Abstract
Long-duration space exploration, including missions to the Moon and Mars, demands strategies to preserve astronauts’ emotional well-being for optimal performance. This study combines behavioral phenotyping, multimodal MRI, in vivo calcium imaging, and brain-wide genomics to bridge macroscopic brain function with mesoscopic neural activity and microscopic genetic processes, providing a dynamic characterization of the mouse connectome under simulated spaceflight conditions. We observed a reduction in gray matter volume, particularly in the prefrontal cortex, with prolonged exposure. Simulated space composite environment (SSCE) disrupted multi-scale functional connectivity and altered the macro-organizational functional gradient, reversing the relationship between brain function and emotional behaviors. Neural activity in the medial prefrontal cortex demonstrated exposure-time-dependent changes across emotional tasks, while genetic analyses linked SSCE-induced alterations in functional profiles to synaptic function and ion channel activity. Our findings reveal how extreme environments impact emotional behaviors, brain networks, and neural activity, offering insights for interventions to maintain brain integrity during extended space missions.
Similar content being viewed by others
Introduction
Long-term space exploration, including trips to the Moon in 2030 and subsequently to Mars in 2040, demands a thorough focus on astronauts’ mental health. Emotions, which are inherently dynamic and evolve over time, are a critical aspect of psychological well-being in space. Extended missions can profoundly affect astronauts’ emotional states, with the impact intensifying as mission durations increase. Understanding the progression of emotional changes and their neurobiological underpinnings during prolonged space travel is vital for the success of future lunar and Martian expeditions. Collecting robust evidence on these changes, particularly during extended missions and stays on the International Space Station (ISS), is crucial for effective mission planning and astronaut health management.
To uncover how emotional behaviors-neurodevelopmental outcome disfunction during spaceflight, a comprehensive approach that integrates both spatial and temporal scales is essential. Whole-brain magnetic resonance imaging (MRI) provides non-invasive window into brain structure and spontaneous activity [1,2,3]. Research by the National Aeronautics and Space Administration (NASA) on 13 astronauts who spent 14 days in space, and 14 astronauts who completed six-month ISS missions, revealed a decrease in gray matter volume in the prefrontal cortex [4, 5]. Ground-based simulations corroborate these findings, showing reductions in gray matter in the frontal and temporal lobes, parahippocampal gyrus, and hippocampus following 30 days of head-down bed rest [4]. Additionally, after just 72 h of this bed rest, neural activity in the left hippocampus and caudate nucleus significantly declined [6]. These findings highlight structural and functional changes in brain regions associated with diverse behaviors, including emotional regulation and cognitive performance [7, 8]. However, most studies have focused on isolated time points and specific regions, overlooking the broader neurobiological mechanisms that underpin functional connectivity across brain regions over time. Furthermore, current research often neglects the dynamic interactions between brain areas, limiting our understanding of the functional network characteristics critical to emotional and cognitive adaptations during prolonged spaceflight.
Despite its noninvasive nature and relatively high spatiotemporal resolution, one limitation of mouse functional magnetic resonance imaging (fMRI) is that current technology does not fully enable data acquisition during awake behavioral tasks [9, 10]. In contrast, in vivo calcium imaging is emerging as a powerful tool for monitoring neuronal activity in specific brain regions during behavioral tasks. The medial prefrontal cortex (mPFC) is a crucial regulator of emotional behavior in both humans and rodents [11, 12]. Research utilizing advanced genetic techniques has revealed that mPFC neurons projecting to the nucleus accumbens (NAc) are involved in social discrimination [13, 14], and activation of the mPFC-NAc circuit can yield antidepressant effects [15]. This study seeks to utilize calcium imaging to observe real-time changes in mPFC neuronal activity during emotion-related tasks, with a focus on tracking how these neural responses evolve with increasing durations of simulated spaceflight.
The molecular basis of neural function is intricately linked to gene regulation. Brain structure and function are shaped by gene expression [16,17,18], with variations in expression contributing to neural phenotypic differences [19, 20]. Integrating genomics with neuroimaging techniques, such as rs-fMRI, has provided valuable insights into brain structure and function [21, 22]. Several studies using rs-fMRI have identified gene expression patterns that underlie human brain function [23,24,25]. Thus, combining data from brain imaging, neuronal activity, and gene expression holds the potential to deepen our understanding of the physiological and pathological mechanisms underlying emotional expressions in the unique environment of spaceflight.
In this study, we integrate behavioral phenotyping, MRI, in vivo calcium imaging, and genomics to comprehensively investigate the SSCE-induced alterations in whole-brain structure and function. We assessed changes in brain structure and functional connectome at two key time points—two and four weeks—under spaceflight conditions, along with mPFC-related neuronal activity during emotion-related tasks. Next, we linked brain-wide gene expression to neuroimaging data and employed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses to identify statistically enriched terms associated with SSCE-induced changes in functional profiles. Our findings provide a multi-scale perspective of the mouse brain connectome, emphasizing the neural alterations associated with prefrontal cortex (PFC) dysfunction in the spaceflight environment. To our knowledge, this represents the first systematic investigation of the neurobiological mechanisms underlying emotional changes within the context of whole-brain functional network, neuronal activity, and gene expression under the unique stressors of spaceflight.
Methods and materials
Animals
Adult (6–8 weeks old) male C57BL/6 J mice were used for experiments. Mice of similar body weight within 10 percent differences were housed in cages of 4–5 for at least a week to acclimate before undergoing various experiments. The mice were kept in the following conditions: under a 12-h light/dark cycle (lights on at 08:00 pm), the temperature at 22 ± 2 °C and the humidity at 50 ± 10%, and given access to food and water ad libitum. All the animals were randomly assigned. Among them, for the 2-week simulated space composite environment (SSCE) exposure, 9 CON mice and 15 SSCE mice were included, and for the 4-week SSCE exposure, 9 CON mice and 9 SSCE mice were included in MRI data collection. In the behavioral and calcium imaging experiments, a total of 10 mice were included, consisting of 5 CON mice and 5 SSCE mice. All experiments were conducted according to the guidelines of the Animal Care and Use Committee of Tianjin University. From the start of the experiment to its conclusion, the health status of the mice was regularly monitored, including their body weight, fur damage, and wounds. Any animals exhibiting abnormal health conditions were excluded from the experiment. During this process, the researchers were unaware of the group allocation during the experiment and/or when evaluating the results.
Simulated space composite environment exposure
The simulated space composite environment mouse model was an improved version based on previous models [26,27,28]. Simply put, 30° tail suspension was used to simulate microgravity. The mice were secured in a restrainer, with only their tails exposed, which were washed with warm water and dried with a paper towel. The tail was wrapped with medical gauze, and one end of a nylon string was fixed to the tail using medical tape and breathable gauze, while the other end was attached to a keyring secured to the crossbeam of a 23*30*18 cm3 cubic cage. By adjusting the length of the nylon string, the mouse’s body formed a 30° angle with the ground. The keyring allowed the mouse to rotate 360° horizontally, and they had free access to water and food. Additionally, other composite factors included the mouse being housed individually (social isolation), confined to a small space, exposed to “Tiangong-2” real background noise at 60–75 dB, and subjected to a 90-min day-night cycle. The total duration of the model was four weeks.
MRI acquisition
All MRI scans were acquired at Tianjin Medical University General Hospital on a Bruker 9.4 T MRI scanner (Bruker BioSpin, Ettlingen, Germany) equipped with a 1H transmit-receive volume coil with active detuning and an inner diameter of 86 mm (Bruker). Raw MRI data were reconstructed using ParaVision 360 V3.0 (Bruker). Before imaging, mice were anesthetized in an induction chamber with 5% isoflurane in oxygen for 3 min. They were then placed in the MRI-compatible coil with the brain positioned at the center of the coil. During acquisition, a small animal monitoring system was used to continuously track respiration and heart rate. The Isoflurane concentration was adjusted (to approximately 1%) to maintain a respiratory rate of 60–80 breaths per minute.
For each session, high-in-plane-resolution T2-turboRARE images were acquired for anatomical reference (TR = 2562 ms, TE = 33 ms, RARE-factor = 8, FA = 180°, 32 coronal slices, slice thickness = 0.5 mm, no interslice gap, image size = 256 × 256, FOV = 20 × 20mm2). Resting-state blood oxygen level-dependent (BOLD) images were subsequently collected using a T2*-weighted free induction decay-echo planar imaging (FID-EPI) sequence with total acquisition time of 10 min (TR = 1000 ms, TE = 14 ms, FA = 70°, bandwidth = 200 KHz, 18 coronal slices, slice thickness = 0.5 mm, no interslice gap, image size =64 × 64, FOV = 19.2 × 19.2mm2, repetitions = 600).
Further details can be found in the supplementary materials.
Genetic enrichment analysis
To explore SSCE-induced differences in functional profiles, we analyzed gene transcriptomics using publicly available volumetric gene expression data from the Allen Institute for Brain Science (AIBS) (http://mouse.brain-map.org). This data is part of the Allen Mouse Brain Atlas (AMBA), a genome-wide, high-resolution atlas of gene expression throughout the adult mouse brain, in which the expression levels of approximately 20000 genes have been quantified and registered to a 3D anatomical template based on the Common Coordinate Framework (CCF) version 3.0 of the Allen Institute. Further details can be found in Reference [29]. For our analysis, we focused on genes with restricted expression patterns in the coronal section, as provided by Brain Gene Expression Analysis toolbox [30]. For computational purposes, these gene-expression data were arranged into a voxel-by-gene data matrix of size 49742 × 4104.
Further details can be found in the supplementary materials.
Stereotaxic surgeries
The mice were anesthetized with 1% isoflurane and placed in a stereotaxic apparatus (RWD Life Technology Co. Ltd, China) over a heating pad. Erythromycin ointment was applied to their eyes to prevent dryness. After exposing the scalp, we cleaned and disinfected the area with iodine. A midline incision was made on the scalp using fine surgical scissors to expose the skull. A microdrill was used to perform craniotomy over the regions where the viral vector would be injected and the optical fiber ferrule implanted. All injections were done using a microsyringe (Hamilton, Switzerland), with the virus injected at a rate of 50–100 nL/min. The needle (700 KH, Hamilton, Switzerland) was left in place for 10 min to allow the virus to diffuse fully into the target brain region before being slowly withdrawn. The scalp incision was closed with surgical sutures and tissue adhesive (Vetbond, 3 M). The optical fiber cannula (diameter: 200 μm, Newdoon, China) was secured using dental cement, and all stereotaxic coordinates were measured relative to bregma. Post-surgery, lincomycin hydrochloride was injected to relieve pain in the animals, and then the mice were placed in a clean recovery cage with a heating pad until they awoke from anesthesia. They were allowed to recover for at least two to three weeks before any further experiments were conducted.
Further details can be found in the supplementary materials.
Fiber photometry
All fiber photometry experiments were conducted using the fiber photometry system (Thinker Tech Nanjing Biotech Co. Ltd) delivers a beam of 470 nm laser light. The light source of the fluorescence excitation system is a 488 nm wavelength laser (OBIS 488LS; Coherent). Light emitted from the source is attenuated by a 1% neutral density filter (Daheng Optics) and then reflected at a right angle by a dichroic mirror (MD498, Thorlabs). This beam is focused onto the core end of the optical fiber patch cord through a 10x magnification objective lens (numerical aperture 0.3, Olympus). The optical fiber patch cord is connected to the optical fiber ferrule implanted in the animal’s head using a ceramic ferrule connector. The excitation light travels through the optical fiber patch cord and the animal’s head ferrule to reach the mPFC region of the brain, where it excites green fluorescent signals. And the GCaMP fluorescence signals were acquired at a sampling rate of 50 Hz. The light intensity at the fiber tip during recording should be controlled between 10–30 μW to reduce fluorescence bleaching. The overall brightness of the environment can affect the fiber recording system, so the laboratory should use dim yellow lights as the light source during fiber recordings. Additionally, an optical signal commutator (Doric Lenses) connected to one end of the fiber patch cord (200 μm, ceramic outside diameter 1.25 mm, 2 meters long) can prevent the fiber from twisting or tangling during recording. Using behavior-tracking cameras to record fiber photometry during tail suspension and social interaction experiments, for synchronizing behavioral testing with neural activities recording.
Behavioral tests
Further details can be found in the supplementary materials.
Histology and immunohistochemistry
The immunofluorescence staining method was modified based on previous studies [31]. In brief, adult mice were perfused transcardially with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Afterward, the brain was extracted and dehydrated in a series of sucrose solutions with concentrations of 20%, 30%, and 30%. Once dehydration was complete, 30 µm thick brain sections were prepared using a cryostat and mounted onto slides. The sections were coverslipped using DAPI solution (Beyotime, China) containing an anti-fade agent, and images were captured using a confocal microscope (Nikon, Japan).
Fiber photometry analysis
Data analysis was performed using open-source MATLAB toolboxes. The tail suspension test (struggling and immobile behaviors) and social interaction test (sniffing an empty box and test mouse behaviors) were synchronized to time zero for each behavior’s onset. We calculated normalized fluorescence changes (ΔF/F) by subtracting baseline fluorescence from the fiber fluorescence at each time point, and then dividing that value by the baseline fluorescence. A 2-second stable calcium signal was selected as the baseline fluorescence signal for the environment to process the data, validated by synchronizing behavioral video recordings to exclude motion artifacts. The baseline stability is confirmed by low signal variance ( <10% ΔF/F fluctuation) and alignment with behavioral states, ensuring reliable normalization against transient neural activity during tasks. Viral expression and fiber implantation sites were confirmed using immunofluorescence staining, and mice that showed no fiber targeting or failed viral expression were excluded after verification.
Data collection and statistical analyses
The number of animal repetitions was reported, and statistical analysis was performed using Origin 2021 (OriginLab Corporation, United States) and SPSS Statistics 20 (IBM, United States). For n sizes, the number of animals or cells were provided in the Figure legends. Before conducting parametric hypothesis testing, all data were tested for homogeneity of variance and normal distribution (using the Kolmogorov-Smirnov and Shapiro-Wilk tests). One-way ANOVA was used for multiple comparisons, with Bonferroni or LSD (assuming equal variances) and Tamhane’s T2 or Games-Howell (assuming unequal variances) to assess the effects of group definitions on behavior, calcium signals, and immunofluorescence data. Repeated measures ANOVA was used to test changes in data across different time points in the model, while two-way ANOVA was used for multi-group experimental analyses. Data are presented as mean ± standard error of the mean (SEM). The significance levels are indicated as CON vs. SSCE, ∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001; pre. vs. 2 weeks, pre vs. 4 weeks, # p < 0.05, ## p < 0.01, ### p < 0.001; 2 weeks vs. 4 weeks, & p < 0.05, & & p < 0.01, & & & p < 0.001. Comprehensive information on statistical analysis is included in the main text and figure legends.
Results
Decreased GMV in mice with simulated space composite environment exposure
Based on high-resolution structural MRI and TMBTA parcellation, we examined the whole-brain GMV across four groups. In mice exposed to a 2-week SSCE, several brain regions showed reduced volumes compared to the CON group, primarily within the cerebral nuclei. These areas included the amygdala nucleus, caudoputamen, and nucleus accumbens (Fig. 1a, b and Supplementary table 1). After 4 weeks of SSCE exposure, however, GMV reductions extended more broadly across both cortical and subcortical regions when compared to the corresponding CON group. Notably affected areas included the sensorimotor cortex, prefrontal cortex, insular cortex, orbital area, and cerebral nuclei (Fig. 1a, b and Supplementary table 2). The prefrontal cortex, in particular, displayed significantly lower grey matter volumes in all four subregions in the 4-week SSCE group, a change that was not statistically significant in the 2-week comparison. (Fig. 1c).
a, b Schematic (a) and statistical results (b) of GMV differences between SSCE and CON after 2-week and 4-week modeling. c Difference in grey matter volumes of whole brain and prefrontal subregions. The statistical threshold is set as p < 0.05. Asterisks indicate significance from two-sample t-tests (*p < 0.05; ** p < 0.01; ***p < 0.001).
Multiscale functional connectivity alterations in mice with simulated space composite environment exposure
To investigate the impact of SSCE on the FC of the mouse brain, we extracted the intrinsic FC from anesthetized BOLD fMRI (Fig. 2a, b and Supplementary Fig. 1a and b). Mice exposed to both 2-week and 4-week SSCE showed a pronounced decrease in functional connectivity strength as compared with the respective control groups (Fig. 2c and Supplementary Fig. 1b). Specifically, after 2 weeks of SSCE, decreased FC strength was mainly observed in the somatosensory cortex, visual cortex, amygdala, and hypothalamus (Supplementary Fig. 1c and e). Notably, reductions in resting-state connectivity were identified in the primary sensorimotor area and hypothalamic lateral zone in both conditions (Supplementary Fig. 1e and f). In addition, decreased FC strength was observed in the right ventral striatum and left pallidum after 4 weeks of SSCE exposure (Supplementary Fig. 1d and f).
a To calculate the pairwise FC, 160 regions of interest (ROIs) were defined based on the TMBTA reference, with 80 in each hemisphere. Pearson correlations were calculated between the BOLD time series of all unique ROI pairs. A group-averaged region-wise FC matrix for the 2-week CON group is shown, reordered by network assignments. b The FC matrix is represented as a weighted network graph, with ROIs as nodes and their pairwise correlations as edges. For clarity, only the strongest 10% of edges are shown, with edge thickness and opacity scaling with the correlation value and node radius scaling with the weighted degree (the weighted sum of all edges connected to the node). c The regional functional connectivity strength is violin plotted and also projected on the brain surface for each group. Each dot represents a single region. Asterisks indicate the significance from paired t-tests after adjusting for multiple comparisons (*p < 0.05; ** p < 0.01; ***p < 0.001). d Edge-wise FC differences between SSCE and CON groups are represented with graph for both 2-week (top) and 4-week (bottom) modeling. Edge thickness and opacity scale with the effect size (red: SSCE > CON, blue: SSCE < CON); node radius scales with the weighted sum of effect size. For visualization, a statistical threshold of p < 0.001 is applied. e The LME model was performed to illustrate the interaction of condition (SSCE or CON) × time on FC. Chord diagram highlights edges with the significant interaction of modeling time and group (p < 0.001). f Network-wise FC differences between SSCE and CON groups for both 2-week (left) and 4-week (right) modeling are represented in matrix form. Significant network pairs are further highlighted in a chord diagram (red: SSCE > CON, blue: SSCE < CON, * p < 0.05). g The interaction of condition × timing on FC is visualized at the network level (* p < 0.05). h PFC-specific FC differences between SSCE and CON groups for both 2-week (top) and 4-week (bottom) modeling are represented with graphs. Edge thickness and opacity scale with the effect size (red: SSCE > CON, blue: SSCE < CON); node radius scales with the weighted sum of effect size. i PFC voxel-wise FC strength is displayed in raincloud plots and sagittal brain view for each group. Each dot represents a voxel within the PFC. Asterisks indicate the significance of paired t-tests after multiple comparisons adjustments (*p < 0.05; ** p < 0.01; ***p < 0.001).
Next, we examined FC at the edge-wise level. In mice exposed to 2-week SSCE, functional hyperconnectivity was mainly observed between cortical and subcortical regions, including the frontal, visual, olfactory, hippocampal and hypothalamic areas (Fig. 2dtop and Supplementary Fig. 2a). Conversely, functional hypoconnectivity was primarily observed between the olfactory area and the frontal, motor, and somatosensory cortices. After 4 weeks of SSCE, functional hyperconnectivity was noted between the frontal, visual cortex and subcortical nuclei, while functional hypoconnectivity was more prominent in connections involving the primary sensory area, olfactory area and cortical subplate (Fig. 2dbottom and Supplementary Fig. 2a). To highlight changes in FC over time, the LME model was performed (Fig. 2e and Supplementary Fig. 2a). Significant interactions of condition × timing were mostly observed in cortical-cortical connections, primarily related to regions in the frontal, somatosensory, visual, insular and olfactory cortices.
To provide a systematic view, we also examined network-wise functional connectivity alterations. In the 2-week SSCE group, functional hyperconnectivity was evident between the frontal cortex and cerebellum (Fig. 2fleft). While, functional hypoconnectivity was mainly observed in edges connecting the motor, auditory, insular, hippocampus, cortical subplate, thalamus, hypothalamus and cerebellum. In the 4-week SSCE group, functional hyperconnectivity was observed between the olfactory cortex and cerebellum (Fig. 2fright). While, functional hypoconnectivity was observed between the auditory cortex and cortical subplate, as well as between the cingular, retrosplenial cortex and cerebellum. The LME model further emphasize significant FC differences over time (Fig. 2g and Supplementary Fig. 2b and c), with notable connections between the frontal and visual cortices, frontal cortex and cerebellum, auditory cortex and hippocampus, insular and retrosplenial cortices, retrosplenial and olfactory cortices, retrosplenial cortex and cerebellum, cortical subplate and thalamus, and within the thalamus.
Thereafter, we focused on PFC-specific FC differences. In mice with 2-week SSCE exposure, functional hyperconnectivity was observed in edges connecting the PFC with the left primary somatosensory barrel field and left lateral septal complex (Fig. 2htop). Functional hypoconnectivity was observed in edges connecting the PFC with the left posteromedial visual area, right posterior agranular insular, right gustatory areas, right piriform area, right Cortical amygdalar area and right hippocampo-amygdalar transition area. In mice with 4-week SSCE, functional hyperconnectivity was observed in edges connecting the PFC with the left caudoputamen, right temporal association areas, right ventral auditory area, right perirhinal area, right lateral amygdalar nucleus, right endopiriform nucleus and right basolateral amygdalar nucleus (Fig. 2hbottom). Functional hypoconnectivity was observed in edges connecting the PFC with the right dorsal and ventral retrosplenial area, right ventral striatum and right Hemispheric region. Finally, we calculated the voxel-wise functional connectivity strength of the PFC and observed a decrease only in mice with 2-week SSCE as compared with the respective CON group (Fig. 2i).
Altered gradients of macroscale functional organization in mice with simulated space composite environment exposure
Considering the high-dimensional complexity of functional connectivity derived from fMRI data, particularly when conducting voxel-wise analyses, we applied a dimensionality reduction approach to the voxel-wise FC matrixes to compute large-scale functional gradients. These gradients provide a neurocognitively meaningful representation of cortical organization and have recently been introduced as a framework for understanding brain topology [32, 33]. In line with previously reported fine-grained descriptions of the mouse brain connectome with voxel resolution [34], we identified two primary cortical gradients reflecting the hierarchical organization of the cortex in healthy mice (Fig. 3b, Supplementary Fig. 3). Specifically, the principal gradient (gradient 1) captured a sensory-to-fugal transition between unimodal sensorimotor regions and higher-order transmodal areas. The secondary gradient (gradient 2) extended across unimodal visual and auditory cortices to primary motor-sensory regions, reflecting a regional differentiation between sensorimotor modalities. Interestingly, similar hierarchical properties were observed in mice with SSCE, with the alignment of their gradients mirroring those of healthy mice (Fig. 3a, c and Supplementary Fig. 4). These findings support the notion that cortical gradients represent fundamental properties of brain topography both in health and in altered conditions such as SSCE exposure [32, 35].
a The principal (top) and secondary (bottom) functional gradients are projected onto the brain surface mesh. b The explanation ratio of connectome gradients (components) derived from the template FC. c Scatter plots of whole-brain voxel-wise gradient score for each of the two functional gradients across different groups. Each dot represents a voxel, colored according to its functional system assignment. d The whole-brain voxel-wise gradient dispersion is violin plotted for each group. Each dot represents a single voxel. Asterisks indicate the significance of paired t-tests after adjusting for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001). e System-level distributions of the principal gradients for the 2-week group pair (left) and 4-week group pair (right). Bubble plots on the right represent the group differences in the gradient scores for each brain system, corrected for multiple comparisons (FWE-corrected at p < 0.05). The color of the circle represents the T-value of the paired t-test (red: SSCE > CON, blue: SSCE < CON). The size of the circle indicates the significance of the corresponding paired t-test. Radar plots of the T-values are also provided. f Pairwise comparison of within-network gradient dispersion for the 2-week group pair (left) and 4-week group pair (right). g Scatter plots of voxel-wise gradient scores of the PFC for the first two functional gradients in different groups. Each dot represents a voxel, colored according to its regional assignment. h The voxel-wise gradient dispersion in the PFC is violin plotted for each group. Each dot represents a single voxel. Asterisks indicate the significance of paired t-tests after adjusting for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001, FWE-corrected).
Further whole-brain gradient comparisons revealed increased gradient dispersion in mice exposed to 2 weeks of SSCE compared to the corresponding CON group, but decreased gradient dispersion in mice exposed to 4 weeks of SSCE (Fig. 3d). Specifically, the increased principal functional gradient score in the 2-week SSCE group was primarily observed in transmodal networks positioned at the positive extreme end of the gradient axis, whereas, unimodal networks located at the negative extreme end showed decreased principal functional gradient score. Conversely, the 4-week SSCE group exhibited decreased scores in transmodal networks and increased scores in unimodal networks (Fig. 3e). A similar expansion in range was observed for the secondary functional gradient (Supplementary Fig. 5).
Pairwise comparisons revealed that all networks showed higher within-network dispersion in mice with 2-week SSCE exposure, except for the frontal network with reduced dispersion and the retrosplenial network with no significance (p < 0.05, FWE corrected) (Fig. 3fleft), suggesting overall lower within-network connectedness in the 2-week SSCE group. In contrast, mice with 4-week SSCE exposure showed higher within-network dispersion in the frontal, olfactory, and cerebral nuclei networks, while networks such as the motor, somatosensory, visual, cingulate, hippocampus, thalamus, and cerebellum exhibited reduced within-network dispersion (Fig. 3fright). Comparable results were obtained when considering the first three gradients for within-network dispersion calculation (Supplementary Fig. 6).
Focusing on the PFC alone (Fig. 3g), a consistent gradient pattern across different groups was identified, with the first gradient transitioning between the ventral and dorsal anterior cingulate area, and the second gradient distinguishing the limbic region from the anterior cingulate area. In both the 2-week and 4-week SSCE group, an increased gradient dispersion in the PFC was observed as compared with the corresponding CON group (Fig. 3h), indicating a sustained reduction in functional integration within the PFC.
Reversed brain-emotional behavior relationships in mice with simulated space composite environment exposure
In the previous section, functional gradient analysis highlighted the altered functional integration across various networks in mice exposed to SSCE. To further investigate whether these alterations correlate with emotional and behavioral outcomes, we examined the relationship between FC and emotional behaviors. In control mice, we found that FC between the frontal network and motor and hippocampal regions was negatively associated with EPM performance, while FC between the olfactory network and thalamus was positively correlated (Fig. 4aleft). However, in SSCE-exposed mice, none of these correlations were observed. For TST performance, negative correlations in control mice were found primarily between the frontal network and hypothalamus, between the visual network and both thalamus and hypothalamus, and between the cingulate and insular cortices. Positive correlations were observed within auditory networks and cortical subplate regions, as well as between somatosensory and cerebellar regions. In contrast, SSCE-exposed mice showed only a positive correlation between FC in the somatosensory network and cerebellum with TST performance (Fig. 4amiddle). In the social test, control mice exhibited negative correlations between behavioral scores and FC within the frontal, somatosensory, motor, auditory, cerebral nuclei, and cerebellar networks, while positive correlations were observed within somatosensory, cortical subplate, and thalamic regions, as well as between the frontal and insular cortices, and between cortical subplate and cerebral nuclei (Fig. 4aright). Nonetheless, in mice exposed to SSCE, negative correlations were found in FC between the frontal network and cortical subplate, between retrosplenial and cerebral nuclei and between thalamus and cingular cortex. Conversely, positive correlations were found in FC between retrosplenial and frontal cortices, between somatosensory and both cortical subplate and hypothalamus, and between cortical subplate and auditory cortex. Notably, subsequent examination revealed highly contrasting brain–behavioral relationship patterns between the SSCE and CON groups in both the EPM and Social tests (Fig. 4b), indicating distinct neural and behavioral responses under SSCE exposure. Additionally, the PFC-specific and the PL-specific brain-behavior relationship patterns also showed significant negative correlations between the two groups (Fig. 4c, d and Supplementary Fig. 7). These findings suggest that the SSCE and CON groups may interpret or respond to emotional and social cues differently, potentially reflecting altered mechanisms of cognitive processing, emotional regulation, or social cognition under the influence of SSCE exposure.
a Spearman’s correlation patterns between network-wise functional connectivity and three behavioral measures: the percentage of time in the open arms in the EPM: EPM-percentage (left), Stillness duration in the TST: TST-still (middle), sniffing time in the social test: Social-sniff (right). Note that, the upper and lower triangles of the matrix respectively represent the CON and SSCE groups after 4 weeks of modeling. Asterisks indicate correlations that passed the significance threshold of 0.05. b Contrasting network-wise brain-behavior relationships between the SSCE and the CON groups in terms of EPM and Social tests. c Circular heatmaps summarizing spearman’s correlations between PFC-specific ROI-wise FC and the three behavioral measures. The inner and outer arcs respectively represent the CON and SSCE groups after 4 weeks of modeling. The blocks outlined in black indicate correlations that passed the significance threshold of 0.05. d PFC-specific brain-behavior relationships in the SSCE group are the opposite of those in the CON group in terms of EPM and Social tests.
Simulated space composite environment exposure can cause individuals’ emotional behaviors to undergo self-reconstruction
After clarifying the relationship between brain function in the resting state and behaviors. To explore the temporal changes in emotion-related behaviors under SSCE, we conducted two classic behavioral tests at different time points (Fig. 5a, Pre., 2-week SSCE exposure and 4-week SSCE exposure) for observation. In the TST, which measures depressive-like behavior (Fig. 5b), there were significant differences in the duration of immobility among the different groups over time (Fig. 5c, repeated measurement ANOVA, F = 7.903, p < 0.01), with group and time showing an interaction effect (F = 12.196, p < 0.01). Further observations revealed that during exposure, the immobility time in the control group remained at baseline levels, while the SSCE group showed a significant increase in immobility time after two weeks of exposure (p < 0.01), and this change persisted through the four-week exposure period (p < 0.01). The results from between-group comparisons were consistent with the above findings (Fig. 5d), reflecting that under the unique stress conditions of SSCE, the learned helplessness behavior persisted as exposure time lengthens.
a Schematic of the SSCE paradigm. b, e Experimental paradigms for TST (b) and SDT (e). c, d In the TST, the time that mice remain immobile within 5 min (Pre., pre-SSCE; 2 weeks, SSCE exposure for two weeks; 4 weeks, SSCE exposure for four weeks; CON: n = 5, SSCE: n = 5). f In stage 1 of SDT, the mouse’s sniffing time of empty boxes (CON: n = 5, SSCE: n = 5). g-i In stages 2 and 3 of SDT, the discrimination index of the sniffing of familiar (g, h) and novel mice (i). j, m, o Different viruses tracing strategy. k, n, p Injection site at NAc (k, n) and mPFC (p), tracing neurons in mPFC (n) and axon terminal in NAc (p), enlarged image. l Statistical results of the number of brain regions and their neurons projected into the NAc (mPFC, n = 9 slices/3 mice; AIV, Agranular insular cortex, ventral part, n = 4 slices/3 mice; Cg2, Cingulate cortex, n = 5 slices/3 mice; PV, Paraventricular thalamic nucleus, n = 6 slices/3 mice; BLA, Basolateral amygdala, n = 5 slices/3 mice).
Afterwards, to further explore social interaction abilities, we designed a similar social discrimination test to examine the impact on social functions, including social preference, social memory, and social novelty recognition (Fig. 5e). First, during the adaptation phase, we observed that neither group of mice showed any location preference at any time point under SSCE exposure, allowing us to proceed with subsequent experiments (Fig. 5f, p > 0.05). Then, we found that under normal conditions, the test mice preferred to interact with the experimental mice rather than staying in the corner (Discrimination Index>0). However, after two weeks of SSCE exposure, this social preference disappeared (Discrimination Index<0). At different levels of social ability, the discrimination index of SSCE-exposed mice varied significantly with the modeling time (Fig. 5g: F = 9.819, p < 0.01; Fig. 5h: F = 11.244, p < 0.01; Fig. 5i: F = 13.757, p < 0.001), and there was an interaction effect between group and time (Fig. 5g: F = 9.229, p < 0.01; Fig. 5h: F = 6.053, p < 0.05; Fig. 5i: F = 7.301, p < 0.01). This indicated that SSCE exposure indeed impaired various aspects of social abilities in mice, and these effects persisted. Interestingly, as the exposure period extended, the mice showed some recovery in social preference ability, but it did not return to normal levels (2 weeks vs. 4 weeks, p < 0.05). This suggested that the impact of SSCE on social abilities was heterogeneous. Our results indicated that the effect of SSCE exposure on emotion-related behaviors was a dynamic and self-restructuring process, where animals continuously adjust their behavior, adapt to external stress, and meet internal demands to reshape their behavioral patterns.
Simulated space composite environment exposure persistently suppresses mPFCNAc neuronal activity during active avoidance behavior
The relationship between the mPFC and NAc has been reported in previous studies [36,37,38]. To identify which brain regions project to the NAc and clarify the specific projections between the mPFC and NAc, we injected rAAV2/Retro-CaMKIIα-GCaMP6m-WPRE-pA into the NAc (Fig. 5j, k). Three weeks later, we found AAV-infected neurons were limited to specific upstream brain regions, including the mPFC, AIV, Cg2, PV, and BLA (Supplementary Fig. 8a), with the highest number of pyramidal neurons projecting from the mPFC to the NAc (Fig. 5l, one-way ANOVA, F(4,24) = 29.577, p < 0.001).
To further confirm this projection, we used CTB-488 dye and a viral retrograde tracing technique (Supplementary Fig. 8b, Fig. 5m), which showed that specific neurons in the mPFC were infected (Supplementary Fig. 8c, Fig. 5n). We also injected rAAV-CaMKIIα-GCaMP6m-WPRE-pA into the mPFC (Fig. 5o), and three weeks later, excitatory axon terminals were detected in the NAc (Fig. 5p). These results, from both anterograde and retrograde perspectives, clearly show that the mPFC has a monosynaptic excitatory projection to the NAc.
Then, we used fiber photometry to record the activity of GCaMP6m-expressing excitatory neurons in the subjected to the TST to experimentally link mPFCNAc neuronal activity to the depression-like behaviors observed during the SSCE process (Fig. 6a–c). Previous studies have reported that the activity of excitatory neurons in the mPFC alternates between low calcium signals (during the mice’s immobile period) and high calcium signals (during the struggle period) [31]. We also observed such a pattern effect for mPFCNAc neurons (Fig. 6d–g, independent-sample t-test, t(16.389) = −8.806, p < 0.001). This indicated that the active avoidance of the mice was significantly associated with the activity of mPFCNAc neurons. Additionally, during the 5-min TST (Fig. 6h, i), the calcium signal intensity in mice exposed to SSCE for two weeks was significantly lower than that of the control group (Fig. 6j–m, repeated measurement ANOVA, CON vs. SSCE, p < 0.01). This phenomenon was also present during the fourth week of exposure (CON vs. SSCE, p < 0.01), suggesting that the activity of mPFCNAc neurons in SSCE-treated mice was continuously suppressed during active avoidance behavior.
a Schematic illustration of fiber photometry recording in vivo. b, c Representative images validate GCaMP6m expression in mPFCNAc neurons and optical fiber tract above the mPFC (b). c. Enlarged image boxed in (b). d Schematic of fiber photometry recording in TST. e, f Representative image (e) and peri-event plots (f) of ΔF/F Ca2+ signal changes from mPFCNAc neuron during immobility and struggling in TST. g Comparison of averaged ΔF/F of mPFCNAc signals in response to TST during the immobility and struggling. h, i Heatmap (h) and peri-event plots (i) of Ca2+ transients of mPFCNAc neuron during the struggling in SSCE (CON: n = 5 mice; SSCE: n = 5 mice). j-m The mean (j, k) and peak (l, m) Ca2+ signal change (ΔF/F) within 2 s during the struggling.
Simulated space composite environment exposure exhibits temporal specificity in the low-excitable mPFCNAc neuronal activity in response to distinct social behaviors
To investigate the functional impacts of mPFCNAc neurons on social behavior, we employed fiber photometry techniques, injecting retrograde GCaMP6m viruses into the NAc under the control of the CaMKII promoter. This approach enabled us to monitor changes in the activity of mPFCNAc somas via fiber optics. Previous studies have provided limited insight into the role of mPFCNAc neurons in social behavior, prompting us to conduct a social discrimination test with the test mice. Our findings revealed that the calcium signal intensity was significantly higher when the test mice interacted with familiar mice compared to their behavior in an empty box under normal circumstances (Fig. 7a–d, one-way ANOVA, t(25) = −6.559, p < 0.001). Interestingly, when a novel mouse was introduced, the test mice naturally spent more time interacting with the novel mouse rather than the familiar one. However, we observed no significant difference in neuronal activity during interactions with either type of mouse (Fig. 7e–h, one-way ANOVA, t(26) = 0.853, p > 0.05) .
a, e Schematic of fiber photometry recording in stage 2 (a) and stage 3 (e) of SDT. b, c, f, g Representative image (b, f) and peri-event plots (c, g) of ΔF/F Ca2+ signal changes from mPFCNAc neuron during sniffing the empty box, familiar mouse and novel mouse in SDT. d, h Comparison of averaged ΔF/F of mPFCNAc signals in response to SDT during sniffing the empty box, familiar mouse and novel mouse. i, j Heatmap (i) and peri-event plots (j) of Ca2+ transients of mPFCNAc neuron during sniffing the familiar mouse in stage 2 of SDT (CON: n = 5 mice; SSCE: n = 5 mice). l, m Heatmap (l) and peri-event plots (m) of Ca2+ transients of mPFCNAc neuron during sniffing the novel mouse in stage 3 of SDT. k, n The peak Ca2+ signal change (ΔF/F) within 5 s during sniffing the familiar mouse in stage 2 (k) and sniffing the novel mouse in stage 3 (n).
In a subsequent phase of our study, we found that after two weeks of SSCE exposure, the neuronal activity of the test mice was significantly suppressed during interactions with familiar mice, regardless of the presence of a novel mouse (Fig. 7i–k, Supplementary 9, repeated measurement ANOVA, pre. vs. 2 weeks, p < 0.05). Notably, there was some recovery in neuronal activity by the fourth week (2 weeks vs. 4 weeks, p > 0.05). However, their activity remained suppressed when interacting with the novel mouse (Fig. 7l–n, repeated measurement ANOVA, pre. vs. 2 weeks, p < 0.01; 2 weeks vs. 4 weeks, p < 0.05). These results suggested that the mPFCNAc neuronal activity in test mice exhibited temporal specificity in response to different interaction partners following SSCE treatment.
Transcriptomic association with differences in PL-specific functional profiles
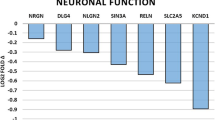
Given our results so far about the alterations in macroscale functional organization and behavioral responses, we hypothesized that a molecular mechanism could underlie the functional reconfiguration of the brain, shaping distinct psychological and neural outcomes. To this end, we utilized volumetric gene expression data provided by the Allen Institute for Brain Science, which is part of the Allen Mouse Brain Atlas (AMBA), a genome-wide gene expression database built upon in situ hybridization images. We performed Spearman’s correlations between regional differences in PL-specific functional profiles for both group pairs (i.e., as depicted in the effect size maps, Fig. 8b) and the expression profiles of 4104 genes (Fig. 8a). After adjusting for multiple comparisons with false discovery rate (FDR) correction, we identified 2423 genes significantly correlated with 2-week effect size map and 1481 genes significantly correlated with 4-week effect size map (Fig. 8c, d). Of these, a set of 956 genes were significantly correlated with both effect size maps. The mRNA expression of the related genes has been verified (Supplementary table 3, 4, Independent sample t test, Supplementary Fig. 10a, t(15) = 2.378, p < 0.05; Supplementary Fig. 10b, t(9.570) = 2.656, p < 0.05; Supplementary Fig. 10c, t(8.857) = 4.857, p < 0.001; Supplementary Fig. 10d, t(16) = 1.312, p > 0.05; Supplementary Fig. 10e, t(16) = −1.624, p > 0.05; Supplementary Fig. 10f, t(16) = −0.249, p > 0.05; Supplementary Fig. 10g, t(16) = 1.135, p > 0.05; Supplementary Fig. 10h, t(15) = 2.760, p < 0.05; Supplementary Fig. 10i, t(14) = 0.544, p > 0.05; Supplementary Fig. 10j, t(15) = 3.184, p < 0.01;Supplementary Fig. 10k, t(16) = 4.159, p < 0.01; Supplementary Fig. 10l, t(16) = 3.557, p < 0.01).
a Normalized gene expression levels across 157 ROIs. b Brain surface projections of effect size values for the 2-week and 4-week comparison pairs (left) and gene expression levels. c Number of genes significantly correlated with the 2-week and 4-week effect size maps. d Significant genes are ranked in descending order according to their correlation coefficients. Scatter plots illustrate representative correlations between effect size values and gene expression levels. e GO enrichment analysis of genes significantly correlated with differences in PL-specific functional profiles for the 2-week (left) and 4-week (right) comparison pairs. Enrichment in cellular components (orange), biological processes (blue) and molecular functions (green) is shown. Note that, q value represents the FDR-corrected p value. The color of the circle represents the enrichment score of each term. The size of the circle indicates the number of genes significantly enriched in each term.
To further investigated the putative function of these gene sets, we conducted GO and KEGG enrichment analyses (Fig. 8e and Supplementary Fig. 11). GO analysis revealed enrichment in cellular components critical for synaptic activity and neural signaling, including “synaptic membrane,” “dendrite,” and “axon.” Biological processes such as “cell-cell signaling,” “behavior,” and “regulation of membrane potential” were prominent, suggesting roles in nervous system development and synaptic organization. Molecular functions highlighted transmembrane transporter and ion channel activities, essential for maintaining ion homeostasis and neurotransmitter dynamics. KEGG analysis identified pathways like “circadian entrainment,” “glutamatergic synapse,” “neuroactive ligand-receptor interaction,” “calcium signaling pathway,” and “pathway in cancer”, indicating SSCE-induced neurobiological and cellular shifts impacting mental health, cognitive function, and possibly long-term cellular health.
We also performed GO and KEGG enrichment analyses for genes specific to 2-week and 4-week effect size maps, as well as for the overlapping genes (Supplementary Fig. 12–14). Despite differences in the timing of the exposure, the results consistently highlighted the same key terms related to synaptic activity, cell signaling, and ion channel function. Taken together, our results provide compelling evidence for a set of genes that might underlie structural and functional adaptations in response to SSCE exposure, potentially contributing to the unique psychological and behavioral outcomes observed in these mice.
Discussion
The Artemis program by NASA aims to achieve manned lunar missions by 2030 and to complete its first crewed Mars mission and to establish a base by 2040 [39, 40]. The space environment presents unique challenges due to a combination of stressors, with microgravity as the primary factor, alongside noise, circadian rhythm changes, and social isolation [41,42,43]. To better approximate the authentic work and living conditions of astronauts, we constructed simulated space composite environment encompassing these factors. As mission duration extends, astronauts’ emotional states undergo dynamic changes. Long-duration space exploration necessitates careful consideration of astronauts’ psychological well-being [34, 44]. A study on space environment proposed a four-phase emotional change model, transitioning from tension to calm and from distress to adaptation [35, 45]. We investigated how brain networks are involved in this process, focusing on two critical time points in the SSCE: two weeks and four weeks. Our findings revealed a progressive reduction in gray matter volume across various brain regions with prolonged over time, notably in the prefrontal cortex. The SSCE induced changes in multiscale functional connectivity and macro-organizational functional gradients, significantly altering the relationship between brain activity and emotional behaviors. Furthermore, the mPFC exhibited time-specific changes in neural activity across various emotional tasks. Finally, we discussed potential genetic mechanisms underlying the SSCE-induced neural network alterations, which may relate to changes in synaptic function and ion channel activity.
Here, we utilized BOLD fMRI in anesthetized mouse to map brain-wide connectivity architecture and examine how functional topology reconfigures in response to the space environment [46,47,48]. Through two MRI scans of C57BL/6 J mice, we investigated the impact of SSCE exposure on FC and observed significant reductions in FC strength in key brain regions, including the somatosensory cortex, visual cortex, amygdala, and hypothalamus, particularly after 2 weeks of exposure. These disruptions, which persisted through 4 weeks, suggest impairments in sensory processing, emotional regulation, and homeostatic control. In addition, we observed hyperconnectivity in cortical-subcortical networks alongside hypoconnectivity in sensory and motor areas. While such hyperconnectivity patterns may suggest compensatory network reorganization [49, 50], alternative interpretations—such as maladaptive plasticity or circuit dysregulation due to chronic environmental stress—should be considered in light of the current cross-sectional design and lack of functional validation. Longitudinal imaging and circuit-level interventions (e.g., chemogenetics and optogenetics) are necessary to determine the causal significance and functional outcomes of this reorganization. Furthermore, by focusing on the prefrontal cortex, we observed dynamic FC changes with other brain regions across the SSCE exposure period, suggesting that SSCE may impact higher-order cognitive processes, such as emotional regulation [51], social behavior [52], and stress adaptation [53]. To our knowledge, these observations provide novel insight into brain-wide network reconfiguration under simulated space conditions.
Our findings align with previous studies examining the effects of microgravity or spaceflight on the human brain [54,55,56], in which functional changes have been observed in brain regions such as the cerebellum and cortical sensorimotor and somatosensory areas—regions closely associated with motor control, proprioception, and cognitive regulation. The observed FC alterations may reflect adaptive neural changes in response to the stressors of SSCE exposure. However, prolonged exposure to this condition could lead to functional impairments. Our results underscore the need for further research into the long-term effects of space-like environments on brain connectivity and cognition, as well as potential interventions to mitigate these effects in future space exploration.
The results of functional gradients reveal that SSCE exposure alters the macroscale functional organization of the mouse brain in a time-dependent manner. We observed increased gradient dispersion after 2 weeks of SSCE exposure, particularly in transmodal networks, suggesting a disruption in the typical functional connectivity between higher-order and unimodal sensorimotor regions. However, after 4 weeks of SSCE exposure, a compensatory reorganization occurred, with unimodal networks showing increased functional gradient scores and transmodal networks displaying decreased scores, indicating adaptive changes in response to prolonged SSCE exposure.
Further analysis of within-network dispersion revealed a reduction in overall functional connectedness, especially in the 2-week SSCE group, which showed higher dispersion across most brain networks. The PFC was notably affected, exhibiting increased gradient dispersion in both the 2-week and 4-week groups, indicative of reduced functional integration within this area. This reduction in PFC connectivity may underlie the behavioral changes observed, such as altered anxiety-like and social behaviors, highlighting the impact of SSCE on higher-order cognitive functions. These findings suggest that while short-term SSCE exposure disrupts brain connectivity, prolonged exposure may induce adaptive reorganization, albeit with potentially lasting effects on key regions critical for complex cognitive processing.
However, conducting MRI on small animals presents methodological challenges: it requires the animals to remain motionless for extended periods, necessitating restraints and acclimatization to head fixation and the noise of the MRI, which can be labor-intensive for researchers and often induces stress in the animals. Consequently, anesthesia is commonly employed [57, 58]. Unfortunately, the use of anesthetics can confound fMRI measurements in several ways, potentially altering neuronal activity and affecting neurovascular coupling—this may also represent a limitation of the current study [9, 10]. Nonetheless, some research indicates that isoflurane anesthesia yields brain activity data that is closest to the animal’s resting state, thereby enhancing the credibility of this study. In future studies, functional MRI during awake tasks could be considered.
Subsequently, we identified significant alterations in brain-emotional behavior relationships in SSCE-exposed mice, characterized by reversed or absent correlations between FC and emotional/social behaviors compared to controls, potentially suggesting disrupted brain network integration. Notably, changes in brain-emotional coupling in regions such as the PFC and hippocampus, which are critical for emotional regulation and social cognition, point to maladaptive reorganization of these circuits [59, 60]. The reversal of FC–behavior associations raises the possibility that SSCE induces aberrant reconfiguration of key regulatory networks, potentially through altered signaling efficiency [61], neurochemical imbalances such as dysregulated hypothalamic-pituitary-adrenal (HPA) axis activity [62], or biased network dynamics favoring subcortical threat-processing systems (e.g., amygdala-centered circuits) over cortical control regions [53]. Such disruptions may reflect a breakdown in the brain’s capacity to integrate internal states with external stimuli, a hallmark of stress-related dysfunction. However, possible confounding factors such as cross-sectional design, separate conditions for behavioral and imaging data collection, and altered arousal levels under anesthesia should be considered in interpreting these findings. Future longitudinal studies with integrated awake imaging and behavioral assessments will be critical to determine whether these altered brain-behavior relationships represent a permanent maladaptation [63].
Does the PFC, as the brain’s executive center responsible for regulating higher cognitive and emotional processes [64, 65], also exhibit these functional impairments under specific emotional task conditions? Recent studies have investigated the role of the PFC in various neuropsychiatric disorders. Dysregulation of synaptic transmission in the PFC is closely associated with social deficits, emotional disorders, and memory loss in neuropsychiatric conditions such as autism [66], schizophrenia [67,68,69], depression [70, 71], and Alzheimer’s disease [72, 73]. The NAc is generally recognized for its role in integrating reward and motivational inputs and translating them into motor outputs [74,75,76]. Excitatory glutamatergic inputs from the PFC, which convey information about context, cues, and emotional behavioral control, converge onto the NAc [37, 77]. Our research indicated that after two weeks of exposure to SSCE, the activity of excitatory neurons projecting from the mPFC to the NAc decreased and persisted until the fourth week, correlating with the manifestation of depressive-like behaviors in these mice. Additionally, we investigated the role of the mPFC-NAc circuit in social behavior and found it to be involved in social preference, social memory, and novelty-seeking behaviors [14, 37, 78]. However, recent studies have reported that optogenetic manipulation of the excitatory/inhibitory balance in the mPFC can impair social interactions in mice [79]. Inactivation of the PL neurons projecting to the NAc disrupts social recognition without affecting social preference [37], findings that contradict our results. This discrepancy may arise from our specific use of the SSCE stress model, which exhibits adaptive recovery over time, with behavioral phenotypic changes influenced by multiple factors. These include not only the mPFC-NAc neural circuit we focus on but also local microcircuits involving glutamatergic principal neurons and GABAergic interneurons within the mPFC [80], as well as long-range connections with other brain regions [81], all of which are functionally related to specific behaviors [82, 83]. Multi-faceted techniques such as optogenetics, in vivo electrophysiological recordings, and calcium imaging have revealed that activation of mPFC PV+ neurons can rescue social memory deficits induced by ventral hippocampus inhibition [84], while optogenetic activation of excitatory neurons in the BLA projecting to the mPFC can increase anxiety-like behavior and reduce social interactions [85, 86]. Importantly, our analysis is largely data-driven and exploratory, and interpretations here are inevitably influenced by our perspectives and the references consulted. An alternative explanation may also be valid.
To our knowledge, while significant progress has been made in understanding the effects of spaceflight on the brain through MRI and in vivo calcium imaging, the underlying genetic regulatory mechanisms remain largely unknown. Identifying patterns of gene expression is fundamental for measuring brain functional activity, and coupling functional activity with gene expression may reveal genes that exhibit significant changes during spaceflight [87,88,89]. The PFC is a key contributor to interpreting rs-fMRI gene associations, and our gene expression model yields promising results. After two weeks of SSCE exposure, approximately 2423 DEGs in the PFC show a strong correlation with fMRI parameters. After four weeks, 1481 DEGs are significantly correlated, predominantly in synaptic-related pathways and ion channel activity. Furthermore, one possible explanation is that differential modulation of synaptic function underlies emotional information processing in the mouse PFC [90, 91]. Additionally, pathways related to circadian rhythms, glutamatergic synapses, and calcium signaling point to broader neurobiological shifts that may influence mental health and long-term cellular integrity. Overall, our evidence reveals a close relationship between the intrinsic functional organization of the mouse PFC and the temporal and spatial differences in gene expression with increased SSCE exposure time. And these findings highlight both immediate and adaptive molecular responses to SSCE exposure, which may contribute to altered anxiety-like and social behaviors. Further research is needed to fully understand the long-term effects of such environmental stressors on brain function and behavior. Finally, any functional interpretations of the identified genes should be approached with caution, as our assessment of the relationship between gene expression and rs-fMRI in cortical regions is based on correlation, which does not imply causation. The above results comprehensively indicate that 2 weeks may represent an acute phase where initial neuroplastic responses to stressors like microgravity and isolation manifest, while 4 weeks likely reflects a chronic adaptation phase, where compensatory reorganization or persistent dysfunction becomes evident. In addition, our study only used male C57BL/6 J mice, and the results may not be applicable to females. This is a limitation that should be acknowledged.
In summary, we have demonstrated a multi-scale developmental pattern of brain networks from short-term to long-term exposure in a space-specific environment. Our study provides a precise dynamic characterization of the multiscale connectome in mice under these conditions, linking macro-level functions with mesoscopic neural activity and microscopic gene expression. We describe the correlated trajectories of structural and functional changes in the brain during spaceflight, investigating whether these heterogeneous changes relate to gene expression in cortical regions. Furthermore, we emphasize that the value of these model environments extends beyond practical understanding and prediction of spaceflight’s effects on psychological behavior. They offer profound insights for neuroscience, including theoretical perspectives on how emotional behavior and brain networks are affected in unique environments, and propose new strategies for interventions aimed at preserving and altering brain integrity throughout the flight.
Data availability
All analyzed data is included in the article and its supplementary information files. If anyone needs to access the original data, please contact the corresponding authors (bain@tju.edu.cn, jiajia.yang@tju.edu.cn, richardming@tju.edu.cn).
References
Amemiya S, Takao H, Hanaoka S, Ohtomo K. Global and structured waves of rs-fMRI signal identified as putative propagation of spontaneous neural activity. NeuroImage. 2016;133:331–40.
Yue J, Han SW, Liu X, Wang S, Zhao WW, Cai LN, et al. Functional brain activity in patients with amnestic mild cognitive impairment: an rs-fMRI study. Front Neurol. 2023;14:1244696.
Raimondo L, Oliveira LAF, Heij J, Priovoulos N, Kundu P, Leoni RF, et al. Advances in resting state fMRI acquisitions for functional connectomics. NeuroImage. 2021;243:118503.
Liao Y, Lei M, Huang H, Wang C, Duan J, Li H, et al. The time course of altered brain activity during 7-day simulated microgravity. Front Behav Neurosci. 2015;9:124.
Koppelmans V, Bloomberg JJ, Mulavara AP, Seidler RD. Brain structural plasticity with spaceflight. npj Microgravity. 2016;2:2.
Cassady K, Koppelmans V, Reuter-Lorenz P, Dios YD, Gadd N, Wood S, et al. Effects of a spaceflight analog environment on brain connectivity and behavior. NeuroImage. 2016;141:18–30.
Greicius MD, Krasnow B, Reiss A, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8.
Liégeois R, Li J, Kong R, Orban C, Van De Ville D, Ge T, et al. Resting brain dynamics at different timescales capture distinct aspects of human behavior. Nat Commun. 2019;10:2317.
Masamoto K, Kanno I. Anesthesia and the quantitative evaluation of neurovascular coupling. J Cereb Blood Flow Metab. 2012;32:1233–47.
Pan WJ, Billings JCW, Grooms JK, Shakil S, Keilholz SD. Considerations for resting state functional MRI and functional connectivity studies in rodents. Front Neurosci. 2015;9:269.
Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93.
Klune CB, Jin B, DeNardo LA. Linking mPFC circuit maturation to the developmental regulation of emotional memory and cognitive flexibility. eLife. 2021;10:e64567.
Papp M, Gruca P, Litwa E, Lason M, Willner P. Optogenetic stimulation of transmission from prelimbic cortex to nucleus accumbens core overcomes resistance to venlafaxine in an animal model of treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2023;123:110715.
Choi TY, Jeon H, Jeong S, Kim EJ, Kim J, Jeong YH, et al. Distinct prefrontal projection activity and transcriptional state conversely orchestrate social competition and hierarchy. Neuron. 2024;112:611–27.e8.
Happ DF, Wegener G, Tasker RA. Effect of ischemic lesions in medial prefrontal cortex and nucleus accumbens on affective behavior in rats. Behav Brain Res. 2020;378:112234.
Hawrylycz M, Miller JA, Menon V, Feng D, Dolbeare T, Guillozet-Bongaarts AL, et al. Canonical genetic signatures of the adult human brain. Nature Neuroscience. 2015;18:1832–44.
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–9.
Lein ES, Belgard TG, Hawrylycz M, Molnár Z. Transcriptomic perspectives on neocortical structure, development, evolution, and disease. Annu Rev Neurosci. 2017;40:629–52.
Arnatkevičiūtė A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–67.
Fornito A, Arnatkevičiūtė A, Fulcher BD. Bridging the gap between connectome and transcriptome. Trends Cogn Sci. 2019;23:34–50.
Patel Y, Shin J, Drakesmith M, Evans J, Pausova Z, Paus T. Virtual histology of multi-modal magnetic resonance imaging of cerebral cortex in young men. NeuroImage. 2020;218:116968.
Edwards LJ, McColgan P, Helbling S, Zarkali A, Vaculčiaková L, Pine KJ, et al. Quantitative MRI maps of human neocortex explored using cell type-specific gene expression analysis. Cereb Cortex. 2023;33:5704–16.
Liu F, Gong X, Yao X, Cui L, Yin Z, Li C, et al. Variation in the CACNB2 gene is associated with functional connectivity of the Hippocampus in bipolar disorder. BMC Psychiatry. 2019;19:62.
Hou Z, Liu X, Jiang W, Hou Z, Yin Y, Xie C, et al. Effect of NEUROG3 polymorphism rs144643855 on regional spontaneous brain activity in major depressive disorder. Behav Brain Res. 2021;409:113310.
Wang J, Braskie MN, Hafzalla GW, Faskowitz J, McMahon KL, Zubicaray GI, et al. Relationship of a common OXTR gene variant to brain structure and default mode network function in healthy humans. NeuroImage. 2017;147:500–6.
Liang R, Wang L, Yang Q, Xu Q, Sun SF, Zhou HC, et al. Time-course adaptive changes in hippocampal transcriptome and synaptic function induced by simulated microgravity associated with cognition. Front Cell Neurosci. 2023;17:1275771.
Xu Q, Liang R, Gao J, Fan YY, Dong JR, Wang L, et al. rTMS Ameliorates time-varying depression and social behaviors in stimulated space complex environment associated with VEGF signaling. Life Sci Space Res (Amst). 2024;42:17–26.
Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985). 2002;92:1367–77.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76.
Grange, P, Bohland, JW, Hawrylycz, M, Mitra, PP Brain Gene Expression Analysis: a MATLAB toolbox for the analysis of brain-wide gene-expression data. Neurons and Cognition (2017).
Liang R, Wang L, Li XY, Liu XH, Chang HN, Xu Q, et al. Repetitive transcranial magnetic stimulation rescues simulated space complex environment-induced emotional and social impairments by enhancing neuronal excitability in the medial prefrontal cortex. Cereb Cortex. 2023;33:7148–62.
Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci USA. 2016;113:12574–9.
Haak KV, Marquand AF, Beckmann CF. Connectopic mapping with resting-state fMRI. NeuroImage. 2018;170:83–94.
Krittanawong C, Singh NK, Scheuring RA, Urquieta E, Bershad EM, Macaulay TR, et al. Human health during space travel: state-of-the-art review. Cells. 2022;12:40.
Gushin VI, Kholin SF, Ivanovsky YR. Chapter 2 Soviet psychophysiological investigations of simulated isolation: some results and prospects. Adv Space Biol Med. 1993;3:5–14.
Murugan M, Jang HJ, Park M, Miller EM, Cox J, Taliaferro JP, et al. Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell. 2017;171:1663–77.
Park G, Ryu C, Kim S, Jeong SJ, Koo JW, Lee YS, et al. Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice. Cell Rep. 2021;35:109104.
Shao Z, Xu Y, Chen L, Wang S, Zhang M, Liu S, et al. Dysfunction of the NAc-mPFC circuit in insomnia disorder. Neuroimage Clin. 2020;28:102474.
Evans ME, Graham LD. A flexible lunar architecture for exploration (FLARE) supporting NASA’s artemis program. Acta Astronaut. 2020;177:351–72.
Shearer CK, McCubbin FM, Eckley S, Simon SB, Meshik A, McDonald F, et al. Apollo Next Generation Sample Analysis (ANGSA): an Apollo Participating Scientist Program to Prepare the Lunar Sample Community for Artemis. Space Sci Rev. 2024;220:62.
De Pascale S, Arena C, Aronne G, Micco VD, Pannico A, Paradiso R, et al. Biology and crop production in Space environments: challenges and opportunities. Life Sci Space Res (Amst). 2021;29:30–37.
Marshall-Goebel K, Damani R, Bershad EM. Brain physiological response and adaptation during spaceflight. Neurosurgery. 2019;85:E815–21.
Mann V, Sundaresan A, Mehta SK, Crucian B, Doursout MF, Devakottai S. Effects of microgravity and other space stressors in immunosuppression and viral reactivation with potential nervous system involvement. Neurol India. 2019;67:S198–S203.
Hockey GR. Compensatory control in the regulation of human performance under stress and high workload; a cognitive-energetical framework. Biol Psychol. 1997;45:73–93.
Styf JR, Hutchinson K, Carlsson SG, Hargens AR. Depression, mood state, and back pain during microgravity simulated by bed rest. Psychosom Med. 2001;63:862–4.
Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19:1523–36.
Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7.
van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–34.
Hillary FG, Grafman JH. Injured brains and adaptive networks: the benefits and costs of hyperconnectivity. Trends Cogn Sci. 2017;21:385–401.
Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19:701–10.
Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2022;1251:E1–24.
Adolphs R. The social brain: neural basis of social knowledge. Ann Rev of Psycholog. 2009;60:693–716.
Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22.
Demertzi A, Van Ombergen A, Tomilovskaya E, Jeurissen B, Pechenkova E, Perri CD, et al. Cortical reorganization in an astronaut’s brain after long-duration spaceflight. Brain Struct Funct. 2016;221:2873–6.
Van Ombergen A, Laureys S, Sunaert S, Tomilovskaya E, Parizel PM, Wuyts FL. Spaceflight-induced neuroplasticity in humans as measured by MRI: what do we know so far? npj Microgravity. 2017;3:2.
Van Ombergen A, Wuyts FL, Jeurissen B, Sijbers J, Vanhevel F, Jillings S, et al. Intrinsic functional connectivity reduces after first-time exposure to short-term gravitational alterations induced by parabolic flight. Sci Rep. 2017;7:3061.
Sirmpilatze N, Baudewig J, Boretius S. Temporal stability of fMRI in medetomidine-anesthetized rats. Sci Rep. 2019;9:16673.
Paasonen J, Stenroos P, Salo RA, Kiviniemi V, Gröhn O. Functional connectivity under six anesthesia protocols and the awake condition in rat brain. NeuroImage. 2018;172:9–20.
Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–31.
Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527:179–85.
Hermans EJ, Henckens MAJ, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–14.
McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29.
Tye KM. Neural circuit motifs in valence processing. Neuron. 2018;100:436–52.
Dang TP, Mattan BD, Kubota JT, Cloutier J. The ventromedial prefrontal cortex is particularly responsive to social evaluations requiring the use of person-knowledge. Sci Rep. 2019;9:5054.
Fukuda H, Ma N, Suzuki S, Harasawa N, Ueno K, Gardner JL, et al. Computing social value conversion in the human brain. J Neurosci. 2019;39:5153–72.
Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370:1209–19.
Pagani M, Bertero A, Liska A, Galbusera A, Sabbioni M, Barsotti N, et al. Deletion of autism risk gene Shank3 disrupts prefrontal connectivity. J Neurosci. 2019;39:5299–310.
Qin L, Ma K, Yan K. Chemogenetic activation of prefrontal cortex in Shank3-Deficient mice ameliorates social deficits, NMDAR hypofunction, and Sgk2 downregulation. iScience. 2019;17:24–35.
Harony-Nicolas H, Kay M, Hoffmann J, Klein ME, Bozdagi-Gunal O, Riad M, et al. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. elife. 2017;6:e18904.
Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–43.
Belleau EL, Treadway MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. 2019;85:443–53.
Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, et al. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–3.
Beckman D, Ott S, Donis-Cox K, Janssen WG, Bliss-Moreau E, Rudebeck PH, et al. Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc Natl Acad Sci USA. 2019;116:26239–46.
Yang H, W de Jong J, Tak Y, Peck J, Bateup HS, Lammel S. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron. 2018;97:434–49.e4.
Chen G, Lai S, Bao G, Ke J, Meng X, Lu S, et al. Distinct reward processing by subregions of the nucleus accumbens. Cell Rep. 2023;42:112069.
Castro DC, Bruchas MR. A motivational and neuropeptidergic hub: anatomical and functional diversity within the nucleus accumbens shell. Neuron. 2019;102:529–52.
Wu XB, Zhu Q, Gao MH, Yan SX, Gu PY, Zhang PF, et al. Excitatory projections from the prefrontal cortex to nucleus accumbens core D1-MSNs and κ opioid receptor modulate itch-related scratching behaviors. J Neurosci. 2023;43:1334–47.
McGrath AG, Briand LA. Post-weaning social isolation causes sex-specific alterations to dendritic spine density in subregions of the prefrontal cortex and nucleus accumbens of adult mice. Brain Res. 2022;1777:147755.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8.
Gamo NJ, Lur G, Higley MJ, Wang M, Paspalas CD, Vijayraghavan S, et al. Stress impairs prefrontal cortical function via D1 dopamine receptor interactions with hyperpolarization-activated cyclic nucleotide-gated channels. Biol Psychiatry. 2015;78:860–70.
Arnsten AFT. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–99.
Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68.
Stokes MG. ‘Activity-silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci. 2015;19:394–405.
Sun Q, Li X, Li A, Zhang J, Ding Z, Gong H, et al. Ventral hippocampal-prefrontal interaction affects social behavior via parvalbumin positive neurons in the medial prefrontal cortex. iScience. 2020;23:100894.
Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209.
Sun T, Song Z, Tian Y, Tian W, Zhu C, Ji G, et al. Basolateral amygdala input to the medial prefrontal cortex controls obsessive-compulsive disorder-like checking behavior. Proc Natl Acad Sci USA. 2019;116:3799–804.
Konopka G. Cognitive genomics: Linking genes to behavior in the human brain. Netw Neurosci. 2017;1:3–13.
Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, et al. BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:1241–4.
Wang GZ, Belgard TG, Mao D, Chen L, Berto S, Preuss TM, et al. Correspondence between resting-state activity and brain gene expression. Neuron. 2015;88:659–66.
Yan Z, Rein B. Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: pathophysiological implications. Mol Psychiatry. 2022;27:445–65.
Yamada D, Takahashi J, Iio K, Nagase H, Saitoh A. Modulation of glutamatergic synaptic transmission and neuronal excitability in the prelimbic medial prefrontal cortex via delta-opioid receptors in mice. Biochem Biophys Res Commun. 2021;560:192–8.
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (2023YFF1204004), Tianjin Natural Science Foundation (24JCQNJC01080), National Natural Science Foundation of China (81925020, 82371886, 82202797, 82202249), and the Lingang Laboratory (LG-TKN-202204-01).
Author information
Authors and Affiliations
Contributions
L.R. and F.T.Y. conceived the study, designed the experiment, analyzed and interpreted the data, and wrote the paper. The above two authors contributed equally to this work. W. L., R.J, M.L.H. and Z.M.L designed experiments. Z.C.G. and F.Q.Y provided research ideas and gave suggestions during the experiments. C.Y.Y., Y.J.J. and M.D. supervised the whole work, and participated in the revision of the original draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments on animal were conducted in accordance with the Declaration of Helsinkis. All animal experiments comply with the ARRIVE guidelines. All animal procedures involving animals were conducted in accordance with the approved protocol for the care and use of laboratory animals the Animal Care and Use Committee of Tianjin University (Animal license number: TJUE: 2023-105). All the authors compliance with all relevant ethical regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, R., Fang, T., Wang, L. et al. Multi-connectomics underpin emotional dysfunction in mouse exposed to simulated space composite environment. Transl Psychiatry 15, 359 (2025). https://doi.org/10.1038/s41398-025-03538-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03538-6