Abstract
Recreational use of nitrous oxide (N2O) has risen dramatically over the past decades. This study aimed to examine its rewarding effect and the underlying mechanisms. The exposure of mice to a subanesthetic concentration (20%) of N2O for 30 min for 4 consecutive days paired with N2O in the morning and paired with the air in the afternoon produced apparent rewarding behavior in the conditioned place preference (CPP) paradigm. This was abrogated by microinjection into the nucleus accumbens (NAc) of the dopamine (DA) D1 receptor antagonist SCH23390, but not the D2 antagonist haloperidol. N2O robustly enhanced DAergic neuronal activity of the ventral tegmental area (VTA) and the concentration of DA in the NAc. The repeated N2O exposure also upregulated the expression of brain-derived neurotrophic factor (BDNF) in the VTA and its multiple downstream mediators in the NAc. Conversely, VTA focal knockdown of BDNF and the inhibition of the downstream mediators suppressed the N2O-induced rewarding effect and the DAergic neuronal activity of the VTA. Further, the combined intervention of BDNF knockdown and D1 antagonist significantly inhibited the N2O-induced rewarding effect in mice, which was greater than that of BDNF knockdown alone, but was not significantly different from that of D1 antagonist alone. These results indicate that the rewarding properties of N2O at subanesthetic concentration are associated with its upregulation of the VTA-NAc DA reward pathway probably via mediation of D1 receptor and BDNF/TrkB signaling. Among them, the modulation of BDNF may be the upstream of D1 receptor involved in N2O rewarding effect.
Similar content being viewed by others
Introduction
Nitrous oxide (N2O), often known as laughing gas, is an inhalational anesthetic and analgesic which has been used in dentistry and emergency care for almost 250 years [1, 2]. Inhalation of N2O can cause rapid but short-lived euphoria, relaxation, and even hallucination [3]. Due to the convenience and cost effectiveness, N2O use for recreational purpose has risen dramatically worldwide over the past decades, becoming the seventh most commonly used recreational drug [4, 5]. The N2O abuse is particularly serious in adolescents of European countries and the Unites States, with an estimated prevalence of 2–15.8% [6, 7]. However, given that the neurological damage and rapid antidepressant effects of N2O have been extensively reported [8,9,10], little is known about the underlying mechanisms of such addiction-like rewarding effects [11, 12].
Dopaminergic (DA) projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), namely the VTA-NAc dopamine (DA) pathway, is an essential mesolimbic reward circuitry for drug addiction [13]. Early studies have revealed that N2O exposure markedly increased DA level in the rat NAc and had no effects on ketamine-induced increase of DA level [14], but suppressed morphine- and cocaine-induced elevation of extracellular DA level in the NAc [15, 16]. One study reported that repeated N2O exposure caused rewarding effects in the conditioned place preference (CPP), a standard behavioral paradigm for studying drug rewarding effects; this N2O-induced reward behavior was attenuated by systemic administration of the D1 receptor antagonist SCH23390, but not the D2 antagonist haloperidol [17]. Besides, brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin-related kinase B (TrkB) are widely distributed in the brain, including the VTA and NAc [18]. The coexistence of BDNF in DAergic neurons of the VTA has been observed [19]. The BDNF/TrkB- extracellular signal-regulated kinases (ERK)/ cAMP response element-binding protein (CREB) downstream signaling pathway of the VTA-NAc dopamine reward circuit is heavily involved in cocaine, morphine, methamphetamine, alcohol dependent and addictions [20,21,22,23,24,25,26]. The upregulation of BDNF/TrkB-ERK/CREB signaling was considered to be associated with rapid antidepressant effects of N2O [27].
The compiling evidence prompted us to investigate the precise mechanisms underlying the VTA-NAc dopamine reward pathway involved in rewarding effect of N2O. We first applied orthogonal design to optimize the N2O exposure pattern and established the rewarding effects of N2O in mice. Regional microinjection and focal knockdown in the brain of genetically engineered mice were utilized for optic-fiber recording and monitoring real-time neurochemical effects of N2O exposure. Our finding demonstrated that the addictive effect of N2O was associated with the upregulation of the VTA-NAc dopamine reward pathway via the mediation of D1 receptor and BDNF/TrkB-ERK-CREB signaling.
Methods and materials
Stereotaxic brain surgery
Prior to the N2O/air exposure and CPP test (see below), all animals received stereotaxic brain surgery on the VTA and NAc for microinjection of AAV virus, implant of optic-fiber cannula and drug delivery cannula for pharmacological intervention. Mice were anesthetized by 2% isoflurane for surgery in the stereotactic instrument (RWD, Shenzhen, China). The intended stereotaxic coordinates were ±0.45 ML, −3.4 mm AP, −4.2 mm DV for VTA; ±0.75 ML, 1.25 mm AP, −4.4 mm DV for NAc. For microinjection of AAV virus, each brain site was injected with a volume of 300 nL (Vehicle: Sterile PBS buffer) of purified and mixed AAV (1012 IU/mL) at an injection rate of 100 nL/min using a 32-gauge needles connected to a 1 μL microsyringe (Hamilton, Nevada, USA). rAAV-hSyn-DA2h was injected into the NAc and rAAV-hSyn-NLS-Cre-P2A-mCherry was injected into the VTA. Optic-fiber cannula (200 μm in diameter) was implanted into the two brain sites. The drug delivery cannula was implanted into NAc and fixed with dental cement. The virus was injected at 21 days prior to the N2O/air exposure (see below). The optic-fiber cannula and drug delivery cannula were implanted 7 days prior to the N2O/air exposure. Such that, animals were allowed for at least 7 days to recover from surgery.
In vivo optic-fiber recording
In vivo optic-fiber recording was used to measure calcium activity of DA-labelled neurons in the VTA and DA concentration in the NAc. Fluorescence signals were recorded using a Fiber Photometry system equipped with a 470- and 410- excitation lasers (RWD, Shenzhen, China). The wavelength of 470 nm was used to excite fluorescence from the genetically encoded indicator of GCaMPs and DA sensor. The 410 nm served control for movement and bleaching. Fluorescence signals were recorded using a script provided by Inper Technology Corporation, LTD. The 470- and 410- signals were independently processed and normalized to baseline signals to determine ΔF/F based on the formula ΔF/F = (F–F0)/F0, where F is the fluorescence value at a given time, F0 is the mean value of the pre-stimulus fluorescence signals over a period (2 s). The baseline correction and motion-correction strategies were used in the process of data analysis. ΔF/F values are presented as heatmaps and curve graphs.
CPP paradigm
The procedure consisted of the four phases: acclimatization (3 days), pre-test (1 day), conditioning (4 days), and test (1 day) (Fig. 1B). The acclimatization was aimed at allowing animals to adapt to the environment and eliminating stress. For acclimatization, an animal was placed in the aisle with the guillotine doors open and allowed free access to all three compartments for 10 mins for 3 consecutive days.
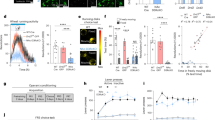
A The schematic diagram of CPP apparatus. B The timeline of CPP procedure. C Effect of N2O on the acquisition of CPP in mice (n = 12 / group). D Effects of D1 and D2 antagonists on N2O-induced increase of CPP score (n = 6 / group). E Effect of N2O exposure on the expression of NAc’s D1R (n = 3 / group). F Effect of repeated N2O exposure on relative intensity of D1R in neurons of the NAc (n = 3 / group). Scale bar: 50 μm. All data are means ± SEM. *P < 0.05, **P < 0.01.
In the pre-test phase, the two guillotine doors were removed. Individual mice were allowed to explore the entire CPP apparatus for 15 mins. Time spent in each chamber was recorded to determine their initial preferences between the two chambers. CPP scores were calculated by using the following formula:
The ratio was calculated by dividing time spent in the N2O or air chamber by the sum of time spent in both chambers. Only animals with a ratio of 40–60% in either chamber were retained for further experiments.
In the conditioning phase, the guillotine doors were closed. A mouse was placed in the N2O chamber for 30 mins in morning and subsequently in the air chamber for 30 mins in afternoon for 4 consecutive days. For N2O exposure, gas containing 20% N2O and 20–21% oxygen supplied from either the air or the oxygen concentrator was delivered into the N2O chamber. For control exposure, the air was delivered to the N2O chamber. For pharmacological interventions, DA antagonists, inhibitors of BDNF downstream mediators, or vehicle were microinjected into the NAc 20 mins prior to the exposure for 4 consecutive days.
In the test day, the two guillotine doors were removed. The mouse was initially placed in the aisle and allowed to explore the entire CPP apparatus for 15 min. The CPP scores were calculated.
The detailed methods in other experiments are described in the supplementary materials.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software 9.0 (GraphPad Software Inc., CA, USA) and results are expressed as mean ± SEM. Student t test was used to examine differences between the two groups. One-way analysis of variance (ANOVA) was used to determine statistical significance of multiple groups, followed by Bonferroni post hoc multiple comparisons to detect between-group differences. Two-way ANOVA was used to examine the statistical differences across the groups in changes in CPP scores, followed by Bonferroni post hoc multiple comparisons to detect between-group differences. All statistical tests were two-tailed. Statistical significance was defined as P < 0.05.
Results
N2O exposure induces CPP
Two groups of C57BL/6 J mice were exposed to 20% N2O and air for 30 mins for 4 days (experimental parameters was determined by orthogonal experiment described in the supplementary materials), respectively. Upon exposure to N2O once a day for 4 days, there was a significant group × time interaction (F (1, 44) = 7.638, P = 0.0083), with significant main effect of group (F (1, 44) = 7.620, P = 0.0084) and time (F (1, 44) = 16.23, P = 0.0002). Thus, under the optimized conditions, N2O-exposed mice had an approximately 6.5-fold greater CPP score than those air-exposed mice (P = 0.0019) (Fig. 1C), at the same time produced pronounced locomotion in the CPP paradigm (Fig. S2).
N2O enhances DAergic activity in VTA-NAc pathway
We next sought to identify the role of dopamine receptors in N2O-mediated CPP in mice. Four groups of C57BL/6 J mice were included to identify DA receptor subtypes that were involved in the addictive effects of N2O. One group served as controls by exposing to the air; the other three groups respectively received NAc microinjection of vehicle, the D1 antagonist SCH23390 (1 µg/0.5 µl/side), and the D2 antagonist haloperidol (1 µg/0.5 µl/side) 20 mins prior to the N2O exposure once a day for 4 days during the conditioning phase (Fig. 1B). Two-way ANOVA showed a marked group × time interaction (F (3, 40) = 4.220, P = 0.0110) with significant main effect of group (F (3, 40) = 3.405, P = 0.0266) and time (F (1, 40) = 46.85, P < 0.0001). The CPP score of the D1 antagonist-treated mice markedly decreased compared to the vehicle-treated group (P = 0.0326), but did not differ between the vehicle- and D2 antagonist-treated groups (P > 0.9999) (Fig. 1D). Then, to further explore the effect of N2O on D1R, the expression of D1R were measured by western blot and immunofluorescence. The results were showed in Fig. 1E, F. N2O exposure to mice upregulated the expression level and relative intensity of D1R in NAc (P ≤ 0.0237).
Mice upon N2O exposure were further subject to DAergic neurons labelling by double immunofluorescence staining of NeuN/TH. The DAergic neurons in the VTA were counted and compared between the N2O- and air-exposed groups. The number of NeuN/TH-labelled DAergic neurons in the VTA of the N2O-exposed mice was dramatically increased compared with those air-exposed mice (P = 0.0002) (Fig. 2A, B). Meanwhile, the mRNA levels of TH in VTA were detected. N2O exposure upregulated the TH expression in mRNA level in VTA (P = 0.0003) (Fig. 2C).
A, B Effects of repeated N2O exposure on NeuN/TH positive neurons of the VTA (n = 3 / group). Scale bar: 50 μm. C Effect of N2O exposure on the expression of VTA’s TH mRNA (n = 3 / group) D, E The localization of rAAV-hSyn-DA2h microinjection site. F The heatmap of DA2h fluorescence in NAc in response to N2O induction (n = 5 / group). G The Average ΔF/F of DA2h fluorescence in NAc in response to N2O induction (n = 5 / group). H, I The localization of rAAV-EF1α-DIO-GCaMP6s microinjection site. Scale bar: 50 μm. J The heatmap of GCaMP fluorescence in VTA in response to N2O induction (n = 5 / group). K The Average ΔF/F of GCaMP fluorescence in VTA in response to N2O induction (n = 5 / group). All data are means ± SEM. ***P < 0.001.
To further evaluate the effects of N2O on NAc dopamine release and VTA dopamine neuronal activity of the VTA-NAc pathway, in vivo optic-fiber recording was applied to synchronously record changes over 15 s of the concentration of DA in the NAc and the intensity of Ca2+ signals of the TH-labelled DAergic neurons in the VTA. C57BL/6 J mice with rAAV-hSyn-DA2h injection in the NAc were exposed to 20% N2O or the air for 15 s, while the changes in the fluorescence signal intensity of the DA sensor in the NAc were recorded by an optical fiber recorder followed by the fluorescence validation of microinjection site (Fig. 2D, E). The N2O exposure induced a progressive and pronounced enhancement of fluorescence intensity and reached the peak in 6–8 s (Fig. 2F, G), which reflected an increase in DA concentration. The microinjection site was localized within the NAc (Fig. 2D, E).
For Ca2+ signals in the VTA, the DAT-Cre micewith VTA microinjection of rAAV-EF1α-DIO-GCaMP6s was exposed to 20% N2O or the air for 15 s. The intensity of Ca2+ signals was synchronously recorded, followed by the validation of microinjection site using GCaMP/TH double immunofluorescence labelling of DAergic neurons (Fig. 2H, I). The intensity of Ca2+ signals increased steeply and rapidly reached the peak in the first few seconds of the N2O exposure, followed by multiple fluctuations throughout the remaining times (Fig. 2J, K). All GCaMP/TH double labelled neuronal cells were localized within the VTA (Fig. 2H, I). Thus, these data demonstrated that N2O enhances DAergic activity in VTA-NAc pathway.
VTA-derived BDNF drives N2O-induced rewarding effect and DAergic activity
We next sought to identify the role of BDNF in N2O-induced rewarding effects. A serial of CPP tests was conducted to examine the effects of inhibition of the BDNF downstream mediators, TrkB, ERK, and CREB on N2O-induced rewarding effect. The three inhibitors, which could inhibit the three downstream regulators mentioned above respectively, ANA-12 (3 µg/0.5 µL/side), SCH772984 (3 µg/0.5 µL/side), and KG501 (3 µg/0.5 µL/side), were injected into the NAc 20 mins prior to the N2O exposure once a day for 4 days during the conditioning phase. Four groups of C57BL/6 J mice were included for each mediator. One group served as controls by injecting vehicle and exposing to the air; one group were injected with an inhibitor and exposed to the air; and the other two groups were respectively injected with vehicle and the inhibitor, and then were exposed to N2O. Two-way ANOVA revealed pronounced differences existing group × time interaction (ANA-12, (F (3, 64) = 4.931, P = 0.0038) with significant main effect of group (F (3, 64) = 6.742, P = 0.0005) and time (F (1, 64) = 14.92, P = 0.0003); SCH772984, (F (3, 64) = 5.607, P = 0.0018) with significant main effect of group (F (3, 64) = 4.295, P = 0.0080) and time (F (1, 64) = 6.768, P = 0.0115); KG501, (F (3, 64) = 2.347, P = 0.0819) with significant main effect of group (F (3, 64) = 8.018, P = 0.0001) and time (F (1, 64) = 20.35, P < 0.0001)) (Fig. 3A–C). CPP scores of all the groups pretreated with any inhibitor and exposed to the air were not statistically different from those of the controls (P > 0.9999). CPP scores of the groups pretreated with the three inhibitors and exposed to N2O were only approximately 32–40% of those pretreated with vehicle and exposed to N2O (ANA-12, P = 0.0349; SCH772984, P = 0.0491; KG501, P = 0.0109).
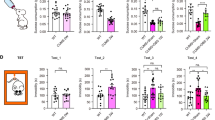
A Effect of ANA-12 on N2O-induced CPP behaviors in mice (n = 9 / group). B Effect of SCH 772984 on N2O-induced CPP behaviors in mice (n = 9 / group). C Effect of KG 501 on N2O-induced CPP behaviors in mice (n = 9 / group). D Effect of N2O exposure on the expression of VTA’s BDNF mRNA (n = 3 / group). E Effect of N2O exposure on the expression of VTA’s BDNF protein (n = 3 / group). F Effect of N2O exposure on the expression of NAc’s p-Trkb protein (n = 3 / group). G Effect of N2O exposure on the expression of NAc’s p-ERK1/2 protein (n = 3 / group). H Effect of N2O exposure on the expression of NAc’s p-CREB protein (n = 3 / group). I, J Effect of N2O exposure on NeuN/p-ERK1/2 positive neurons of the NAc (n = 3 / group). Scale bar: 50 μm. K, L Effect of N2O exposure on NeuN/c-Fos positive neurons of the NAc (n = 3 / group). Scale bar: 50 μm. All data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
We then determined the expression of BDNF in the VTA of mice exposed to N2O or air, following the CPP tests as described above. In addition, the downstream mediators, including p-TrkB/TrkB, p-ERK/ERK, p-CREB/CREB and c-Fos in the NAc were examined by Western blot and immunofluorescence staining (Fig. 3D–L). qPCR analysis revealed significantly greater mRNA expression of BDNF in the VTA of N2O-exposed mice than those air-exposed mice (P = 0.0293) (Fig. 3D). The protein levels were further supported by Western blot analysis of BDNF in VTA (P = 0.0319), p-TrkB/TrkB (P = 0.0399), p-ERK/ERK (P = 0.0224), and p-CREB/CREB (P = 0.0069) in the NAc (Fig. 3E–H). Semi-quantitative immunofluorescence analysis showed that the number of the double-labelled neurons in the NAc of the N2O-exposed mice was nearly 8-fold higher on NeuN/p-ERK (P = 0.0009) (Fig. 3I, J) and 3-fold higher on NeuN/c-fos (P = 0.0007) than those air-exposed mice (Fig. 3K, L). Thus, we validated the activation of BDNF cascade upon N2O exposure.
To identify the importance of regional BDNF in N2O-induced rewarding effects, mice were injected with rAAV-hSyn-NLS-Cre-P2A-mCherry at VTA to knockdown BDNF in situ, followed by exposure to the air and N2O for 30 mins a day for 4 days. The controlled Flox groups were injected with vehicle in the VTA were respectively exposed to the air and N2O in the same manner (Fig. 4). A striking difference was present of the group factor (F (3, 64) = 4.734, P = 0.0048). Under the air exposure, the CPP scores did not differ between BDNF knockdown and control Flox groups (P > 0.9999). Under the N2O exposure, the CPP score of the BDNF knockdown mice was only approximately half of the control Flox mice (P = 0.0190) (Fig. 4A).
A Effects of BDNF knockdown on N2O-induced CPP behaviors in mice (n = 9 / group). B Effect of BDNF knockdown on the expression of VTA’s BDNF mRNA (n = 3 / group). C The schematic of rAAV-hSyn-NLS-Cre-P2A-mCherry microinjection in VTA. D–G Effect of BDNF knockdown on the expression of NAc’s p-Trkb, p-ERK1/2 and p-CREB proteins (n = 3 / group). H, I Effect of BDNF knockdown on exposure of NeuN/c-Fos positive neurons of the NAc (n = 3 / group). Scale bar: 50 μm. J, K Effect of BDNF knockdown on exposure of NeuN/TH positive neurons of the VTA (n = 3 / group). Scale bar: 50 μm. All data are means ± SEM. *P < 0.05, **P < 0.01 and ****P < 0.0001.
Following the completion of CPP test, the mice were randomly chosen for further measurement of the expression of BDNF mRNA of the VTA using q-PCR (n = 3 for each group) and the downstream mediators, p-TrkB/TrkB, p-ERK1/2/ERK1/2, p-CREB/CREB, c-fos in the NAc, and DAergic neurons in the VTA using Western blot and immunofluorescence (n = 3 each group). q-PCR analysis showed a remarkable decrease of BDNF mRNA level in the knockdown group compared to the controls (P = 0.0405) (Fig. 4B, C). Western blot analysis displayed significantly greater values of p-TrkB/TrkB, p-ERK1/2/ERK1/2, and p-CREB/CREB in the N2O-exposed control Flox mice than those exposed to the air (P ≤ 0.0485). Under the N2O exposure, the BDNF knockdown group had obviously lower expression levels of the three mediators than the control Flox group (P ≤ 0.0467), but did not distinguish from the BDNF knockdown and control Flox groups exposed to the air (P > 0.9999) (Fig. 4D–G).
On semi-quantitative immunofluorescence analysis, the number of the c-Fos positive neurons in the NAc of the N2O-exposed control Flox group was approximately 28-fold greater than the air-exposed control Flox group (P < 0.0001). Under the N2O exposure, the number of the c-Fos positive neurons of the BDNF knockdown group was only 43% of the control Flox group (P < 0.0001) (Fig. 4H, I).
The number of the NeuN/TH positive neurons in the VTA of the N2O-exposed control Flox group was approximately 9-fold greater than the air-exposed control Flox group (P < 0.0001). Under the N2O exposure, the number of the NeuN/TH positive neurons of the BDNF knockdown group was significantly lower (about 54%) than the control Flox group (P = 0.0015) (Fig. 4J, K).
A combination of D1 antagonist and BDNF knockdown completely abolishes N2O-induced rewarding effect
The above results encouraged us to explore whether BDNF plays an equivalent role in the regulation of N2O reward with D1 antagonism. Two-way ANOVA showed a prominent difference in changes in CPP score among the group × time interaction (F (4, 50) = 12.36, P < 0.0001) with significant main effect of group (F (4, 50) = 14.52, P < 0.0001) and time (F (1, 50) = 35.54, P < 0.0001) (Fig. 5A). The N2O exposure caused an approximately 16-fold increase of CPP score in the control Flox group (P < 0.0001). Both D1 antagonist-treated and BDNF knockdown group had strikingly lower CPP scores than the control Flox group under the N2O exposure (P ≤ 0.0011). Notably, D1 antagonism further decreased the CPP scores of the BDNF knockdown group (P = 0.0459). Together, our findings indicate that D1 receptor activation drives N2O-induced addictive effects in mice, with BDNF being upstream of D1 receptor regulation.
A Effects of a combination of D1 antagonist and BDNF knockdown on N2O-induced CPP behaviors in mice (n = 6 / group). *P < 0.05, **P < 0.01, ****P < 0.0001 and ns is not significant. B Graphic abstract. Putative mechanisms of addictive effects of N2O. (BDNF brain-derived neurotrophic factor, c-Fos Fos proto-oncogene, CREB cAMP response element-binding protein, DA dopamine, D1R dopamine 1-like receptor, ERK extracellular signal-regulated kinas, GABA γ-aminobutyric acid neurons, NAc nucleus accumbens, N2O nitrous oxide, NMDA N-methyl-D-aspartate neurons, Trkb tropomyosin-related kinase B, VTA ventral tegmental area).
Discussion
The present study represents a systematic investigation of addictive properties and the underlying mechanisms of N2O with the employment of behavioral, focal knockdown, molecular, and in vivo neurochemical approaches.
The orthogonal design revealed that the exposure to 20% N2O for 30 min a day for 4 consecutive days with 4 pairings of N2O in morning and 4 pairings of the air in afternoon produced pronounced rewarding effects and locomotion in the CPP paradigm. Although this exposure pattern was different from the previous studies, in which rats were exposed to 60% N2O for 40 min daily for 4 days paired with N2O and 4 days paired with placebo gas alternately [14, 17, 28], the severity of the 20% N2O-induced addictive behavior was comparable to and even greater than that of 60% N2O. The concentration of N2O for clinical analgesia and anesthesia is generally 30–60% [3]. The subanesthetic concentration of N2O exerted more apparent psychotropic effects, including euphoria and relaxation [3]. Likewise, the ketamine-like effects of N2O on hippocampal synaptic function were observed at a subanesthetic, but not therapeutically relevant concentration [29]. Subanesthetic dose of the intravenous anesthetic propofol, but not the anesthetic dose, enhanced synaptic transmission and biochemical changes in the VTA-NAc circuit [30]. It appeared that anesthetic agents with subanesthetic dose may be more effective in acquiring addictive behaviors. Furthermore, exposure to N2O led to the increase of locomotion activity, this may be due to the increase in dopamine levels caused by N2O stimulation, which is consistent with previous research results[31, 32].
The current study revealed that the N2O-induced rewarding effect was largely abolished by focal injection into the NAc of the D1 antagonist, but not the D2 antagonist. This was consistent with the results obtained in systemic administration of the DA antagonists [17]. Moreover, the repeated N2O exposure caused a dramatic increase of the number of TH-labelled DAergic neurons in the VTA. Previous studies have shown that TH serves as the rate-limiting enzyme for dopamine synthesis, and changes in TH activity or expression levels lead to changes in the activity of presynaptic dopaminergic neurons. Experiments have shown that L-theanine inhibits the rewarding effect of nicotine in the mouse CPP model by inhibiting the expression of TH and the production of dopamine in the mouse midbrain [33]. Presynaptic dopamine activity in the dorsal striatum decreases during adolescence, possibly due to decreased TH expression [34]. At the same time, studies have found that rats raised in social isolation show increased TH expression levels and increased dopamine release, leading to enhanced dopamine system responses. This results in greater ethanol and cocaine intake and an increased risk of addiction vulnerability [35]. Regarding the distribution of neurons in VTA subregions, previous studies showed that the lVTA projects dopamine signals through dopamine neurons to regulate the NAc shell and other brain regions, thereby forming a neural circuit for reward [36, 37]. When we analyzed the changes in TH fluorescence signals in the VTA brain region, we chose the lVTA, which is consistent with our research. However, for other VTA subregions, such as the role of the mVTA in N2O-induced reward behavior, or the changes in other subregions induced by N2O, our current data cannot reflect this and requires further research in the future.
In vivo optic-fiber recording showed that DAergic neuronal activity of the VTA and the concentration of DA in the NAc were robustly enhanced upon the N2O exposure. An early microdialysis study also observed N2O-induced increase of DA level in the rat NAc [14]. It seems that the N2O may in parallel act at the two sites of the VTA-NAc dopamine reward pathway, i.e., directly or indirectly facilitating the excitability of DAergic neurons of the VTA and the concentration of DA in the NAc, and activating postsynaptic D1 receptors in the NAc, ultimately evoking addiction-related behaviors. The D1 receptors of the mesolimbic dopaminergic reward pathways are thought to play a positive role in the development of substance abuse and addictive behaviors [38]. This study provides direct evidence proving an involvement of the D1 receptor-mediated VTA-NAc reward pathway in the acquisition of N2O-induced rewarding effect.
As critical mediators, the BDNF/TrkB signaling is extensively involved in drug abuse and addiction [39]. In this study, the repeated N2O exposure not only upregulated the expression of BDNF mRNA and protein in the VTA, but also strongly enhanced the phosphorylation of the BDNF intracellular downstream mediators, TrkB, ERK, CREB and the expression of the transcription factor c-fos in the NAc. Nonetheless, the N2O-induced addictive behaviors were suppressed by focal VTA knockdown of BDNF and individual microinjection into the NAc of the three inhibitors (ANA-12, SCH772984, and KG501) respectively against the BDNF downstream mediators (TrkB, ERK, and CREB). The BDNF knockdown further inhibited the expression of BDNF mRNA of the VTA, the phosphorylation of TrkB, ERK, CREB and the expression of c-fos in the NAc. It is implicated that the acquisition of the N2O-related rewarding effect is widely associated with the enhancement of the BDNF/TrkB-ERK/CREB signaling of the VTA-NAc pathway, i.e., N2O upregulated BDNF-containing neuronal activities in the VTA and facilitated its binding to postsynaptic TrkB, in turn, leading to the activation of intracellular ERK/CREB cascade in the NAc. The antidepressant effects of N2O are also associated with its enhancement of BDNF/TrkB-ERK/CREB signaling in the medial prefrontal cortex (mPFC) [40, 41]. It thus appears that, while N2O has wide and positive effects in modulating the BDNF/TrkB-ERK/CREB signaling of the brain, the psychotropic consequences achieved depend upon brain regions it acts.
Furthermore, while both D1 antagonism and BDNF knockdown resulted in pronounced reduction of rewarding behaviors under the repeated N2O exposure, the mice intervened with a combination of the D1 antagonism and the BDNF knockdown even exhibited markedly lower CPP score than the BDNF knockdown group, suggesting a synergistic effect of the D1 receptor and BDNF/TrkB signaling in the acquisition of the N2O-induced rewarding effect. In parallel, the BDNF knockdown potently suppressed the expression of TH-labelled DAergic neurons in the VTA. It is well documented that BDNF widely coexists in DAergic neurons of the VTA [19]. The co-expression of BDNF in mesencephalic DAergic neurons occurred from prenatal period throughout adult life [42]. Infusion of BDNF into the VTA induced long-lasting potentiation of cocaine seeking [43]. One injection or single infusion of BDNF into the VTA caused bidirectional switches between DA-dependent and -independent nicotine, opiate, and alcohol motivation [44,45,46]. These studies suggest that the activation of BDNF/TrkB signaling bidirectionally or reversibly modulated the functional states of the VTA-NAc DA reward pathway. The present study further confirms that the addictive properties of N2O are acquired probably in part via synergistic effects of the D1 receptor and BDNF/TrkB signaling in the VTA-NAc DA reward pathway.
Limitations should be considered in this study. Firstly, it is believed that anesthetic and analgesic effects of N2O are principally related to its inhibition of the N-methyl-D-aspartate (NMDA) receptor as an antagonist and biphasic modulation of γ-aminobutyric acid (GABA) transmission in multiple brain regions [11, 47]. Blockade of ventral midbrain NMDA receptors increased DA impulse flow and release in the NAc, and enhanced brain stimulation reward [48,49,50], whereas the activation of VTA GABA neurons caused place aversion and interrupted reward behavior [51, 52]. Whether the addictive properties of N2O are associated with its antagonism of NMDA receptors and disinhibition of VTA GABA neurons remains for further exploration (Fig. 5). Secondly, it has been reported that the antidepressant effects of N2O are associated with its modulation of glutamatergic transmission, BDNF/TrkB signaling, and neuronal nitric oxide synthase (nNOS) in the mPFC [29, 40, 41]. Further examination of neuroanatomic and neurochamical links between the addictive and antidepressant effects of N2O could provide a deeper inght into psychotropic mechanisms of N2O. Thirdly, the results in this study were produced from male mice aged 6–8 weeks, but may not be necessarily applied in mice of other ages and gender. Both clinical and preclinical studies have shown that gender differences run through various stages of drug addiction. The impact of sex hormones on medication should not be underestimated. For example, estradiol promotes the positive subjective effects of drugs, while progesterone and its metabolites, weaken drug related reactions and protect females from the adverse effects of drugs [53, 54]. Moreover, the differential outcomes were observed mice of different ages. A study on the relationship between social failure and cocaine addiction showed that in adult mice with repeated social failures, the expression of proBDNF and TrkB receptors in NAc was reduced, while such parameters in adolescent mice remained unchanged [55]. Therefore, whether our findings also operate in female mice or mice of other ages require further study. In addition, our results indicated that the efficiency of BDNF knockdown efficiency is around 40%. The knockdown efficiency of the Cre virus combined with Flox mice could fluctuate depending on several variables, including injection volume, titer, infection duration and injection site of the virus. The efficiency presented in our present study was similar to other groups [56, 57]. Nonetheless, the results from complete deletion of BDNF in situ may vary. After complete BDNF knockout, it is uncertain whether there is a difference in CPP results between the D1 antagonist group and the combination group. However, according to our existing results, it can still be concluded that BDNF is the upstream of regulating D1 receptors involved in N2O rewarding effect. However, the knockdown efficiency as above mentioned does pose limitations that require further research.
Collectively, the repeated exposure of N2O at a subanesthetic concentration produced apparent addictive effects in the CPP paradigm. The addictive properties of N2O are associated with the upregulation of the VTA-NAc dopamine reward pathway probably via mediation of BDNF/TrkB signaling and D1 receptor. Among them, the modulation of BDNF in VTA may be the upstream of D1 receptor in NAc involved in N2O rewarding effect.
Data availability
Data are available from the corresponding author upon request.
References
Irwin MG, Trinh T, Yao CL. Occupational exposure to anaesthetic gases: a role for TIVA. Expert Opin Drug Saf. 2009;8:473–83.
Savage S, Ma D. The neurotoxicity of nitrous oxide: The facts and “putative” mechanisms. Brain Sci. 2014;4:73–90.
Gillman MA. What is better for psychiatry: titrated or fixed concentrations of nitrous oxide? Front Psychiatry. 2022;13:773190.
Kaar SJ, Ferris J, Waldron J, Devaney M, Ramsey J, Winstock AR. Up: The rise of Nitrous Oxide abuse. an international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30:395–401.
Team TGCR. Global drugs survey 2016. An overview of our key findings. 2019. https://www.drugsandalcohol.ie/25667/. Accessed 2019-08-04.
Garland EL, Howard MO, Perron BE. Nitrous oxide inhalation among adolescents: prevalence, correlates, and co-occurrence with volatile solvent inhalation. J Psychoactive Drugs. 2009;41:337–47.
Gernez E, Lee GR, Niguet JP, Zerimech F, Bennis A, Grzych G. Nitrous oxide abuse: clinical outcomes, pharmacology, pharmacokinetics, toxicity and impact on metabolism. Toxics. 2023;11:962.
Zheng D, Ba F, Bi G, Guo Y, Gao Y, Li W. The sharp rise of neurological disorders associated with recreational nitrous oxide use in China: a single-center experience and a brief review of Chinese literature. J Neurol. 2020;267:422–9.
Xiang Y, Li L, Ma X, Li S, Xue Y, Yan P, et al. Recreational nitrous oxide abuse: prevalence, neurotoxicity, and treatment. Neurotox Res. 2021;39:975–85.
Rech P, Custodio RM, Rodrigues Uggioni ML, Silveira Prestes G, Marcal F, Silveira VP, et al. Use of nitrous oxide in the treatment of major depressive disorder and treatment-resistant major depressive disorder: a systematic review and meta-analysis nitrous oxide in depressive disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2024;129:110869.
Brunt TM, van den Brink W, van Amsterdam J. Mechanisms involved in the neurotoxicity and abuse liability of nitrous oxide: a narrative review. Int J Mol Sci. 2022;23:14747.
Fidalgo M, Prud’homme T, Allio A, Bronnec M, Bulteau S, Jolliet P, et al. Nitrous oxide: what do we know about its use disorder potential? results of the french monitoring centre for addiction network survey and literature review. Subst Abus. 2019;40:33–42.
Niehaus JL, Cruz-Bermudez ND, Kauer JA. Plasticity of addiction: a mesolimbic dopamine short-circuit? Am J Addict. 2009;18:259–71.
Sakamoto S, Nakao S, Masuzawa M, Inada T, Maze M, Franks NP, et al. The differential effects of nitrous oxide and xenon on extracellular dopamine levels in the rat nucleus accumbens: a microdialysis study. Anesth Analg. 2006;103:1459–63.
Benturquia N, Le Guen S, Canestrelli C, Lagente V, Apiou G, Roques BP, et al. Specific blockade of morphine- and cocaine-induced reinforcing effects in conditioned place preference by nitrous oxide in mice. Neuroscience. 2007;149:477–86.
Benturquia N, Le Marec T, Scherrmann JM, Noble F. Effects of nitrous oxide on dopamine release in the rat nucleus accumbens and expectation of reward. Neuroscience. 2008;155:341–4.
Yang T, Yue G, Ge Y, Zhang Y, Xu P, Wang Y, et al. SCH 23390 inhibits the acquisition of nitrous oxide-induced conditioned place preference and the changes in ERK phosphorylation expression in nucleus accumbens of mice. Neurosci Lett. 2022;781:136674.
Li Y, Li F, Qin D, Chen H, Wang J, Wang J, et al. The role of brain derived neurotrophic factor in central nervous system. Front Aging Neurosci. 2022;14:986443.
Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–34.
Guo N, Zhang L, Fan W, Bai L, Zhang X, Shi Z, et al. Inhibition of Geranylgeranylacetone on cholecystokinin-B receptor, BDNF and dopamine D1 receptor induced by morphine. Biochem Biophys Res Commun. 2022;588:23–8.
Keshavarzi S, Kermanshahi S, Karami L, Motaghinejad M, Motevalian M, Sadr S. Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: The role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology. 2019;72:74–84.
Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015;1628:60–67.
Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–37.
Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, et al. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701.
Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–45.
Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, et al. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem. 2012;120:202–9.
Kohtala S, Rantamaki T. Rapid-acting antidepressants and the regulation of TrkB neurotrophic signalling-insights from ketamine, nitrous oxide, seizures and anaesthesia. Basic Clin Pharmacol Toxicol. 2021;129:95–103.
Ramsay DS, Watson CH, Leroux BG, Prall CW, Kaiyala KJ. Conditioned place aversion and self-administration of nitrous oxide in rats. Pharmacol Biochem Behav. 2003;74:623–33.
Izumi Y, Hsu FF, Conway CR, Nagele P, Mennerick SJ, Zorumski CF. Nitrous oxide, a rapid antidepressant, has ketamine-like effects on excitatory transmission in the adult hippocampus. Biol Psychiatry. 2022;92:964–72.
Nagata I, Sasaki M, Miyazaki T, Saeki K, Ogawa KI, Kamiya Y. Subanesthetic dose of propofol activates the reward system in rats. Anesth Analg. 2022;135:414–26.
Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology. 1999;143:39–46.
Kaiyala KJ, Ramsay DS. Concentration-related metabolic rate and behavioral thermoregulatory adaptations to serial administrations of nitrous oxide in rats. PLoS One. 2018;13:e0194794.
Di X, Yan J, Zhao Y, Chang Y, Zhao B. L-theanine inhibits nicotine-induced dependence via regulation of the nicotine acetylcholine receptor-dopamine reward pathway. Sci China Life Sci. 2012;55:1064–74.
Matthews M, Bondi C, Torres G, Moghaddam B. Reduced presynaptic dopamine activity in adolescent dorsal striatum. Neuropsychopharmacology. 2013;38:1344–51.
Karkhanis AN, Leach AC, Yorgason JT, Uneri A, Barth S, Niere F, et al. Chronic social isolation stress during peri-adolescence alters presynaptic dopamine terminal dynamics via augmentation in accumbal dopamine availability. ACS Chem Neurosci. 2019;10:2033–44.
Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, Lammel S. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron. 2018;97:434–49.e434.
Cross EA, Borland JM, Shaughnessy EK, Lee SD, Vu V, Sambor EA et al. Distinct subcircuits within the mesolimbic dopamine system encode the salience and valence of social stimuli. BioRxiv. 2024;https://doi.org/10.1101/2024.07.23.604824.
Baik JH. Dopamine signaling in reward-related behaviors. Front Neural Circuits. 2013;7:152.
Collo G, Cavalleri L, Spano P. Structural plasticity in mesencephalic dopaminergic neurons produced by drugs of abuse: critical role of BDNF and dopamine. Front Pharmacol. 2014;5:259.
Kohtala S, Theilmann W, Rosenholm M, Penna L, Karabulut G, Uusitalo S, et al. Cortical Excitability and activation of TrkB signaling during rebound slow oscillations are critical for rapid antidepressant responses. Mol Neurobiol. 2019;56:4163–74.
Liu W, Li Q, Ye B, Cao H, Shen F, Xu Z, et al. Repeated nitrous oxide exposure exerts antidepressant-like effects through neuronal nitric oxide synthase activation in the medial prefrontal cortex. Front Psychiatry. 2020;11:837.
Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–9.
Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–11.
Grieder TE, Yee M, Vargas-Perez H, Maal-Bared G, George S, Ting AKR, et al. Administration of BDNF in the ventral tegmental area produces a switch from a nicotine-non-dependent D1R-mediated motivational state to a nicotine-dependent-like D2R-mediated motivational state. Eur J Neurosci. 2022;55:714–24.
Ting AKR, Vargas-Perez H, Bufalino MR, Bahi A, Dreyer JL, Tyndale RF, et al. Infusion of brain-derived neurotrophic factor into the ventral tegmental area switches the substrates mediating ethanol motivation. Eur J Neurosci. 2013;37:996–1003.
Vargas-Perez H, Ting AKR, Walton CH, Hansen DM, Razavi R, Clarke L, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–4.
Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54:9–18.
French ED, Mura A, Wang T. MK-801, phencyclidine (PCP), and PCP-like drugs increase burst firing in rat A10 dopamine neurons: comparison to competitive NMDA antagonists. Synapse. 1993;13:108–16.
Mathe JM, Nomikos GG, Schilstrom B, Svensson TH. Non-NMDA excitatory amino acid receptors in the ventral tegmental area mediate systemic dizocilpine (MK-801) induced hyperlocomotion and dopamine release in the nucleus accumbens. J Neurosci Res. 1998;51:583–92.
Bergeron S, Rompre PP. Blockade of ventral midbrain NMDA receptors enhances brain stimulation reward: a preferential role for GluN2A subunits. Eur Neuropsychopharmacol. 2013;23:1623–35.
Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, et al. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–83.
Bouarab C, Thompson B, Polter AM. VTA GABA neurons at the interface of stress and reward. Front Neural Circuits. 2019;13:78.
Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56.
Zhou Y, Zhao M, Zhou C, Li R. Sex differences in drug addiction and response to exercise intervention: from human to animal studies. Front Neuroendocrinol. 2016;40:24–41.
Montagud-Romero S, Nunez C, Blanco-Gandia MC, Martinez-Laorden E, Aguilar MA, Navarro-Zaragoza J, et al. Repeated social defeat and the rewarding effects of cocaine in adult and adolescent mice: dopamine transcription factors, proBDNF signaling pathways, and the TrkB receptor in the mesolimbic system. Psychopharmacology. 2017;234:2063–75.
Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–70.
Heldt SA, Zimmermann K, Parker K, Gaval M, Weinshenker D, Ressler KJ. BDNF deletion or TrkB impairment in amygdala inhibits both appetitive and aversive learning. J Neurosci. 2014;34:2444–50.
Acknowledgements
This study was supported by grants from Health and Medical Research Fund (HMRF) of the Food and Health Bureau of Hong Kong (No.: 12133711) and General Research Fund (GRF) of Research Grant Council of HKSAR (17115017). We thank Ms. Ju-Ping Xing for her assisting in data collection, conducting imaging & Flow cytometry and fluorescence confocal microscopy.
Author information
Authors and Affiliations
Contributions
ZZJ conceptualized and developed the project, supervised experiments, and substantially revised the manuscript. WQL designed and conducted experiments and drafted the initial manuscript. XL supervised experiments, revised the manuscripts, and provided critical comments. SNL and SCY performed experiments and collected data. WQL and SNL analyzed data and constructed the figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments were performed following the approved protocol according to the guidelines of the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong (CULATR ref: 22–121). All animal procedures were performed in strict accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, WQ., Liu, SN., Yang, SC. et al. Nitrous oxide exerts rewarding effect via regulating D1 receptor and BDNF pathway in ventral tegmental area-nucleus accumbens dopamine circuit. Transl Psychiatry 15, 34 (2025). https://doi.org/10.1038/s41398-025-03257-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03257-y