Abstract
Real-time fMRI (rtfMRI) neurofeedback (NF) is a novel noninvasive technique that permits individuals to voluntarily control brain activity. The crucial role of the insula in emotional and salience processing makes it one of the most commonly targeted regions in previous rtfMRI studies. To provide an overview of progress in the field, the present review identified 25 rtfMRI insula studies and systematically reviewed key characteristics and findings in these studies. We found that rtfMRI-based NF training is efficient for modulating insula activity and its associated behavioral/symptom-related and neural changes. Furthermore, we also observed a maintenance effect of self-regulation ability and sustained symptom improvement, which is of importance for clinical application. However, training success of insula regulation was not consistently paralleled by behavioral/symptom-related changes, suggesting a need for optimizing the NF training protocol enabling more robust training effects. Principles including inclusion of a well-designed control group/condition, statistical analyses and reporting results following common criteria and a priori determination of sample and effect sizes as well as pre-registration are also highly recommended. In summary, we believe our review will inspire and inform both basic research and therapeutic translation of rtfMRI NF training as an intervention in mental disorders particularly those with insula dysfunction.
Similar content being viewed by others
Introduction
The insula, known as the “Island of Reil”, is located deep in the lateral sulcus [1] and extensively interconnected with other brain regions [1, 2]. Supported by an extensive network of connections, the insula represents an integrative hub in the emotion and salience network [3, 4] and plays a vital role in the integration of external-oriented sensory input and internal-oriented awareness [5, 6]. The insula is a cytoarchitectonically and functionally heterogenous region organized along a posterior-to-anterior gradient such that the anterior insula (AI) is mainly involved in interoceptive processing [7,8,9], emotion [10], risky decision-making [11] and empathy [12, 13], while the posterior insula (PI) primarily integrates somatosensory, vestibular, and motor information by receiving afferent projections from the spinal cord and brainstem via the thalamus [1]. Functional and structural dysregulations of the insular cortex are closely associated with various mental disorders, including anxiety disorder [14, 15], major depression disorder (MDD) [16,17,18], autism spectrum disorder [19,20,21], schizophrenia [22,23,24], addiction [25,26,27], alexithymia [28, 29] and Parkinson’s disease [30, 31]. Thus, a noninvasive brain modulation approach that can directly target the insula and normalize its dysregulations may represent a novel and transdiagnostically promising therapeutic strategy.
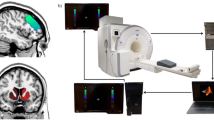
Real-time fMRI (rtfMRI) neurofeedback (NF) training capitalizes on recent progress in fMRI techniques and represents a noninvasive approach to modulate brain signals [32,33,34,35]. More specifically, rtfMRI NF can assist participants to gain voluntary control over brain activity by transforming the BOLD signal into real-time visual feedback. Previous studies have demonstrated that this approach allows individuals to gain self-regulation of most brain regions, including the amygdala [36, 37], the anterior cingulate cortex (ACC) [38, 39], the prefrontal cortex [40,41,42], the hippocampus [43], as well as the insula. The clinical application potential of rtfMRI NF training has also been supported by accumulating studies in clinical populations, including MDD [44, 45], anxiety disorder [46, 47], borderline personality disorder [48, 49], addiction [50, 51], post-traumatic stress disorder (PTSD) [52, 53], and attention deficit hyperactivity disorder [39, 54].
Based on the promising potential in both basic research and therapeutic application the rtfMRI NF field has progressed rapidly during the last decade. However, despite the promising translational potential of rtfMRI most therapeutic applications remain in the experimental stage. Thus, a systematic review of key characteristics of NF training and its associated behavioral and neural changes is important both for optimizing NF training protocol and facilitating the translation of rtfMRI into therapeutic application. Recent reviews focused on general progress in the field of rtfMRI (e.g. [32, 34, 55]), the therapeutic potential of rtfMRI NF training across different mental disorders (e.g. [56, 57]) or in specific disorders, such as eating disorders [58], schizophrenia [59], and addiction [60]. Although these reviews help us obtain a comprehensive understanding of progress in the field, very few reviews focus on the rtfMRI modulation of a specific target region. The two previous region-oriented reviews focused on the amygdala as a target region for emotion regulation [36] and therapeutic target in MDD [61] and suggest that a region-oriented systematic review can contribute to understanding characteristics of region-specific NF modulation and facilitating therapeutic translations in mental disorders characterized by region-specific dysregulation.
Given the crucial role of the insula in emotional and salience processing [4, 10], it has been one of the most popular regions that have been investigated in the field of rtfMRI [62]. Successful insula rtfMRI regulation has been repeatedly reported both in healthy and clinical populations (Table 1). Surprisingly, to date no study has systematically reviewed progress in the field of rtfMRI-based insula modulation. Against this background, we conducted a systematic review aiming to provide an overview of progress in this field, which may inspire researchers in optimizing NF training protocol design and thus facilitate therapeutic translations. More specifically, the main objectives of the present review are to provide a systematic overview addressing the following questions: (1) What characteristics/parameters (e.g., training protocol design, feedback types, regulation strategies, etc.) were used in rtfMRI NF training studies of insula regulation? (2) Can rtfMRI NF training consistently lead to successful voluntary control over the insula activity, and if so, can this training success be maintained to a “transfer” run without NF (i.e. the maintenance effect of self-regulation ability)? (3) What behavioral/symptom-related and neural changes can be induced by rtfMRI NF training of insula regulation?
Methods
Literature search and inclusion criteria
A literature search was conducted by two authors independently following the PRISMA guidelines [63, 64] (Fig. S1). Specifically, literature on rtfMRI NF training on the insula was searched using the Web of Science, Science Direct and PubMed databases with the search terms “neurofeedback” and “fMRI” or “functional magnetic resonance imaging” or “functional MRI” and “insula” or “insular”. A second search was then performed in these 3 databases with the following keywords: “rtfMRI” or “real time” or “real-time” and “fMRI” or “functional magnetic resonance imaging” or “functional MRI” and “insula” or “insular”. Literature search was undertaken on April 2, 2024 and resulted in 865 articles (247 articles in PubMed, 138 articles in Science Direct and 480 articles in Web of Science). To avoid possible omission and mitigate potential publication bias [64, 65], we further searched on clinicaltrials.gov for possible trial registrations, comprehensive study protocol preregistrations, and registered reports and found 34 potential studies. The resulted 899 studies were further screened by analyzing titles and abstracts based on the following eligibility criteria: (1) written in English; (2) utilized rtfMRI-NF training (rather than other NF modalities such as electroencephalography, functional near-infrared spectroscopy or electrophysiology); (3) used BOLD signal as NF sources; (4) reported original research (i.e. reviews or commentaries were excluded). Based on these criteria, 40 articles were remained and entered into the next stage for full-text analyses, in which another 15 articles were excluded and resulted in 25 articles for the final analysis (details see Supplementary Materials).
Data extraction and publication bias
The following characteristics were extracted from each of the 25 qualified articles including (1) demographic information: population (healthy/clinical), sample size, age and gender distribution; (2) feedback characteristics: target regions and feedback types; (3) regulation strategies and directions; (4) localization approaches; (5) protocol design: number of training runs, number and duration of regulation blocks, practice and transfer run, control group/condition, and stimuli; (6) outcome measures and main findings. Training success was determined by comparing differences between the experimental and control groups, between regulation and rest blocks, or between the first and last runs. In addition, in line with inclusion criteria of recent rtfMRI meta-analysis studies [66, 67], 11 studies with available T or F values and degrees of freedom or other source data (e.g., mean values, standard deviation) were selected for conducting a quantitative effect size assessment of training success using the comprehensive meta-analysis software [68]. Training success in this exploratory analysis was defined by the difference in insula activity between the first and the last training runs in the NF training group, which led to the most qualified studies (see NF training success on insula regulation). An exploratory publication bias assessment was also conducted based on these 11 studies using the Egger’s test and showed a significant publication bias (t = 5.79, p < 0.001; Figure S3). Furthermore, we conducted a quality assessment based on the Consensus on the Reporting and Experimental Design of Clinical and Cognitive-Behavioural FMRI-neurofeedback Studies (CRED-NF checklist; [69]) and showed a moderate quality of qualified studies included in the present study with an averaged consistency about 0.61 (see Supplementary Table 1).
Results
Participants
The final 25 qualified articles consisted of 14 studies in healthy and 11 in clinical populations (Table 1 and Fig. 1A). Clinical studies included individuals with schizophrenia [70], disruptive behavior disorder (DBD) [71], obsessive-compulsive disorder (OCD) [72], spider phobia [47], MDD [73, 74], alcohol and tobacco addiction [75,76,77,78], and criminal psychopaths [79]. Significant training effects on insula regulation were found in 13 of 14 studies (92.86%) in healthy subjects and 8 of 11 studies in clinical populations (72.72%) (Fig. S2). Of note the 2 clinical studies showing no significant training effects were conducted in comparably small samples and in patient groups characterized by marked regulatory control deficits, i.e. OCD patients (n = 3; [72]) and criminal psychopaths (n = 4; [79]).
Population (A) and gender distribution (B) in the qualified 25 studies. (C) Feedback types categorized by target regions. (D) Types of regulation strategies categorized by regulation directions. AI anterior insula, b bilateral, C continuous feedback, I intermittent feedback, Insula-dmFC functional connectivity between the insula and dorsomedial frontal cortex, l left, PI posterior insula, r right, * Studies showing significant effects on insula regulation; # Nonsignificant training effects on insula regulation.
There were 599 participants in total and sample size ranged from 3 to 84, with only 6 studies more than 30 (Fig. 1B). For 22 studies information on gender was available and the percentage of females was 45.74% with 20 studies including both genders while 2 studies only recruited male [80] or female participants [47]. The remaining 3 studies did not report gender information [75, 78, 79]. For age, 3 studies only reported age range and one study did not report specific age information [75, 78, 81, 82]. The mean age of the remaining 21 studies is 28.81 years old. While the youngest sample (mean = 11.6) was observed in a study examining the rtfMRI training effects on the emotion regulation network in adolescents [83], the most senior sample (mean = 48.44) was found in a clinical study on patients with MDD [73]. Detailed demographics are presented in Table 1 and Fig. 1.
Feedback types and target regions
RtfMRI NF is normally delivered in a visual form (e.g., a thermometer bar or magnitude of monetary reward) intermittently or continuously (e.g. [78, 82, 84]). All included studies displayed feedback based on regional-specific BOLD signal amplitude of the insula, which included 7 studies focusing on the right AI, 6 studies focusing on the left AI, 5 studies focusing on bilateral AI, one study focusing on the left PI, and 6 studies focusing on the whole insula (Table 1 and Fig. 1C). With respect to the feedback type, 21 studies employed continuous feedback, which was continuously presented to participants in regulation/rest blocks. The remaining 4 studies used intermittent feedback presented as the mean activity of a whole block at the end of each regulation/rest block (Table 1). Significant training effects were found in 3 of 4 studies (75%) using intermittent feedback and 18 of 21 studies (85.71%) using continuous feedback (Fig. S2).
Regulation strategies and direction
Efficient NF training commonly depends on valid regulation strategies which can be specific following of instructions or freely developed by participants themselves (i.e. free strategies). While 10 studies used free strategies, 15 studies provided specific strategies with mental imagery being the most popular strategy (12 studies) (Table 1 and Fig. S2). Significant training effects were observed in 8 of 10 studies (80%) using free strategies and in 13 of 15 studies (86.67%) using specific strategies (Fig. S2). To bring brain activation back to baseline level, participants were asked to close their eyes and rest, relax, focus on a cross, count numbers, or view neutral stimuli during rest blocks (Table 1).
With respect to regulation direction, there were 15 studies on up-regulation, 7 studies on down-regulation, and 3 studies on both up- and down-regulation (Table 1 and Fig. S2). Twelve of the 15 (80%) up-regulation studies [70, 73, 74, 80,81,82, 85,86,87,88,89,90] and 6 of 7 (85.71%) down-regulation studies [47, 75,76,77,78, 91] reported successful regulation of insula activity. For the 3 studies involving both up- and down-regulation, all of them reported successful up-regulation [83, 92, 93] and one of them reported successful down-regulation [93] of the insula activity. Thus, in total there were 15 studies reporting training success (83.33%) among the 18 studies involving up-regulation and 7 studies reporting training success (70%) among the 10 studies involving down-regulation (Fig. 1D). In contrast to 4 out of 18 up-regulation studies applying free regulation strategies, most of the down-regulation studies used free regulation strategies (7 in 10 studies) (details see Table 1 and Fig. 1D).
Localizing approaches
Precise localization of the target region is vital for obtaining desired feedback. In the present study, there were 8 studies employing anatomical localization, 14 studies applying functional localization, and 3 studies combining both approaches to define the insula (Table 2 and Fig. S2). We categorized functional localization tasks into 3 main types, including emotional recall tasks, passive observation tasks (emotional/substance-related pictures) and other specific tasks (see Table 2). While all of the 8 studies using anatomical localization showed significant training effects, 13 of the 14 studies (92.86%) applying functional localization reported significant training effects. The remaining 3 studies using both approaches reported no significant training effects (Fig. S2). Note that 2 of the 3 studies showing no significant training effects were the same as the 2 studies with low sample sizes in OCD patients (n = 3) and criminal psychopaths (n = 4) as discussed in the “Participants” section [72, 79].
Protocol design
Training runs
Training protocols in the 25 qualified studies employed between 3 and 24 training runs (mean = 8.92), with 12 studies incorporating 3 or 4 runs performed on a single day and 13 studies (including 9 clinical studies) comprising 6 or more runs separated into 2–10 days (Table 2). Clinical studies included more training runs separated on different days than healthy subject studies (11.73 vs. 6.71 runs respectively). Each run included 4–12 regulation blocks (mean = 6.2) alternating with rest blocks with a single regulation block ranging from 9–45 s (mean = 28.64). Given the hemodynamic nature of the BOLD signal, the duration of each block in most of the studies (23 studies) ranged from 20–45 s and only 2 studies had shorter blocks of 9 s and 12.5 s respectively [47, 92].
Practice and transfer runs
While a practice run is set to help participants familiarize the training procedure before formal NF training, a transfer run is applied to examine the maintenance of training effects without feedback. In the 25 studies, only 4 included a practice run [47, 84, 90, 93] and 3 of them reported training success [47, 90, 93]. Eleven studies included transfer runs (Table 2) and 9 of them tested maintenance effects in a transfer run immediately following NF training (Table 2). More specifically, 3 studies reported significant maintenance effects in transfer runs immediately [88, 89] or 2 days following NF training [82]. The last study also reported that changes in rsFC of the AI but not in the behavioral performance induced by NF training were also maintained in the transfer run [82].
Control group/condition
Inclusion of a control group/condition can exclude the possibility that the NF training effect is not specific to NF. In the 25 qualified studies, 13 studies included a control group in which 8 studies used sham feedback from an unspecific or task-irrelevant region [74,75,76,77, 82, 87,88,89], followed by 2 studies with no feedback [47, 73] and 3 studies using 2 control groups with sham or no feedback [85, 86, 90]. Sham control regions included a whole top slice far from the insula [82, 85, 86, 88, 89], the primary visual cortex [90], the parahippocampal place area [74], the cuneus [75], the parietal cortex [76, 87], the occipital cortex [77], and the middle temporal gyrus [89]. Among the other 12 studies without a control group, there were 2 studies comprising 2 NF training groups comparing training differences between two different target ROIs (AI vs. ACC [91]) or ability in self-regulation of the insula between lean and obese individuals [80]. In addition to sham control groups, the bidirectional regulation (up- and down-regulation) in a within-group design was applied as a control condition in 3 studies [83, 92, 93].
Stimuli
In the 25 qualified studies, 21 studies presented stimuli to participants and 4 studies did not use any stimuli [80, 84, 85, 90]. More specifically, 18 studies presented participants with different visual stimuli, including emotional faces [70, 83], emotional/threat-related stimuli [73, 74, 79, 81, 86, 87, 92], affective video clips [71], pain empathy-evoking pictures [82, 89], symptom-related aversive scenes [47, 72], and substance-related pictures [75,76,77,78]. Among these 18 studies, 13 of them presented visual stimuli from the International Affective Picture System (IAPS) [72,73,74,75,76,77, 79, 81, 82, 86, 87, 89, 92], one study displayed video stimuli from the Emotional Movie Database [71], 2 studies used stimuli from the NimStim Face Stimulus Set [70, 83], one study used custom-designed stimuli [47], and one study did not report the source of stimuli used [78], although some of them additionally used custom-designed stimuli or stimuli from the Internet [72, 75,76,77]. The remaining 3 studies used stimuli of other modalities, with 2 studies using painful heat stimulation to induce the insula activity during regulation [91, 93] and one study presenting emotional auditory stimuli to aid participants in implementing regulation strategies [88]. Details of specific stimulus sources used and presentation time points (e.g., during the localizer or NF training task) are presented in Table 2. Furthermore, while there were 9 studies in the 10 down-regulation studies presenting stimuli during regulation/rest blocks, only 4 in 18 up-regulation studies did the same.
Outcome measurements
NF training success on insula regulation
Training success of insula regulation was mainly determined by comparing the difference in insula activity between the NF training and control groups, between the first and the last training runs, or between regulation and rest blocks. Successful insula regulation was reported in 21 of the 25 qualified studies (84%) based on these different comparison approaches. The most consistent training success was determined by a significant difference in insula activity between the first and the last training runs in the NF training group, which was found in 15 studies (10 studies in healthy populations; .). Seven of the studies (some studies reported training success based on more than one comparison) reported significant differences between the regulation and rest blocks in the NF training group and 3 studies reported significant differences between the NF training and control groups (details see Table 3). A further meta-analysis of 11 studies with available statistics showed a medium effect size of NF training based on the comparison between the first and the last training runs in the NF group (SMD = 0.633, 95%CI = 0.370–0.896, Z = 4.721, p < 0.0001; Fig. 2).
Behavioral outcomes associated with training success
Behavioral effects associated with NF training were measured in 22 studies using different types of behavioral/symptom-related questionnaires/ratings/tasks (Table 4). Similar to measurement of training success, significant behavioral effects were also determined by these 3 comparison approaches. Significant behavioral effects were found in 6 studies in healthy and 7 studies in clinical populations. In the 6 healthy population studies, while one study of AI up-regulation showed significantly higher pain empathy ratings in the NF compared with the control groups [82], the other 3 studies reported increased valence ratings to aversive pictures in the last compared with the first training run [86] and increased reaction time, suggestive of improved alertness in the post-training relative to pre-training measurement in a cognitive attention task [90] during up-regulation, but decreased pain perception ratings to heat stimulation in the last training run relative to the functional localizer task during down-regulation [91]. In the remaining 2 studies, they showed NF training affected participants’ subjective perception of regulation strategy implementation or training success such that one study of AI up-regulation reported increased rating scores for degrees of regulation strategy implementation in the NF training compared with the control groups [89] and the other one of AI bidirectional regulation showed significantly increased ratings of perceived training success over runs within the NF group [92].
In 2 clinical studies on up-regulation, NF training induced symptom relief in depression as measured by mood and symptom severity questionnaires post-training relative to pre-training [73] and improvement in recognition accuracy to disgust faces but impaired recognition accuracy to happy faces in schizophrenia patients in the regulation compared with the rest blocks [70]. In the remaining 5 clinical studies on down-regulation, while 2 studies showed improvement in anger control and impulsiveness of patients with alcohol dependence [77] or lower anxiety levels to spider pictures in individuals with spider phobia [47] in the NF training relative to the control groups, the other 3 studies reported decreased disgust levels to symptom-evoking stimuli in OCD [72], improvement in depression symptoms in depression patients in the post- relative to pre-training tests [74], or decreased craving scores in addicted smokers [78]. All details of behavioral outcome measurements are shown in Table 4.
Seven studies included follow-up measurements varying from 2 days to 6 months after NF training [47, 72, 74, 77, 78, 82, 90], with one study in healthy participants showing a sustained improvement in alertness, as measured by a cognitive attention task [90], and 3 clinical studies reporting a sustained decrease in disgust ratings to symptom-evoking stimuli in OCD [72], craving scores in addicted smokers [78], or symptom levels in individuals with spider phobia [47] (Table 4).
Neural outcomes associated with training success
Neural outcomes associated with training success were measured by changes in functional connectivity during NF training or resting-state recording. Nine studies examined functional connectivity changes during NF training and only found increased functional connectivity of the insula mainly with frontal regions and additionally with the cingulate cortex and amygdala (Table 3). Another study, in which only one out of 4 subjects finished all of the training runs, showed causal functional connectivity changes mainly driven by the posterior cingulate cortex (Table 3) and thus findings in this respect should be interpreted with caution given the small sample size [79].
For rsFC changes, 5 studies recorded resting-state data pre- and post-training [75,76,77,78, 82] and 2 studies reported significant rsFC changes, with one study in healthy subjects reporting increased rsFC of the insula mainly with regions of the empathic network/mirror neuron system (MNS) but decreased rsFC with regions of the default mode network [82] and the other one in patients with alcohol use disorder showing increased rsFC of the insula mainly with frontal regions [75].
Brain-behavior associations
Brain-behavior associations were reported in 10 studies. Five studies in healthy subjects demonstrated significant associations between NF-induced changes of the insula activity and different behavioral measurements including changes in valence ratings to aversive pictures [86], pain empathy ratings [82], unpleasantness ratings to painful heat stimulation [93], interoceptive accuracy measured following NF training [89], and dispositional empathy levels as measured by the Interpersonal Reactivity Index [88]. In another 4 clinical studies, NF-induced insula activity was found to be significantly correlated with improvement in recognition accuracy of disgust faces [70], rule-breaking behavior as measured by the Child Behavior Checklist [71], fear/belief of spiders as measured by the Fear of Spider Questionnaire and Spider Belief Questionnaire [47], and depressive symptom severity as measured by the Hamilton Rating Scale for Depression and mood as measured by the Positive and Negative Affect Schedule [73] (Table 4). The last study did not find any significant correlations [90].
Discussion
In the present systematic review, we obtained 25 qualified studies on insula regulation based on rtfMRI NF training. Participants’ demographics, feedback types, regulation strategies and direction, localizing approaches, protocol design, outcome measurements and main findings were systematically reviewed to provide a comprehensive overview of progress in the field of rtfMRI NF training of insula regulation.
In the 25 qualified studies, rtfMRI NF training of insula regulation has been applied in both healthy individuals and clinical populations with schizophrenia, DBD, OCD, spider phobia, MDD, addiction, and criminal psychopaths. In healthy individuals (14 studies), NF training of insula regulation was applied to modulate their behavioral responses in the domain of emotional (valence), attentional, empathic, and pain processing that are closely associated with the insula function [82, 86, 90, 91]. Similarly in clinical studies (11 studies), NF training was applied to normalize altered emotional/cognitive responses or to improve symptom severity in disorders characterized by insula dysfunction [1, 94]. Based on different comparison approaches, 21 of the 25 qualified studies (84%) reported successful self-regulation of the insula, with 13 of 14 studies in healthy (92.86%) and 8 of 11 studies in clinical populations (72.73%). The average age of participants varied from 11.6–48.44 years including both males and females (Table 1). Training success as measured by comparison between the first and the last training runs in the NF group reflecting improvement of self-regulation ability with progress over training runs was the most consistently reported method (15 of the 21 studies). A further meta-analysis showed a medium effect size of training success based on this comparison in 11 studies with available statistics. Comparable training efficacy has been reported in recent review/meta-analysis studies on different target regions [56, 67]. Although the training success rate may be inflated by publication bias, these findings can still constitute ample evidence for the feasibility of rtfMRI NF in adding both healthy and clinical individuals to acquire volitionally control over the insula activity.
Successful insula regulation can be achieved with between 3 and 24 training runs. While 12 studies incorporated 3 or 4 training runs performed on a single day in healthy subjects, the remaining 13 studies (including 9 clinical studies) comprised 6 or more runs separated into 2 to 10 days (Table 2). Although the average number of training runs in clinical studies was more than in healthy subject studies (11.73 vs. 6.71 runs), clinical studies preferred to separate training runs into different single days, possibly in consideration of decreasing the training time on each day to avoid fatigue of patients on the one hand and to repeat training for a more robust and long-lasting effect at the clinical level on the other. In addition to active training runs, 13 studies included a practice/pre-training run or a transfer run without feedback (Table 2). Inclusion of a practice run can help subjects familiarize the training procedure and has been identified as a key factor in influencing training performance [95]. It has also been suggested that training success can be more reliable when a practice run is provided [36]. In the 25 qualified studies, 11 studies measured the maintenance effect in a transfer run although only two study explored maintenance effects lasting for days [74, 82] and 9 studies tested it immediately following NF training. Only 3 studies reported significant maintenance effects either in a transfer run given immediately [88, 89] or 2 days after NF training [82]. The maintenance of self-regulation ability without NF is of tremendous significance by suggesting the possibility of independent self-regulation on brain activity without continuous dependence on the MRI scanner and thus is key for a promising potential in clinical translational settings. Token together, more emphasis thus should be paid on benefits of including a practice run and how to achieve more robust maintenance effect in future studies.
Similar to other rtfMRI studies, the insula was also defined structurally, functionally, or by combining both approaches. Functional localization tasks included emotional recall tasks (e.g., retrieval of autobiographical memory), passive observation of emotional/substance-related pictures, and other more specific tasks (Table 2). Although there is no evidence for the superiority of different localization approaches, a combination of structural and functional localization can take advantage of both more precise localization structurally and higher sensitivity functionally. After localization, another important step is selection of regulation strategies. In the present review, the majority of studies provided subjects with specific insula regulation strategies (15 studies), with mental imagery being the most popular strategy (12 studies). On the one hand, the advantage of providing a specific strategy is that it can provide a specific direction for subjects to work on and consequently may reduce trial and error efforts, whereas it may have disadvantages where the provided strategy is invalid or not suitable for the individual or the specific population (e.g. [96]). On the other hand, free strategies allow subjects to develop their own strategy, consequently leading to the most efficient strategy, however this approach comes at the cost of longer trial and error periods and the risk that subjects may fail to find an efficient strategy. Furthermore, a 7T fMRI study has shown that different cognitive strategies used for pain attenuation can induce different patterns of brain activity including the insula [97]. Thus, in the context of decreasing inter-subject variability induced by the use of different regulation strategies, a specific strategy, with an efficacy validated before application, may be more suitable, although it should be emphasized that currently there is no empirical evidence to confirm its superiority. Selection of regulation strategies should also take different functions of different subregions and the lateralization of the insula into consideration [98,99,100]. In addition, while specific strategies were used in most up-regulation studies (14 in 18 studies), free strategies were used in most down-regulation studies (7 in 10 studies), suggesting that determination of a specific regulation strategy may be more difficult for down-regulation. Regulation direction also affected the choice of whether stimuli were presented/delivered such that while stimuli were simultaneously presented during regulation/rest blocks to induce the insula activity in most down-regulation studies (9 of 10 studies), only 4 in 18 studies did this in up-regulation studies (Table 2). A variety of different types of stimuli including emotional pictures or videos, symptom-evoked pictures or painful heat stimulation were used in different studies depending on research purposes (see Table 2). It should also be noted that, for studies using the functional localization approach to define the insula (17 studies), although stimuli from the IAPS were used most frequently (10 studies), sources of stimuli were still heterogeneous. How to balance the use of validated stimulus datasets (e.g., standardized open datasets) and specific research purposes should also be considered in future studies.
In this review, the majority of the studies (21 studies) applied continuous NF, which can provide subjects the real-time NF information during the whole block and enable them to adjust their use of regulation strategies in real-time. The remaining 4 studies used intermittent feedback presented at the end of each regulation/rest block [47, 72, 78, 90], which can exclude effects of the hemodynamic response and provide individuals global information of their regulation performance [69]. This also makes intermittent feedback more suitable for MVPA-based NF aiming to modulate distributed brain patterns, including decoded NF (see [101]) and representational similarity analysis-based NF [102, 103]. Although there are no studies having explored the superiority of the two types of feedback, intermittent feedback has been found to be superior in regulating the premotor cortex and amygdala as compared to continuous feedback [104, 105]. A pilot study in 14 tinnitus patients has revealed that continuous feedback is more suitable for long-term NF training with multiple runs and intermittent feedback is more efficient for a single run NF training with respect to voluntary control over the auditory cortex [106]. However, given the limited evidence, the superiority of continuous vs. intermittent feedback should be inferred with caution and future studies are needed (cf [107, 108]).
In contrast to other factors, inclusion of a control group/condition does not affect training performance per se but is crucial to determine whether the training effect is NF-specific. In this review, half of the studies included a control group. It has been recommended that selection of a control group should be based on the specific goal of an NF training study [109]. Although it is ideal to control as many confounding effects as possible by using more control groups, it is costly and sometimes not feasible in practice. Control groups in the 13 studies used sham feedback from an unspecific or control region [74,75,76,77, 82, 87,88,89], no feedback [47, 73], or both of the sham and no feedback controls [85, 86, 90]. Although an entire top slice of the brain was conventionally used as the sham control region in rt-fMRI insula studies [82, 85, 86, 88, 89, 92], our recent work showed that it was not a proper control region when an interoceptive regulation strategy was used for AI up-regulation [89]. This entire top slice overlaps with the supplementary motor area which is a hub of the interoceptive network [8, 110] and thus may confound the NF training effect. This underlines the importance of proper selection of the sham control region depending on specific regulation strategies used for NF training. A control group with matched regulation strategies is a more common method that not only can minimize the number of control groups and maximize statistic power but also can simultaneously control for both placebo/unspecific effects and the use of regulation strategies (cf [109]). In addition, another way to control for confounding effects is using bidirectional regulation in a within-group design [109] which has been applied in 3 studies [83, 92, 93]. Successful bidirectional regulation together with corresponding directional behavioral/clinical changes can provide a well control for motivational and placebo effects [109].
So what behavioral and neural changes can be induced by rtfMRI NF training on insula regulation? As a prerequisite for the therapeutic potential of rtfMRI NF, behavioral/symptom-related changes associated with training success have been measured in 22 among the 25 qualified studies. However, significant behavioral/symptom-related changes were only found in 13 of them, including ratings to emotional stimuli, painful stimulation, or perceived regulation success in healthy subjects [82, 86, 89,90,91,92] and improvement in symptom-related indices in psychiatric disorders including spider phobia [47], schizophrenia [70], OCD [72], MDD [73, 74], and addictions [77, 78]. Importantly, behavioral changes were also found to be correlated with NF-induced changes in insula activity in 9 studies involving both healthy [82, 86, 88, 89, 93] and clinical populations [47, 70, 72, 73]. Significant brain-behavior correlations can provide further evidence that behavioral/symptom-related changes are specific to NF training. There is also evidence for a sustained decrease in symptom severity in patients with OCD [72], smoking addiction [78] and spider phobia [47] varying from 1 day to 6 months after NF training in follow-up measurements (Table 4). However, in contrast to the majority of studies (21 studies) showing training success of insula regulation, there are no comparable studies demonstrating parallel behavioral/symptom-related changes (13 studies). These findings on the one hand verify the therapeutic potential of rtfMRI NF training but on the other hand highlight the need to optimize NF training protocol for more robust behavioral/symptom-related changes.
In addition to changes at the behavioral level, 10 studies have also examined neural changes indexed by functional connectivity and all of them revealed increased functional connectivity of the AI, mainly with the medial and dorsal lateral prefrontal regions and the cingulate cortex within the regulatory control network. Acquisition of voluntary control over brain activity by NF training is particularly involved in the regulation learning process and consequently the underlying regulatory and learning networks [111]. Similar increased functional connectivity with these networks has also been found in previous rtfMRI studies targeting other regions [46, 112, 113]. These findings suggest that successful regulation may share a similar neural modulation mechanism underpinned by functional coupling between different target regions and similar regulation and learning networks. In contrast to the more convergent findings on increased functional connectivity during NF training, preliminary findings in 2 studies on rsFC changes induced by NF training are divergent [75, 82]. Changes of rsFC induced by NF training have also been found in previous rtfMRI studies targeting other regions either in healthy [114] or clinical populations [46, 61, 115, 116], indicating possibilities that NF training can also alter intrinsic organization of brain networks.
There are some limitations in the present study. First, characteristics such as feedback types, training protocols, and definition of training success in different studies were highly heterogeneous, which did not allow a fully quantitative meta-analysis and therefore mainly a qualitative review was conducted. Although we conducted an exploratory meta-analysis and showed a medium effect size of NF training, any inferences related to this finding should be made with caution given the small sample size of qualified studies (11 studies). Second, given the nature of rtfMRI studies, training efficacy is mainly determined by estimating activation changes in a pre-defined insula region and therefore coordinate-based meta-analyses employing the activation likelihood estimation approach requiring whole-brain results are also not appropriate for the scope of the present study. Third, the potential of publication bias did not allow statistical inferences with respect to determining the most efficient training characteristics. Similar to publication bias in other fields [117, 118], most of available rtfMRI insula publications were based on positive results. Indeed our exploratory analysis using the Egger’s test showed a significant publication bias, which can not be excluded and may also further inflate the effect size of our exploratory meta-analysis [65, 119]. To mitigate publication bias we additionally conducted a search for registered reports [65, 120, 121], but found no registered report that targeted the insula for rtfMRI NF training. Hence, the ratio of training success can still be an inflated indicator of training efficacy and comparisons between specific characteristics that may affect training success are therefore of limited information (cf [36]). This further did not allow us to draw specific conclusions on how to optimize selection of characteristics and principles of NF protocol design.
In conclusion, the present review provides a comprehensive overview of progress in the field of insula regulation based on rtfMRI NF training based on 25 qualified studies. Results showed that rtfMRI NF is an effective approach to assist individuals in learning voluntary regulation of insula activity in both healthy and clinical populations, particularly given the evidence of the maintenance effect of self-regulation ability observed in transfer runs without NF. Successful insula regulation can induce corresponding changes not only in behavioral/symptom-related indices that are closely associated with insula function but also in neural responses such as functional connectivity. Together with the sustained symptom severity improvement as revealed by follow-up measurements, these findings suggest a promising clinical application potential of rtfMRI NF in normalizing alterations in psychiatric disorders characterized by insula dysfunction. Of note, there are fewer studies showing parallel behavioral/symptom-related changes compared with studies showing training success of insula regulation, possibly due to high heterogeneity of characteristics used across studies and indicating a need for optimizing the NF training protocol enabling more robust training effects particularly in clinical populations. Those may be achieved via developing novel regulation strategies that are more suitable for patients, determining the optimal setup of training runs separated into different days, or optimizing individualized localization of target subregions associated with clinical symptoms. Although design of training protocols such as whether a transfer run and stimuli are used should be based on specific research purposes, principles for inclusion of a well-designed control group/condition, statistical analyses and reporting results following standardized criteria and a priori determination of sample and effect sizes as well as pre-registration of studies are highly recommended (see also [63, 69, 120,121,122]). While pre-registration studies and particularly comprehensive study preregistrations entail a complete analysis plan and document any deviations between the preregistration and the final results and registered reports are peer-reviewed based on a comprehensive preregistration protocol as required by specific journals. They therefore allow to increase the transparency, credibility, and reproducibility of studies [65, 121, 123, 124]. However, comprehensive study preregistrations and registered reports are still rare in the rtfMRI NF field (e.g., [125, 126]). Taken together, we believe our review will inspire and inform both fundamental research and therapeutic translation of rtfMRI NF training as an intervention in mental disorders particularly those with insula dysfunction.
References
Flynn FG, Benson DF, Ardila A. Anatomy of the insula - functional and clinical correlates. Aphasiology. 1999;13:55–78.
Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–69.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8.
Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67.
Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66.
Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70.
Caseras X, Murphy K, Mataix-Cols D, Lopez-Sola M, Soriano-Mas C, Ortriz H, et al. Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Hum Brain Mapp. 2013;34:1220–9.
Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA. 2004;101:6333–4.
Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34:2944–58.
Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–91.
Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–22.
Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. 2011;35:903–11.
Valentini E. The role of anterior insula and anterior cingulate in empathy for pain. J Neurophysiol. 2010;104:584–6.
Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89:273–6.
Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302.
Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me”. World J Biol Psychiatry. 2010;11:538–49.
Wiebking C, de Greck M, Duncan NW, Tempelmann C, Bajbouj M, Northoff G. Interoception in insula subregions as a possible state marker for depression-an exploratory fMRI study investigating healthy, depressed and remitted participants. Front Behav Neurosci. 2015;9:82.
Xu X, Dai J, Liu C, Chen Y, Xin F, Zhou F, et al. Common and disorder-specific neurofunctional markers of dysregulated empathic reactivity in major depression and generalized anxiety disorder. Psychother Psychosom. 2020;89:114–6.
Ebisch SJH, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32:1013–28.
Francis SM, Camchong J, Brickman L, Goelkel-Garcia L, Mueller BA, Tseng A, et al. Hypoconnectivity of insular resting-state networks in adolescents with Autism Spectrum Disorder. Psychiatry Res Neuroimaging. 2019;283:104–12.
Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33:1198–203.
Caldiroli A, Buoli M, van Haren N, de Nijs J, Altamura AC, Cahn W. Social cognition in schizophrenia patients and their siblings: the role of insula. Eur Neuropsychopharmacol. 2017;27:S695.
Tuominen L, DeCross S, Nasr S, Boeke E, Wolthusen R, Tootell R, et al. Abnormal anterior insula activity during fear generalization in schizophrenia. Schizophr Bull. 2017;43:S27.
Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123:93–104.
Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci. 2015;19:414–20.
Klugah-Brown B, Di X, Zweerings J, Mathiak K, Becker B, Biswal B. Common and separable neural alterations in substance use disorders: a coordinate-based meta-analyses of functional neuroimaging studies in humans. Hum Brain Mapp. 2020;41:4459–77.
Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67.
Ernst J, Böker H, Hättenschwiler J, Schüpbach D, Northoff G, Seifritz E, et al. The association of interoceptive awareness and alexithymia with neurotransmitter concentrations in insula and anterior cingulate. Soc Cogn Affect Neurosci. 2013;9:857–63.
Li J, Xu L, Zheng X, Fu M, Zhou F, Xu X, et al. Common and dissociable contributions of alexithymia and autism to domain-specific interoceptive dysregulations: A dimensional neuroimaging approach. Psychother Psychosom. 2019;88:187–9.
Christopher L, Koshimori Y, Lang AE, Criaud M, Strafella AP. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain. 2014;137:2143–54.
Criaud M, Christopher L, Boulinguez P, Ballanger B, Lang AE, Cho SS, et al. Contribution of insula in Parkinson’s disease: a quantitative meta-analysis study. Hum Brain Mapp. 2016;37:1375–92.
Paret C, Goldway N, Zich C, Keynan JN, Hendler T, Linden D, et al. Current progress in real-time functional magnetic resonance-based neurofeedback: methodological challenges and achievements. NeuroImage. 2019;202:116107.
Watanabe T, Sasaki Y, Shibata K, Kawato M. Advances in fMRI real-time neurofeedback. Trends Cogn Sci. 2017;21:997–1010.
Weiskopf N. Real-time fMRI and its application to neurofeedback. Neuroimage. 2012;62:682–92.
Zhao Z, Yao S, Li K, Sindermann C, Zhou F, Zhao W, et al. Real-time functional connectivity-informed neurofeedback of amygdala-frontal pathways reduces anxiety. Psychother Psychosom. 2019;88:5–15.
Barreiros AR, Almeida I, Baia BC, Castelo-Branco M. Amygdala modulation during emotion regulation training with fMRI-based neurofeedback. Front Hum Neurosci. 2019;13:89.
Young KD, Siegle GJ, Zotev V, Phillips R, Misaki M, Yuan H, et al. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. Am J Psychiatry. 2017;174:748–55.
Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum Brain Mapp. 2011;32:22–31.
Zilverstand A, Sorger B, Slaats-Willemse D, Kan CC, Goebel R, Buitelaar JK. fMRI neurofeedback training for increasing anterior cingulate cortex activation in adult attention deficit hyperactivity disorder. An exploratory randomized, single-blinded study. PLoS One. 2017;12:e0170795.
Kohl SH, Veit R, Spetter MS, Gunther A, Rina A, Luhrs M, et al. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: a randomized controlled trial in overweight and obese subjects. Neuroimage. 2019;191:596–609.
Mayeli A, Misaki M, Zotev V, Tsuchiyagaito A, Zoubi O, Phillips R, et al. Self-regulation of ventromedial prefrontal cortex activation using real-time fMRI neurofeedback—Influence of default mode network. Hum Brain Mapp. 2020;41:342–52.
Sherwood MS, Kane JH, Weisend MP, Parker JG. Enhanced control of dorsolateral prefrontal cortex neurophysiology with real-time functional magnetic resonance imaging (rt-fMRI) neurofeedback training and working memory practice. Neuroimage. 2016;124:214–23.
Zhu Y, Gao H, Tong L, Li Z, Wang L, Zhang C, et al. Emotion regulation of hippocampus using real-time fMRI neurofeedback in healthy human. Front Hum Neurosci. 2019;13:242.
Young KD, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, et al. Real-time fMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One. 2014;9:e88785.
Zotev V, Mayeli A, Misaki M, Bodurka J. Emotion self-regulation training in major depressive disorder using simultaneous real-time fMRI and EEG neurofeedback. Neuroimage Clin. 2020;27:102331.
Scheinost D, Stoica T, Saksa J, Papademetris X, Constable RT, Pittenger C, et al. Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Transl Psychiatry. 2013;3:e250.
Zilverstand A, Sorger B, Sarkheil P, Goebel R. fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front Behav Neurosci. 2015;9:148.
Paret C, Kluetsch R, Zaehringer J, Ruf M, Demirakca T, Bohus M, et al. Alterations of amygdala-prefrontal connectivity with real-time fMRI neurofeedback in BPD patients. Soc Cogn Affect Neurosci. 2016;11:952–60.
Zaehringer J, Ende G, Santangelo P, Kleindienst N, Ruf M, Bertsch K, et al. Improved emotion regulation after neurofeedback: a single-arm trial in patients with borderline personality disorder. Neuroimage Clin. 2019;24:102032.
Canterberry M, Hanlon CA, Hartwell KJ, Li XB, Owens M, LeMatty T, et al. Sustained reduction of nicotine craving with real-time neurofeedback: exploring the role of severity of dependence. Nicotine Tob Res. 2013;15:2120–4.
Kirschner M, Sladky R, Haugg A, Stampfli P, Jehli E, Hodel M, et al. Self-regulation of the dopaminergic reward circuit in cocaine users with mental imagery and neurofeedback. EBioMedicine. 2018;37:489–98.
Nicholson AA, Rabellino D, Densmore M, Frewen PA, Paret C, Kluetsch R, et al. The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp. 2017;38:541–60.
Zweerings J, Pflieger EM, Mathiak KA, Zvyagintsev M, Kacela A, Flatten G, et al. Impaired voluntary control in PTSD: probing self-regulation of the ACC with real-time fMRI. Front Psychiatry. 2018;9:219.
Alegria AA, Wulff M, Brinson H, Barker GJ, Norman LJ, Brandeis D, et al. Real-time fMRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Hum Brain Mapp. 2017;38:3190–209.
Thibault RT, MacPherson A, Lifshitz M, Roth RR, Raz A. Neurofeedback with fMRI: a critical systematic review. Neuroimage. 2018;172:786–807.
Dudek E, Dodell-Feder D. The efficacy of real-time functional magnetic resonance imaging neurofeedback for psychiatric illness: a meta-analysis of brain and behavioral outcomes. Neurosci Biobehav Rev. 2021;121:291–306.
Taschereau-Dumouchel V, Cushing CA, Lau H. Real-time functional MRI in the treatment of mental health disorders. Annu Rev Clin Psychol. 2022;18:125–54.
Bartholdy S, Musiat P, Campbell IC, Schmidt U. The potential of neurofeedback in the treatment of eating disorders: a review of the literature. Eur Eat Disord Rev. 2013;21:456–63.
Gandara V, Pineda JA, Shu IW, Singh F. A systematic review of the potential use of neurofeedback in patients with schizophrenia. Schizophr Bull. 2020;1:sgaa005.
Martz ME, Hart T, Heitzeg MM, Peltier SJ. Neuromodulation of brain activation associated with addiction: a review of real-time fMRI neurofeedback studies. Neuroimage Clin. 2020;27:102350.
Young KD, Siegle GJ, Misaki M, Zotev V, Phillips R, Drevets WC, et al. Altered task-based and resting-state amygdala functional connectivity following real-time fMRI amygdala neurofeedback training in major depressive disorder. Neuroimage Clin. 2018;17:691–703.
Fede SJ, Dean SF, Manuweera T, Momenan R. A guide to literature informed decisions in the design of real time fMRI neurofeedback studies: a systematic review. Front Hum Neurosci. 2020;14:60.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Scheel AM, Schijen MRMJ, Lakens D. An excess of positive results: comparing the standard psychology literature with registered reports. Adv Methods Pract Psychol Sci. 2021;4:25152459211007467.
Fernández-Alvarez J, Grassi M, Colombo D, Botella C, Cipresso P, Perna G, et al. Efficacy of bio- and neurofeedback for depression: a meta-analysis. Psychol Med. 2022;52:201–16.
Goldway N, Jalon I, Keynan JN, Hellrung L, Horstmann A, Paret C, et al. Feasibility and utility of amygdala neurofeedback. Neurosci Biobehav Rev. 2022;138:104694.
Brüggemann P, Rajguru K. Comprehensive Meta-Analysis (CMA) 3.0: a software review. J Mark Anal. 2022;10:425–9.
Ros T, Enriquez-Geppert S, Zotev V, Young KD, Wood G, Whitfield-Gabrieli S, et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies CRED-nf checklist. Brain. 2020;143:1674–85.
Ruiz S, Lee S, Soekadar SR, Caria A, Veit R, Kircher T, et al. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum Brain Mapp. 2013;34:200–12.
Böttinger BW, Aggensteiner PM, Hohmann S, Heintz S, Ruf M, Glennon J, et al. Exploring real-time functional magnetic resonance imaging neurofeedback in adolescents with disruptive behavior disorder and callous unemotional traits. J Affect Disord. 2024;345:32–42.
Buyukturkoglu K, Roettgers H, Sommer J, Rana M, Dietzsch L, Arikan EB, et al. Self-regulation of anterior insula with real-time fMRI and its behavioral effects in obsessive-compulsive disorder: a feasibility study. PLoS One. 2015;10:e0135872.
Linden DE, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, et al. Real-time self-regulation of emotion networks in patients with depression. PLoS One. 2012;7:e38115.
Mehler DMA, Sokunbi MO, Habes I, Barawi K, Subramanian L, Range M, et al. Targeting the affective brain-a randomized controlled trial of real-time fMRI neurofeedback in patients with depression. Neuropsychopharmacology. 2018;43:2578–85.
Karch S, Keeser D, Hummer S, Paolini M, Kirsch V, Karali T, et al. Modulation of craving related brain responses using real-time fMRI in patients with alcohol use disorder. PLoS One. 2015;10:e0133034.
Karch S, Paolini M, Gschwendtner S, Jeanty H, Reckenfelderbaumer A, Yaseen O, et al. Real-time fMRI neurofeedback in patients with tobacco use disorder during smoking cessation: functional differences and implications of the first training session in regard to future abstinence or relapse. Front Hum Neurosci. 2019;13:65.
Karch S, Krause D, Lehnert K, Konrad J, Haller D, Rauchmann BS, et al. Functional and clinical outcomes of FMRI-based neurofeedback training in patients with alcohol dependence: a pilot study. Eur Arch Psychiatry Clin Neurosci. 2022;272:557–69.
Rana M, Ruiz S, Corzo AS, Muehleck A, Eck S, Salinas CZ, et al. Use of real-time functional magnetic resonance imaging-based neurofeedback to downregulate insular cortex in nicotine-addicted smokers. J Vis Exp. 2020.
Sitaram R, Caria A, Veit R, Gaber T, Ruiz S, Birbaumer N. Volitional control of the anterior insula in criminal psychopaths using real-time fMRI neurofeedback: a pilot study. Front Behav Neurosci. 2014;8:344.
Frank S, Lee S, Preissl H, Schultes B, Birbaumer N, Veit R. The obese brain athlete: self-regulation of the anterior insula in adiposity. PLoS One. 2012;7:e42570.
Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE. Neurofeedback: a promising tool for the self-regulation of emotion networks. Neuroimage. 2010;49:1066–72.
Yao S, Becker B, Geng Y, Zhao Z, Xu X, Zhao W, et al. Voluntary control of anterior insula and its functional connections is feedback-independent and increases pain empathy. Neuroimage. 2016;130:230–40.
Cohen Kadosh K, Luo Q, de Burca C, Sokunbi MO, Feng J, Linden DEJ, et al. Using real-time fMRI to influence effective connectivity in the developing emotion regulation network. Neuroimage. 2016;125:616–26.
Berman BD, Horovitz SG, Hallett M. Modulation of functionally localized right insular cortex activity using real-time fMRI-based neurofeedback. Front Hum Neurosci. 2013;7:638.
Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, et al. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage. 2007;35:1238–46.
Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry. 2010;68:425–32.
Lawrence EJ, Su L, Barker GJ, Medford N, Dalton J, Williams SC, et al. Self-regulation of the anterior insula: reinforcement learning using real-time fMRI neurofeedback. Neuroimage. 2014;88:113–24.
Kanel D, Al-Wasity S, Stefanov K, Pollick FE. Empathy to emotional voices and the use of real-time fMRI to enhance activation of the anterior insula. Neuroimage. 2019;198:53–62.
Zhang Y, Zhang Q, Wang J, Zhou M, Qing Y, Zou H, et al. “Listen to your heart”: a novel interoceptive strategy for real-time fMRI neurofeedback training of anterior insula activity. Neuroimage. 2023;284:120455.
Popovova J, Mazloum R, Macauda G, Stampfli P, Vuilleumier P, Fruhholz S, et al. Enhanced attention-related alertness following right anterior insular cortex neurofeedback training. iScience. 2024;27:108915.
Emmert K, Breimhorst M, Bauermann T, Birklein F, van de Ville D, Haller S. Comparison of anterior cingulate vs. insular cortex as targets for real-time fMRI regulation during pain stimulation. Front Behav Neurosci. 2014;8:350.
Veit R, Singh V, Sitaram R, Caria A, Rauss K, Birbaumer N. Using real-time fMRI to learn voluntary regulation of the anterior insula in the presence of threat-related stimuli. Soc Cogn Affect Neurosci. 2012;7:623–34.
Rance M, Ruttorf M, Nees F, Schad LR, Flor H. Neurofeedback of the difference in activation of the anterior cingulate cortex and posterior insular cortex: two functionally connected areas in the processing of pain. Front Behav Neurosci. 2014;8:357.
Namkung H, Kim SH, Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 2017;40:200–7.
Haugg A, Renz FM, Nicholson AA, Lor C, Gotzendorfer SJ, Sladky R, et al. Predictors of real-time fMRI neurofeedback performance and improvement-a machine learning mega-analysis. Neuroimage. 2021;237:118207.
Sepulveda P, Sitaram R, Rana M, Montalba C, Tejos C, Ruiz S. How feedback, motor imagery, and reward influence brain self-regulation using real-time fMRI. Hum Brain Mapp. 2016;37:3153–71.
Schulz E, Stankewitz A, Witkovsky V, Winkler AM, Tracey I. Strategy-dependent modulation of cortical pain circuits for the attenuation of pain. Cortex. 2019;113:255–66.
Duerden EG, Arsalidou M, Lee M, Taylor MJ. Lateralization of affective processing in the insula. Neuroimage. 2013;78:159–75.
Montalbano MJ, Shane Tubbs R Lateralization of the Insular Cortex. In: Turgut, M, Yurttaş, C, Tubbs, R (editors) Island of Reil (Insula) in the Human Brain. Cham: Springer. 2018.
Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34:300–6.
Shibata K, Lisi G, Cortese A, Watanabe T, Sasaki Y, Kawato M. Toward a comprehensive understanding of the neural mechanisms of decoded neurofeedback. Neuroimage. 2019;188:539–56.
Ciarlo A, Russo AG, Ponticorvo S, di Salle F, Luhrs M, Goebel R, et al. Semantic fMRI neurofeedback: a multi-subject study at 3 tesla. J Neural Eng. 2022;19:036020.
Russo AG, Luhrs M, Di Salle F, Esposito F, Goebel R. Towards semantic fMRI neurofeedback: navigating among mental states using real-time representational similarity analysis. J Neural Eng. 2021;18:046015.
Hellrung L, Dietrich A, Hollmann M, Pleger B, Kalberlah C, Roggenhofer E, et al. Intermittent compared to continuous real-time fMRI neurofeedback boosts control over amygdala activation. Neuroimage. 2018;166:198–208.
Johnson KA, Hartwell K, LeMatty T, Borckardt J, Morgan PS, Govindarajan K, et al. Intermittent “real-time” fMRI feedback is superior to continuous presentation for a motor imagery task: a pilot study. J Neuroimaging. 2012;22:58–66.
Emmert K, Kopel R, Koush Y, Maire R, Senn P, van de Ville D, et al. Continuous vs. intermittent neurofeedback to regulate auditory cortex activity of tinnitus patients using real-time fMRI - A pilot study. Neuroimage Clin. 2017;14:97–104.
Pamplona G, Zweerings J, Lor C, de Erney L, Roecher E, Taebi A et al. Neural mechanisms of feedback processing and behavioral adaptation during neurofeedback training. bioRxiv: [Preprint]. 2024. Available from: https://www.biorxiv.org/content/10.1101/2024.08.19.608543v2.
Oblak EF, Lewis-Peacock JA, Sulzer JS. Self-regulation strategy, feedback timing and hemodynamic properties modulate learning in a simulated fMRI neurofeedback environment. PLoS Comput Biol. 2017;13:e1005681.
Sorger B, Scharnowski F, Linden DEJ, Hampson M, Young KD. Control freaks: towards optimal selection of control conditions for fMRI neurofeedback studies. Neuroimage. 2019;186:256–65.
Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007;1141:178–87.
Emmert K, Kopel R, Sulzer J, Bruhl AB, Berman BD, Linden DEJ, et al. Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: How is brain regulation mediated? Neuroimage. 2016;124:806–12.
Haller S, Birbaumer N, Veit R. Real-time fMRI feedback training may improve chronic tinnitus. Eur Radiol. 2010;20:696–703.
Zotev V, Krueger F, Phillips R, Alvarez RP, Simmons WK, Bellgowan P, et al. Self-regulation of amygdala activation using real-time fMRI neurofeedback. PLoS One. 2011;6:e24522.
Li Z, Tong L, Guan M, He W, Wang L, Bu H, et al. Altered resting-state amygdala functional connectivity after real-time fMRI emotion self-regulation training. Biomed Res Int. 2016;2016:2719895.
Misaki M, Phillips R, Zotev V, Wong CK, Wurfel BE, Krueger F, et al. Real-time fMRI amygdala neurofeedback positive emotional training normalized resting-state functional connectivity in combat veterans with and without PTSD: a connectome-wide investigation. Neuroimage Clin. 2018;20:543–55.
Yuan H, Young KD, Phillips R, Zotev V, Misaki M, Bodurka J. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connect. 2014;4:690–701.
Kicinski M, Springate DA, Kontopantelis E. Publication bias in meta-analyses from the cochrane database of systematic reviews. Stat Med. 2015;34:2781–93.
Jennions MD, Møller AP. Publication bias in ecology and evolution: an empirical assessment using the ‘trim and fill’ method. Biol Rev Camb Philos Soc. 2002;77:211–22.
Algermissen J, Mehler DMA. May the power be with you: are there highly powered studies in neuroscience, and how can we get more of them? J Neurophysiol. 2018;119:2114–7.
Allen C, Mehler DMA. Open science challenges, benefits and tips in early career and beyond. PLoS Biol. 2019;17:e3000246.
Reducing publication bias with Registered Reports. Nat Neurosci. 2024;27:1635.
Beyer F, Flannery J, Gau R, Janssen L, chaare L, Hartmann H, et al. A fMRI pre-registration template. PsychArchives. 2021.
Chambers CD, Tzavella L. The past, present and future of registered reports. Nat Hum Behav. 2022;6:29–42.
Chambers CD. Registered reports: a new publishing initiative at cortex. Cortex. 2013;49:609–10.
Mehler DMA, Williams AN, Whittaker JR, Krause F, Lührs M, Kunas S, et al. Graded fMRI neurofeedback training of motor imagery in middle cerebral artery stroke patients: a preregistered proof-of-concept study. Front Hum Neurosci. 2020;14:226.
Hohn VD, Tiemann L, Bott FS, May ES, Fritzen C, Nickel MM, et al. Neurofeedback and attention modulate somatosensory alpha oscillations but not pain perception. PLoS Biol. 2025;23:e3002972.
Funding
This study was supported by the National Natural Science Foundation of China (grant number: 32471139) and the Humanity and Social Science Foundation of Ministry of Education of China (grant number: 22XJC190003).
Author information
Authors and Affiliations
Contributions
Author contributions are as follows: Data curation, visualization, and paper drafting: YZ; Supervision: SY, and QZ; Paper revising: YZ, SY, QZ, BB, and KMK.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Becker, B., Kendrick, K.M. et al. Self-navigating the “Island of Reil”: a systematic review of real-time fMRI neurofeedback training of insula activity. Transl Psychiatry 15, 170 (2025). https://doi.org/10.1038/s41398-025-03382-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03382-8