inhibits EMT, viability, migration, and invasion of laryngeal cancer cells, thereby suppressing laryngeal cancer LNM.472 Li et al. demonstrated that MEOX1 promotes LNM in ovarian cancer by regulating multiple biological processes, including proliferation, EMT, lymphangiogenesis, and ECM remodeling. These findings suggest that MEOX1 may serve as a potential biomarker for ovarian cancer LNM diagnosis and treatment.473 In gastric cancer, RPRD1B promotes fatty acid uptake and synthesis, as well as LNM through activating the c-Jun/c-Fos/SREBP1 axis. This highlights RPRD1B as a potential target for gastric cancer LNM treatment.474
Therapeutic strategies targeting tumor metastasis demonstrate innovative pathways across multiple layers and dimensions. These advancements encompass a spectrum of approaches, ranging from precise attacks on metastatic cancer cells to intricate modulation of the complex TME, culminating in personalized treatments tailored to specific organ metastasis (Table 4). Each advance illustrates a profound understanding of tumor biology and a precise approach to treatment. With ongoing advancements in fundamental research and technology, these targeted therapies will continue to be refined and integrated, offering patients with cancer more efficacious, safer, and tailored treatment regimens. In conclusion, this progression promises to significantly extend patient survival and enhance QOL.
Emerging therapeutic technologies
The accelerated advancement of science and technology has facilitated the application of numerous emerging technologies in tumor metastasis research and treatment. These innovative methodologies enhance therapeutic efficacy, minimize side effects, and offer personalized and precise solutions to the complexities of metastatic cancers (Table 5). This progress brings new hope and potential for the treatment of tumor metastasis.
Nanotechnology and nanomaterials
In recent years, the application of nanotechnology in medicine, particularly in treating tumor metastasis, has attracted considerable attention. Nanomaterials, with their distinctive properties and capabilities, present novel avenues for the early detection, diagnosis, and treatment of tumor metastasis. Nanomaterials can be used as contrast agents to enhance the resolution and sensitivity of imaging techniques, thereby improving the accuracy of tumor metastasis detection.475,476 Nanomaterial-based targeted drug delivery systems use surface-modified nanoparticles to selectively bind to tumor cells, facilitating precise drug release and enhancing efficacy.477,478 This approach addresses the limitations of traditional treatments, including their poor selectivity and high toxicity. Nanomaterials have the potential to construct an integrated diagnostic and therapeutic platform, thereby synchronizing disease diagnosis and treatment, and improving medical efficiency and therapeutic efficacy.479,480 The application of nanotechnology has markedly advanced research on the complex interplay between TME, metabolism, and therapeutic interventions, thereby facilitating the development of innovative therapeutic strategies. Some nanomaterials can release antitumor drugs in response to specific conditions such as pH, temperature, and enzyme activity within the TME.481,482,483 Moreover, nanomaterials have the potential to impede tumor cell invasion and metastasis by influencing cell adhesion and matrix remodeling, among other mechanisms.484,485,486 Furthermore, nanomaterials can be combined with therapeutic strategies such as immunotherapy and radiotherapy to facilitate the effectiveness of combined therapy.480,487,488,489 Novel nanomaterials and techniques have led to the expectation that nanotechnology will facilitate transformative advances in cancer therapy. Nevertheless, it is imperative to underscore the biosafety, long-term effects, and potential side effects of nanomaterials.490
Gene-editing technologies
Gene-editing technology, particularly CRISPR/Cas9, has significantly advanced life science research, offering immense promise for advancing precision tumor medicine.491,492,493 In the context of tumor metastasis, gene editing has the potential to inhibit tumor growth and metastasis by knocking out or repairing oncogenes and mutated genes.494,495,496 Furthermore, gene editing can identify and characterize genes associated with tumor metastasis, which may offer potential therapeutic targets for antitumor metastasis treatments.497,498 Moreover, gene editing facilitates the identification of genes responsible for tumor drug resistance, thereby offering potential targets for addressing this significant challenge in cancer therapy.499,500 Furthermore, it can modify the expression of cells or molecules within the TME, thereby inhibiting tumor invasion and metastasis.501,502,503 Beyond direct gene editing, the application of nano-biomaterials and stimulus-responsive delivery strategies offers innovative approaches to enhance gene-editing efficiency and precisely control release,504,505 thereby mitigating toxicity and side effects. Nevertheless, gene-editing technologies still face significant challenges, including ensuring specificity and safety, avoiding off-target effects, and addressing ethical and legal concerns.506,507
Cancer vaccines
Cancer vaccines, which stimulate effective antitumor immune responses by activating the immune system and immune cells, have the potential to inhibit tumor growth and metastasis.508 These vaccines can be classified into two main categories: prophylactic and therapeutic vaccines. In addition, they can be further distinguished based on their composition or carrier, which includes whole-cell tumor vaccines,509 tumor antigen vaccines,510 peptide vaccines,511 RNA vaccines,512 DNA vaccines,513 dendritic cell vaccines,514,515 nanovaccines,516 in situ vaccines,517,518 and bacterial nanovaccines.519 The integration of nanotechnology,520,521 biomaterials,522 and gene-editing technology,523 among other techniques, has the potential to further reduce off-target effects and enhance antitumor immune responses, thereby expanding the scope of treatment of tumor metastasis. Cancer vaccines can facilitate the aggregation of antigens in lymph nodes, thereby enhancing antigen presentation and initiating effective antitumor immune responses.524,525,526,527 Moreover, cancer vaccines can directly activate immune cells, thereby eliciting antitumor immunity.528 Furthermore, these vaccines can augment the efficacy of tumor cell destruction by modulating immunosuppressive mechanisms.513,529,530 The development of vaccines based on specific tumor cell antigens from individual patients allows for a personalized treatment approach.509,531 Moreover, cancer vaccines with visualization and real-time monitoring are rapidly evolving, facilitating a deeper understanding of the mechanisms and principles of vaccines in vivo. This enhances the efficacy of the cancer vaccines.525,532 Nevertheless, cancer vaccines face several challenges, including immune evasion of tumor cells, optimization of vaccine design, and immunosuppression of the TME.
Artificial intelligence
Artificial intelligence (AI) technologies, including machine learning and artificial neural networks, can be utilized for tumor risk stratification, metastasis detection, and treatment response. These technologies are undergoing rapid development for the treatment of tumor metastasis.533,534,535 AI has the potential to markedly enhance the diagnostic accuracy and efficiency of clinicians through its assistance in medical image analysis, thereby reducing diagnostic time and minimizing the risk of missed diagnosis.536,537 Moreover, AI can analyze vast biological data to predict drug targets, thereby accelerating the development of new drugs and reducing associated costs.538,539 Furthermore, AI can scrutinize patients’ genomic and clinical data, facilitating the identification of gene mutations, assessment of cancer risk, drug sensitivity stratification, treatment response prediction, and prognostic modeling, thereby enabling precision medicine.540,541,542,543,544,545,546 The advent of large language models, such as ChatGPT, is anticipated to facilitate the application of generative AI (GAI) in several critical areas, including clinical document writing and data management,547,548,549,550 as well as clinical decision assistance551,552 and personal healthcare.553 Nevertheless, several challenges remain to be addressed, including the interpretability of AI, data bias, and protection of privacy.554
Bioinformatics
Bioinformatics is an interdisciplinary field that draws on knowledge from biology, computer science, and mathematics to address complex biological issues.555 It plays a pivotal role in research on tumor metastasis. Bioinformatics can analyze and interpret a vast quantity of biological data, which serves as the foundation for uncovering the biological characteristics, metastatic mechanisms, and regulatory mechanisms of tumor metastasis.556,557,558 Furthermore, bioinformatics facilitates the discovery and identification of potential biomarkers for the early diagnosis, treatment monitoring, and prognosis assessment of tumor metastasis.559,560 By examining the molecular characteristics of cancer cells, bioinformatics can identify potential new therapeutic targets that can inform the design and optimization of novel anticancer drugs.561,562,563,564 Moreover, bioinformatics can integrate patients’ genomic, transcriptomic, and proteomic data with medical imaging and treatment response data.565 This integrated approach provides a valuable reference for formulating personalized treatment plans. However, the application of bioinformatics also encounters challenges, including data quality and availability, the accurate annotation of information, fairness, and ethical considerations.566,567,568
Challenges of studying cancer metastasis
Knowledge gaps in understanding tumor metastasis
The dissemination of metastasis raises several significant questions, particularly regarding the timing of this process. It is postulated that dissemination may commence in the initial stages of tumor progression, with the asymptomatic establishment of PMN occurring at this stage. This early and often undetectable phase presents challenges for clinical diagnosis and early intervention, thus complicating studies on PMN in human patient samples. Consequently, it is difficult to understand how advancements in our knowledge of PMN could translate into clinical benefits for cancer treatment.
Recent genomic analyses and lineage-tracing experiments have revealed that numerous metastasis evolve through a metastasis-to-metastasis route.68,569,570,571,572,573 In bone metastasis, Zhang et al. discovered that tumors metastasizing to the bone enhance their stemness and metastatic capabilities via epigenetic modifications.574 However, comprehensive studies comparing transcriptomic, epigenetic, and genetic alterations between tumor cells from primary and secondary metastasis are lacking. This limitation arises because current methodologies cannot reliably differentiate the cellular origin of CTCs, whether from surgical operations during cell line injections, primary metastasis, or further advanced metastasis.
The phenomenon of metastasis organ tropism, coupled with the observation of metastasis-to-metastasis, indicates that tumor progression occurs with host tissue evolution. A single type of cancer can metastasize to various tissues, whereas different cancers can metastasize to the same organ. However, there is a paucity of comprehensive studies comparing metastatic ecosystems across various tissues from the same cancer type or different cancer types that metastasize to the same organ. Some research groups have identified this knowledge gap and have focused on delineating tumor-host co-evolution. For example, Xu et al. comprehensively analyzed lung cancer metastatic TME in various organs, including the bone, liver, and brain.575 Similarly, Liu et al. examined the diverse immune archetypes of bone metastasis colonized by different cancer types.576 Both studies used single-cell sequencing, underscoring the need for more extensive high-throughput analyses. Such profiling could enhance our understanding of cell-cell signaling and immune-tumor interactions within the metastatic TME, elucidate organ tropism mechanisms, and inform potential treatments for metastasis. These studies are vital for developing early interventions to prevent metastasis, identifying biomarkers for prognosis, and tailoring personalized therapies to improve patient outcomes. The paucity of high-throughput screening of metastatic TMEs is attributable to several factors. The establishment of organ-specific spontaneous metastasis in mouse models is challenging because of the lack of ideal models that eliminate the influence of surgical interventions and excessive immune response to cell line tags. Moreover, in vivo metastatic models are inherently difficult to control. Obtaining matched primary and metastatic samples from patients is a significant challenge, particularly given the invasive nature of the procedures involved and the necessity for coordination across diverse healthcare systems. Furthermore, patients who undergo surgical procedures often introduce substantial batch effects, which can complicate data analysis and interpretation.
Single-cell sequencing techniques have been widely used in studies on the TME. However, tumor metastasis is dynamic, with significant cellular and molecular kinetics constantly evolving. Profiling samples from a specific time point provides only a partial representation of the entire metastatic process. Despite the availability of sophisticated analytical tools, such as monocle 3577,578,579,580 and RNA velocity,581,582 which facilitate the estimation of cellular state transitions at high resolution across experimental conditions, these techniques remain inadequate for fully capturing the actual lineage trajectories. The accuracy of trajectory inference is challenging to evaluate, given the extensive reprogramming events that occur in the TME. Furthermore, data preprocessing of single-cell datasets has the potential to eliminate rare cell populations, which could result in analyses skewed by dominant cell populations.
Obstacles of cancer metastasis in clinical trials
Clinical trials are an indispensable component in the process of acquiring high-quality therapeutic evidence and assessing the efficacy and safety of unlisted drugs. They play a pivotal role in accelerating the development of antitumor metastatic drugs, thereby meeting the needs of patients with cancer.583 Nevertheless, clinical trials are confronted with a multitude of challenges that impede the acquisition of superior medical evidence and profoundly affect the diagnosis and treatment of tumor metastasis.
Experimental design
Randomized controlled trials remain the gold standard for evaluating treatment regimens and efficacy in tumor metastasis, providing robust evidence for developing clinical treatment protocols.584,585,586 Organ-specific tumor treatment strategies have yielded notable therapeutic outcomes in numerous tumor types. This success prompted the design of numerous clinical trials based on primary tumors to achieve optimal efficacy. However, metastatic tumors frequently exhibit greater complexity than their primary counterparts, rendering primary tumor-based treatment regimens ineffective in the context of metastatic disease.380,587 Therapeutic regimens specifically tailored to the characteristics of metastasis have demonstrated favorable outcomes.387 One such example is the use of bone modifiers to treat bone metastasis. Moreover, in light of the difficulties encountered when attempting to achieve optimal therapeutic effects with a single-treatment approach, combination therapy has increasingly become the primary focus of tumor metastasis treatment.588 Therefore, well-designed clinical trials targeting the distinctive characteristics of tumor metastasis should be conducted to enhance efficacy in patients with tumor metastasis and obtain high-quality evidence-based medical evidence.
Tumor heterogeneity
The heterogeneous nature of tumors is a consequence of the selective pressure exerted by the TME. Primary tumor cells and distant metastatic cells exhibit differential metabolic processes, immune responses, growth rates, and other characteristics that collectively contribute to tumor heterogeneity.589 This phenomenon is a crucial indicator of cancer progression.590,591 Tumor heterogeneity enhances cancer cell invasiveness and promotes drug resistance, which is a significant factor in the development of drug resistance.589,592,593,594 Targeted therapy represents a strategy to address drug resistance in cancer cells; however, it is also susceptible to drug resistance.379,380,587,595,596 The results of clinical trials have revealed notable discrepancies in the efficacy of treatment regimens when administered to subgroups of patients with disparate genetic profiles.585,593 This indicates that a significant reason for the failure of targeted therapy for tumor metastasis may be attributed to the fact that targeted therapy is based on the distinctive gene expression of the primary tumor rather than metastatic cancer cells.597,598 Consequently, when conducting clinical trials about the treatment of tumor metastasis, it is imperative that researchers consider tumor heterogeneity and utilize biomarker-based methodologies to identify patient subgroups that respond to targeted pharmaceuticals, thereby optimizing the therapeutic outcomes.599
Non-experimental factors
In the context of clinical trials, investigators must consider a multitude of non-experimental factors, including the composition of the research team, availability of patient resources, financial constraints, accessibility of equipment, and prevailing ethical regulations. If these factors are not effectively managed, they can impede the standard conduct of clinical trials. The availability of patient resources is a prerequisite for clinical research. The successful implementation of clinical trials is contingent upon the enrollment of eligible patients and the attainment of a specified sample size and scale.600,601,602,603 Moreover, the considerable financial costs associated with conducting these trials are a significant factor.604 A substantial number of clinical trials require costly infrastructure to facilitate their studies. Consequently, the applicability of their conclusions is contingent on cost-effectiveness.605 It is becoming increasingly common for different institutions and countries to collaborate to reduce costs and accelerate patient recruitment.585 Furthermore, the lack of specialized personnel, inadequate equipment availability, prolonged turnaround times, and discrepancies in ethical regulations can impede the appropriate conduct of clinical trials.601,606 Therefore, investigators must consider and manage these non-experimental factors during the design and execution of clinical trials to promote the normal, safe, and orderly implementation of clinical trials.
Conclusions and future direction
In recent decades, significant advancements have been made in tumor metastasis research, particularly in malignant diseases. Researchers have elucidated intricate mechanisms and emphasized the pivotal role of the TME as a crucial regulator in this dynamic process. From the intricate modulation of cellular signaling pathways to the complex interplay within the ECM and the intricate cellular and molecular dynamics across various host organs, all have been identified as key factors influencing tumor metastasis. The particular characteristics of multiorgan metastasis, including those to the lungs, liver, brain, and bones, along with their underlying molecular mechanisms, have further enhanced our comprehensive understanding of the landscape of tumor metastasis. Recent clinical trials have not only deepened our understanding of the intricate relationships between tumor metastasis, primary tumor characteristics, treatment responses, and patient outcomes but have also fueled the development and application of diverse strategies targeting specific signaling pathways and innovative therapeutic targets. These efforts have enhanced the efficacy of therapeutic interventions and have the potential to extend patient survival and improve QOL. Further research is required to gain a deeper understanding of the mechanisms by which the TME influences tumor metastasis. A comprehensive examination of the intricate interactions between tumors and various host tissues, organs, immune cells, vascular networks, and other microenvironmental components is required to identify novel therapeutic targets and intervention strategies. Concurrently, we seek to gain molecular-level insights into the entire process of tumor metastasis by applying advanced bioinformatics, genomics, and proteomics technologies, thereby enabling more precise monitoring and intervention in disease progression. As data accumulates and technology advances at an unprecedented pace, the vision of personalized medicine is becoming increasingly realized. The implementation of customized treatment regimens based on individual genetic profiles, protein expression patterns, and other biomarkers will facilitate a more precise match with patients’ therapeutic needs, thereby enhancing treatment efficacy while minimizing adverse effects. This paradigm shift in treatment modalities undoubtedly offers hope to patients with cancer, heralding a new era of tumor therapy. In conclusion, a comprehensive and intensive study of tumor metastasis has deepened our understanding of its complex biological processes and laid a solid foundation for the development of more effective and individualized treatment strategies. These endeavors have the potential to markedly enhance patients’ survival rates and QOL, thereby contributing to the global initiative to surmount this formidable health challenge.
References
Ganesh, K. & Massague, J. Targeting metastatic cancer. Nat. Med. 27, 34–44 (2021).
Liu, M., Yang, J., Xu, B. & Zhang, X. Tumor metastasis: Mechanistic insights and therapeutic interventions. MedComm. (2020) 2, 587–617 (2021).
Welch, D. R. & Hurst, D. R. Defining the Hallmarks of Metastasis. Cancer Res 79, 3011–3027 (2019).
Massague, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Quintanal-Villalonga, A. et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 17, 360–371 (2020).
Liu, W. et al. Microenvironmental influences on metastasis suppressor expression and function during a metastatic cell’s journey. Cancer Microenviron. 7, 117–131 (2014).
Li, X., Jin, L. & Tan, Y. Different roles of matrix metalloproteinase 2 in osteolysis of skeletal dysplasia and bone metastasis (Review). Mol. Med. Rep. 23, 70 (2021).
Xu, S. et al. Efficacy of percutaneous vertebroplasty for the relief of osteoblastic spinal metastasis pain. Exp. Ther. Med. 22, 727 (2021).
Muto, A. et al. Lineage-committed osteoclast precursors circulate in blood and settle down into bone. J. Bone Min. Res. 26, 2978–2990 (2011).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Nguyen, D. X., Bos, P. D. & Massague, J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 (2009).
Izraely, S. & Witz, I. P. Site-specific metastasis: A cooperation between cancer cells and the metastatic microenvironment. Int. J. Cancer 148, 1308–1322 (2021).
Sacks, P. & Rahman, M. Epidemiology of brain metastases. Neurosurg. Clin. N. Am. 31, 481–488 (2020).
Walker, A. E., Robins, M. & Weinfeld, F. D. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology 35, 219–226 (1985).
Percy, A. K., Elveback, L. R., Okazaki, H. & Kurland, L. T. Neoplasms of the central nervous system. Epidemiologic considerations. Neurology 22, 40–48 (1972).
Counsell, C. E., Collie, D. A. & Grant, R. Incidence of intracranial tumours in the Lothian region of Scotland, 1989-90. J. Neurol. Neurosurg. Psychiatry 61, 143–150 (1996).
Benna, M. et al. Brain metastases epidemiology in a Tunisian population: trends and outcome. CNS Oncol. 7, 35–39 (2018).
Schouten, L. J., Rutten, J., Huveneers, H. A. & Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94, 2698–2705 (2002).
Barnholtz-Sloan, J. S. et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 22, 2865–2872 (2004).
Johnson, J. D. & Young, B. Demographics of brain metastasis. Neurosurg. Clin. N. Am. 7, 337–344 (1996).
Valiente, M. et al. The evolving landscape of brain metastasis. Trends Cancer 4, 176–196 (2018).
Stemmler, H. J. et al. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast 15, 219–225 (2006).
Yau, T. et al. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 45, 196–201 (2006).
Coleman, R. E. et al. Bone metastases. Nat. Rev. Dis. Prim. 6, 83 (2020).
Coleman, R. E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12, 6243s–6249s (2006).
Yang, W. et al. Research progress of bone metastases: From disease recognition to clinical practice. Front Oncol. 12, 1105745 (2022).
Hernandez, R. K. et al. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer 18, 44 (2018).
Yang, Y. et al. A multicenter, retrospective epidemiologic survey of the clinical features and management of bone metastatic disease in China. Chin. J. Cancer 35, 40 (2016).
Lehrer, E. J. et al. Trends in diagnosis and treatment of metastatic cancer in the United States. Am. J. Clin. Oncol. 44, 572–579 (2021).
Weinfurt, K. P. et al. Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med. Care 42, 164–175 (2004).
Flora, D. R. et al. Assessment of bone health awareness and education in breast cancer patients with bone metastasis in the USA. J. Cancer Educ. 38, 1522–1530 (2023).
Liu, D. et al. Prognosis of prostate cancer and bone metastasis pattern of patients: a SEER-based study and a local hospital based study from China. Sci. Rep. 10, 9104 (2020).
Cetin, K. et al. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer 86, 247–254 (2014).
Horn, S. R. et al. Epidemiology of liver metastases. Cancer Epidemiol. 67, 101760 (2020).
Hendriks, L. E. et al. Single organ metastatic disease and local disease status, prognostic factors for overall survival in stage IV non-small cell lung cancer: Results from a population-based study. Eur. J. Cancer 51, 2534–2544 (2015).
Kulaylat, A. N. et al. Overall survival by pattern of recurrence following curative intent surgery for colorectal liver metastasis. J. Surg. Oncol. 110, 1011–1015 (2014).
Manfredi, S. et al. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 244, 254–259 (2006).
Wang, S. et al. Incidence and prognosis of liver metastasis at diagnosis: a pan-cancer population-based study. Am. J. Cancer Res. 10, 1477–1517 (2020).
Clark, A. M. et al. Liver metastases: Microenvironments and ex-vivo models. Exp. Biol. Med. 241, 1639–1652 (2016).
Bosch, F. X., Ribes, J., Diaz, M. & Cleries, R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 127, S5–S16 (2004).
Hallet, J. et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 121, 589–597 (2015).
Chen, H. et al. The Epidemiology of Lung Metastases. Front. Med. 8, 723396 (2021).
Mitry, E. et al. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut 59, 1383–1388 (2010).
Riihimaki, M. et al. Metastatic sites and survival in lung cancer. Lung Cancer 86, 78–84 (2014).
Riihimaki, M., Hemminki, A., Sundquist, J. & Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 6, 29765 (2016).
Griffeth, L. K. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc. 18, 321–330 (2005).
Hart, I. R. & Fidler, I. J. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 40, 2281–2287 (1980).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Fidler, I. J. & Poste, G. The “seed and soil” hypothesis revisited. Lancet Oncol. 9, 808 (2008).
Cochran, A. J. et al. Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 6, 659–670 (2006).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Leong, S. P. et al. Molecular mechanisms of cancer metastasis via the lymphatic versus the blood vessels. Clin. Exp. Metastasis 39, 159–179 (2022).
Hess, K. R. et al. Metastatic patterns in adenocarcinoma. Cancer 106, 1624–1633 (2006).
Shuman Moss, L. A., Jensen-Taubman, S. & Stetler-Stevenson, W. G. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am. J. Pathol. 181, 1895–1899 (2012).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Peng, W. et al. Integrated transcriptomics, proteomics, and glycomics reveals the association between up-regulation of Sialylated N-glycans/Integrin and breast cancer brain metastasis. Sci. Rep. 9, 17361 (2019).
McHugh, K. P. et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 105, 433–440 (2000).
Liapis, H., Flath, A. & Kitazawa, S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn. Mol. Pathol. 5, 127–135 (1996).
Ross, F. P. et al. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J. Biol. Chem. 268, 9901–9907 (1993).
Crippes, B. A. et al. Antibody to beta3 integrin inhibits osteoclast-mediated bone resorption in the thyroparathyroidectomized rat. Endocrinology 137, 918–924 (1996).
Pantano, F. et al. Integrin alpha5 in human breast cancer is a mediator of bone metastasis and a therapeutic target for the treatment of osteolytic lesions. Oncogene 40, 1284–1299 (2021).
Mortezaee, K. CXCL12/CXCR4 axis in the microenvironment of solid tumors: A critical mediator of metastasis. Life Sci. 249, 117534 (2020).
Huang, J. et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target Ther. 6, 153 (2021).
Chakraborty, S. & Banerjee, S. Understanding crosstalk of organ tropism, tumor microenvironment and noncoding RNAs in breast cancer metastasis. Mol. Biol. Rep. 50, 9601–9623 (2023).
Rashed, W. M. et al. Pediatric diffuse intrinsic pontine glioma: where do we stand? Cancer Metastasis Rev. 38, 759–770 (2019).
Yousefi, M. et al. Organ-specific metastasis of breast cancer: molecular and cellular mechanisms underlying lung metastasis. Cell Oncol. 41, 123–140 (2018).
Wrenn, E., Huang, Y. & Cheung, K. Collective metastasis: coordinating the multicellular voyage. Clin. Exp. Metastasis 38, 373–399 (2021).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Turajlic, S. & Swanton, C. Metastasis as an evolutionary process. Science 352, 169–175 (2016).
Ulintz, P. J. et al. Lymph node metastases in colon cancer are polyclonal. Clin. Cancer Res 24, 2214–2224 (2018).
Cheung, K. J. et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863 (2016).
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 376, 2109–2121 (2017).
Al Bakir, M. et al. The evolution of non-small cell lung cancer metastases in TRACERx. Nature 616, 534–542 (2023).
Hu, Z., Li, Z., Ma, Z. & Curtis, C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat. Genet. 52, 701–708 (2020).
Reiter, J. G. et al. Lymph node metastases develop through a wider evolutionary bottleneck than distant metastases. Nat. Genet. 52, 692–700 (2020).
Siraj, S. et al. Clonal evolution and timing of metastatic colorectal cancer. Cancers 12, 2938 (2020).
Echeverria, G. V. et al. High-resolution clonal mapping of multi-organ metastasis in triple negative breast cancer. Nat. Commun. 9, 5079 (2018).
Naxerova, K. et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 357, 55–60 (2017).
Maddipati, R. & Stanger, B. Z. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 5, 1086–1097 (2015).
Huang, X. et al. Mutational characteristics of bone metastasis of lung cancer. Ann. Palliat. Med. 10, 8818–8826 (2021).
Kim, M. Y. et al. Tumor self-seeding by circulating cancer cells. Cell 139, 1315–1326 (2009).
Yang, H. et al. Identification and characterization of TM4SF1(+) tumor self-seeded cells. Cell Rep. 43, 114512 (2024).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2011).
Horimoto, Y., Polanska, U. M., Takahashi, Y. & Orimo, A. Emerging roles of the tumor-associated stroma in promoting tumor metastasis. Cell Adh. Migr. 6, 193–202 (2012).
Fang, T. et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 9, 191 (2018).
Loric, S. et al. Extracellular vesicles in breast cancer: from biology and function to clinical diagnosis and therapeutic management. Int J. Mol. Sci. 24, 7208 (2023).
Jeppesen, D. K. et al. Reassessment of exosome composition. Cell 177, 428–445 e418 (2019).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Ren, G., Esposito, M. & Kang, Y. Bone metastasis and the metastatic niche. J. Mol. Med. 93, 1203–1212 (2015).
Liu, H. et al. Reprogrammed marrow adipocytes contribute to myeloma-induced bone disease. Sci. Transl. Med. 11, eaau9087 (2019).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Gong, Z. et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity 55, 1483–1500.e1489 (2022).
Li, H. et al. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 182, 240–249 (2009).
Wang, M. et al. Tumor-derived exosomes drive pre-metastatic niche formation in lung via modulating CCL1(+) fibroblast and CCR8(+) Treg cell interactions. Cancer Immunol. Immunother. 71, 2717–2730 (2022).
Li, P. et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat. Immunol. 21, 1444–1455 (2020).
Hao, X. et al. Osteoprogenitor-GMP crosstalk underpins solid tumor-induced systemic immunosuppression and persists after tumor removal. Cell Stem Cell 30, 648–664 e648 (2023).
Welte, T. et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat. Cell Biol. 18, 632–644 (2016).
Sun, H. et al. Hypoxia-inducible exosomes facilitate liver-tropic premetastatic niche in colorectal cancer. Hepatology 74, 2633–2651 (2021).
Seubert, B. et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 61, 238–248 (2015).
Wang, D. et al. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 77, 3655–3665 (2017).
Steele, C. W. et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal Adenocarcinoma. Cancer Cell 29, 832–845 (2016).
Yamamoto, M. et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 68, 9754–9762 (2008).
Kitamura, T. et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc. Natl Acad. Sci. USA 107, 13063–13068 (2010).
Yu, Y. et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br. J. Cancer 110, 724–732 (2014).
Calon, A. et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584 (2012).
Giannoni, E. et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 70, 6945–6956 (2010).
Miles, F. L. & Sikes, R. A. Insidious changes in stromal matrix fuel cancer progression. Mol. Cancer Res. 12, 297–312 (2014).
Yamaguchi, H. & Sakai, R. Direct interaction between carcinoma cells and cancer associated fibroblasts for the regulation of cancer invasion. Cancers 7, 2054–2062 (2015).
Zhang, D. et al. Metabolic reprogramming of cancer-associated fibroblasts by IDH3alpha downregulation. Cell Rep. 10, 1335–1348 (2015).
Guido, C. et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-beta drives tumor growth: connecting TGF-beta signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle 11, 3019–3035 (2012).
Liberti, M. V. & Locasale, J. W. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 41, 211–218 (2016).
Fu, S. et al. Impaired lipid biosynthesis hinders anti-tumor efficacy of intratumoral iNKT cells. Nat. Commun. 11, 438 (2020).
Yang, K. et al. Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-kappaB activation via GPR81-mediated signaling. Front Immunol. 11, 587913 (2020).
Biswas, S. K. Metabolic reprogramming of immune cells in cancer progression. Immunity 43, 435–449 (2015).
Condamine, T., Ramachandran, I., Youn, J. I. & Gabrilovich, D. I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 66, 97–110 (2015).
Wang, Y. et al. Metabolic modulation of immune checkpoints and novel therapeutic strategies in cancer. Semin Cancer Biol. 86, 542–565 (2022).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Clavel, C. et al. Immunolocalization of Matrix Metallo-Proteinases and their tissue inhibitor in human mammary pathology. Bull. Cancer 79, 261–270 (1992).
Sangaletti, S. et al. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Res. 68, 9050–9059 (2008).
Tan, G. J., Peng, Z. K., Lu, J. P. & Tang, F. Q. Cathepsins mediate tumor metastasis. World J. Biol. Chem. 4, 91–101 (2013).
Sato, T. et al. Neutrophil elastase and cancer. Surg. Oncol. 15, 217–222 (2006).
Roelofs, A. J., Thompson, K., Gordon, S. & Rogers, M. J. Molecular mechanisms of action of bisphosphonates: current status. Clin. Cancer Res 12, 6222s–6230s (2006).
Rohan, T. E. et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J. Natl Cancer Inst. 106, dju136 (2014).
Roh-Johnson, M. et al. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33, 4203–4212 (2014).
Condeelis, J. & Pollard, J. W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266 (2006).
Sharma, V. P. et al. Live tumor imaging shows macrophage induction and TMEM-mediated enrichment of cancer stem cells during metastatic dissemination. Nat. Commun. 12, 7300 (2021).
Harney, A. S. et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 5, 932–943 (2015).
Lin, E. Y. et al. Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Mol. Oncol. 1, 288–302 (2007).
Mazzieri, R. et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19, 512–526 (2011).
Wyckoff, J. B. et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67, 2649–2656 (2007).
Arwert, E. N. et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 23, 1239–1248 (2018).
Opdenakker, G. & Van Damme, J. The countercurrent principle in invasion and metastasis of cancer cells. Recent insights on the roles of chemokines. Int J. Dev. Biol. 48, 519–527 (2004).
Piccard, H., Muschel, R. J. & Opdenakker, G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit. Rev. Oncol. Hematol. 82, 296–309 (2012).
Nieswandt, B., Hafner, M., Echtenacher, B. & Männel, D. N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59, 1295–1300 (1999).
Labelle, M., Begum, S. & Hynes, R. O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590 (2011).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010).
Ao, Z. et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 75, 4681–4687 (2015).
Demers, M. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl Acad. Sci. USA 109, 13076–13081 (2012).
Szczerba, B. M. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 (2019).
Spiegel, A. et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 6, 630–649 (2016).
Casbon, A. J. et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc. Natl Acad. Sci. USA 112, E566–E575 (2015).
Zea, A. H. et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65, 3044–3048 (2005).
De Santo, C. et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat. Immunol. 11, 1039–1046 (2010).
Shao, B. et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 118, 4015–4023 (2011).
Barthel, S. R. et al. Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res. 73, 942–952 (2013).
Sipkins, D. A. et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 (2005).
Schneider, J. G., Amend, S. R. & Weilbaecher, K. N. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone 48, 54–65 (2011).
Lorger, M. et al. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc. Natl Acad. Sci. USA 106, 10666–10671 (2009).
Taichman, R. S. et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 62, 1832–1837 (2002).
Ha, H., Debnath, B. & Neamati, N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics 7, 1543–1588 (2017).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Terpos, E., Ntanasis-Stathopoulos, I., Gavriatopoulou, M. & Dimopoulos, M. A. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 8, 7 (2018).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Aguirre-Ghiso, J. A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 7, 834–846 (2007).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Andrejeva, G. & Rathmell, J. C. Similarities and Distinctions of Cancer and Immune Metabolism in Inflammation and Tumors. Cell Metab. 26, 49–70 (2017).
Yang, L. & Zhang, Y. Tumor-associated macrophages: from basic research to clinical application. J. Hematol. Oncol. 10, 58 (2017).
Penny, H. L. et al. Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology 5, e1191731 (2016).
Huh, S. J. et al. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 70, 6071–6082 (2010).
Liang, S. & Dong, C. Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions. Am. J. Physiol. Cell Physiol. 295, C701–C707 (2008).
Park, J. et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 8, 361ra138 (2016).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 (2013).
Najmeh, S. et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1-integrin mediated interactions. Int. J. Cancer 140, 2321–2330 (2017).
Hiratsuka, S. et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2, 289–300 (2002).
Kitamura, T. et al. Monocytes differentiate to immune suppressive precursors of metastasis-associated macrophages in mouse models of metastatic breast cancer. Front. Immunol. 8, 2004 (2017).
Yan, H. H. et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70, 6139–6149 (2010).
Barkan, D. et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 (2008).
Malladi, S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016).
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D. & Ossowski, L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 63, 1684–1695 (2003).
Canon, J. R. et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin. Exp. Metastasis 25, 119–129 (2008).
Shevde, L. A. & Samant, R. S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 37, 131–141 (2014).
Delpla, A. et al. Role of thermal ablation in colorectal cancer lung metastases. Cancers. 13, 908 (2021).
Giancotti, F. G. Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764 (2013).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018).
Toiyama, Y. et al. C-reactive protein as predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer Res. 33, 5065–5074 (2013).
Teng, M. W. et al. Immune-mediated dormancy: an equilibrium with cancer. J. Leukoc. Biol. 84, 988–993 (2008).
Yang, H. et al. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 37, 266 (2018).
Boyerinas, B. et al. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood 121, 4821–4831 (2013).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Kersten, K. et al. Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1beta in tumor-associated macrophages. Oncoimmunology 6, e1334744 (2017).
Coffelt, S. B. et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Qian, B. et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 4, e6562 (2009).
Chen, Q., Zhang, X. H. & Massague, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20, 538–549 (2011).
Hsu, Y. L. et al. CXCL17-derived CD11b(+)Gr-1(+) myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res. 21, 23 (2019).
Yan, H. H. et al. CCL9 Induced by TGFbeta signaling in myeloid cells enhances tumor cell survival in the premetastatic organ. Cancer Res. 75, 5283–5298 (2015).
Gerstberger, S., Jiang, Q. & Ganesh, K. Metastasis. Cell 186, 1564–1579 (2023).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Steeg, P. S. Targeting metastasis. Nat. Rev. Cancer 16, 201–218 (2016).
Wang, H., Zhang, W., Bado, I. & Zhang, X. H. Bone tropism in cancer metastases. Cold Spring Harb. Perspect. Med. 10, a036848 (2020).
Clezardin, P. et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol. Rev. 101, 797–855 (2021).
Boxer, D. I., Todd, C. E., Coleman, R. & Fogelman, I. Bone secondaries in breast cancer: the solitary metastasis. J. Nucl. Med. 30, 1318–1320 (1989).
Raymaekers, K., Stegen, S., van Gastel, N. & Carmeliet, G. The vasculature: a vessel for bone metastasis. Bonekey Rep. 4, 742 (2015).
Johnson, R. W., Sowder, M. E. & Giaccia, A. J. Hypoxia and bone metastatic disease. Curr. Osteoporos. Rep. 15, 231–238 (2017).
Todd, V. M. & Johnson, R. W. Hypoxia in bone metastasis and osteolysis. Cancer Lett. 489, 144–154 (2020).
Woelfle, U. et al. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res. 63, 5679–5684 (2003).
Liao, J., Schneider, A., Datta, N. S. & McCauley, L. K. Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 66, 9065–9073 (2006).
Tuffour, A. et al. Role of the calcium-sensing receptor (CaSR) in cancer metastasis to bone: Identifying a potential therapeutic target. Biochim. Biophys. Acta Rev. Cancer 1875, 188528 (2021).
Sanders, J. L. et al. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology 141, 4357–4364 (2000).
Mamillapalli, R., VanHouten, J., Zawalich, W. & Wysolmerski, J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J. Biol. Chem. 283, 24435–24447 (2008).
Kim, W. et al. Calcium-sensing receptor promotes breast cancer by stimulating intracrine actions of parathyroid hormone–related protein. Cancer Res. 76, 5348–5360 (2016).
Zhang, X. H. et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154, 1060–1073 (2013).
Wu, Q. et al. SCUBE2 mediates bone metastasis of luminal breast cancer by modulating immune-suppressive osteoblastic niches. Cell Res. 33, 464–478 (2023).
Wu, K. et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat. Commun. 12, 5196 (2021).
Hashimoto, K. et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl Acad. Sci. USA 115, 2204–2209 (2018).
Siddiqui, J. A. et al. GDF15 promotes prostate cancer bone metastasis and colonization through osteoblastic CCL2 and RANKL activation. Bone Res. 10, 6 (2022).
Mortezaee, K. Organ tropism in solid tumor metastasis: an updated review. Future Oncol. 17, 1943–1961 (2021).
Thomas, R. J. et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140, 4451–4458 (1999).
Sethi, N., Dai, X., Winter, C. G. & Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19, 192–205 (2011).
Valkenburg, K. C., de Groot, A. E. & Pienta, K. J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 15, 366–381 (2018).
Rieunier, G. et al. Bad to the bone: the role of the insulin-like growth factor axis in osseous metastasis. Clin. Cancer Res. 25, 3479–3485 (2019).
Boire, A., Brastianos, P. K., Garzia, L. & Valiente, M. Brain metastasis. Nat. Rev. Cancer 20, 4–11 (2020).
Steeg, P. S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 18, 696–714 (2021).
Basnet, H. et al. Flura-seq identifies organ-specific metabolic adaptations during early metastatic colonization. ELife 8, e43627 (2019).
Zhang, L. et al. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 71, 645–654 (2011).
Sevenich, L. et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 16, 876–888 (2014).
Yonemori, K. et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 116, 302–308 (2010).
Zhang, B. et al. Adhesion to the brain endothelium selects breast cancer cells with brain metastasis potential. Int J. Mol. Sci. 24, 7087 (2023).
Zhou, W. et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25, 501–515 (2014).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
DeBerardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 (2011).
Gao, Y. et al. Metastasis organotropism: redefining the congenial soil. Dev. Cell 49, 375–391 (2019).
Fischer, G. M. et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 9, 628–645 (2019).
Cordero, A. et al. FABP7 is a key metabolic regulator in HER2+ breast cancer brain metastasis. Oncogene 38, 6445–6460 (2019).
Pan, Y. & Monje, M. Neuron-Glial interactions in health and brain cancer. Adv. Biol. 6, e2200122 (2022).
Koh, W., Kwak, H., Cheong, E. & Lee, C. J. GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 24, 523–539 (2023).
Neman, J. et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl Acad. Sci. USA 111, 984–989 (2014).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Chen, Q. et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016).
Philips, R. L. et al. The JAK-STAT pathway at 30: Much learned, much more to do. Cell 185, 3857–3876 (2022).
Lee, J. W. et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567, 249–252 (2019).
Fares, J. et al. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct. Target Ther. 5, 28 (2020).
Vidal-Vanaclocha, F. The prometastatic microenvironment of the liver. Cancer Microenviron. 1, 113–129 (2008).
MacPhee, P. J., Schmidt, E. E. & Groom, A. C. Intermittence of blood flow in liver sinusoids, studied by high-resolution in vivo microscopy. Am. J. Physiol. 269, G692–G698 (1995).
Alix-Panabières, C. et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 59, 1384–1392 (2013).
Jewell, A. P. Is the liver an important site for the development of immune tolerance to tumours? Med. Hypotheses 64, 751–754 (2005).
Rossetto, A. et al. Carcinogenesis and metastasis in liver: cell physiological basis. Cancers 11, 1731 (2019).
Clayton, A. et al. Cancer Exosomes express CD39 and CD73, which suppress T cells through Adenosine production. J. Immunol. 187, 676–683 (2011).
Yang, L. et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583, 133–138 (2020).
Elowitz, M. B., Levine, A. J., Siggia, E. D. & Swain, P. S. Stochastic gene expression in a single cell. Science 297, 1183–1186 (2002).
Bu, P. et al. Aldolase B-mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis. Cell Metab. 27, 1249–1262 e1244 (2018).
Zhang, S. et al. SERPINE2 promotes liver cancer metastasis by inhibiting c‐Cbl‐mediated EGFR ubiquitination and degradation. Cancer Commun. 44, 384–407 (2024).
Song, G. et al. Hypermethylation of GNA14 and its tumor-suppressive role in hepatitis B virus-related hepatocellular carcinoma. Theranostics 11, 2318–2333 (2021).
Besic, N. & Gazic, B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid 23, 709–713 (2013).
Riihimäki, M. et al. Metastatic spread in patients with gastric cancer. Oncotarget 7, 52307–52316 (2016).
Wang, C. & Luo, D. The metabolic adaptation mechanism of metastatic organotropism. Exp. Hematol. Oncol. 10, 30 (2021).
Davis, R. T. et al. Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat. Cell Biol. 22, 310–320 (2020).
McGovern, M. et al. A “latent niche” mechanism for tumor initiation. Proc. Natl Acad. Sci. USA 106, 11617–11622 (2009).
Oskarsson, T. et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874 (2011).
Tichet, M. et al. Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat. Commun. 6, 6993 (2015).
Müller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Wang, L. et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol. Cancer 18, 86 (2019).
Mao, X. et al. Nidogen 1-enriched extracellular vesicles facilitate extrahepatic metastasis of liver cancer by activating pulmonary fibroblasts to secrete tumor necrosis factor Receptor 1. Adv. Sci. 7, 2002157 (2020).
Beird, H. C. et al. Osteosarcoma. Nat. Rev. Dis. Prim. 8, 77 (2022).
Mazumdar, A. et al. Exploring the role of Osteosarcoma-derived extracellular vesicles in pre-metastatic niche formation and metastasis in the 143-B Xenograft Mouse Osteosarcoma Model. Cancers 12, 3457 (2020).
Stamatopoulos, A. et al. Mesenchymal stromal cells for bone sarcoma treatment: Roadmap to clinical practice. J. Bone Oncol. 16, 100231 (2019).
Gross, A. C. et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight 3, e99791 (2018).
Mazumdar, A. et al. Osteosarcoma-derived extracellular vesicles induce lung fibroblast reprogramming. Int J. Mol. Sci. 21, 5451 (2020).
Zhang, W. et al. Adaptive fibrogenic reprogramming of osteosarcoma stem cells promotes metastatic growth. Cell Rep. 24, 1266–1277 (2018).
Ren, L. et al. Dysregulation of ezrin phosphorylation prevents metastasis and alters cellular metabolism in osteosarcoma. Cancer Res. 72, 1001–1012 (2012).
Navet, B. et al. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases. Cancers 10, 398 (2018).
Ji, H. et al. Lymph node metastasis in cancer progression: molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduct. Target Ther. 8, 367 (2023).
Follain, G. et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat. Rev. Cancer 20, 107–124 (2020).
Pelon, F. et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 11, 404 (2020).
Nathanson, S. D., Shah, R. & Rosso, K. Sentinel lymph node metastases in cancer: Causes, detection and their role in disease progression. Semin Cell Dev. Biol. 38, 106–116 (2015).
Reticker-Flynn, N. E. et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 185, 1924–1942 e1923 (2022).
Motohara, T. et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 38, 2885–2898 (2018).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011).
Sheehan, J. P. et al. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J. Neurosurg. 97, 1276–1281 (2002).
Yousefi, M. et al. Lung cancer-associated brain metastasis: Molecular mechanisms and therapeutic options. Cell Oncol. 40, 419–441 (2017).
O’Dowd, P. D., Sutcliffe, D. F. & Griffith, D. M. Oxaliplatin and its derivatives – An overview. Coord. Chem. Rev. 497, 215439 (2023).
Gerstner, E. R. & Fine, R. L. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J. Clin. Oncol. 25, 2306–2312 (2007).
Schuette, W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 45, S253–S257 (2004).
Regina, A. et al. Multidrug resistance in brain tumors: roles of the blood-brain barrier. Cancer Metastasis Rev. 20, 13–25 (2001).
Zakaria, N. et al. Targeting lung cancer stem cells: research and clinical impacts. Front Oncol. 7, 80 (2017).
Li, L. & Bhatia, R. Stem cell quiescence. Clin. Cancer Res. 17, 4936–4941 (2011).
Nassar, D. & Blanpain, C. Cancer stem cells: basic concepts and therapeutic implications. Annu. Rev. Pathol. 11, 47–76 (2016).
Xu, Z. Y. et al. 5-Fluorouracil chemotherapy of gastric cancer generates residual cells with properties of cancer stem cells. Int J. Biol. Sci. 11, 284–294 (2015).
Robey, R. W. et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 18, 452–464 (2018).
Moitra, K. Overcoming multidrug resistance in cancer stem cells. Biomed. Res. Int. 2015, 635745 (2015).
Bleau, A. M. et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 4, 226–235 (2009).
Plaks, V., Kong, N. & Werb, Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16, 225–238 (2015).
Hu, Y. et al. Fibroblast-derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS One 10, e0125625 (2015).
Korkaya, H. et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell 47, 570–584 (2012).
Dieras, V. et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 18, 732–742 (2017).
Olson, E. M. et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann. Oncol. 24, 1526–1533 (2013).
von Minckwitz, G. et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 377, 122–131 (2017).
von Minckwitz, G. et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 380, 617–628 (2019).
Freedman, R. A. et al. TBCRC 022: A Phase II trial of Neratinib and Capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 37, 1081–1089 (2019).
Ma, F. et al. Phase I Study and biomarker analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase inhibitor, in patients with human epidermal growth factor Receptor 2–Positive metastatic breast cancer. J. Clin. Oncol. 35, 3105–3112 (2017).
Xuhong, J. C., Qi, X. W., Zhang, Y. & Jiang, J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am. J. Cancer Res. 9, 2103–2119 (2019).
Murthy, R. K. et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med 382, 597–609 (2020).
Tolaney, S. M. et al. A Phase II Study of Abemaciclib in patients with brain metastases secondary to hormone receptor–positive breast cancer. Clin. Cancer Res. 26, 5310–5319 (2020).
Shepherd, F. A. et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 353, 123–132 (2005).
Fukuoka, M. et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J. Clin. Oncol. 21, 2237–2246 (2003).
Sato, J. D. et al. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol. Biol. Med. 1, 511–529 (1983).
Wu, Y. L. et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann. Oncol. 24, 993–999 (2013).
Alexander, M., Kim, S. Y. & Cheng, H. Update 2020: Management of non-small cell lung cancer. Lung 198, 897–907 (2020).
You, L. et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene 23, 6170–6174 (2004).
Ippen, F. M. et al. The Dual PI3K/mTOR pathway inhibitor GDC-0084 achieves antitumor activity in PIK3CA-mutant breast cancer brain metastases. Clin. Cancer Res. 25, 3374–3383 (2019).
Ippen, F. M. et al. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 21, 1401–1411 (2019).
Choi, Y. J. et al. Phase II Study of Dovitinib in patients with castration-resistant prostate cancer (KCSG-GU11-05). Cancer Res. Treat. 50, 1252–1259 (2018).
Fallon, M. et al. A Randomized Placebo-controlled trial of the anti-nerve growth factor antibody Tanezumab in subjects with cancer pain due to bone metastasis. Oncologist 28, e1268–e1278 (2023).
Challita-Eid, P. M. et al. Enfortumab Vedotin antibody-drug conjugate targeting Nectin-4 Is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76, 3003–3013 (2016).
Francisco, L. M., Sage, P. T. & Sharpe, A. H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242 (2010).
Hellmann, M. D., Friedman, C. F. & Wolchok, J. D. Combinatorial cancer immunotherapies. Adv. Immunol. 130, 251–277 (2016).
Goldberg, S. B. et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 17, 976–983 (2016).
Long, G. V. et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 19, 672–681 (2018).
Zhou, Y. et al. First-in-maintenance therapy for localized high-grade Osteosarcoma: An open-label Phase I/II trial of the anti-PD-L1 Antibody ZKAB001. Clin. Cancer Res. 29, 764–774 (2023).
Long, G. V. et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 23, 1378–1388 (2022).
Boye, K. et al. Pembrolizumab in advanced osteosarcoma: results of a single-arm, open-label, phase 2 trial. Cancer Immunol. Immunother. 70, 2617–2624 (2021).
Velev, M. et al. Efficacy and safety of nivolumab in bone metastases from renal cell carcinoma: Results of the GETUG-AFU26-NIVOREN multicentre phase II study. Eur. J. Cancer 182, 66–76 (2023).
Tawbi, H. A. et al. Combined Nivolumab and Ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med 379, 722–730 (2018).
Priceman, S. J. et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2(+) breast cancer metastasis to the brain. Clin. Cancer Res. 24, 95–105 (2018).
Pastorino, U. et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc Surg. 113, 37–49 (1997).
Fong, Y. et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Ann. Surg. 230, 309–318 (1999).
Beckham, T. H., Yang, T. J., Gomez, D. & Tsai, C. J. Metastasis-directed therapy for oligometastasis and beyond. Br. J. Cancer 124, 136–141 (2020).
Gillespie, E. F. et al. Prophylactic radiation therapy versus standard of care for patients with high-risk asymptomatic bone metastases: a multicenter, randomized phase ii clinical trial. J. Clin. Oncol. 42, 38–46 (2024).
Shady, W. et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology 278, 601–611 (2016).
de Baere, T. et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann. Oncol. 26, 987–991 (2015).
Rusthoven, K. E. et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J. Clin. Oncol. 27, 1579–1584 (2009).
Ito, K. et al. Phase 2 clinical trial of stereotactic body radiation therapy for painful nonspine bone metastases. Pr. Radiat. Oncol. 11, e139–e145 (2021).
Milano, M. T. et al. Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: Some patients survive longer than a decade. Radiother. Oncol. 131, 45–51 (2019).
Lou, Y. et al. Study on the correlation between pain and cytokine expression in the peripheral blood of patients with bone metastasis of malignant cancer treated using external radiation therapy. Pain. Res Manag 2022, 1119014 (2022).
Huisman, M. et al. International consensus on use of focused ultrasound for painful bone metastases: Current status and future directions. Int J. Hyperth. 31, 251–259 (2015).
Rieke, V. & Butts Pauly, K. MR thermometry. J. Magn. Reson Imaging 27, 376–390 (2008).
Napoli, A. et al. MR-guided high-intensity focused ultrasound: current status of an emerging technology. Cardiovasc Interv. Radio. 36, 1190–1203 (2013).
Napoli, A. et al. Focused ultrasound and external beam radiation therapy for painful bone metastases: A phase II clinical trial. Radiology 307, e211857 (2023).
Napoli, A. et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics 33, 1555–1568 (2013).
Bitton, R. R. et al. MRI-guided focused ultrasound of osseous metastases: treatment parameters associated with successful pain reduction. Invest Radio. 56, 141–146 (2021).
Baselga, J. The EGFR as a target for anticancer therapy–focus on cetuximab. Eur. J. Cancer 37, S16–S22 (2001).
Liu, Z. et al. Candidate tumour suppressor CCDC19 regulates miR‐184 direct targeting of C‐Myc thereby suppressing cell growth in non‐small cell lung cancers. J. Cell Mol. Med 18, 1667–1679 (2014).
Kyriakopoulos, C. E. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. J. Clin. Oncol. 36, 1080–1087 (2018).
Heery, C. R. et al. Samarium-153-EDTMP (Quadramet(R)) with or without vaccine in metastatic castration-resistant prostate cancer: A randomized Phase 2 trial. Oncotarget 7, 69014–69023 (2016).
Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 29, 15–18 (2002).
Riechelmann, R. & Grothey, A. Antiangiogenic therapy for refractory colorectal cancer: current options and future strategies. Ther. Adv. Med. Oncol. 9, 106–126 (2017).
Hey, S. P. et al. A systematic review and meta-analysis of Bevacizumab in first-line metastatic breast cancer: lessons for research and regulatory enterprises. J. Natl Cancer Inst. 112, 335–342 (2020).
Fujita, H. et al. The novel VEGF receptor/MET-targeted kinase inhibitor TAS-115 has marked in vivo antitumor properties and a favorable tolerability profile. Mol. Cancer Ther. 12, 2685–2696 (2013).
Mackey, J. R. et al. Primary results of ROSE/TRIO-12, a randomized placebo-controlled phase III trial evaluating the addition of ramucirumab to first-line docetaxel chemotherapy in metastatic breast cancer. J. Clin. Oncol. 33, 141–148 (2015).
Bostrom, J. et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science 323, 1610–1614 (2009).
Olsson, A. et al. Tasquinimod triggers an early change in the polarization of tumor associated macrophages in the tumor microenvironment. J. Immunother. Cancer 3, 53 (2015).
Sleeboom, J. J. F. et al. The extracellular matrix as hallmark of cancer and metastasis: From biomechanics to therapeutic targets. Sci. Transl. Med. 16, eadg3840 (2024).
Secli, L., Fusella, F., Avalle, L. & Brancaccio, M. The dark-side of the outside: how extracellular heat shock proteins promote cancer. Cell Mol. Life Sci. 78, 4069–4083 (2021).
Armstrong, H. K. et al. Dysregulated fibronectin trafficking by Hsp90 inhibition restricts prostate cancer cell invasion. Sci. Rep. 8, 2090 (2018).
Ferreira, S., Saraiva, N., Rijo, P. & Fernandes, A. S. LOXL2 inhibitors and breast cancer progression. Antioxidants 10, 312 (2021).
Chen, L.-C. et al. Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway. Breast Cancer Res. Treat. 134, 989–1004 (2012).
Leung, L. et al. Anti-metastatic inhibitors of Lysyl Oxidase (LOX): Design and structure-activity relationships. J. Med Chem. 62, 5863–5884 (2019).
Chang, J. et al. Pre-clinical evaluation of small molecule LOXL2 inhibitors in breast cancer. Oncotarget 8, 26066–26078 (2017).
Tang, H. et al. Lysyl oxidase drives tumour progression by trapping EGF receptors at the cell surface. Nat. Commun. 8, 14909 (2017).
Desgrosellier, J. S. et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat. Med. 15, 1163–1169 (2009).
Ross, M. H. et al. Bone-induced expression of Integrin beta3 enables targeted nanotherapy of breast cancer metastases. Cancer Res. 77, 6299–6312 (2017).
Halama, N. et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by Anti-CCR5 therapy in cancer patients. Cancer Cell 29, 587–601 (2016).
Lee, Y., Park, H. R., Chun, H. J. & Lee, J. Silibinin prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease via mitochondrial stabilization. J. Neurosci. Res. 93, 755–765 (2015).
Henrik Heiland, D. et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 10, 2541 (2019).
Nenkov, M., Ma, Y., Gassler, N. & Chen, Y. Metabolic reprogramming of colorectal cancer cells and the microenvironment: implication for therapy. Int J. Mol. Sci. 22, 6262 (2021).
Kroemer, G. & Pouyssegur, J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13, 472–482 (2008).
Xing, B. C., Wang, C., Ji, F. J. & Zhang, X. B. Synergistically suppressive effects on colorectal cancer cells by combination of mTOR inhibitor and glycolysis inhibitor, Oxamate. Int J. Clin. Exp. Pathol. 11, 4439–4445 (2018).
Park, G. B., Chung, Y. H. & Kim, D. 2-Deoxy-D-glucose suppresses the migration and reverses the drug resistance of colon cancer cells through ADAM expression regulation. Anticancer Drugs 28, 410–420 (2017).
Matés, J. M. et al. Therapeutic targeting of glutaminolysis as an essential strategy to combat cancer. Semin Cell Dev. Biol. 98, 34–43 (2020).
Chen, Z. et al. Novel 1,3,4-Selenadiazole-Containing Kidney-Type Glutaminase inhibitors showed improved cellular uptake and antitumor activity. J. Med. Chem. 62, 589–603 (2019).
Momcilovic, M. et al. Targeted Inhibition of EGFR and Glutaminase induces metabolic crisis in EGFR mutant lung cancer. Cell Rep. 18, 601–610 (2017).
Gross, M. I. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 13, 890–901 (2014).
Zaytseva, Y. Lipid metabolism as a targetable metabolic vulnerability in colorectal cancer. Cancers 13, 301 (2021).
Pandurangan, A. K. & Esa, N. M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac. J. Cancer Prev. 15, 5501–5508 (2014).
Newman, A. C. & Maddocks, O. D. K. One-carbon metabolism in cancer. Br. J. Cancer 116, 1499–1504 (2017).
Raje, N. et al. Evaluating results from the multiple myeloma patient subset treated with denosumab or zoledronic acid in a randomized phase 3 trial. Blood Cancer J. 6, e378 (2016).
Smith, M. R. et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann. Oncol. 26, 368–374 (2015).
Diel, I. J. et al. The role of denosumab in the prevention of hypercalcaemia of malignancy in cancer patients with metastatic bone disease. Eur. J. Cancer 51, 1467–1475 (2015).
Stopeck, A. et al. Cost-effectiveness of denosumab for the prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J. Med. Econ. 23, 37–47 (2020).
Lin, Y. et al. Biosimilarity of HS-20090 to Denosumab in healthy Chinese subjects: a randomized, double-blinded, pharmacokinetics/pharmacodynamics study. Expert Opin. Investig. Drugs 31, 1125–1132 (2022).
Li, H. et al. Efficacy and safety of Denosumab Biosimilar QL1206 Versus Denosumab in patients with bone metastases from solid tumors: a randomized phase III trial. BioDrugs 37, 259–269 (2023).
Hussain, M. et al. Differential effect on bone lesions of targeting integrins: Randomized Phase II trial of Abituzumab in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 22, 3192–3200 (2016).
Grullich, C. Cabozantinib: a MET, RET, and VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res. 201, 207–214 (2014).
Yu, K. J. et al. Cabozantinib-induced osteoblast secretome promotes survival and migration of metastatic prostate cancer cells in bone. Oncotarget 8, 74987–75006 (2017).
Leibowitz-Amit, R. et al. Changes in plasma biomarkers following treatment with cabozantinib in metastatic castration-resistant prostate cancer: a post hoc analysis of an extension cohort of a phase II trial. J. Transl. Med. 14, 12 (2016).
Crabb, S. J. et al. Pan-AKT Inhibitor Capivasertib with Docetaxel and Prednisolone in metastatic castration-resistant prostate cancer: a randomized, Placebo-Controlled Phase II Trial (ProCAID). J. Clin. Oncol. 39, 190–201 (2021).
Oswald, A. J. et al. Aromatase inhibition plus/minus Src inhibitor saracatinib (AZD0530) in advanced breast cancer therapy (ARISTACAT): a randomised phase II study. Breast Cancer Res. Treat. 199, 35–46 (2023).
Vander Ark, A., Cao, J. & Li, X. Mechanisms and approaches for overcoming Enzalutamide resistance in prostate cancer. Front. Oncol. 8, 180 (2018).
Evans, C. P. et al. The PREVAIL Study: Primary outcomes by site and extent of baseline disease for Enzalutamide-treated men with chemotherapy-naive metastatic castration-resistant prostate cancer. Eur. Urol. 70, 675–683 (2016).
Graff, J. N. et al. Efficacy and safety of enzalutamide in patients 75 years or older with chemotherapy-naive metastatic castration-resistant prostate cancer: results from PREVAIL. Ann. Oncol. 27, 286–294 (2016).
David, K. et al. Changes in bone and mineral homeostasis after short-term androgen deprivation therapy with or without androgen receptor signalling inhibitor - substudy of a single-centre, double blind, randomised, placebo-controlled phase 2 trial. EBioMedicine 97, 104817 (2023).
Wallander, M., Axelsson, K. F., Lundh, D. & Lorentzon, M. Patients with prostate cancer and androgen deprivation therapy have increased risk of fractures-a study from the fractures and fall injuries in the elderly cohort (FRAILCO). Osteoporos. Int. 30, 115–125 (2019).
von Moos, R. et al. Management of bone health in solid tumours: From bisphosphonates to a monoclonal antibody. Cancer Treat. Rev. 76, 57–67 (2019).
Coleman, R. et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 31, 1650–1663 (2020).
Alzahrani, M. et al. Symptomatic skeletal-related events in patients receiving longer term bone-modifying agents for bone metastases from breast and castration resistant prostate cancers. Support Care Cancer 30, 3977–3984 (2022).
Chee, C. E. et al. A Phase I, first-in-human study of PRL3-zumab in advanced, refractory solid tumors and hematological malignancies. Target Oncol. 18, 391–402 (2023).
De Bono, J. et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020).
Pasqualini, R. et al. Targeting the interleukin-11 receptor alpha in metastatic prostate cancer: A first-in-man study. Cancer 121, 2411–2421 (2015).
Nilsson, S. Radium-223 dichloride for the treatment of bone metastatic castration-resistant prostate cancer: an evaluation of its safety. Expert Opin. Drug Saf. 14, 1127–1136 (2015).
Hoskin, P. et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 15, 1397–1406 (2014).
Parker, C. et al. Effect of radium-223 dichloride (Ra-223) on hospitalisation: An analysis from the phase 3 randomised Alpharadin in Symptomatic Prostate Cancer Patients (ALSYMPCA) trial. Eur. J. Cancer 71, 1–6 (2017).
Nilsson, S. et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann. Oncol. 27, 868–874 (2016).
Srivastava, S. C. et al. The development and in-vivo behavior of tin containing radiopharmaceuticals–I. Chemistry, preparation, and biodistribution in small animals. Int J. Nucl. Med. Biol. 12, 167–174 (1985).
Myint, Z. W. et al. A single arm phase II study of bone-targeted Sn-117 m-DTPA in symptomatic castration-resistant prostate cancer with skeletal metastases. BMC Cancer 22, 415 (2022).
Chen, P. et al. Efficacy and safety of 188Re-HEDP in lung cancer patients with bone metastases: a randomized, multicenter, multiple-dose phase IIa study. Int J. Clin. Oncol. 26, 1212–1220 (2021).
Eary, J. F. et al. Samarium-153-EDTMP biodistribution and dosimetry estimation. J. Nucl. Med. 34, 1031–1036 (1993).
Thang, S. P. et al. Clinical outcomes of 177lutetium-prostate-specific membrane antigen therapy in advanced prostate cancer-a prospective pilot study in an Asian population. Nucl. Med. Commun. 41, 618–628 (2020).
Taheri, M. et al. 153Sm-EDTMP and 177Lu-EDTMP are equally safe and effective in pain palliation from skeletal metastases. Nuklearmedizin 57, 174–180 (2018).
Tayyeb, B. & Parvin, M. Pathogenesis of breast cancer metastasis to brain: a comprehensive approach to the signaling network. Mol. Neurobiol. 53, 446–454 (2016).
Patchell, R. A. et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280, 1485–1489 (1998).
Chidel, M. A. et al. Treatment outcome for patients with primary nonsmall-cell lung cancer and synchronous brain metastasis. Radiat. Oncol. Investig. 7, 313–319 (1999).
Suh, J. H. Stereotactic radiosurgery for the management of brain metastases. N. Engl. J. Med. 362, 1119–1127 (2010).
Aoyama, H. et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295, 2483–2491 (2006).
Fortin, D. The blood-brain barrier: its influence in the treatment of brain tumors metastases. Curr. Cancer Drug Targets 12, 247–259 (2012).
Welsh, J. W. et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 31, 895–902 (2013).
Mok, T. S. et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 376, 629–640 (2017).
Ceresoli, G. L. et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann. Oncol. 15, 1042–1047 (2004).
Gadgeel, S. M. et al. Pooled analysis of CNS response to Alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 34, 4079–4085 (2016).
Crino, L. et al. Multicenter Phase II study of whole-body and intracranial activity with Ceritinib in patients With ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and Crizotinib: Results from ASCEND-2. J. Clin. Oncol. 34, 2866–2873 (2016).
Camidge, D. R. et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 379, 2027–2039 (2018).
Wang, Y., Ye, F., Liang, Y. & Yang, Q. Breast cancer brain metastasis: insight into molecular mechanisms and therapeutic strategies. Br. J. Cancer 125, 1056–1067 (2021).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15, 1452–1459 (2009).
Lin, N. U. et al. Phase II Trial of Lapatinib for Brain Metastases in Patients With Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer. J. Clin. Oncol. 26, 1993–1999 (2008).
Bachelot, T. et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 14, 64–71 (2013).
Park, Y. H. et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br. J. Cancer 100, 894–900 (2009).
Singh, K. et al. Update on the management of brain metastasis. Neurotherapeutics 19, 1772–1781 (2022).
Davies, M. A. et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 18, 863–873 (2017).
Robert, C. et al. Five-year outcomes with Dabrafenib plus Trametinib in metastatic Melanoma. N. Engl. J. Med. 381, 626–636 (2019).
Drago, J. Z. et al. Clinical experience with combination BRAF/MEK inhibitors for melanoma with brain metastases: a real-life multicenter study. Melanoma Res. 29, 65–69 (2019).
Holbrook, K. et al. Intracranial antitumor activity with encorafenib plus binimetinib in patients with melanoma brain metastases: A case series. Cancer 126, 523–530 (2020).
Al Bandar, M. H. & Kim, N. K. Current status and future perspectives on treatment of liver metastasis in colorectal cancer (Review). Oncol. Rep. 37, 2553–2564 (2017).
Watanabe, T. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J. Clin. Oncol. 17, 1–29 (2011).
Ismaili, N. Treatment of colorectal liver metastases. World J. Surg. Oncol. 9, (2011).
Pawlik, T. M. et al. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J. Gastrointest. Surg. 10, 240–248 (2006).
Altendorf-Hofmann, A. & Scheele, J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg. Oncol. Clin. N. Am. 12, 165–192 (2003).
Mendez Romero, A. & de Man, R. A. Stereotactic body radiation therapy for primary and metastatic liver tumors: From technological evolution to improved patient care. Best. Pr. Res Clin. Gastroenterol. 30, 603–616 (2016).
Leporrier, J. et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br. J. Surg. 93, 465–474 (2006).
Zakaria, S. et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann. Surg. 246, 183–191 (2007).
Scheithauer, W. et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 306, 752–755 (1993).
Saltz, L. B. et al. Irinotecan plus Fluorouracil and Leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 343, 905–914 (2000).
An, X. et al. Short term results of neoadjuvant chemoradiotherapy with fluoropyrimidine alone or in combination with oxaliplatin in locally advanced rectal cancer: A meta analysis. Eur. J. Cancer 49, 843–851 (2013).
Saltz, L. B. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 26, 2013–2019 (2008).
Bokemeyer, C. et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 27, 663–671 (2009).
Johdi, N. A. & Sukor, N. F. Colorectal cancer immunotherapy: options and strategies. Front. Immunol. 11, 1624 (2020).
Franke, A. J. et al. Immunotherapy for colorectal cancer: a review of current and novel therapeutic approaches. J. Natl Cancer Inst. 111, 1131–1141 (2019).
Overman, M. J. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Antoniotti, C. et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 23, 876–887 (2022).
Xue, T. et al. PTX promotes breast cancer migration and invasion by recruiting ATF4 to upregulate FGF19. Cell Signal 122, 111309 (2024).
Wu, C. et al. Reactive myelopoiesis and FX-expressing macrophages triggered by chemotherapy promote cancer lung metastasis. JCI Insight 8, e167499 (2023).
Liang, S. et al. Co-expression of CD44v6 and MMP2 predicts lung metastasis and unfavorable prognosis in osteosarcoma. Future Oncol. 20, 1799–1806 (2024).
Cheng, S. et al. RGCC-mediated PLK1 activity drives breast cancer lung metastasis by phosphorylating AMPKalpha2 to activate oxidative phosphorylation and fatty acid oxidation. J. Exp. Clin. Cancer Res. 42, 342 (2023).
Peng, J. et al. Upregulation of collagen type X alpha 1 promotes the progress of triple-negative breast cancer via Wnt/beta-catenin signaling. Mol. Carcinog. 63, 1588–1598 (2024).
Liu, J. et al. Type X collagen knockdown inactivate ITGB1/PI3K/AKT to suppress chronic unpredictable mild stress-stimulated triple-negative breast cancer progression. Int J. Biol. Macromol. 273, 133074 (2024).
Wang, R. et al. Antiplatelet drug ticagrelor suppresses triple negative breast cancer metastasis by targeting PI3K. Biochem. Pharm. 226, 116408 (2024).
Ganesan, K. et al. Ononin inhibits triple-negative breast cancer lung metastasis by targeting the EGFR-mediated PI3K/Akt/mTOR pathway. Sci. China Life Sci. 67, 1849–1866 (2024).
Zou, W. et al. The circadian gene ARNTL2 promotes nasopharyngeal carcinoma invasiveness and metastasis through suppressing AMOTL2-LATS-YAP pathway. Cell Death Dis. 15, 466 (2024).
Zhou, Q. et al. PLUNC inhibits invasion and metastasis in nasopharyngeal carcinoma by inhibiting NLRP3 inflammasome activation. Biochim Biophys. Acta Mol. Basis Dis. 1870, 167352 (2024).
Lin, C. Y. et al. NAMPT enhances LOX expression and promotes metastasis in human chondrosarcoma cells by inhibiting miR-26b-5p synthesis. J. Cell Physiol. 239, e31345 (2024).
Wang, M. et al. Arenobufagin inhibits lung metastasis of colorectal cancer by targeting c-MYC/Nrf2 axis. Phytomedicine 127, 155391 (2024).
Ma, M. et al. Low expression of ZFP36L1 in osteosarcoma promotes lung metastasis by inhibiting the SDC4-TGF-beta signaling feedback loop. Oncogene 43, 47–60 (2024).
Deng, Z. et al. Ziyuglycoside II, a triterpene glycoside compound in Sanguisorbae officinalis l. extract, suppresses metastasis in osteosarcoma via CBX4-mediated Wnt/beta-catenin signal pathway. Phytomedicine 132, 155716 (2024).
Hou, C.-H., Chen, W.-L. & Lin, C.-Y. Targeting nerve growth factor-mediated osteosarcoma metastasis: mechanistic insights and therapeutic opportunities using larotrectinib. Cell Death Dis. 15, 381 (2024).
Lee, B. M., Choi, J. Y. & Seong, J. Efficacy of local treatment in lymph node metastasis from hepatocellular carcinoma. Liver Cancer 12, 218–228 (2023).
Caivano, D. et al. Stereotactic body radiation therapy for the treatment of lymph node metastases: a retrospective mono-institutional study in a large cohort of patients. Front. Oncol. 13, 1163213 (2023).
Lin, C. et al. Clinical treatment considerations in the intensity-modulated radiotherapy era for parotid lymph node metastasis in patients with nasopharyngeal carcinoma. Radiother. Oncol. 186, 109802 (2023).
Saddawi-Konefka, R., Schokrpur, S. & Gutkind, J. S. Let it be: Preserving tumor-draining lymph nodes in the era of immuno-oncology. Cancer Cell 42, 930–933 (2024).
Reticker-Flynn, N. E. & Engleman, E. G. Lymph nodes: at the intersection of cancer treatment and progression. Trends Cell Biol. 33, 1021–1034 (2023).
Wu, H. et al. RACK1 promotes the invasive activities and lymph node metastasis of cervical cancer via galectin-1. Cancer Lett. 469, 287–300 (2020).
Xu, L. et al. Receptor for activated C kinase 1 promotes cervical cancer lymph node metastasis via the glycolysis‑dependent AKT/mTOR signaling. Int J. Oncol. 61, 83 (2022).
Du, Q. et al. FASN promotes lymph node metastasis in cervical cancer via cholesterol reprogramming and lymphangiogenesis. Cell Death Dis. 13, 488 (2022).
Mei, X. et al. DHCR7 promotes lymph node metastasis in cervical cancer through cholesterol reprogramming-mediated activation of the KANK4/PI3K/AKT axis and VEGF-C secretion. Cancer Lett. 584, 216609 (2024).
Zhu, T. et al. SOAT1 promotes gastric cancer lymph node metastasis through lipid synthesis. Front. Pharm. 12, 769647 (2021).
Liu, P. et al. MicroRNA-431-5p inhibits angiogenesis, lymphangiogenesis, and lymph node metastasis by affecting TGF-beta1/SMAD2/3 signaling via ZEB1 in gastric cancer. Mol. Carcinog. 63, 1378–1391 (2024).
Kong, Y. et al. circNFIB1 inhibits lymphangiogenesis and lymphatic metastasis via the miR-486-5p/PIK3R1/VEGF-C axis in pancreatic cancer. Mol. Cancer 19, 82 (2020).
Wang, Y. et al. Long Noncoding RNA VESTAR regulates Lymphangiogenesis and lymph node metastasis of esophageal squamous cell carcinoma by enhancing VEGFC mRNA stability. Cancer Res. 81, 3187–3199 (2021).
Yan, S. et al. MiR-182-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-C. Cancer Lett. 488, 18–26 (2020).
Zhang, C. et al. Upregulated miR‑411‑5p levels promote lymph node metastasis by targeting RYBP in head and neck squamous cell carcinoma. Int J. Mol. Med. 47, 36 (2021).
Moon, J. H., Lee, S. H. & Lim, Y. C. Wnt/beta-catenin/Slug pathway contributes to tumor invasion and lymph node metastasis in head and neck squamous cell carcinoma. Clin. Exp. Metastasis 38, 163–174 (2021).
Chen, L. et al. Upregulation of microRNA-141 suppresses epithelial-mesenchymal transition and lymph node metastasis in laryngeal cancer through HOXC6-dependent TGF-beta signaling pathway. Cell Signal 66, 109444 (2020).
Li, J. et al. Unraveling the molecular mechanisms of lymph node metastasis in ovarian cancer: focus on MEOX1. J. Ovarian Res. 17, 61 (2024).
Jia, Y. et al. Long non-coding RNA NEAT1 mediated RPRD1B stability facilitates fatty acid metabolism and lymph node metastasis via c-Jun/c-Fos/SREBP1 axis in gastric cancer. J. Exp. Clin. Cancer Res. 41, 287 (2022).
Yang, Z. et al. Folic acid-mediated hollow Mn3O4 nanocomposites for in vivo MRI/FLI monitoring the metastasis of gastric cancer. Biomed. Eng. Online 23, 53 (2024).
Wang, X. et al. A TMVP1-modified near-infrared nanoprobe: molecular imaging for tumor metastasis in sentinel lymph node and targeted enhanced photothermal therapy. J. Nanobiotechnol. 21, 130 (2023).
Sheikh, A. et al. Understanding the novel approach of nanoferroptosis for cancer therapy. Nanomicro Lett. 16, 188 (2024).
Zhang, W. et al. The progress and perspective of nanoparticle-enabled tumor metastasis treatment. Acta Pharm. Sin. B 10, 2037–2053 (2020).
Yang, Y. et al. Semiconducting polymer nanoparticles as theranostic system for near-Infrared-II fluorescence imaging and photothermal therapy under safe laser fluence. ACS Nano 14, 2509–2521 (2020).
Tang, X. et al. Heterogeneous-structure-based AuNBs@TiO(2) nano-photosensitizers for computed Tomography Imaging Guided NIR-II photodynamic therapy and cancer metastatic prevention. Adv. Health. Mater. 13, e2304209 (2024).
Wei, D., Sun, Y., Zhu, H. & Fu, Q. Stimuli-responsive polymer-based nanosystems for cancer theranostics. ACS Nano 17, 23223–23261 (2023).
Mao, L. et al. Stimuli-responsive polymeric nanovaccines toward next-generation immunotherapy. ACS Nano 17, 9826–9849 (2023).
Huang, Q. et al. Tumor microenvironment–responsive versatile “Trojan horse” theranostic nanoplatform for magnetic resonance imaging–guided multimodal synergistic antitumor treatment. Acta Biomater. 147, 270–286 (2022).
Ashrafizadeh, M. et al. (Nano)platforms in breast cancer therapy: Drug/gene delivery, advanced nanocarriers and immunotherapy. Med. Res. Rev. 43, 2115–2176 (2023).
Cai, Q. et al. Nanomaterial-based strategies for preventing tumor metastasis by interrupting the metastatic biological processes. Adv. Health. Mater. 13, e2303543 (2024).
Wu, J., Long, Y., Li, M. & He, Q. Emerging nanomedicine-based therapeutics for hematogenous metastatic cascade inhibition: Interfering with the crosstalk between “seed and soil. Acta Pharm. Sin. B 11, 2286–2305 (2021).
Gu, J. et al. Radioactive hybrid semiconducting polymer nanoparticles for imaging-guided tri-modal therapy of breast cancer. J. Mater. Chem. B 12, 6091–6101 (2024).
Wu, L. et al. Diagnosis and treatment status of inoperable locally advanced breast cancer and the application value of inorganic nanomaterials. J. Nanobiotechnol. 22, 366 (2024).
Li, J. et al. Hybrid nanomaterials for cancer immunotherapy. Adv. Sci. 10, e2204932 (2022).
Huang, Y. et al. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: prevention and treatment. Signal Transduct. Target Ther. 9, 34 (2024).
Schambach, A. et al. A new age of precision gene therapy. Lancet 403, 568–582 (2024).
Xing, H. & Meng, L. H. CRISPR-cas9: a powerful tool towards precision medicine in cancer treatment. Acta Pharm. Sin. 41, 583–587 (2020).
Daley, G. Q. Welcoming the era of gene editing in medicine. N. Engl. J. Med 390, 1642–1645 (2024).
Zhao, G. et al. EIF5A2 controls ovarian tumor growth and metastasis by promoting epithelial to mesenchymal transition via the TGFbeta pathway. Cell Biosci. 11, 70 (2021).
Wang, Y. et al. Co-delivery of Cas9 mRNA and guide RNAs for editing of LGMN gene represses breast cancer cell metastasis. Sci. Rep. 14, 8095 (2024).
Marayati, R. et al. PIM3 kinase promotes tumor metastasis in hepatoblastoma by upregulating cell surface expression of chemokine receptor cxcr4. Clin. Exp. Metastasis 39, 899–912 (2022).
Liao, L. et al. Anti-HIV Drug Elvitegravir suppresses cancer metastasis via increased proteasomal degradation of m6A Methyltransferase METTL3. Cancer Res. 82, 2444–2457 (2022).
Zhao, H. et al. STIM1 is a metabolic checkpoint regulating the invasion and metastasis of hepatocellular carcinoma. Theranostics 10, 6483–6499 (2020).
Wu, C. et al. AKR1C3-dependent lipid droplet formation confers hepatocellular carcinoma cell adaptability to targeted therapy. Theranostics 12, 7681–7698 (2022).
Zhang, J. et al. DSTYK promotes metastasis and chemoresistance via EMT in colorectal cancer. Front. Pharm. 11, 1250 (2020).
Munsterberg, J. et al. ALCAM contributes to brain metastasis formation in non-small-cell lung cancer through interaction with the vascular endothelium. Neuro Oncol. 22, 955–966 (2020).
Li, M. et al. Knockdown of hypoxia-inducible factor-1 alpha by tumor targeted delivery of CRISPR/Cas9 system suppressed the metastasis of pancreatic cancer. J. Control Release 304, 204–215 (2019).
Mammadova-Bach, E. et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood 135, 1146–1160 (2020).
Chae, S.-Y. et al. Rationally designed nanoparticle delivery of Cas9 ribonucleoprotein for effective gene editing. J. Control Release 345, 108–119 (2022).
Cheng, H., Zhang, F. & Ding, Y. CRISPR/Cas9 delivery system engineering for genome editing in therapeutic applications. Pharmaceutics 13, 1649 (2021).
Smith, D. J. et al. CRISPR-Cas9 potential for identifying novel therapeutic targets in muscle-invasive bladder cancer. Nat. Rev. Urol. 22, 55–65 (2025).
Ormond, K. E. et al. The clinical application of gene editing: ethical and social issues. Per Med. 16, 337–350 (2019).
Romero, P. et al. The Human Vaccines Project: A roadmap for cancer vaccine development. Sci. Transl. Med. 8, 334ps339 (2016).
Yang, C. et al. Photodynamic therapy derived personalized whole cell tumor vaccine prevents postsurgery tumor recurrence and metastasis. Small 20, e2308456 (2024).
Oltmanns, F. et al. Mucosal tumor vaccination delivering endogenous tumor antigens protects against pulmonary breast cancer metastases. J. Immunother. Cancer 12, e008652 (2024).
Xie, C. et al. A nanovaccine based on adjuvant Peptide FK-13 and l-Phenylalanine Poly(ester amide) Enhances CD8(+) T cell-mediated antitumor immunity. Adv. Sci. 10, e2300418 (2023).
Dong, L. et al. Stimuli-responsive mRNA vaccines to induce robust CD8(+) T cell response via ROS-mediated innate immunity boosting. J. Am. Chem. Soc. 146, 19218–19228 (2024).
Curcio, C. et al. PI3Kgamma inhibition combined with DNA vaccination unleashes a B-cell-dependent antitumor immunity that hampers pancreatic cancer. J. Exp. Clin. Cancer Res. 43, 157 (2024).
Wang, T. et al. Antigen self-presented personalized nanovaccines boost the immunotherapy of highly invasive and metastatic tumors. ACS Nano 18, 6333–6347 (2024).
Mahadevan, K. K. et al. Type I conventional dendritic cells facilitate immunotherapy in pancreatic cancer. Science 384, eadh4567 (2024).
Gurunathan, S. et al. Nanovaccines: An effective therapeutic approach for cancer therapy. Biomed. Pharmacother. 170, 115992 (2024).
Zou, Y. et al. Cold nanozyme for precise enzymatic antitumor immunity. ACS Nano 16, 21491–21504 (2022).
Li, Y. et al. Antigen capture and immune modulation by bacterial outer membrane vesicles as in situ vaccine for cancer immunotherapy post-photothermal therapy. Small 18, e2107461 (2022).
Chen, L. et al. An emerging antibacterial nanovaccine for enhanced chemotherapy by selectively eliminating tumor-colonizing bacteria. Sci. Bull. 69, 2565–2579 (2024).
Guo, J. et al. Engineering customized nanovaccines for enhanced cancer immunotherapy. Bioact. Mater. 36, 330–357 (2024).
Wang, Q. et al. Lymph node-targeting nanovaccines for cancer immunotherapy. J. Control Release 351, 102–122 (2022).
Wu, Y. & Feng, L. Biomaterials-assisted construction of neoantigen vaccines for personalized cancer immunotherapy. Expert Opin. Drug Deliv. 20, 323–333 (2023).
Banda, A. et al. Precision in action: the role of clustered regularly interspaced short palindromic repeats/cas in gene therapies. Vaccines 12, 636 (2024).
Jin, L. et al. In situ programming of nanovaccines for lymph node-targeted delivery and cancer immunotherapy. ACS Nano 16, 15226–15236 (2022).
Xiao, B. et al. An MRI-trackable therapeutic nanovaccine preventing cancer liver metastasis. Biomaterials 274, 120893 (2021).
Li, Q. et al. Elastic nanovaccine enhances dendritic cell-mediated tumor immunotherapy. Small 18, e2201108 (2022).
Su, Q. et al. Facile preparation of a metal-phenolic network-based lymph node targeting nanovaccine for antitumor immunotherapy. Acta Biomater. 158, 510–524 (2023).
Li, T. et al. Manganese oxide-constructed multifunctional biomimetic nanovaccine for robust tumor-specific T cell priming and chemodynamic therapy. Biomaterials 309, 122626 (2024).
Liu, Y. et al. Metal-organic framework-based nanovaccine for relieving immunosuppressive tumors via hindering efferocytosis of macrophages and promoting pyroptosis and cuproptosis of cancer cells. ACS Nano 18, 12386–12400 (2024).
Wang, C. et al. Tumor-associated myeloid cells selective delivery of a therapeutic tumor nano-vaccine for overcoming immune barriers for effective and long-term cancer immunotherapy. Adv. Healthc. Mater. 13, e2401416 (2024).
Wang, H. et al. Hybrid Ginseng-derived Extracellular Vesicles-Like Particles with Autologous Tumor Cell Membrane for Personalized Vaccination to Inhibit Tumor Recurrence and Metastasis. Adv. Sci. (Weinh.) 11, e2308235 (2024).
He, A. et al. Nanovaccine-based strategies for lymph node targeted delivery and imaging in tumor immunotherapy. J. Nanobiotechnol. 21, 236 (2023).
Kann, B. H., Hosny, A. & Aerts, H. Artificial intelligence for clinical oncology. Cancer Cell 39, 916–927 (2021).
Corti, C. et al. Artificial intelligence in cancer research and precision medicine: Applications, limitations and priorities to drive transformation in the delivery of equitable and unbiased care. Cancer Treat. Rev. 112, 102498 (2023).
Wu, T. et al. Research trends in the application of artificial intelligence in oncology: a bibliometric and network visualization study. Front. Biosci. 27, 254 (2022).
Zheng, Q. et al. Artificial intelligence performance in detecting tumor metastasis from medical radiology imaging: A systematic review and meta-analysis. EClinicalMedicine 31, 100669 (2021).
Retamero, J. A. et al. Artificial intelligence helps pathologists increase diagnostic accuracy and efficiency in the detection of breast cancer lymph node metastases. Am. J. Surg. Pathol. 48, 846–854 (2024).
Carini, C. & Seyhan, A. A. Tribulations and future opportunities for artificial intelligence in precision medicine. J. Transl. Med. 22, 411 (2024).
Tong, X. et al. Deep representation learning of chemical-induced transcriptional profile for phenotype-based drug discovery. Nat. Commun. 15, 5378 (2024).
Shao, J. et al. Predicting gene mutation status via artificial intelligence technologies based on multimodal integration (MMI) to advance precision oncology. Semin Cancer Biol. 91, 1–15 (2023).
Yala, A. et al. Multi-institutional validation of a mammography-based breast cancer risk model. J. Clin. Oncol. 40, 1732–1740 (2022).
Wang, Y. J. et al. Advancing presurgical non-invasive molecular subgroup prediction in medulloblastoma using artificial intelligence and MRI signatures. Cancer Cell 42, 1239–1257 e1237 (2024).
Hoang, D. T. et al. A deep-learning framework to predict cancer treatment response from histopathology images through imputed transcriptomics. Nat. Cancer 5, 1305–1317 (2024).
Hamamoto, R. et al. Introducing AI to the molecular tumor board: one direction toward the establishment of precision medicine using large-scale cancer clinical and biological information. Exp. Hematol. Oncol. 11, 82 (2022).
Zhang, Z. & Wei, X. Artificial intelligence-assisted selection and efficacy prediction of antineoplastic strategies for precision cancer therapy. Semin Cancer Biol. 90, 57–72 (2023).
He, X. et al. Artificial intelligence-based multi-omics analysis fuels cancer precision medicine. Semin. Cancer Biol. 88, 187–200 (2023).
Patel, S. B. & Lam, K. ChatGPT: the future of discharge summaries? Lancet Digit Health 5, e107–e108 (2023).
Huang, J. et al. A critical assessment of using ChatGPT for extracting structured data from clinical notes. npj Digit. Med. 7, 106 (2024).
Staubli, S. M. et al. Decoding the Clavien-Dindo classification: Artificial Intelligence (AI) as a novel tool to grade postoperative complications. Ann. Surg. Published online (2024).
Zaretsky, J. et al. Generative Artificial Intelligence to transform inpatient discharge summaries to patient-friendly language and format. JAMA Netw. Open 7, e240357 (2024).
Benary, M. et al. Leveraging Large Language Models for Decision Support in Personalized Oncology. JAMA Netw. Open 6, e2343689 (2023).
Savage, T. et al. Diagnostic reasoning prompts reveal the potential for large language model interpretability in medicine. NPJ Digit Med. 7, 20 (2024).
Zhou, S. et al. The performance of large language model powered chatbots compared to oncology physicians on colorectal cancer queries. Int. J. Surg. 110, 6509–6517 (2024).
Zhu, L. et al. Harnessing artificial intelligence for prostate cancer management. Cell Rep. Med. 5, 101506 (2024).
Branco, I. & Choupina, A. Bioinformatics: new tools and applications in life science and personalized medicine. Appl. Microbiol. Biotechnol. 105, 937–951 (2021).
Chen, C. et al. Exosomal circTUBGCP4 promotes vascular endothelial cell tipping and colorectal cancer metastasis by activating Akt signaling pathway. J. Exp. Clin. Cancer Res. 42, 46 (2023).
Wang, L. A. et al. The potential significance of the EMILIN3 gene in augmenting the aggressiveness of low-grade Gliomas is noteworthy. Cancer Manag Res. 16, 711–730 (2024).
Xie, R. et al. N6-methyladenosine modification of OIP5-AS1 promotes glycolysis, tumorigenesis, and metastasis of gastric cancer by inhibiting Trim21-mediated hnRNPA1 ubiquitination and degradation. Gastric Cancer 27, 49–71 (2023).
Li, H. et al. Identification and verification of anoikis-related gene markers to predict the prognosis of patients with bladder cancer and assist in the diagnosis and treatment of bladder cancer. Transl. Cancer Res. 13, 579–593 (2024).
Yuan, J. et al. Long intergenic non-coding RNA DIO3OS promotes osteosarcoma metastasis via activation of the TGF-beta signaling pathway: a potential diagnostic and immunotherapeutic target for osteosarcoma. Cancer Cell Int 23, 215 (2023).
Sun, H., Li, L., Yan, J. & Huang, T. Prioritization of drug targets for thyroid cancer: a multi-omics Mendelian randomization study. Endocrine 86, 732–743 (2024).
Li, Z. et al. Development of a macrophage-related risk model for metastatic melanoma. Int J. Mol. Sci. 24, 13752 (2023).
Li, Z. W. et al. Identifying potential anti-metastasis drugs for prostate cancer through integrative bioinformatics analysis and compound library screening. J. Gene Med. 25, e3548 (2023).
Cai, F.-f et al. ADT-OH inhibits malignant melanoma metastasis in mice via suppressing CSE/CBS and FAK/Paxillin signaling pathway. Acta Pharm. Sin. 43, 1829–1842 (2021).
Bojmar, L. et al. Multi-parametric atlas of the pre-metastatic liver for prediction of metastatic outcome in early-stage pancreatic cancer. Nat. Med. 30, 2170–2180 (2024).
Jager, N. Bioinformatics workflows for clinical applications in precision oncology. Semin. Cancer Biol. 84, 103–112 (2022).
Singer, J. et al. Bioinformatics for precision oncology. Brief. Bioinform. 20, 778–788 (2019).
Tang, A., Woldemariam, S., Roger, J. & Sirota, M. Translational bioinformatics to enable precision medicine for all: elevating equity across molecular, clinical, and digital realms. Yearb. Med. Inf. 31, 106–115 (2022).
Patel, S. A., Rodrigues, P., Wesolowski, L. & Vanharanta, S. Genomic control of metastasis. Br. J. Cancer 124, 3–12 (2020).
Ullah, I. et al. Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J. Clin. Invest. 128, 1355–1370 (2018).
Brown, D. et al. Phylogenetic analysis of metastatic progression in breast cancer using somatic mutations and copy number aberrations. Nat. Commun. 8, 14944 (2017).
Blasco, M. T., Espuny, I. & Gomis, R. R. Ecology and evolution of dormant metastasis. Trends Cancer 8, 570–582 (2022).
El-Kebir, M., Satas, G. & Raphael, B. J. Inferring parsimonious migration histories for metastatic cancers. Nat. Genet. 50, 718–726 (2018).
Zhang, W. et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184, 2471–2486 e2420 (2021).
Xu, Z. et al. Unbiased metastatic niche-labeling identifies estrogen receptor-positive macrophages as a barrier of T cell infiltration during bone colonization. bioRxiv. Preprint at https://www.biorxiv.org/content/10.1101/2024.05.07.593016v1 (2024).
Liu, F., Ding, Y. & Xu, Z. Single cell profiling of bone metastasis ecosystems from multiple cancer types reveals convergent and divergent mechanisms of bone colonization. bioRxiv. Preprint at https://www.biorxiv.org/content/10.1101/2024.05.07.593027v1 (2024).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Bergen, V. et al. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Qiu, X. et al. Mapping transcriptomic vector fields of single cells. Cell 185, 690–711.e645 (2022).
Chen, D. & Qi, E. Y. Innovative highlights of clinical drug trial design. Transl. Res. 224, 71–77 (2020).
Norback, K., Hoglund, A. T., Godskesen, T. & Frygner-Holm, S. Ethical concerns when recruiting children with cancer for research: Swedish healthcare professionals’ perceptions and experiences. BMC Med. Ethics 24, 23 (2023).
Cescon, D. & Siu, L. L. Cancer clinical trials: the rear-view mirror and the crystal ball. Cell 168, 575–578 (2017).
Bothwell, L. E. et al. Assessing the Gold Standard — Lessons from the History of RCTs. N. Engl. J. Med. 374, 2175–2181 (2016).
Schott, A. F. et al. Phase II studies of two different schedules of dasatinib in bone metastasis predominant metastatic breast cancer: SWOG S0622. Breast Cancer Res. Treat. 159, 87–95 (2016).
Spurr, L. F. et al. Highly aneuploid non-small cell lung cancer shows enhanced responsiveness to concurrent radiation and immune checkpoint blockade. Nat. Cancer 3, 1498–1512 (2022).
Vitale, I., Shema, E., Loi, S. & Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 27, 212–224 (2021).
Li, Z., Seehawer, M. & Polyak, K. Untangling the web of intratumour heterogeneity. Nat. Cell Biol. 24, 1192–1201 (2022).
Gavish, A. et al. Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature 618, 598–606 (2023).
Marusyk, A., Janiszewska, M. & Polyak, K. Intratumor heterogeneity: The Rosetta stone of therapy resistance. Cancer Cell 37, 471–484 (2020).
Khan, S. U., Fatima, K., Aisha, S. & Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal 22, 109 (2024).
Shi, Z. D. et al. Tumor cell plasticity in targeted therapy-induced resistance: mechanisms and new strategies. Signal Transduct. Target Ther. 8, 113 (2023).
Taplin, M. E. et al. Androgen receptor modulation optimized for response-splice variant: A Phase 3, randomized trial of Galeterone Versus Enzalutamide in Androgen Receptor Splice Variant-7-expressing Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 76, 843–851 (2019).
Khushalani, N. I. et al. Final results of urelumab, an anti-CD137 agonist monoclonal antibody, in combination with cetuximab or nivolumab in patients with advanced solid tumors. J. Immunother. Cancer 12, e007364 (2024).
Pearson, A. et al. High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov. 6, 838–851 (2016).
Filho, O. M. et al. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: Phase II neoadjuvant clinical trial of T-DM1 combined with Pertuzumab. Cancer Discov. 11, 2474–2487 (2021).
Diamond, G. A. TWEedledum. Am. J. Cardiol. 62, 1152 (1988).
Morris, P. G. et al. Phase II Study of Paclitaxel and Dasatinib in metastatic breast cancer. Clin. Breast Cancer 18, 387–394 (2018).
Davidson, B. et al. Liver resection surgery compared with thermal ablation in high surgical risk patients with colorectal liver metastases: the LAVA international RCT. Health Technol. Assess. 24, 1–38 (2020).
Huey, R. W. et al. Feasibility and value of genomic profiling in cancer of unknown primary: real-world evidence from prospective profiling study. J. Natl Cancer Inst. 115, 994–997 (2023).
Briel, M. et al. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J. Clin. Epidemiol. 80, 8–15 (2016).
Bothwell, L. E., Greene, J. A., Podolsky, S. H. & Jones, D. S. Assessing the Gold Standard–Lessons from the History of RCTs. N. Engl. J. Med. 374, 2175–2181 (2016).
Even-Sapir, E. et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J. Nucl. Med. 47, 287–297 (2006).
Thomson, S. The challenge of completing a randomised clinical trial. Br. J. Neurosurg. 38, 1–2 (2024).
Ryan, C. et al. Epidemiology of bone metastases. Bone 158, 115783 (2022).
Walker, A. E., Robins, M. & Weinfeld, F. D. Epidemiology of brain tumors. Neurology 35, 219–219 (1985).
Erichsen, R. et al. Time trends in incidence and prognosis of primary liver cancer and liver metastases of unknown origin in a Danish region, 1985–2004. Eur. J. Gastroenterol. Hepatol. 20, 104–110 (2008).
Li, S. L. et al. Exosomes from LNCaP cells promote osteoblast activity through miR-375 transfer. Oncol. Lett. 17, 4463–4473 (2019).
Nakai, Y. et al. Efficacy of an orally active small-molecule inhibitor of RANKL in bone metastasis. Bone Res. 7, 1 (2019).
Yoneda, T. et al. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J. Bone Min. Res. 16, 1486–1495 (2001).
Chen, F., Han, Y. & Kang, Y. Bone marrow niches in the regulation of bone metastasis. Br. J. Cancer 124, 1912–1920 (2021).
Archer Goode, E., Wang, N. & Munkley, J. Prostate cancer bone metastases biology and clinical management (Review). Oncol. Lett. 25, 163 (2023).
Umer, M., Mohib, Y., Atif, M. & Nazim, M. Skeletal metastasis in renal cell carcinoma: A review. Ann. Med. Surg. 27, 9–16 (2018).
Mikami, S. et al. Invasion and metastasis of renal cell carcinoma. Med. Mol. Morphol. 47, 63–67 (2014).
Satcher, R. L. & Zhang, X. H. Evolving cancer-niche interactions and therapeutic targets during bone metastasis. Nat. Rev. Cancer 22, 85–101 (2022).
Polavaram, N. S. et al. Tumor- and osteoclast-derived NRP2 in prostate cancer bone metastases. Bone Res. 9, 24 (2021).
The choice of non-steroidal anti-inflammatory drugs in the rheumatic diseases. Drug Ther Bull. 15, 93-95, (1977).
Wu, S. et al. Current progress and mechanisms of bone metastasis in lung cancer: a narrative review. Transl. Lung Cancer Res. 10, 439–451 (2021).
He, Y. et al. IL-20RB mediates tumoral response to osteoclastic niches and promotes bone metastasis of lung cancer. J. Clin. Invest. 132, e157917 (2022).
Wang, Z. et al. Bone-metastatic lung adenocarcinoma cells bearing CD74-ROS1 fusion interact with macrophages to promote their dissemination. Oncogene 43, 2215–2227 (2024).
Xu, H. et al. Transcription factors in colorectal cancer: molecular mechanism and therapeutic implications. Oncogene 40, 1555–1569 (2021).
Hung, J. Y. et al. Colony-stimulating factor 1 potentiates lung cancer bone metastasis. Lab. Invest. 94, 371–381 (2014).
Richardsen, E., Uglehus, R. D., Johnsen, S. H. & Busund, L. T. Macrophage-colony stimulating factor (CSF1) predicts breast cancer progression and mortality. Anticancer Res. 35, 865–874 (2015).
Chen, W. C. et al. Bone sialoprotein promotes lung cancer osteolytic bone metastasis via MMP14-dependent mechanisms. Biochem. Pharm. 211, 115540 (2023).
Grunwald, V. et al. An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma. Nat. Rev. Urol. 15, 511–521 (2018).
Brown, J. et al. Implications of bone metastasis on response to systemic therapy in patients with advanced renal cell carcinoma: A systematic literature review. Cancer Treat. Rev. 129, 102792 (2024).
Canellas-Socias, A., Sancho, E. & Batlle, E. Mechanisms of metastatic colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 21, 609–625 (2024).
Ferraro, G. B. et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat. Cancer 2, 414–428 (2021).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Avraham, H. K. et al. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J. Pathol. 232, 369–381 (2014).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Quail, D. F. & Joyce, J. A. The microenvironmental landscape of brain tumors. Cancer Cell 31, 326–341 (2017).
Argaw, A. T. et al. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 177, 5574–5584 (2006).
Engelhardt, S., Patkar, S. & Ogunshola, O. O. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br. J. Pharm. 171, 1210–1230 (2014).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Lyle, L. T. et al. Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin. Cancer Res. 22, 5287–5299 (2016).
Gril, B. et al. Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases. Nat. Commun. 9, 2705 (2018).
Li, Q. et al. IntegrinB5 upregulated by HER2 in gastric cancer: a promising biomarker for liver metastasis. Ann. Transl. Med. 8, 451 (2020).
Tabaries, S. et al. Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes. Mol. Cell Biol. 32, 2979–2991 (2012).
Elliott, J. A., Osterlind, K., Hirsch, F. R. & Hansen, H. H. Metastatic patterns in small-cell lung cancer: correlation of autopsy findings with clinical parameters in 537 patients. J. Clin. Oncol. 5, 246–254 (1987).
Brodt, P. Liver Metastasis: Biology and Clinical Management. (Springer, Dordrecht, 2011).
Tsilimigras, D. I. et al. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: A systematic review of the current evidence. Surg. Oncol. 27, 280–288 (2018).
Schwarz, R. E. et al. Systemic cytotoxic and biological therapies of colorectal liver metastases: expert consensus statement. HPB 15, 106–115 (2013).
Margonis, G. A. et al. Association between specific mutations in KRAS Codon 12 and colorectal liver metastasis. JAMA Surg. 150, 722–729 (2015).
Margonis, G. A. et al. KRAS mutation status dictates optimal surgical margin width in patients undergoing resection of colorectal liver metastases. Ann. Surg. Oncol. 24, 264–271 (2017).
Piperno-Neumann, S. et al. Prospective study of surveillance testing for metastasis in 100 high-risk uveal melanoma patients. J. Fr. Ophtalmol. 38, 526–534 (2015).
Zheng, Z., Jia, S., Shao, C. & Shi, Y. Irradiation induces cancer lung metastasis through activation of the cGAS-STING-CCL5 pathway in mesenchymal stromal cells. Cell Death Dis. 11, 326 (2020).
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015).
Wang, J. et al. A synthetic metastatic niche reveals antitumor neutrophils drive breast cancer metastatic dormancy in the lungs. Nat. Commun. 14, 4790 (2023).
Yoshimura, T., Li, C., Wang, Y. & Matsukawa, A. The chemokine monocyte chemoattractant protein-1/CCL2 is a promoter of breast cancer metastasis. Cell Mol. Immunol. 20, 714–738 (2023).
Loo, S. Y. et al. Fatty acid oxidation is a druggable gateway regulating cellular plasticity for driving metastasis in breast cancer. Sci. Adv. 7, eabh2443 (2021).
Li, Z. et al. PRMT2 promotes RCC tumorigenesis and metastasis via enhancing WNT5A transcriptional expression. Cell Death Dis. 14, 322 (2023).
Tian, F. et al. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat. Commun. 12, 2536 (2021).
Liu, Y. et al. Formation of pre-metastatic niches induced by tumor extracellular vesicles in lung metastasis. Pharm. Res. 188, 106669 (2023).
Xiao, P. et al. Enzyme/pH dual stimuli-responsive nanoplatform co-deliver disulfiram and doxorubicin for effective treatment of breast cancer lung metastasis. Expert Opin. Drug Deliv. 20, 1015–1031 (2023).
Wang, X. et al. Specific and long-term luminescent monitoring of hydrogen peroxide in tumor metastasis. Adv. Mater. 35, e2210948 (2023).
Long, Y. et al. Self-delivery micellar nanoparticles prevent premetastatic niche formation by interfering with the early recruitment and vascular destruction of granulocytic myeloid-derived suppressor cells. Nano Lett. 20, 2219–2229 (2020).
Lu, Z. et al. Micellar nanoparticles inhibit breast cancer and pulmonary metastasis by modulating the recruitment and depletion of myeloid-derived suppressor cells. Nanoscale 14, 17315–17330 (2022).
Pei, P. et al. Radioactive nano-oxygen generator enhance anti-tumor radio-immunotherapy by regulating tumor microenvironment and reducing proliferation. Biomaterials 280, 121326 (2022).
Zhang, J. et al. Photothermal-controlled NO-releasing Nanogels reverse epithelial-mesenchymal transition and restore immune surveillance against cancer metastasis. J. Control Release 371, 16–28 (2024).
Ganesan, K. et al. Cryoprotective isoliquiritigenin-zein phosphatidylcholine nanoparticles inhibits breast cancer-bone metastasis by targeting JAK-STAT signaling pathways. Chem. Biol. Interact. 396, 111037 (2024).
Xia, C. et al. Sponge-like nano-system suppresses tumor recurrence and metastasis by restraining myeloid-derived suppressor cells-mediated immunosuppression and formation of pre-metastatic niche. Acta Biomater. 158, 708–724 (2023).
Gu, J. et al. Glycopolymer-grafted nanoparticles as glycosaminoglycan mimics with cell proliferation and anti-tumor metastasis activities. Int J. Biol. Macromol. 253, 126975 (2023).
Bai, Y. et al. A silver-induced absorption red-shifted dual-targeted nanodiagnosis-treatment agent for NIR-II photoacoustic imaging-guided photothermal and ROS simultaneously enhanced immune checkpoint blockade antitumor therapy. Adv. Sci. 11, e2306375 (2024).
Guo, X. et al. Decomposable nanoagonists Enable NIR-Elicited cGAS-STING activation for Tandem-amplified photodynamic-metalloimmunotherapy. Adv. Mater. 36, e2313029 (2024).
Gu, W. et al. A bioactive nanocomposite integrated specific TAMs target and synergistic TAMs repolarization for effective cancer immunotherapy. Bioact. Mater. 38, 472–485 (2024).
Liu, H. et al. Metal-organic framework-mediated multifunctional nanoparticles for combined chemo-photothermal therapy and enhanced immunotherapy against colorectal cancer. Acta Biomater. 144, 132–141 (2022).
Qiu, Y. et al. Nano Ultrasound contrast agent for synergistic chemo-photothermal therapy and enhanced immunotherapy against liver cancer and metastasis. Adv. Sci. 10, e2300878 (2023).
Xu, W. W. et al. Genome-wide CRISPR/Cas9 screening identifies a targetable MEST-PURA interaction in cancer metastasis. EBioMedicine 92, 104587 (2023).
Hu, H. F. et al. LINC00982-encoded protein PRDM16-DT regulates CHEK2 splicing to suppress colorectal cancer metastasis and chemoresistance. Theranostics 14, 3317–3338 (2024).
Rinella, L. et al. Dickkopf-1 (DKK1) drives growth and metastases in castration-resistant prostate cancer. Cancer Gene Ther. 31, 1266–1279 (2024).
Pizzolato, G. et al. The tumor suppressor p53 is a negative regulator of the carcinoma-associated transcription factor FOXQ1. J. Biol. Chem. 300, 107126 (2024).
Hu, Y. et al. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Clin. Cancer Res. 27, 2764–2772 (2021).
Mo, Y. et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol. Cancer 22, 4 (2023).
Zhang, D. et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 17, 777–787 (2022).
Wei, C. G. et al. Calcium phosphate-based nanomedicine mediated CRISPR/Cas9 delivery for prostate cancer therapy. Front. Bioeng. Biotechnol. 10, 1078342 (2022).
Wan, T. et al. A Duplex CRISPR-Cas9 Ribonucleoprotein nanomedicine for colorectal cancer gene therapy. Nano Lett. 21, 9761–9771 (2021).
Han, D. et al. Redirecting antigens by engineered photosynthetic bacteria and derived outer membrane vesicles for enhanced cancer immunotherapy. ACS Nano 17, 18716–18731 (2023).
Li, C. et al. Overcoming neutrophil-induced immunosuppression in postoperative cancer therapy: Combined sialic acid-modified liposomes with scaffold-based vaccines. Asian J. Pharm. Sci. 19, 100906 (2024).
Song, H. et al. Antigen epitope-TLR7/8a conjugate as self-assembled carrier-free nanovaccine for personalized immunotherapy. Acta Biomater. 141, 398–407 (2022).
Huang, T. et al. Biomimetic dual-target theranostic nanovaccine enables magnetic resonance imaging and chemo/chemodynamic/immune therapy of Glioma. ACS Appl Mater. Interfaces 16, 27187–27201 (2024).
Wang, Z. et al. Laser-activatable in situ vaccine enhances cancer-immunity cycle. Adv. Mater. 35, e2307193 (2023).
Lu, M. Y. et al. AI-based pathology predicts origins for cancers of unknown primary. Nature 594, 106–110 (2021).
Tian, F. et al. Prediction of tumor origin in cancers of unknown primary origin with cytology-based deep learning. Nat. Med. 30, 1309–1319 (2024).
Zhang, M. B. et al. Cervical lymph node metastasis prediction from papillary thyroid carcinoma US videos: a prospective multicenter study. BMC Med. 22, 153 (2024).
Zhou, H. et al. AI-guided histopathology predicts brain metastasis in lung cancer patients. J. Pathol. 263, 89–98 (2024).
Liu, L. et al. Automated machine learning for predicting liver metastasis in patients with gastrointestinal stromal tumor: a SEER-based analysis. Sci. Rep. 14, 12415 (2024).
Fu, Y. et al. Longitudinal ultrasound-based AI model predicts axillary lymph node response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Eur. Radio. 34, 7080–7089 (2024).
Fan, J. et al. MEAI: an artificial intelligence platform for predicting distant and lymph node metastases directly from primary breast cancer. J. Cancer Res Clin. Oncol. 149, 9229–9241 (2023).
Wu, S. et al. Artificial intelligence-based model for lymph node metastases detection on whole slide images in bladder cancer: a retrospective, multicentre, diagnostic study. Lancet Oncol. 24, 360–370 (2023).
Luo, H. et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 20, 1645–1654 (2019).
Uema, R. et al. A novel artificial intelligence-based endoscopic ultrasonography diagnostic system for diagnosing the invasion depth of early gastric cancer. J. Gastroenterol. 59, 543–555 (2024).
Caruso, C. M., Guarrasi, V., Ramella, S. & Soda, P. A deep learning approach for overall survival prediction in lung cancer with missing values. Comput. Methods Prog. Biomed. 254, 108308 (2024).
Cilla, S. et al. Explainable machine learning model to predict overall survival in patients treated with palliative radiotherapy for bone metastases. JCO Clin. Cancer Inf. 8, e2400027 (2024).
Hou, X. et al. Triple-negative breast cancer survival prediction using artificial intelligence through integrated analysis of tertiary lymphoid structures and tumor budding. Cancer 130, 1499–1512 (2024).
Hwang, J., Lee, Y., Yoo, S. K. & Kim, J. I. Image-based deep learning model using DNA methylation data predicts the origin of cancer of unknown primary. Neoplasia 55, 101021 (2024).
Wang, Y. et al. Identification of metastasis-related genes for predicting prostate cancer diagnosis, metastasis and immunotherapy drug candidates using machine learning approaches. Biol. Direct 19, 50 (2024).
Kwok, T., Yeguvapalli, S. & Chitrala, K. N. Identification of genes crucial for biological processes in breast cancer liver metastasis relapse. Int. J. Mol. Sci. 25, 5439 (2024).
Wu, J. et al. Expression and potential molecular mechanism of TOP2A in metastasis of non-small cell lung cancer. Sci. Rep. 14, 12228 (2024).
Liu, Y., Yin, Z., Wang, Y. & Chen, H. Exploration and validation of key genes associated with early lymph node metastasis in thyroid carcinoma using weighted gene co-expression network analysis and machine learning. Front Endocrinol. 14, 1247709 (2023).
Du, W. et al. Regulation of tumor metastasis and CD8(+) T cells infiltration by circRNF216/miR-576-5p/ZC3H12C axis in colorectal cancer. Cell Mol. Biol. Lett. 29, 19 (2024).
Li, Z. et al. Dihydroartemisinin inhibits melanoma migration and metastasis by affecting angiogenesis. Phytother. Res. Published online (2023).
Si, T. et al. Ruangan Lidan decoction inhibits the growth and metastasis of liver cancer by downregulating miR-9-5p and upregulating PDK4. Cancer Biol. Ther. 24, 2246198 (2023).
Luo, B. et al. Jinfukang inhibits lung cancer metastasis by regulating T cell receptors. J. Ethnopharmacol. 318, 116885 (2024).
Sun, S. et al. Endosomal protein DENND10/FAM45A integrates extracellular vesicle release with cancer cell migration. BMC Biol. 22, 154 (2024).
Li, Z. et al. LINC00909 up-regulates pluripotency factors and promotes cancer stemness and metastasis in pancreatic ductal adenocarcinoma by targeting SMAD4. Biol. Direct 19, 24 (2024).
Zhu, J. et al. RNF115 aggravates tumor progression through regulation of CDK10 degradation in thyroid carcinoma. Cell Biol. Toxicol. 40, 14 (2024).
Lv, Y. et al. SOCS2 inhibits hepatoblastoma metastasis via downregulation of the JAK2/STAT5 signal pathway. Sci. Rep. 13, 21814 (2023).
Yin, L. et al. Construction and validation of a risk model based on the key SNARE proteins to predict the prognosis and immune microenvironment of gliomas. Front. Mol. Neurosci. 16, 1304224 (2023).
Acknowledgements
This work was supported by the Top Young and Middle-aged Medical Talent of Chongqing, Top Young and Middle-aged Medical Studio of Chongqing, Chongqing Science and Health Joint Fund for Top Young and Middle-aged Talent (2023GDRC007), the Key Project for Clinical Innovation of Army Medical University (CX2019LC107), the Second Affiliated Hospital of Army Military Medical University Discipline Talent Construction Special Project (2023XKRC007).
Author information
Authors and Affiliations
Contributions
L.Y.X., L.F.S, C.Q.J., and D.L.J. performed the literature search, prepared tables and figures, and wrote the manuscript. J.Z., Z.X. and O.Y.Q. reviewed and revised the manuscript. All the authors listed have made a substantial contribution to this work. All authors have read and approved this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Liu, F., Cai, Q. et al. Invasion and metastasis in cancer: molecular insights and therapeutic targets. Sig Transduct Target Ther 10, 57 (2025). https://doi.org/10.1038/s41392-025-02148-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41392-025-02148-4
This article is cited by
-
TRV130 inhibits colon cancer progression via suppressing the Hedgehog signaling pathway: in vitro and in vivo evidence
Hereditas (2026)
-
Comprehensive pan-cancer analysis and experimental verification of the roles of SCP2 in colon adenocarcinoma
BMC Cancer (2026)
-
HDACi induces apoptosis of osteosarcoma cells by inhibiting the MAPK pathway
Scientific Reports (2026)
-
The complex role of nutrients in cancer spread
Nature (2026)
-
Polystyrene Microplastics Disrupt Anticancer Proteins: An In Silico and In Vitro Study
Toxicology and Environmental Health Sciences (2026)