Abstract
There is insufficient evidence to guide dose and frequency optimization with repeated-dose ketamine for depression. This study assessed the value of symptomatic non-improvement after the first few ketamine infusions as a predictor of overall non-response in depression for early decision-making to discontinue treatment. A total of 135 individuals with major depressive disorder or bipolar disorder experiencing a current major depressive episode were administered six repeated doses of intravenous ketamine. Depressive symptoms were assessed using the Montgomery-Åsberg Depression Rating Scale (MADRS) at baseline, 4 h after the first infusion, and 24 h after each infusion. Improvement, partial response, and response were defined as a reduction rate of ≥ 20%, 30%, and 50% in MADRS scores, respectively. This study examined the relationship between improvement (as opposed to non-improvement after each infusion or consecutive non-improvements after the first few infusions) and partial response and response after the sixth infusion. This analysis was summarized using sensitivity, specificity, and other diagnostic test parameters. The sensitivities of improvement at 24 h post-infusion 4 and improvement at 24 h post-infusion 3, vs. three consecutive non-improvements, as predictors for overall partial response and response exceeded 90%. No significant reduction in depressive symptoms was seen in non-improvers following the remaining infusions after the above-identified point. Our study suggests that non-improvement after four infusions, or more conservatively three consecutive non-improvements after three infusions, could serve as a signal of overall non-response to repeated-dose intravenous ketamine for depression and that subsequent treatments would not be warranted.
Similar content being viewed by others
Introduction
Ketamine has demonstrated rapid and robust antidepressant effects in patients with treatment-resistant depression (TRD) [1] or suicidality [2]. The antidepressant effect of a single dose of ketamine is relatively short-lived, usually ranging from 3 to 7 days post-infusion [3]. To prolong the efficacy of ketamine, various strategies (e.g., use of riluzole, lithium, or rapamycin) have been attempted, but none have been proven effective in randomized controlled studies [4,5,6]. Repeated-dose ketamine has been shown to maintain and even amplify the antidepressant effects of a single dose of ketamine for up to 2–3 weeks [7, 8]. The regimen recommended and typically used for repeated-dose intravenous ketamine is 0.5 mg/kg over 40 min twice or thrice weekly for 4–6 sessions. However, there remains a lack of sufficient evidence to guide dose [9, 10] and frequency [11] optimization with repeated-dose intravenous ketamine.
We have previously reported the overall safety and effectiveness of six repeated doses of intravenous ketamine for TRD or depression with suicidality (n = 97; 77 with major depressive disorder [MDD] and 20 with bipolar disorder [BD]) in a single-arm, open-label study in the Chinese population [12]. The patients showed higher response and remission rates after six infusions (68% and 51%, respectively) than after the first infusion (14% and 9%, respectively) [12]. This pattern was also seen in other randomized controlled studies, open-label studies, and case series of repeated infusions [11, 13,14,15]. We also confirmed a previous finding that the rapid antidepressant response of ketamine after the first infusion was associated with the subsequent antidepressant response [7]. These findings suggest that there may be different trajectory patterns in response to ketamine, with some patients responding or even remitting early, some patients gradually achieving a response after multiple infusions, and some patients not responding throughout the treatment. Given the relatively serious condition and suicidal risk of the patients receiving ketamine, early identification of those in whom repeated-dose ketamine is ineffective is of great importance. Early identification of this subgroup of patients is beneficial to optimize and accelerate treatment decision-making such as by increasing the dose of ketamine, combining therapies, or switching to alternative treatments (e.g., electroconvulsive therapy). Terminating ineffective treatment in advance can also save costs and reduce the side effects or adverse reactions caused by ketamine, which are transient and well tolerated but more common and severe than those caused by conventional antidepressants [16].
As mentioned above, our and others’ previous studies have shown that early response after the first infusion may be a useful predictor to gauge the subsequent antidepressant response to repeated-dose intravenous ketamine [7, 12]. However, no valid practical index has been established to predict the subsequent ineffectiveness of repeated-dose intravenous ketamine. Patients receiving conventional antidepressants who show early symptom improvement (i.e., within 1–4 weeks in antidepressant trials) have been identified as the subgroup most likely to respond and remit in subsequent treatment [17,18,19]. Some studies have also found that a lack of early symptom improvement predicts failure to achieve response/remission at treatment endpoint [20,21,22]. Inspired by observations in conventional antidepressant trials, Lipsitz et al. used early improvement ( ≥ 20% reduction in the 16-item Quick Inventory for Depressive Symptomatology-Self Report [QIDS-SR16] scores) post-infusion 1 and 2 as indexes to predict a reduction in depressive symptoms following four repeated doses of ketamine treatment and found that both were significantly associated with lower QIDS-SR16 scores post-infusion 4 compared to non-early improvers. However, individuals without early improvement still had a high likelihood ( > 58%) of achieving clinically significant symptom reduction ( ≥ 20% reduction in QIDS-SR16 scores) after the fourth infusion [23]. This finding is similar to our and others’ previous studies, which used a less conservative index as a predictor, i.e., early response ( ≥ 50% reduction in rating scale scores of depressive symptoms) [11, 12, 15].
Given that early non-improvement or non-response after the first or second infusion is not a good predictor of the overall ineffectiveness of repeated-dose intravenous ketamine, we sought to describe a complete picture of the overall ineffectiveness (i.e., non-response or non-partial response [< 30% reduction in rating scale scores of depressive symptoms] after the full set of six infusions) predicted by non-improvement after each of the first five infusions and more conservative combined indicators (i.e., consecutive non-improvements after the first few infusions) to determine an appropriate point for the non-improvers to discontinue the treatment. We also further evaluated whether a significant reduction in depressive symptoms followed the remaining infusions in repeated-dose ketamine treatment after the above-identified point.
Materials and methods
Study design
This was a post-hoc analysis of data from a single-arm, open-label clinical study conducted at the Affiliated Brain Hospital of Guangzhou Medical University in China between September 2016 and December 2018. The study protocol was approved by the local ethics committee. All patients provided written informed consent before participation. The trial was registered in the Chinese Clinical Trials Registry (http://www.chictr.org.cn, ChiCTR-OOC-17012239).
The design and procedure of the study have been previously described [12]. The present study included data from three phases: a screening and pretreatment assessment phase, a 2-week repeated-dose ketamine treatment phase, and an assessment phase 4 h after the first infusion and 24 h after each infusion.
Ketamine (0.5 mg/kg) was diluted in 0.9% sodium chloride (40 mL) and administered intravenously over 40 min with a thrice-weekly regimen for six infusions. The dose of 0.5 mg/kg was chosen based on previous clinical trials [1], which is the most commonly used dose. This dose has been confirmed to be superior in subsequent clinical trials [9, 24] and has also been included in later expert opinion [25] and task force recommendations [26]. Patients remained on any pre-study antidepressant and other psychiatric medication at stable dosages throughout the study. Benzodiazepines were allowed, and no patients received heavy sedatives such as phenobarbital. Physical therapies (e.g., electroconvulsive therapy and transcranial magnetic stimulation) were not allowed during the study or within 4 weeks prior to the ketamine treatment.
Patients
This study included male or female patients aged 18–65 years experiencing a current major depressive episode of MDD or BD without psychotic symptoms, as confirmed by the Structured Clinical Interview for DSM-5 (SCID-5). Additional inclusion criteria were TRD, defined as a current and/or history of insufficient response to at least two antidepressants of adequate dosage and duration [27], or suicidality (a score of the Beck Scale for Suicidal Ideation [SSI]-part I ≥ 2 [28]); moderate-to-severe depression at screening (a total score of the 17-item Hamilton Depression Rating Scale [HAMD-17] ≥ 17 [29]). We enrolled patients with current suicidality or TRD because these patients might experience the most severe levels of depression and therefore might be most in need of rapid-acting antidepressant ketamine treatment. Key exclusion criteria were meeting any Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnostic criteria of other serious mental disorder (e.g., schizophrenia, neurodevelopmental disorders, and substance-related and addictive disorders). Comorbidity of anxiety disorder or obsessive-compulsive disorder was permitted if this was not the primary cause of depression within the prior 1-year period. Other exclusion criteria were any unstable medical illness or lifetime history of neurological diseases; current pregnancy, breastfeeding, or planned future pregnancy during the study period; current presence of an urgent risk of suicidal or harmful/homicidal behavior; a positive urine toxicology at screening; inability to communicate, provide written consent, or cooperate with the study procedures.
Outcome measures
Depressive symptom severity was rated using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS) [30] to identify participants who experienced a clinically significant improvement in their symptoms with ketamine and who clinically partially or fully responded to an acute course of treatment. We chose MADRS as the symptom monitoring tool instead of HAMD-17 in the present study because MADRS does not include many items that are less sensitive to short-term changes in depression, such as appetite, weight, and sleep, as does HAMD-17. Most individual items on the MADRS are sensitive to antidepressant response [31], and evidence supports the suitability of the MADRS used every 24 h to assess the rapid onset of antidepressant efficacy in patients [32]. Symptom improvement was defined as a decrease ≥ 20% from baseline in MADRS score, according to Lipsitz et al. [23], based on the criteria commonly applied in related studies with conventional antidepressants [19]. Participants were grouped as either “improvers” or “non-improvers” following infusions 1–5. Partial response and response were defined as a reduction of ≥ 30% and ≥ 50% from baseline in MADRS score, respectively. Participants were grouped as either “partial responders” or “non-partial responders” and as either “responders” or “non-responders” 24 h after the whole treatment.
A 7-day recall period was used for baseline measurements, whereas a 4-h recall period was used for measurements taken 4 h after the first infusion. Additionally, a 24-h recall period was used for assessments conducted 24 h after each of the six doses. Research psychiatrists were trained to complete the ratings. Excellent-to-good interrater reliability was established for both scales in patients with affective symptoms before this study (HAMD-17 and MADRS, with intraclass correlation coefficient > 0.90).
Statistical analysis
According to the type and distribution of the data, the Chi-squared test or Fisher’s exact test for categorical variables, as well as the Student’s t-test or Wilcoxon (Mann–Whitney) rank-sum test for continuous variables, was used to compare the demographic and clinical characteristics at baseline between partial responders and non-partial responders, as appropriate.
The relationship between improvement (as opposed to non-improvement after each infusion or consecutive non-improvements after the first few infusions) and partial response and response after the sixth infusion was summarized with sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), Youden index, and area under curve (AUC) to find an appropriate point at which non-improvers should discontinue treatment. In clinical practice with repeated-dose ketamine for TRD or depression with suicidality, due to the severity and particularity of the patient’s condition, the harm of false negative (i.e., 1-sensitivity) and false positive (i.e., 1-specificity) results in predicting the treatment outcome is not equal. Misdiagnosis of false negatives can lead to premature termination of treatment before adequate relief of depressive symptoms, and the treatment will most likely fail. Therefore, sensitivity was the primary indicator used in this study for prediction. We considered a sensitivity > 90% (i.e., a false negative < 10%) to be acceptable.
A mixed linear model for repeated measurements using compound symmetry covariance structure with restricted maximum likelihood estimation was used to estimate the trajectories of symptom change in the non-improvers, based on MADRS scores. We chose a compound symmetry covariance structure based on the characteristics of our data, which comprised repeated measurements of depressive symptom change of non-improvers within a short period. We assumed that the correlations between any two observations at different time points were equal. Additionally, we calculated the correlation coefficients between any two measurements using the residual covariance matrix based on the unstructured covariance structure model; the numerical distribution and time trend characteristics of the correlation coefficients supported a symmetric covariance structure over other covariance structures. Post-hoc pairwise comparisons were performed to examine whether there was an additional improvement in depressive symptoms after the identified point for non-improvers to discontinue treatment. Multiple comparisons were corrected with Sidak’s correction. Further, we performed item-to-item analyses of MADRS in non-improvers to determine which symptoms changed most from baseline to the identified early discontinuation point using paired t-tests, and the effect sizes were calculated. All data were analyzed using IBM SPSS Statistics 24.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at two-tailed P < 0.05.
Results
Patients
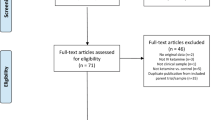
The flow of participants is presented in Fig. 1. A total of 135 patients received ketamine treatment, and 117 (86.7%) patients completed the six infusions. The 18 patients who discontinued treatment tended to have higher MADRS scores (t = −2.455, P = 0.015), a higher proportion of antidepressant-free participants (Fisher’s exact test, P = 0.019), and lower doses of antidepressants than completers (Mann–Whitney U-test, P = 0.008) (Table S1). This implies that a portion of patients with potentially poorer baseline medication compliance and/or more severe depression symptoms withdrew from the study. Our analysis of the reasons for treatment withdrawal also confirms this speculation, with three cases owing to suicidal behavior and three cases due to perceiving the treatment as being ineffective, although an additional 12 cases withdrew informed consent without reporting specific reasons. Of the 117 completers, 90 (76.9%) were MDD, and 27 (23.1%) were BD. Baseline demographics and clinical characteristics grouped by the partial response and non-partial response after the sixth infusion are presented in Table 1, which were generally comparable between the two groups, except that the non-partial responders had a higher proportion of comorbidity of anxiety disorder or obsessive-compulsive disorder (χ2 = 7.2414, P = 0.007). For the whole sample, the mean age was 34.9 years (standard deviation [SD] = 11.9), 51.3% (N = 60) of the patients were women, and 82.9% (N = 97) of the patients had TRD (with or without suicidality). The mean MADRS score at baseline was 32.2 (SD = 7.8). The antidepressants most commonly used at baseline were escitalopram, duloxetine, and venlafaxine. The mean fluoxetine equivalent dose was 37.5 (SD = 22.3) mg/day (Table 1). There was no significant difference in antidepressant use between non-partial responders and partial responders. The specific information regarding patients’ antidepressant use is presented in the supplementary materials (Tables S2 and S3).
Non-improvement and non-response
Early non-improvement in predicting overall non-partial response
Ninety-one (77.8%) participants achieved a partial response at 24 h post-infusion 6, whereas only 34 (29.3%) participants achieved a partial response at 4 h and 24 h post-infusion 1 (see Table S4 for detailed partial response rates at each point). Of the 91 partial responders, 54.4% reported improvements at 4 h post-infusion 1 (i.e., sensitivity was 54.4%), as did 23.1% of the non-partial responders (i.e., specificity was 76.9%), with PPV, NPV, Youden index, and AUC of 0.891, 0.328, 0.314, and 0.657, respectively. The sensitivity, specificity, PPV, NPV, Youden index, and AUC of improvement at 24 h post-infusion 1 in predicting overall partial response were 0.6, 0.885, 0.947, 0.390, 0.485, and 0.742, respectively, and were 0.989, 0.50, 0.874, 0.929, 0.489, and 0.745, respectively, at 24 h post-infusion 5. See Table 2 for details of diagnostic test statistics of infusions 2–4. As shown in Table 2, when using improvement after each of the first five infusions to predict the overall partial response at 24 h post-infusion 6, the sensitivities of improvement at 24 h post-infusion 4 and 5 both exceeded 90%. Analyses of the subsets of MDD, TRD, and suicidality yielded similar results (Tables S5, S6, and S7).
When using two consecutive non-improvement as an indicator, the sensitivities of improvement at 24 h post-infusion 4 (as opposed to two consecutive non-improvements after infusions 3 and 4) and 24 h post-infusion 5 (as opposed to two consecutive non-improvements after infusions 4 and 5) in predicting the overall partial response both exceeded 90%. Similarly, when using three consecutive non-improvements as an indicator, the sensitivities of the improvement at 24 h post-infusion 3 (as opposed to three consecutive non-improvements after infusions 1, 2, and 3), 24 h post-infusion 4 (as opposed to three consecutive non-improvements after infusions 2, 3, and 4), and 24 h post-infusion 5 (as opposed to three consecutive non-improvements after infusions 3, 4, and 5) in predicting the overall partial response exceeded 90%. See Table 3 for detailed diagnostic test statistics.
Early non-improvement in predicting overall non-response
Seventy-one (60.7%) participants achieved a response at 24 h post-infusion 6, whereas only 10 (8.6%) and 16 (13.8%) participants achieved a response at 4 h and 24 h post-infusion 1, respectively (see Table S4 for detailed response rates at each point). Of the 71 responders, 55.7% reported improvements at 4 h post-infusion 1 (i.e., sensitivity was 55.7%), as did 34.8% of the non-responders (i.e., specificity was 65.2%), with PPV, NPV, Youden index, and AUC of 0.709, 0.492, 0.209, and 0.605, respectively. The sensitivity, specificity, PPV, NPV, Youden index, and AUC of the improvement at 24 h post-infusion 1 in predicting the overall response were 0.657, 0.761, 0.807, 0.593, 0.418, and 0.709, respectively, and were 1.000, 0.304, 0.689, 1.000, 0.304, and 0.652, respectively, at 24 h post-infusion 5. See Table S8 for detailed diagnostic test statistics of infusions 2–4. As shown in Table S8, when using improvement after each of the first five infusions to predict the overall response at 24 h post-infusion 6, the sensitivities of the improvement at 24 h post-infusion 4 and 5 both exceeded 90%.
When using either two or three consecutive non-improvement as an indicator, we found that the sensitivities of improvement at 24 h post-infusion 3, 4, and 5 in predicting the overall response exceeded 90%. See Table S9 for detailed diagnostic test statistics.
Symptom reduction in non-improvers
Non-improvement at 24 h post-infusion 4
The linear mixed model demonstrated statistically significant reductions (F = 2.442, degrees of freedom [df] = 126, P = 0.022) in depressive symptoms, as assessed by MADRS scores, from baseline to the end of treatment in patients with no improvement observed at 24 h post-infusion 4. However, post-hoc tests revealed no reduction in depressive symptoms from 24 h post-infusion 4 to the end, after Sidak’s correction (P > 0.3 for all comparisons) (Fig. 2a). Item-to-item analyses using paired t-tests showed that the most-reduced items from baseline to 24 h post-infusion 4 were item 10 (suicide, Cohen’s d = 0.302), item 2 (apparent sadness, Cohen’s d = 0.244), and item 1 (reported sadness, Cohen’s d = 0.239) in non-improvers, but none showed a statistical significance (P > 0.1 for all comparisons, not corrected; Table S10).
Linear mixed models showed significant reductions in depressive symptoms from baseline to the end of treatment in patients with no improvement at 24 h post-infusion 4 (a), two consecutive non-improvements at 24 h post-infusion 3 (b), and three consecutive non-improvements at 24 h post-infusion 3 (c). Post hoc tests, after Sidak’s correction, revealed no significant changes from 24 h post-infusion 4 to the end in non-improvers at 24 h post-infusion 4 (a) and from 24 h post-infusion 3 to the end in those with three consecutive non-improvements at 24 h post-infusion 3 (c). However, depressive symptoms significantly decreased from 24 h post-infusion 3 to the end in patients with two consecutive non-improvements at 24 h post-infusion 3 (b; post-infusion 3 vs. 5: estimated marginal mean difference [EMMD], P = 0.033; post-infusion 3 vs. 6: EMMD = 5.480, P = 0.006). MADRS Montgomery–Åsberg Depression Rating Scale. *Significant difference at the given time point was found when compared to 24 h post-infusion 3 (paired t test, p < 0.05). **Significant difference at the given time point was found when compared to 24 h post-infusion 3 (paired t test, p < 0.01).
Two consecutive non-improvement at 24 h post-infusion 3
The linear mixed model demonstrated statistically significant reductions (F = 8.311, df = 168, P < 0.001) in depressive symptoms, as assessed by MADRS scores, from baseline to the end of treatment in patients who exhibited two consecutive non-improvements at 24 h post-infusion 3. Post-hoc tests revealed reductions in depressive symptoms from 24 h post-infusion 3 to the end, after Sidak’s correction (post-infusion 3 vs. 5, estimated marginal mean difference [EMMD] = 4.800, P = 0.033; post-infusion 3 vs. 6, EMMD = 5.480, P = 0.006) (Fig. 2b).
Three consecutive non-improvement at 24 h Post-Infusion 3
The linear mixed model demonstrated statistically significant reductions (F = 5.540, df = 147, P < 0.001) in depressive symptoms, as assessed by MADRS scores, from baseline to the end of treatment for that subset of patients who had three consecutive non-improvements at 24 h post-infusion 3. However, post-hoc tests revealed no reductions in depressive symptoms from 24 h post-infusion 3 to the end, after Sidak’s correction (P > 0.2 for all comparisons) (Fig. 2c). Item-to-item analyses using paired t-tests showed that the most-reduced items from baseline to 24 h post-infusion 3 were item 2 (apparent sadness, Cohen’s d = 0.492, P = 0.006, not corrected), item 10 (suicide, Cohen’s d = 0.466, P = 0.009, not corrected), and item 1 (reported sadness, Cohen’s d = 0.366, P = 0.057, not corrected) in three consecutive non-improvers (Table S11).
Discussion
To our knowledge, this is the first study to present a complete picture of overall antidepressant ineffectiveness predicted by non-improvement after sequential ketamine infusions to determine an optimal point for non-improvers to discontinue repeated-dose ketamine treatment. We found that the sensitivity of improvement at 24 h post-infusion 4, and that of improvement at 24 h post-infusion 3 vs. three consecutive non-improvements, as predictors for the overall partial response and response, exceeded 90%. These results suggested that if a patient exhibits non-improvement after four infusions, or more conservatively, three consecutive non-improvements after three infusions, then the patient can be deemed nonresponsive and subsequent treatment would not be warranted. We also further confirmed that non-improvers presented no significant reduction in depressive symptoms following the remaining infusions of repeated-dose ketamine treatment after the above-identified early discontinuation point.
In the present study, patients showed a higher partial response rate and response rate 24 h after six infusions (77.8% and 60.7%, respectively) than after the first infusion (29.3% and 13.8%, respectively). This pattern was also seen in other trials of repeated-dose ketamine [13, 14, 33]. Unlike studies that permitted a dose increase in patients who did not respond [14, 33], our study used a fixed dose of 0.5 mg/kg intravenous ketamine. Therefore, the overall response in these early non-responders cannot be explained by a function of dose increase, suggesting that some patients require several infusions to achieve a response. However, our study also found that a subset of patients who did not exhibit early improvement with ketamine remained unresponsive to ketamine throughout the entire treatment period. These findings confirmed the hypothesis that there may be different trajectory patterns in response to ketamine, with some patients responding early, some gradually achieving response after infusions, and some not responding throughout the treatment. The differential response trajectories to ketamine are consistent with those found in previous studies with conventional antidepressants [34, 35]. The early distinction of those patients who do not respond at all from those who exhibit a more delayed response is difficult. Lipsitz et al. [23] found that most (58%) individuals who did not improve post-infusion 1 or 2 with ketamine still experienced an antidepressant response or partial response ( ≥ 20% reduction in QIDS-SR16) post-infusion 4. Although terminating unnecessary, ineffective treatment in advance is beneficial, it is also important to avoid a false premature termination of treatment before adequate relief of symptoms. This is of particular concern because patients receiving repeated-dose ketamine experience TRD or depression with suicidality, and premature termination of treatment usually means treatment failure.
Our data suggested that non-improvement after four infusions, or more conservatively, three consecutive non-improvements, may be an appropriate point to discontinue treatment, with < 10% false negatives. These findings are in line with the latest international expert opinion on ketamine and esketamine use in TRD [25], which recommends that if an individual exhibits minimal response (i.e., ≤ 20% improvement from baseline in total depression symptom severity) after 4–6 ketamine treatments, then that individual can be deemed nonresponsive and subsequent treatments would not be warranted. To the best of our knowledge, no similar research has been reported that offers a complete picture of the overall antidepressant ineffectiveness of ketamine predicted according to non-improvement after sequential infusions. Our study was designed and executed as close to the real clinical environment as possible. Our findings therefore provide a convincing reference for the appropriate timing of early termination of repeated-dose ketamine in clinical practice. Within this naturalistic design, we further explored the reduction of depressive symptoms in the non-improvers after the above-identified points and found no statistical significance. Additional item-to-item analyses showed that even the most-reduced symptoms did not reach a medium effect size (Cohen’s d < 0.50) in non-improvers from baseline to the identified time of early ketamine treatment discontinuation. All these results suggest that most non-improvers by the above-identified points have no significant improvement and would have no subsequent improvement either qualitatively or quantitatively.
Major depression is a heterogeneous condition. The intrinsic non-specificity implies a wide range of etiologies, risk factors, and symptom profiles [36, 37], which likely account for the highly variable response to treatments, including ketamine [1, 38,39,40,41,42]. Patients with TRD are a more heterogeneous and complex group in terms of phenomenology, patterns of comorbidity, and prior history. The mechanism of action underlying the antidepressant effects of ketamine remains unclear. The initially proposed hypothesis of N-methyl-D-aspartate (NMDA)-mediated disinhibition of GABAergic interneurons is being increasingly challenged by a growing body of contrary evidence, which has found that other non-ketamine NMDA receptor antagonists do not exhibit antidepressant effects similar to those of ketamine [43, 44]. Current evidence suggests that the enhanced and sustained activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors is responsible for ketamine’s antidepressant effects [45], possibly by influencing the synthesis and release of brain-derived neurotrophic factor (BDNF) through the mTOR pathway [46, 47]. It could be speculated that the key mechanistic steps mediating symptom relief may differ at the cellular and behavioral levels across different subgroups of responders to ketamine, resulting in different response trajectories. However, specific mechanisms and response trajectories require further research to deepen our understanding of ketamine’s antidepressant effects. Similar to conventional antidepressants, multiple baseline demographic, clinical, and biological predictors of the response to ketamine have been investigated, but few validated [48,49,50]. The reported potential pretreatment predictors include clinical characteristics (i.e., higher body mass index, no history of suicide attempts, positive family history of alcohol use disorder), neurocognitive function (i.e., lower processing speed), peripheral biochemistry (i.e., lower plasma adiponectin levels, higher levels of circulating vitamin B12), sleep electrophysiology (i.e., low delta sleep ratio), neurobiochemistry (i.e., low Glx/glutamate ratio), neuroimaging (i.e., increased pretreatment anterior cingulate cortex activity), and genetic variations (i.e., val66met BDNF allele) [48]. Our previous research also identified pretreatment predictors that may be related to the response to repeated-dose intravenous ketamine treatment, such as lower personal income [12], no psychiatric hospitalization history [12], better visual learning [51], and larger pretreatment volumes of the right thalamus and left subiculum head of the hippocampus [52]. Although these predictors, as well as moderator/mediator biomarkers, provide promising insights into ketamine’s mechanism of action and the differential response trajectories to ketamine, they remain largely exploratory in nature and none are ready for clinical use at present [48, 49]. As Andrade argued [53], it is nice to hope that research will identify patients who might be ketamine responders, but given the poor progress in the identification of response predictors to other treatments in psychiatry, there is no reason to expect that similar studies with ketamine would fare any better. Therefore, predicting the overall antidepressant effect of ketamine based on response to the first few infusions (as the most reliable posttreatment clinical predictor) is currently a more reasonable, practical, and operational approach, which is in line with the idea that treatment with ketamine should be considered an explicit individual therapeutic trial [25]. Our study also suggests that this is feasible, although observation through three ketamine infusions, at minimum, is necessary.
Limitations
The relatively large sample size, inclusive eligibility criteria of participants, and real-world design and execution add value to the present study, which strengthens the external validity of the results to patients in a real clinical environment. However, the results should be interpreted with several limitations in mind. First, this study was a post-hoc examination of data from a single-arm open-label clinical trial not designed to address our study question. Second, this study was performed in the Chinese population, and all included patients were treatment-resistant or had suicidality; also, a portion of patients with potentially poorer baseline medication compliance and/or more severe depression symptoms withdrew from the study, which limits the generalizability of these results. Third, we included patients with comorbidity of anxiety disorder or obsessive-compulsive disorder, if this was not the primary cause of depression within the prior 1-year period, which was found to be a significant pretreatment factor correlated to the treatment response. This might be a confounder when interpreting our results. Fourth, although patients maintained pre-study antidepressants and other psychiatric medications at stable dosages throughout the study, the influence of these medications on depressive symptoms cannot be ruled out. Fifth, data from patients with MDD/BD and TRD/suicidality were analyzed as a whole. Although in our sensitivity analyses, the results with MDD, TRD, and suicidality data analyzed separately were generally consistent with the results when MDD, BD, TRD, and suicidality data were analyzed as a whole, this approach may increase heterogeneity. Sixth, multiple comparisons of the MADRS scores at each point were corrected and could potentially make some of the results somewhat conserved. Finally, symptom improvement was defined a priori based on the criteria commonly used in studies of conventional antidepressants; its rationality requires further empirical investigation and demonstration. Furthermore, we also considered a priori an acceptable sensitivity > 90% (i.e., < 10% false negative) in predicting the antidepressant outcome of ketamine. Which threshold to use to balance the harm of false negatives and false positives requires careful study in future research. It should be noted that the findings of this study are limited to the situation in which ketamine is used as an antidepressant treatment; it is unclear whether the findings apply when ketamine is used in anti-suicidal treatment.
Conclusion
Our study findings suggest that non-improvement after four ketamine infusions, or even a more conservative three consecutive non-improvements after three ketamine infusions, could serve as a signal of non-response in depression, and subsequent treatment would not be warranted. The results of this study may have implications from both research and clinical standpoints, such as informing patients who are not improving whether and when to optimize the dose, discontinue treatment, or switch to alternative treatments. In light of these findings, future hypothesis-driven studies with refined designs are needed to replicate our results and better understand the mechanisms underlying the different response trajectories with ketamine. Further, for patients with TRD who do not respond to ketamine, how to optimize treatment is an understudied topic of great importance.
Data availability
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
References
Marcantoni WS, Akoumba BS, Wassef M, Mayrand J, Lai H, Richard-Devantoy S, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 – January 2019. J Affect Disord. 2020;277:831–41.
Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175:150–8.
Salloum NC, Fava M, Hock RS, Freeman MP, Flynn M, Hoeppner B, et al. Time to relapse after a single administration of intravenous ketamine augmentation in unipolar treatment-resistant depression. J Affect Disord. 2020;260:131–9.
Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82.
Costi S, Soleimani L, Glasgow A, Brallier J, Spivack J, Schwartz J, et al. Lithium continuation therapy following ketamine in patients with treatment resistant unipolar depression: a randomized controlled trial. Neuropsychopharmacol. 2019;44:1812–9.
Abdallah CG, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, et al. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacol. 2020;45:990–7.
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–6.
Kryst J, Kawalec P, Mitoraj AM, Pilc A, Lasoń W, Brzostek T. Efficacy of single and repeated administration of ketamine in unipolar and bipolar depression: a meta-analysis of randomized clinical trials. Pharm Rep. 2020;72:543–62.
Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25:1592–603.
Lijffijt M, Murphy N, Iqbal S, Green CE, Iqbal T, Chang LC, et al. Identification of an optimal dose of intravenous ketamine for late-life treatment-resistant depression: a Bayesian adaptive randomization trial. Neuropsychopharmacol. 2022;47:1088–95.
Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816–26.
Zheng W, Zhou YL, Liu WJ, Wang CY, Zhan YN, Li HQ, et al. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. J Psychiatr Res. 2018;106:61–8.
Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176:401–9.
McIntyre RS, Rodrigues NB, Lee Y, Lipsitz O, Subramaniapillai M, Gill H, et al. The effectiveness of repeated intravenous ketamine on depressive symptoms, suicidal ideation and functional disability in adults with major depressive disorder and bipolar disorder: results from the Canadian Rapid Treatment Center of Excellence. J Affect Disord. 2020;274:903–10.
Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014;155:123–9.
Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78.
Wagner S, Engel A, Engelmann J, Herzog D, Dreimüller N, Müller MB, et al. Early improvement as a resilience signal predicting later remission to antidepressant treatment in patients with major depressive disorder: systematic review and meta-analysis. J Psychiatr Res. 2017;94:96–106.
Olgiati P, Serretti A, Souery D, Dold M, Kasper S, Montgomery S, et al. Early improvement and response to antidepressant medications in adults with major depressive disorder. Meta-analysis and study of a sample with treatment-resistant depression. J Affect Disord. 2018;227:777–86.
Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70:344–53.
Gilaberte I, Romera I, Perez-Sola V, Menchon JM, Schacht A. Different levels of lack of improvement at 4 weeks of escitalopram treatment as predictors of poor 8-week outcome in MDD. J Affect Disord. 2013;146:433–7.
Wagner S, Tadić A, Roll SC, Engel A, Dreimüller N, Engelmann J, et al. A combined marker of early non-improvement and the occurrence of melancholic features improve the treatment prediction in patients with major depressive disorders. J Affect Disord. 2017;221:184–91.
Yuan H, Zhu X, Luo Q, Halim A, Halim M, Yao H, et al. Early symptom non-improvement and aggravation are associated with the treatment response to SSRIs in MDD: a real-world study. Neuropsychiatr Dis Treat. 2019;15:957–66.
Lipsitz O, McIntyre RS, Rodrigues NB, Kaster TS, Cha DS, Brietzke E, et al. Early symptomatic improvements as a predictor of response to repeated-dose intravenous ketamine: results from the Canadian Rapid Treatment Center of Excellence. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110126.
Rodrigues NB, McIntyre RS, Lipsitz O, Lee Y, Cha DS, Nasri F, et al. Safety and tolerability of IV ketamine in adults with major depressive or bipolar disorder: results from the Canadian rapid treatment center of excellence. Expert Opin Drug Saf. 2020;19:1031–40.
McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178:383–99.
Swainson J, McGirr A, Blier P, Brietzke E, Richard-Devantoy S, Ravindran N, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task Force Recommendations for the Use of Racemic Ketamine in Adults with Major Depressive Disorder: Recommendations Du Groupe De Travail Du Réseau Canadien Pour Les Traitements De L’humeur Et De L’anxiété (Canmat) Concernant L’utilisation De La Kétamine Racémique Chez Les Adultes Souffrant De Trouble Dépressif Majeur. Can J Psychiatry. 2021;66:113–25.
Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, et al. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol. 2014;28:536–44.
Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–52.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Santen G, Danhof M, Della Pasqua O. Sensitivity of the Montgomery Asberg depression rating scale to response and its consequences for the assessment of efficacy. J Psychiatr Res. 2009;43:1049–56.
Johnson KM, Devine JM, Ho KF, Howard KA, Saretsky TL, Jamieson CA. Evidence to support Montgomery-Asberg depression rating scale administration every 24 h to assess rapid onset of treatment response. J Clin psychiatry. 2016;77:1681–6.
Cusin C, Ionescu DF, Pavone KJ, Akeju O, Cassano P, Taylor N, et al. Ketamine augmentation for outpatients with treatment-resistant depression: Preliminary evidence for two-step intravenous dose escalation. Aust N Z J Psychiatry. 2017;51:55–64.
Kelley ME, Dunlop BW, Nemeroff CB, Lori A, Carrillo-Roa T, Binder EB, et al. Response rate profiles for major depressive disorder: Characterizing early response and longitudinal nonresponse. Depression Anxiety. 2018;35:992–1000.
Uher R, Muthén B, Souery D, Mors O, Jaracz J, Placentino A, et al. Trajectories of change in depression severity during treatment with antidepressants. Psychol Med. 2010;40:1367–77.
Ghaemi SN, Vohringer PA, Vergne DE. The varieties of depressive experience: diagnosing mood disorders. Psychiatr Clin North Am. 2012;35:73–86.
Carragher N, Adamson G, Bunting B, McCann S. Subtypes of depression in a nationally representative sample. J Affect Disord. 2009;113:88–99.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40.
Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–42.
Barth J, Munder T, Gerger H, Nuesch E, Trelle S, Znoj H, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: a network meta-analysis. PLoS Med. 2013;10:e1001454.
Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, Reynolds CF. 3rd. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: a meta-analysis of direct comparisons. World Psychiatry. 2013;12:137–48.
Wang C, Zhou Y, Zheng W, Liu W, Zhan Y, Li H, et al. Association between depression subtypes and response to repeated-dose intravenous ketamine. Acta Psychiatr Scandinavica. 2019;140:446–57.
Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46:1459–72.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6.
Abelaira HM, Réus GZ, Neotti MV, Quevedo J. The role of mTOR in depression and antidepressant responses. Life Sci. 2014;101:10–4.
Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29:419–23
Rong C, Park C, Rosenblat JD, Subramaniapillai M, Zuckerman H, Fus D, et al. Predictors of response to ketamine in treatment resistant major depressive disorder and bipolar disorder. Int J Environ Res Public Health. 2018;15:771.
Kadriu B, Ballard ED, Henter ID, Murata S, Gerlus N, Zarate CA Jr. Neurobiological biomarkers of response to ketamine. Adv Pharm. 2020;89:195–235.
Meshkat S, Rodrigues NB, Di Vincenzo JD, Ceban F, Jaberi S, McIntyre RS, et al. Pharmacogenomics of ketamine: a systematic review. J Psychiatr Res. 2021;145:27–34.
Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, et al. Neurocognitive effects of six ketamine infusions and the association with antidepressant response in patients with unipolar and bipolar depression. J Psychopharmacol. 2018;32:1118–26.
Zhou YL, Wu FC, Liu WJ, Zheng W, Wang CY, Zhan YN, et al. Volumetric changes in subcortical structures following repeated ketamine treatment in patients with major depressive disorder: a longitudinal analysis. Transl Psychiatry. 2020;10:264.
Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78:e852–e7.
Acknowledgements
We thank the patients for their participation in the trial and LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by Guangzhou Science and Technology Plan Project (CW, grant number 202102020557, 2023A03J0842; Y Zhou, grant number 202103000032, 202201010714), Guangdong Basic and Applied Basic Research Foundation (Y Zhou, grant number 2022A1515011567), Guangdong College Students Innovation and Entrepreneurship Training Project (Y Zhou, grant number S202310570038), Innovative Clinical Technique of Guangzhou (YN), Guangzhou Health Science and Technology Project (SM, grant number 20231A010038), and Guangzhou Research-oriented Hospital. The funding source had no role in the study design, analysis, or interpretation of data or in the preparation of the report or decision to publish.
Author information
Authors and Affiliations
Contributions
CW, Y Zhou, and YN conceived and designed the study. CW, XL, WL, Y Zhan, WZ, XC, GL, SM, and Y Zhou collected and prepared the data. CW performed the statistical analyses and drafted the first manuscript. CW, HL, and RSM interpreted the results and revised the manuscript. Y Zhou and YN supervised the analyses and edited the manuscript. All authors reviewed, contributed to, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
RSM has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie. He is the CEO of Braxia Scientific Corp. All the remaining authors have nothing to disclose.
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University and was conducted in accordance with the ethical principles based on the Declaration of Helsinki. Informed consent was obtained from all participants before the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, C., Lan, X., Liu, W. et al. Non-improvement predicts subsequent non-response to repeated-dose intravenous ketamine for depression: a re-analysis of a 2-week open-label study in patients with unipolar and bipolar depression. Transl Psychiatry 14, 324 (2024). https://doi.org/10.1038/s41398-024-03027-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-024-03027-2