Abstract
Cerebrospinal fluid (CSF) biomarkers have been widely adopted in Alzheimer’s disease (AD) diagnosis. However, no studies focused on the application of CSF biomarkers in the clinical practice of complex and atypical patients with cognitive impairment in China. This study aimed to evaluate the added value of CSF AD biomarkers in cognitively impaired patients with complex conditions in a memory clinical setting. A total of 633 participants were included from the National Center for Neurological Disorders in Shanghai, China. The CSF AD biomarkers were measured with ELISA. Cutoff values were firstly identified using Youden’s index. The neurologists proposed etiology diagnosis with a percentage estimate of their confidence and prescribed medication before and after CSF disclosure. Changes in etiological diagnosis, diagnostic confidence, and management plan were compared across the groups. Of the 633 patients (mean [SD] age, 61.1 [11.3] years; 295 males [46.6%]), 372 (58.8%) were diagnosed with dementia, 103 (16.3%) with mild cognitive impairment, and 158 (24.9%) with subjective cognitive decline. Using those pre-defined cutoffs, we categorized patients into 3 groups: Alzheimer’s continuum (68.1%), non-AD pathologic change (11.1%), and normal AD biomarkers (20.8%). After CSF disclosure, the proposed etiology changed in 158 (25.0%) participants and the prescribed medication changed in 200 (31.6%) patients. Mean diagnostic confidence increased from 69.5–83.0% (+13.5%; P < 0.001). In conclusion, CSF AD biomarkers significantly impacted the diagnosis, diagnostic confidence, and treatment plans for Chinese patients with complex cognitive impairment. CSF AD biomarkers are a useful tool for clinicians beyond routine clinical assessment.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) represents the leading cause of dementia and one of the most common chronic diseases among the elderly [1]. When compared to brain histology, experienced clinicians’ diagnoses of “probable AD” have a sensitivity and specificity of only 71% [2]. Accurate diagnosis of AD remains a challenge, especially for younger patients (early-onset dementia), patients manifesting atypical AD (non-amnestic symptoms such as visuospatial dysfunction, and behavioral abnormality), patients with comorbidity, and patients with rapid progression [3]. Earlier and more accurate diagnosis of AD may help to improve patients’ health status and reduce treatment costs by effectively allocating healthcare resources and maximizing the benefits of treatments and supportive services [4, 5]. Moreover, as disease-modifying medications have been approved for AD [6, 7], timely etiological diagnosis is expected to provide actual health benefits to patients exhibiting cognitive decline.
AD neuropathology is now detectable in vivo by positron emission tomography (PET) or cerebrospinal fluid (CSF) [8]. Therefore, β-amyloid (Aβ), tau, and neurodegeneration (ATN) research framework has become widely adopted to define and diagnose AD since its proposal [9]. In AT(N) framework, “A” refers to biomarkers of Aβ plaques, “T” stands for biomarkers of fibrillar tau, and “N” represents biomarkers of neurodegeneration or neuronal injury. Extensive evidence has demonstrated the significant impact of PET and CSF AD-specific biomarkers on diagnosis, diagnostic confidence, and treatment in cognitively impaired individuals [10,11,12]. In contrast to PET, CSF can simultaneously provide information on Aβ, tau, and neurodegeneration biomarkers and allow for the assessment of additional biomarkers. Therefore, CSF testing is particularly recommended in complex and atypical cases, including patients with diagnostic uncertainty, atypical presentations, rapidly progressive cognitive decline, and an early age of onset [13,14,15,16,17].
CSF biomarkers have been proven to be a helpful additional diagnostic tool for clinicians in many countries [18,19,20,21,22,23]. Previous studies demonstrate that 25% of patients with cognitive impairment have a change in etiologic diagnosis and 31% have an alteration of treatment after using CSF biomarkers [12]. To our knowledge, only one previous study investigated the clinical capability of CSF AT(N) framework for routine clinical assessment in a small sample of the Chinese Han population with cognitive disorders [24]. After disclosing the CSF results, etiological diagnoses were changed in 28% of the patients, and diagnostic confidence increased from 70.6–80.4%. However, this study used the Belgium cutoff values listed on the enzyme-linked immunosorbent assay (ELISA) instructions to classify patients based on CSF biomarkers, which limited the interpretation of the results as racial differences exist in molecular biomarkers for AD [25].
In the present work, we firstly established diagnostic cutoff values for CSF biomarkers indicative of AD, correlating these values with Aβ-PET results. Using these cutoff values, we investigated the contribution of CSF AT(N) framework, in addition to routine assessment, to changes in diagnosis, diagnostic confidence, and treatment plans for patients with complex cognitive impairment. We aimed to address current limitations by providing robust evidence on the clinical utility of CSF AD biomarkers in a memory clinic setting of the National Center for Neurological Disorders in China.
Subjects and methods
Participants
Participants with cognitive impairment were consecutively recruited at the memory center of Huashan Hospital affiliated to Fudan University between March 2020 and December 2023. The cognitive status of the participants ranged from subjective cognitive decline (SCD) to mild cognitive impairment (MCI) and dementia. The included patients presenting with complex conditions met one of the following criteria: (1) atypical cognitive profiles at initial evaluation for cognitive complaints (e.g., deficits in visual, language, executive or behavioral domains); (2) unexpected rate of progression (rapid or slow cognitive decline, or absence of progression); (3) early age of onset (before 65 years); or (4) mixed presentations with co-pathology or comorbid conditions (e.g., vascular brain injury, TDP-43 encephalopathy, α-synuclein pathology related disease, or normal pressure hydrocephalus [NPH]). Patients were excluded if they had unstable medical or psychiatric diseases, or other conditions (e.g., increased intracranial pressure, coagulopathy, scoliosis, or a local infection at the site of injection) that could interfere with the completion of lumbar punctures (LP). The study was approved by the Ethics Committee of Huashan Hospital, and all participants or their authorized caregivers provided written informed consent.
Study design
The study design is illustrated in Figure S1. All patients underwent a standard clinical assessment, including medical history, neurological and neuropsychological evaluation, clinical laboratory testing, electroencephalogram, and structural brain imaging before LP. Details have been described in our previous study [26]. Briefly, pre-CSF diagnoses were determined in a consensus meeting by experienced clinicians based on conventional clinical criteria. The clinicians were asked to determine the cognitive stage (SCD, MCI, or dementia), the most likely etiology diagnosis underlying the cognitive symptoms (eg, AD [27, 28], vascular cognitive impairment [VCI] [29], frontotemporal dementia [FTD] [30, 31], dementia with Lewy Bodies [DLB] [32], Parkinson’s disease dementia [PDD] [33, 34], multiple system atrophy [MSA] [35, 36], progressive supranuclear palsy [PSP] [37], corticobasal degeneration [CBD] [38], Limbic-predominant age-related TDP-43 encephalopathy [LATE] [39], NPH [40, 41], Creutzfeldt–Jakob disease [CJD] [42], and autoimmune encephalitis [AE] [43]), rated diagnostic confidence (on a visual scale ranging from 0% [definitely not] to 100% [very certain], with 5% increments), and define the treatment plan if the patient needed to take cognition-specific medications and/or non-cognition-specific medications. We further classified the diagnoses into the following categories: AD, VCI, FTD, DLB, CJD, other neurodegenerative diseases (OND, e.g., PDD, MSA, PSP, CBD, LATE), non-neurodegenerative diseases (NND, e.g., anxiety, depression, psychiatric disorders, NPH, AE), and undetermined diagnoses (UND).
The CSF biomarker results were obtained within 14 days after LP. Clinicians were required to re-evaluate the final etiological diagnosis, diagnostic confidence, and treatment plan. The outcomes assessed included changes in etiologic diagnosis, diagnostic confidence, and treatment plan. Patient treatment was re-evaluated in terms of (1) initiation or withdrawal of cognition-related medications, including cognition-specific (i.e., acetylcholinesterase inhibitors or memantine), non-cognition specific (i.e., anxiolytics, hypnotics, antidepressants, antipsychotics, and anticonvulsants), and (2) further investigations (e.g., amyloid PET, Tau PET, 18F-fluorodeoxyglucose [FDG] PET, dopamine transporter [DaT] scan, genetic screening, and other CSF analysis).
CSF collection and processing
CSF was obtained by LP early in the morning. CSF was collected after removing the first 2 mL and then transported on dry ice to the Medical Laboratory in polypropylene tubes, where it was aliquoted into 0.5 mL polypropylene tubes within 24 h. The 500 µL aliquot of neat CSF was stored at −80 °C until use without any additional freeze-thaw cycles. The CSF aliquots were thawed at room temperature, and the tubes were vortexed for 5 s for a gentle mixing prior to the analysis. Beta-amyloid 1–42 (Aβ42), beta-amyloid 1–40 (Aβ40), total tau (t-tau), and phosphorylated tau 181 (p-tau181) were quantified directly from the storage tubes. Concentrations of CSF biomarkers were measured using enzyme-linked immunosorbent assay (ELISA) and performed according to the instructions (EUROIMMUN AG, Lubeck, Germany). The quality control process consisted of strict adherence to the pre-analytical protocol [44] in practice and the use of samples to monitor quality.
Cutoff values establishment and application
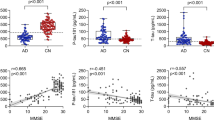
Diagnostic accuracy in identifying or ruling out AD neuropathological change depends on a credible reference standard. Since the current pre-analytical and analytical procedures vary between centers, there are no unified standard cutoff values in the clinical practice [45]. In the present study, we determined the cutoff values using amyloid PET-confirmed AD patients and cognitively unimpaired (CU) controls in our own cohort (eMethod and Table S1). Youden index-based cutoff values were of 759 pg/ml for Aβ42, 50 pg/ml for p-tau181, and 399 pg/ml for t-tau. Cutoff values of Aβ42 ratios that could best distinguish AD from CU were 0.145 for Aβ42/Aβ40 ratio, 0.060 for p-tau181/Aβ42 ratio, and 0.550 for t-tau/Aβ42 ratio (Fig. 1 and Table S2). Accuracy, along with sensitivity and specificity was displayed in Table S2. The best-performing cutoff values of the three Aβ42 ratios and Aβ42 alone were then validated with amyloid PET results in another population from our memory center. These biomarker ratios showed high concordance with amyloid PET (eResults and Figure S2). With Aβ PET-derived cutoffs, any below cutoff of Aβ42 or Aβ42/Aβ40 ratio, or alternatively above the cut-point of p-tau181/Aβ42 or t-tau/Aβ42 ratio were defined as Aβ positive. The biochemical profiles of CSF were interpreted according to an international consensus and categorized into 3 types based on the AT(N) framework (Alzheimer’s continuum, non-AD pathologic change [NAP], or normal AD biomarkers, see Table S3) [9].
A ROC curves of individual CSF Aβ40, Aβ42, p-tau181, t-tau and Aβ42 combined ratios. B indicate scatterplots of CSF levels across AD and CU controls. The Dashed vertical lines showed the cutoff values that yielded the maximum Youden J Index in the ROC analysis for each biomarker alone or ratios. CSF cerebrospinal fluid, AD Alzheimer’s disease, AUC area under the curve, CU cognitively unimpaired, ROC receiver operating characteristic curve.
Statistical analysis
The receiver operating characteristic curve (ROC) analyses were performed to generate area under the curve (AUC) values with 95% confidence intervals and assess the performance of each biomarker. We determined the accuracy, sensitivity, specificity, and optimal cutoff values according to the largest Youden index and mixture modeling.
The distribution of the variables was assessed through histogram inspection and the Shapiro-Wilk normality test. The data are described as mean (standard deviation, SD) or median (interquartile range, IQR) for continuous data and numbers (percentages, %) for categorical data. We assessed the difference with Kruskal-Wallis test, Mann-Whitney U test, or chi-square test as appropriate. Bonferroni corrections were used for the multiple comparisons if the number of groups was larger than 2, and were performed specifically to adjust the post hoc pairwise analyses. For comparison within the group, we used the McNemar test or paired Wilcoxon signed rank tests. A significant level was set at a 2-sided p < 0.05. All computations were performed using SPSS version 26 and R version 4.2.3 (R Foundation for Statistical Computing).
Results
Demographic and clinical characteristics
A total of 633 patients, including 158 (25.0%) patients with initial AD diagnosis, 388 (61.3%) with initial non-AD diagnosis (DLB [n = 15], FTD [n = 29], VCI [n = 46], CJD [n = 67], OND [n = 73], NND [n = 158]) and 87 (13.7%) with initial UND diagnoses, were finally included in our study. Demographic and clinical characteristics across diagnostic groups and cognitive status are presented in Table 1 and Table S4. There was no significant difference in age, sex, or education among people with a baseline diagnosis of AD, non-AD or UND. The mean (SD) age of the whole sample was 61.1 (11.3) years, with 46.6% male individuals, a median of 9 (IQR, 6–12) years of education, and a median disease duration of 1 (IQR, 1–3) years. Among them, the initial UND subgroup progressed faster (0.7 [0.2, 1.3]) than AD (1.8 [1.0, 2.8], p < 0.001) and non-AD (1.0 [0.5, 3.0], p < 0.001). Of all the patients, 16.2% (103/633) were diagnosed with SCD (mean age [SD], 57.6 [12.7] years; 38.8% male), 25.0% (158/633) with MCI (mean age [SD], 62.1 [10.1] years; 56.2% male), and 58.8% (372/633) with dementia (mean age [SD], 61.7 [11.1] years; 46.0% male).
Prevalence of AT(N) profiles
AD continuum profile was the most common (68.1%) in overall patients (Fig. 2), followed by normal (20.8%) and NAP change profiles (11.1%). The pattern was seen in both the MCI and dementia subgroups, but not in the SCD individuals (Table S5). With increasing severity of cognitive status, the prevalence of A − T − N− decreased (38.8% in SCD, 27.2% in MCI, 13.2% in dementia), while A + and A + T + increased (22.3 and 4.9% in SCD, 67.1 and 32.3% in MCI, 81.2 and 36.0% in dementia). NAP profiles were common in SCD (38.8%), but uncommon in MCI (5.7%) and dementia (5.6%). The distribution of AT(N) profiles across the initial etiological diagnosis groups is shown in Figure S3.
Change of diagnosis after CSF biomarkers test
The CSF biomarkers changed the diagnoses of 158 (25.0%) participants (Fig. 3). Diagnoses changed to non-AD or undetermined diagnoses in 43.7% (69/158) participants with an initial diagnosis of AD, including 39 patients (1 patient changed to DLB, 3 patients changed to VCI, 3 patients changed to FTD, 26 patients changed to NND, and 6 patients changed to UND) because of Aβ-negative status and 30 patients (2 patients changed to DLB, 6 patients changed to VCI, 8 patients changed to FTD, 11 patients changed to NND and 3 patients changed to UND) with an A + T- status. CSF biomarkers also changed the diagnoses from non-AD to AD or undetermined diagnoses, or changed the diagnoses within the non-AD subgroup in 17.8% (69/388) patients with an initial diagnosis of non-AD. Among them, 60 patients changed to AD with a biomarker result consistent with AD and 9 patients according to the t-tau status. For patients with an initially undetermined diagnosis, 23.0% (20/87) of them received a certain diagnosis after CSF assessment (15 patients changed to AD with AD continuum and 5 patients changed to CJD with extremely elevated t-tau). The proportion of changed diagnoses showed no significant statistical differences between each two types of CSF profiles (i.e., Alzheimer’s continuum, NAP, or normal AD biomarkers).
The CSF AD biomarkers contributed to the change of the diagnoses in the whole sample A and disaggregating by baseline cognitive stage B, C and D. AD Alzheimer’s disease, CJD Creutzfeldt–Jakob disease, DLB dementia with Lewy Bodies, FTD frontotemporal dementia, MCI mild cognitive impairment, NND non-neurodegenerative diseases, OND other neurodegenerative diseases, SCD subjective cognitive decline, UND undetermined diagnoses, VCI vascular cognitive impairment.
In analyses stratified by cognitive impairment status, etiological diagnoses changed more substantially in the MCI (49/158, 31.0%) and SCD groups (30/103, 29.1%) than in the dementia group (79/372, 21.2%). The difference between the MCI and dementia groups was statistically significant (p = 0.016), while the difference between the SCD and dementia groups was approaching significance (p = 0.09).
Diagnostic confidence increment after CSF biomarkers test
For those patients who did not receive an uncertain etiological diagnosis, the overall confidence of diagnosis significantly increased after the CSF biomarker information, from the mean value of 69.5–83.0% (+13.5%; p < 0.001). Confidence increased more after AD continuum CSF results (+14.6%, from 70.0–84.6%; p < 0.001) and normal CSF biomarkers (+13.7%, from 67.5–81.2%; p = 0.001) than with NAP CSF findings (+6.3%, from 70.5–76.8%). The higher increases in diagnostic confidence were observed in etiologically diagnosed AD participants with a baseline diagnosis of AD (+22.6%, from 69.5–92.1%; p < 0.001; Fig. 4A) and non-AD (+20.2%, from 66.2–86.4%; p < 0.001; Fig. 4C). The confidence also increased in the participants with a pre-CSF AD diagnosis changed to non-AD (+14.7%, from 63.1–77.8%; p < 0.001; Fig. 4B) and in the group who confirmed their initial etiological diagnosis of non-AD (+9.6%, from 71.3–80.9%; p < 0.001; Fig. 4D).
The diagnostic confidence that cognitive impairment due to AD/Non-AD all significantly increased with a confirmed diagnosis in A AD→AD and D Non-AD→Non-AD; the consistent trend was also observed in patients whose diagnosis changed in B AD→Non-AD and C Non-AD→AD. All changes were increased significantly at p < 0.001. AD Alzheimer’s disease, CSF cerebrospinal fluid.
A comparable increase in diagnostic confidence was found in the SCD (+11.9%, from 70.6–82.5%; p < 0.001), MCI (+13.6%, from 67.6–81.2%; p < 0.001) and dementia (+14.0%, from 70.0–84.0%; p < 0.001) subgroups (Figure S4). We further investigated the effect of CSF biomarker classifications on diagnostic confidence across cognitive stages, and the highest increase in diagnostic confidence was observed in the SCD subgroup with normal biomarkers (+20.8%, from 70.5–91.3%; p < 0.001).
Contribution to patient management of CSF biomarkers
CSF biomarker results led to drug use changes for 200 (31.6%) patients (Fig. 5). The overall use of cognition-specific drugs increased significantly from 53.4–60.5% (p < 0.001) in the population, and the use of non-cognition-specific medications increased from 29.4–38.4% (p < 0.001). We noted similar trends in patients with AD continuum CSF profiles, whose use of cognition-specific and non-cognition-specific drugs increased from 58.0–69.0% (p < 0.001) and from 30.4–40.8% (p < 0.001), respectively. In 132 patients with normal CSF biomarker profiles, 15 of them (11.4%, from 30.3–37.9%, p = 0.04) added non-cognition-specific medications. In patients with NAP CSF profiles, no statistically significant differences in medication use were observed.
Changes in the use of cognition-specific (A) and non-cognition-specific (B) medications across the whole sample and stratified by CSF AT(N) profiles. Significant P values are marked with **P < 0.001, *P < 0.05, “NS” indicates negative. AD Alzheimer’s disease, CSF cerebrospinal fluid, NAP non-AD pathologic change.
The changes in management happened in 30 of 103 (29.1%) patients with SCD, 56 of 158 (35.4%) patients with MCI and 114 of 372 (30.6%) patients with dementia (Figure S5). Use of medications increased significantly in MCI (cognition-specific, from 49.4–58.9%, p = 0.014; non-cognition-specific, from 25.9–38.6%, p = 0.001) and dementia subgroups (cognition-specific, from 62.6–72.6%, p < 0.001; non-cognition-specific, from 34.9–43.5%, p < 0.001). There was no statistical difference in medication use in the SCD group before and after CSF disclosure. In the analysis stratified by CSF AT(N) profiles, a similar tendency was observed in AD continuum patients with MCI (cognition-specific medications increased from 44.3–57.5%, p = 0.007; non-cognition-specific medications increased from 19.8–33.0%, p = 0.004) or dementia (cognition-specific medications increased from 64.6–75.2%, p < 0.001; non-cognition-specific medications increased from 34.4–43.7%; p < 0.001), but not in SCD. There was no statistical difference in the use of medications among each cognitive group with NAP or normal CSF profiles.
In 328 (51.8%) patients, further investigations were conducted, including amyloid PET (29 [4.5%]), tau PET (49 [7.7%]), FDG PET (28 [4.4%]), DaT scan (38 [6.0%]), other CSF analysis (123 [19.4%]), genetic screening (74 [11.7%]), and other testing (44 [7.0%]), Table S6). Patients with NAP CSF profiles received more additional examinations (48/70, 68.6%) than AD continuum (223/431, 51.7%, p = 0.009) and normal (57/132, 43.2%, p = 0.001) profiles. Among the additional examinations, other CSF analysis was the most commonly performed, mainly received by the 62 patients with a final diagnosis of UND. Patients needed to receive amyloid PET owing to CSF analysis conflicting with the initial clinical AD diagnosis (13/29, [44.8%]), non-AD diagnosis with T-positive biomarker results (15/29, [51.7%]), and UND diagnosis with T + N+ pathological change (1/29, [3.5%]). Tau PET was used to confirm AD etiology in A + T- cases (3/49, [6.1%]) and to detect tauopathies in non-AD and UND cases (46/49, [93.9%]).
Discussion
This study presented data of a large cohort recruited from a memory clinic setting of the National Center for Neurological Disorders in China. We focused on cognitive-impaired patients with complex conditions (younger patients, patients with complex clinical presentations, patients with mixed pathology, or patients with an unexpected rate of progression), and emphasized the significant impacts of CSF AD biomarkers on the changes in diagnoses and management. Our study is, to our knowledge, the first to evaluate the utility of the CSF AD biomarkers in the Chinese Han population with complex cognitive disorders.
In this study, we first defined the cutoff values of CSF AT(N) biomarkers for AD diagnosis at our center. Among these biomarkers, CSF Aβ42/Aβ40, p-tau181/Aβ42 and t-tau/Aβ42 ratios were superior to Aβ42 alone to differentiate patients with AD from cognitively normal participants, which is consistent with previous studies [46]. CSF p-tau181 showed a higher AUC value than other biomarkers alone (i.e., Aβ42, Aβ40, and t-Tau). Compared with the cutoff values listed on the manufacturer’s guidelines, in which the p-tau181/Aβ42 and t-tau/Aβ42 ratios were not provided, the CSF Aβ42 and Aβ42/Aβ40 ratios in this study were more consistent with Aβ-PET classification (Figure S2). Two previous studies had defined the cutoff values to diagnose AD in the Chinese population [47, 48]. The Chongqing Ageing & Dementia Study (CADS) provided the cutoff values of 933 pg/mL for Aβ42, 48.7 pg/mL for p-tau181, and 313 pg/mL for t-tau [47]. Another study provided the cutoff values for early-onset AD (Aβ42 < 570.9 pg/mL; p-tau181 > 56.49 pg/mL; t-tau > 241.6 pg/mL; t-tau/Aβ42 > 0.529; p-tau181/Aβ42 > 0.0846) from the Peking Union Medical College Hospital (PUMCH) dementia cohort [48]. Variability in the biomarker cutoff levels might be due to differences in enrollment population (our study: AD patients confirmed by Aβ-PET; the CADS study: AD patients without any PET; the PUMCH dementia cohort: early-onset AD patients without any PET) and ELISA kits (our study: EUROIMMUN ELISA kit; the CADS study and PUMCH dementia cohort: INNOTEST ELISA kit).
CSF biomarkers led to an alternative diagnosis in 25.0% of patients and increased diagnostic confidence from 69.5–83.0% (+13.5%) in our study. The results were in line with previous reports which found diagnostic changes ranging between 7–61% and confidence increments ranging from 5–22% [12]. Compared to a previous Chinese study conducted by Ye et al, the overall impact on diagnostic change in our study appears to be relatively lower [24]. The discrepancy was possibly influenced by study design and population enrollment. We used CSF Aβ42 and the ratio of Aβ42/Aβ40, p-tau181/Aβ42, and t-tau/Aβ42 as biomarkers of Aβ deposition, which are considered to be more reliable markers of brain amyloidosis [49, 50]. Moreover, the cutoff values for AD diagnosis vary in populations and centers [45], therefore, the use of center-specific cutoff values to define the CSF biomarker results is a more reasonable approach. Finally, we opted to use a large sample with atypical characteristics, rather than all patients visiting the clinic.
Consistent with previous studies [19, 22, 51,52,53,54,55], our result showed CSF AD biomarkers were helpful in complex and atypical cases for revising etiologic diagnosis and treatment plans. A similar investigation enrolling 28 participants conducted by van den Brink et al showed that CSF analysis changed or better specified the initial clinical diagnosis in 43.0% of atypical cognitively impaired patients [51]. Another multicenter CSF study involving patients with an initial diagnosis of psychiatric disorders exhibited a diagnostic change of 27.5% [19]. Several studies focused on early-onset cognitive impairment indicated that CSF work-up improved diagnostic accuracy, increased diagnostic certainty, and resulted in therapeutic changes [52,53,54]. These data together reflect the important impact of CSF AD biomarkers in daily clinical practice in the diagnostic work-up of complex cognitive impairment.
The value of t-tau and p-tau in CSF in the differential diagnosis of CJD has been reported in various studies [56,57,58]. Extremely elevated t-tau and low p-tau/t-tau ratio were proposed as diagnostic biomarkers for CJD which had a very high diagnostic accuracy in the differentiation of CJD [42]. In this study, we also found that CSF AD biomarkers contributed to a change in CJD diagnosis. Diagnoses changed from CJD to non-CJD in 6 participants (9.0% of participants with a pre-CSF diagnosis of CJD) and from non-CJD to CJD in 8 participants (1.4% of participants with a pre-CSF diagnosis of non-CJD).
This study has several limitations. First, the study was conducted in a tertiary clinical center where patients with complex presentations are usually referred. The relatively high frequencies of complex patients were observed in the present study, therefore limiting the generalizability of the findings to primary care and local memory clinics. Second, we used a non-automated assay of manual ELISA to test CSF biomarkers. The use of automated platforms will help provide more precise and stable CSF AD biomarker measurements and reduce intra- and inter-laboratory variability [59]. We are considering taking the advanced method to improve the performance in future studies. Third, while the sample size was the largest reported to date in China, the trial was conducted as a single-center study at a tertiary memory clinic setting. Immunoassay variability for Aβ and T proteins (t-tau, p-tau) that occurs across centers contributes to the lack of universal cutoff values for the measurement of CSF AD biomarker levels. Such variability will constrain the widespread use of the CSF cutoff values we have established and weaken the confidence of clinicians when evaluating measured levels of biomarkers as diagnostic criteria for AD. Future large-scale multicenter trials are needed to ensure broad generalizability of the findings.
In conclusion, atypical clinical presentations of AD are common in everyday practice and accurate diagnosis is likely to affect therapeutic decisions [60]. CSF AD biomarkers are a useful tool for cognitively impaired patients with complex conditions beyond routine clinical assessment. The use of CSF AD biomarkers results in better etiologic diagnosis and treatment plans. It clearly appears very helpful to achieve a precise differentiation between AD and other conditions, allowing for the provision and adaptation of appropriate treatment while avoiding deleterious therapies and management. Consequently, for the benefit of the patient and the patient’s family, the use of biomarkers improves the precision of the diagnosis and allows the provision of better information and medical care to the patient as well as more tailored supportive care for their relatives. Our data advocate for the increased use of CSF AD biomarkers in memory settings in China.
Data availability
The datasets used during the current study are available from the corresponding author upon reasonable request.
Code availability
All software used in this study is publicly available. The code used in this study can be accessed at https://github.com/Victoria880717/CSF-cognitive-impairment-study.
References
Alzheimer's Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19:1598–695.
Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at national institute on aging alzheimer disease centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71:266–73.
Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021;20:222–34.
Hornberger J, Michalopoulos S, Dai M, Andrade P, Dilla T, Happich M. Cost-effectiveness of Florbetapir-PET in Alzheimer’s disease: a spanish societal perspective. J Ment Health Policy Econ. 2015;18:63–73.
Handels RLH, Wimo A, Dodel R, Kramberger MG, Visser PJ, Molinuevo JL, et al. Cost-utility of using Alzheimer’s disease biomarkers in cerebrospinal fluid to predict progression from mild cognitive impairment to dementia. J Alzheimers Dis. 2017;60:1477–87.
Harris E. Alzheimer drug lecanemab gains traditional FDA approval. JAMA. 2023;330:495.
Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330:512–27.
Wang ZB, Tan L, Gao PY, Ma YH, Fu Y, Sun Y, et al. Associations of the A/T/N profiles in PET, CSF, and plasma biomarkers with Alzheimer’s disease neuropathology at autopsy. Alzheimers Dement. 2023;19:4421–35.
Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Rabinovici GD, Gatsonis C, Apgar C, Chaudhary K, Gareen I, Hanna L, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321:1286–94.
Smith R, Hagerstrom D, Pawlik D, Klein G, Jogi J, Ohlsson T, et al. Clinical utility of tau positron emission tomography in the diagnostic workup of patients with cognitive symptoms. JAMA Neurol. 2023;80:749–56.
Hazan J, Wing M, Liu KY, Reeves S, Howard R. Clinical utility of cerebrospinal fluid biomarkers in the evaluation of cognitive impairment: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2023;94:113–20.
Sorbi S, Hort J, Erkinjuntti T, Fladby T, Gainotti G, Gurvit H, et al. EFNS-ENS guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol. 2012;19:1159–79.
Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, Perret-Liaudet A, et al. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s biomarkers standardization initiative. Alzheimers Dement. 2014;10:808–17.
Miller AM, Begley E, Coen R, Doyle M, Dunne J, Hutchinson S, et al. Clinical consensus guidelines on the application of cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: recommendations of the irish network for biomarkers in neurodegeneration. Ir Med J. 2016;109:483.
Simonsen AH, Herukka SK, Andreasen N, Baldeiras I, Bjerke M, Blennow K, et al. Recommendations for CSF AD biomarkers in the diagnostic evaluation of dementia. Alzheimers Dement. 2017;13:274–84.
Ismail Z, Black SE, Camicioli R, Chertkow H, Herrmann N, Laforce R Jr., et al. Recommendations of the 5th Canadian consensus conference on the diagnosis and treatment of dementia. Alzheimers Dement. 2020;16:1182–95.
Duits FH, Prins ND, Lemstra AW, Pijnenburg YA, Bouwman FH, Teunissen CE, et al. Diagnostic impact of CSF biomarkers for Alzheimer’s disease in a tertiary memory clinic. Alzheimers Dement. 2015;11:523–32.
Paquet C, Magnin E, Wallon D, Troussiere AC, Dumurgier J, Jager A, et al. Utility of CSF biomarkers in psychiatric disorders: a national multicentre prospective study. Alzheimers Res Ther. 2016;8:27.
Gooblar J, Carpenter BD, Coats MA, Morris JC, Snider BJ. The influence of cerebrospinal fluid (CSF) biomarkers on clinical dementia evaluations. Alzheimers Dement. 2015;11:533–40.
Gjerum L, Andersen BB, Bruun M, Simonsen AH, Henriksen OM, Law I, et al. Comparison of the clinical impact of 2-[18F]FDG-PET and cerebrospinal fluid biomarkers in patients suspected of Alzheimer’s disease. PLoS One. 2021;16:e0248413.
Stiffel M, Bergeron D, Mourabit Amari K, Poulin E, Roberge X, Meilleur-Durand S, et al. Use of Alzheimer’s disease cerebrospinal fluid biomarkers in a tertiary care memory clinic. Can J Neurol Sci. 2022;49:203–9.
Sanchez-Soblechero A, Berbel A, Villarejo A, Palmi-Cortes I, Vieira A, Gil-Moreno MJ, et al. Translating NIA-AA criteria into usual practice: report from the ReDeMa project. Alzheimers Dement. 2024;10:e12451.
Ye LQ, Gao PR, Zhang YB, Cheng HR, Tao QQ, Wu ZY, et al. Application of cerebrospinal fluid AT(N) framework on the diagnosis of AD and related cognitive disorders in chinese han population. Clin Interv Aging. 2021;16:311–23.
Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76:264–73.
Chen KL, Wang MY, Wu J, Zuo CT, Huang YY, Wang WY, et al. Incremental value of amyloid PET in a tertiary memory clinic setting in China. Alzheimers Dement. 2024;20:2516–25.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9.
Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28:206–18.
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14.
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89:88–100.
Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines. Mov Disord. 2012;27:349–56.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601.
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6.
Wenning GK, Stankovic I, Vignatelli L, Fanciulli A, Calandra-Buonaura G, Seppi K, et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov Disord. 2022;37:1131–48.
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32:853–64.
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503.
Nelson PT, Dickson DW, Trojanowski JQ, Jack CR Jr, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142:1503–27.
Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S4–16.
Nakajima M, Yamada S, Miyajima M, Ishii K, Kuriyama N, Kazui H, et al. Guidelines for management of idiopathic normal pressure hydrocephalus (Third Edition): endorsed by the japanese society of normal pressure hydrocephalus. Neurol Med Chir. 2021;61:63–97.
Hermann P, Appleby B, Brandel JP, Caughey B, Collins S, Geschwind MD, et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021;20:235–46.
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404.
Hansson O, Batrla R, Brix B, Carrillo MC, Corradini V, Edelmayer RM, et al. The Alzheimer’s association international guidelines for handling of cerebrospinal fluid for routine clinical measurements of amyloid beta and tau. Alzheimers Dement. 2021;17:1575–82.
Delaby C, Teunissen CE, Blennow K, Alcolea D, Arisi I, Amar EB, et al. Clinical reporting following the quantification of cerebrospinal fluid biomarkers in Alzheimer’s disease: an international overview. Alzheimers Dement. 2022;18:1868–79.
Bouwman FH, Frisoni GB, Johnson SC, Chen X, Engelborghs S, Ikeuchi T, et al. Clinical application of CSF biomarkers for Alzheimer’s disease: from rationale to ratios. Alzheimers Dement. 2022;14:e12314.
Fan DY, Jian JM, Huang S, Li WW, Shen YY, Wang Z, et al. Establishment of combined diagnostic models of Alzheimer’s disease in a Chinese cohort: the Chongqing Ageing & Dementia Study (CADS). Transl Psychiatry. 2022;12:252.
Lei D, Mao C, Li J, Huang X, Sha L, Liu C, et al. CSF biomarkers for early-onset Alzheimer’s disease in Chinese population from PUMCH dementia cohort. Front Neurol. 2022;13:1030019.
Mo Y, Stromswold J, Wilson K, Holder D, Sur C, Laterza O, et al. A multinational study distinguishing Alzheimer’s and healthy patients using cerebrospinal fluid tau/Abeta42 cutoff with concordance to amyloid positron emission tomography imaging. Alzheimers Dement. 2017;6:201–9.
Mattsson-Carlgren N, Grinberg LT, Boxer A, Ossenkoppele R, Jonsson M, Seeley W, et al. Cerebrospinal fluid biomarkers in autopsy-confirmed Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2022;98:e1137–e50.
van den Brink SEM, El Amrani L, Therriault J, Gauthier S, Rosa-Neto P, Vitali P. Diagnostic impact of cerebrospinal fluid biomarkers in atypical dementias in Canada. Can J Neurol Sci. 2021;48:312–20.
Balasa M, Sanchez-Valle R, Antonell A, Bosch B, Olives J, Rami L, et al. Usefulness of biomarkers in the diagnosis and prognosis of early-onset cognitive impairment. J Alzheimers Dis. 2014;40:919–27.
Falgas N, Tort-Merino A, Balasa M, Borrego-Ecija S, Castellvi M, Olives J, et al. Clinical applicability of diagnostic biomarkers in early-onset cognitive impairment. Eur J Neurol. 2019;26:1098–104.
Soo SA, Zailan FZ, Tan JY, Sandhu GK, Wong BYX, Wang BZ, et al. Safety and usefulness of lumbar puncture for the diagnosis and management of young-onset cognitive disorders. J Alzheimers Dis. 2022;87:479–88.
Lombardi G, Polito C, Berti V, Ferrari C, Lucidi G, Bagnoli S, et al. Biomarkers study in atypical dementia: proof of a diagnostic work-up. Neurol Sci. 2018;39:1203–10.
Hamlin C, Puoti G, Berri S, Sting E, Harris C, Cohen M, et al. A comparison of tau and 14-3-3 protein in the diagnosis of Creutzfeldt-Jakob disease. Neurology. 2012;79:547–52.
Llorens F, Schmitz M, Karch A, Cramm M, Lange P, Gherib K, et al. Comparative analysis of cerebrospinal fluid biomarkers in the differential diagnosis of neurodegenerative dementia. Alzheimers Dement. 2016;12:577–89.
Chang BK, Day GS, Graff-Radford J, McKeon A, Flanagan EP, Algeciras-Schimnich A, et al. Alzheimer’s disease cerebrospinal fluid biomarkers differentiate patients with Creutzfeldt-Jakob disease and autoimmune encephalitis. Eur J Neurol. 2022;29:2905–12.
Leuzy A, Ashton NJ, Mattsson-Carlgren N, Dodich A, Boccardi M, Corre J, et al. 2020 update on the clinical validity of cerebrospinal fluid amyloid, tau, and phospho-tau as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Eur J Nucl Med Mol Imaging. 2021;48:2121–39.
Gaugler JE, Ascher-Svanum H, Roth DL, Fafowora T, Siderowf A, Beach TG. Characteristics of patients misdiagnosed with Alzheimer’s disease and their medication use: an analysis of the NACC-UDS database. BMC Geriatr. 2013;13:137.
Acknowledgements
We thank all the staff and participants who helped the study. We thank V-Medical Laboratory Co., Ltd for their provision of AD biomarker testing services.
Funding
This study was supported by grants from the STI2030-Major Projects (2022ZD0211600), National Natural Science Foundation of China (92249305), Research Start-up Fund of Huashan Hospital (2022QD002), Excellence 2025 Talent Cultivation Program at Fudan University (3030277001), Shanghai Excellent Young Specialist Training Program 2021 from Shanghai Health and Medical Development Foundation, and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.
Author information
Authors and Affiliations
Contributions
MYW, KLC, YYH, and SFC did the data acquisition, data analysis, chart design, manuscript writing and revision. RZW, YZ, HYH, LZM, JW, and JWX did the data acquisition and analysis. WSL, XZ, MML, and YG did the chart design and interpretation. QD and WC did the concept and design of the study. JTY, YRZ, MC, and LT did the concept and design of the study and provided administrative, technical, or material support. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Huashan Hospital. In accordance with the Declaration of Helsinki, written informed consent was obtained from all participants or their guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, MY., Chen, KL., Huang, YY. et al. Clinical utility of cerebrospinal fluid Alzheimer’s disease biomarkers in the diagnostic workup of complex patients with cognitive impairment. Transl Psychiatry 15, 130 (2025). https://doi.org/10.1038/s41398-025-03345-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03345-z