Abstract
Introduction This study aimed to assess the efficacy and safety of remimazolam for dental intravenous conscious sedation. It focused on evaluating sedation success rate, management of pre-operative anxiety, sedation depth, psychomotor recovery and variations in vital parameters.
Materials and methods This single-centre, prospective cohort study involved 101 patients undergoing dental procedures. Sedation was administered according to the European Medicines Agency guidelines, and outcomes were assessed by: sedation success rates; anxiety reduction, as measured by the Visual Analogue Scale for Anxiety (VAS-A); depth of sedation, as assessed by the Ramsay Sedation Scale; psychomotor recovery, as assessed by the Newman test; and physiologic stability, defined as meeting Post-Anaesthetic Discharge Scoring System criteria.
Results Remimazolam achieved an 100% procedural success rate without rescue sedatives. Pre-operative anxiety (mean MDAS: 16.87 ± 4.80; mean VAS-A: 7.07) was effectively managed, with 69% of patients reporting high or very high anxiolytic effects. Vital parameters remained stable, with variations consistently <20% from baseline values. Psychomotor recovery was rapid, with a mean recovery time of 49 minutes, and most patients were discharged within 60-90 minutes. Most patients (65%) experienced anterograde amnesia, while 74% described their preferred experience during the procedure as ‘sleep' and 26% as ‘calmness'. Minimal adverse effects were reported, and patient satisfaction scores were high (mean VAS satisfaction: 9.73).
Conclusions Remimazolam demonstrated excellent efficacy, safety and patient satisfaction in dental procedural sedation. Its rapid recovery profile, minimal cardiovascular and respiratory impact, and effective anxiolytic properties establish it as a reliable alternative to midazolam, particularly for moderate- to high-risk patients. These findings support the integration of remimazolam into outpatient dental care, with the potential for broader application in paediatric and disabled populations.
Key points
-
Remimazolam demonstrated a 100% success rate in procedural sedation for dental treatments, with minimal adverse effects, making it a reliable sedative choice for outpatient settings.
-
Remimazolam maintained stable cardiovascular and respiratory parameters, even in patients with higher ASA classifications, reducing the need for anaesthesiologist intervention.
-
Patients experienced a mean recovery time of 49 minutes, allowing for discharge within 60-90 minutes, which supports remimazolam's suitability for efficient outpatient care.
Similar content being viewed by others
Introduction
Anaesthesia procedures are essential for dental treatments. However, patients with high anxiety, dental phobia, needle phobia, resistance to local anaesthetics and cognitive disabilities may not be suitable candidates for local, conventional1 or computerised anaesthesia.2 In these cases, sedation techniques are necessary.
Midazolam, a commonly used benzodiazepine, is frequently employed for procedural sedation in dental practice. Although it effectively manages anxiety and discomfort during dental procedures, it has several drawbacks and risks.3 One key limitation is its tendency to accumulate in the body, especially in patients with renal or hepatic failure, or when given in repeated doses. This can lead to prolonged sedation and extended drug effects, potentially affecting post-procedure activities requiring attention and coordination.4
Midazolam may induce respiratory depression, especially at high doses or when combined with other central nervous system depressants.5 This risk is heightened in patients with pre-existing respiratory conditions or in the older population, who may already have compromised pulmonary function and take other medications that potentiate these effects, such as opioids or psychotropic drugs, thereby increasing the risk of complications.6 Additionally, midazolam interacts with the cytochrome P450 enzyme system, potentially affecting the metabolism of other drugs and leading to altered efficacy or safety profiles.7
In March 2023, the European Medicines Agency (EMA) approved remimazolam besylate (Byfavo 20 mg) for procedural sedation.8 Remimazolam offers pharmacokinetic and pharmacodynamic advantages over midazolam, including a shorter half-life and faster onset, enabling more precise sedation control and quicker cognitive recovery.9 It accumulates less in the body, reducing risks of prolonged sedation and respiratory impairment.10,11 Remimazolam's predictable and adjustable sedative effect also allows for tailored sedation levels, minimising the chances of over- or under-sedation.12,13
Remimazolam has shown a favourable safety profile in clinical trials, with minimal severe side effects. However, studies in dentistry remain limited, primarily conducted in China,4,9,14,15 Korea,16 Japan17 and the United States.18 Further research is needed to explore its benefits for anxious or odontophobic patients, individuals with cognitive disabilities, those with a strong gag reflex, patients needing stress reduction, and for lengthy or invasive dental procedures.
This prospective cohort study aimed to assess the success rate of intravenous conscious sedation in dental treatments using remimazolam besylate. It evaluated pre-operative anxiety, sedation depth, psychomotor and physiological recovery, and variation in vital parameters. The study also assessed anaesthesiologist intervention rates, amnesia prevalence, adverse effects and subjective anxiolytic outcomes. To the best of our knowledge, this represents the first European clinical study on remimazolam besylate for intravenous conscious sedation in dentistry.
Materials and methods
Design
This single-centre, non-profit, prospective cohort pharmacological observational study was approved by the local ethics committee (Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Territorial Ethics Committee Lombardy 3) (Trial ID 4996) and adhered to the principles of the Helsinki Declaration (June 1964, amended by the 75th World Medical Association General Assembly held in Helsinki, Finland, from October 16-19, 2024). The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines19 for cohort studies to ensure comprehensive and transparent reporting (online Supplementary Information Table 1). All clinicians administering intravenous conscious sedation were trained and experienced in remimazolam sedation according to the 2023 Intercollegiate Advisory Committee for Sedation in Dentistry (IACSD).20
Participants and setting
A total of 101 patients presenting at the Conscious Dental Sedation Service of the Maxillofacial Surgery and Dental Unit (Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan) were recruited. The prospective cohort included 101 patients who received remimazolam for procedural sedation between February 2024 and October 2024. The prospective design enabled a broader patient assessment under real-life conditions, yielding more generalisable clinical practice data. Inclusion criteria included adults aged ≥18 years, patients needing sedation for minimal co-operation (anxious or odontophobic individuals with Modified Dental Anxiety Scale [MDAS] ≥14 and/or Visual Analogue Scale for Anxiety [VAS-A] ≥5, cognitive disabilities, or gag reflex grade ≥3), frail patients or those with comorbidities (e.g., older person, or with heart disease), and those undergoing invasive or prolonged surgery. Written informed consent was obtained from all participants. Exclusion criteria included contraindications to benzodiazepine sedation for prospective patients and pregnant and breastfeeding individuals. Patient selection and sedation administration followed 2020 IACSD standards for conscious sedation.21 Continuous patient monitoring included pulse oximetry, non-invasive blood pressure measurement and capnography.
Administration of remimazolam besylate for dental procedural sedation
A trained nurse prepared remimazolam besylate (Byfavo 20 mg) by reconstituting it with sodium chloride, yielding a 2.5 mg/mL solution. Based on EMA guidelines,22 an initial intravenous dose of 7 mg was given over one minute for patients <65 years, weighing ≥50 kg, and classified as American Society of Anaesthesiologists (ASA) <III (first group). For patients aged ≥65 years and/or with body weight <50 kg and ASA ≥III, a dose of 2.5-5 mg was administered over one minute (second group). Additional maintenance doses of 2.5 mg over 15 seconds were administered for the first group of patients, while for the second group, maintenance doses of 1.25-2.5 mg over 15 seconds were given as needed, with at least two minutes between doses. Maximum cumulative doses were 33 mg for the first group of patients and 17.5 mg for the second group. If the sedation target was not met after five doses within 15 minutes, sedation failure was declared, and an alternative treatment was considered. Experienced professionals administered remimazolam and monitoring was done by additional staff trained in airway and resuscitation management. Continuous cardiac and respiratory function monitoring, including capnography, was conducted, with emergency equipment, including flumazenil, readily available.
The administration of remimazolam in this study adhered strictly to the 2023 IACSD statement on remimazolam, which defines the legal and practical framework for its use in dental sedation in the UK.20 The sedation technique was classified as an operator-sedation technique, following the same clinical standards and training required for midazolam. In compliance with IACSD guidance, trained sedationists performed the administration of remimazolam pharmacology, dosing and clinical indications.
Outcome variables
The primary outcome was the procedural success rate without rescue sedatives. Secondary outcomes included: pre-operative anxiety (assessed by MDAS, VAS-A)23,24(see online Supplementary Information Table 2); tranquility before, during, and after sedation (Visual Analogue Scale for Tranquility, [VAS-T]25 - target ≥8 intra- and post-operatively); sedation depth (Ramsay sedation scale;26 see online Supplementary Information Table 3); psychomotor recovery (Newman test);27 physiological recovery (Post Anaesthetic Discharge Scoring System [PADSS] score;28 see online Supplementary Information Table 4); vital parameters (systolic blood pressure [SBP], diastolic blood pressure [DBP], mean arterial pressure [MAP], heart rate [HR], respiratory rate [RR], oxygen saturation [SpO2], end-tidal carbon dioxide [EtCO2]); anaesthesiologist intervention rate; prevalence of amnesia; adverse effects; subjective anxiolytic effect; and pain intensity during the procedure. Discharge criteria were aligned with 2020 IACSD standards.21 Patients were only discharged once they had achieved stable vital signs, full psychomotor recovery and had a responsible adult caregiver. Written and verbal post-sedation care instructions were provided, including guidance on avoiding alcohol, operating machinery, or making important decisions for the next 24 hours. In cases where full recovery was delayed beyond two hours, flumazenil administration was considered, as recommended by IACSD.21
Interventions
At the initial visit (T-1), patient selection included recording demographics, medical history, ASA classification, MDAS, VAS-A (except for cognitively disabled patients), and vital signs (SBP, DBP, MAP, HR, SpO2). Patients were instructed to adhere to the 2-4-6-8 fasting rule before the procedure.29 Follow-up calls or visits were arranged for post-operative assessment. On the day of the procedure (T0), patients confirmed their medical history and fasting compliance. Monitoring equipment was set up, and the Newman test (pre-operative score) (Fig. 1) was completed, except for cognitively disabled patients. In cooperating patients, a plexus or mandibular block was administered before sedation to confirm its efficacy and optimise the dosage of remimazolam, given its short half-life. For non-cooperative patients, the block was performed after achieving target sedation scores (VAS-T 8/10, Ramsay 2/6 or 3/6). Vital signs were measured after each remimazolam administration. After the procedure (T1), patients were monitored until a Ramsay score of 2 was reached. If full recovery was delayed beyond two hours, flumazenil was considered. Recorded data included sedation success rate, procedure time, total remimazolam dose, post-operative VAS-T, recovery time, and vital signs at discharge. Comfort and satisfaction levels were assessed verbally (VAS 1-10). Discharge required a PADSS score of 9-10 and acceptable Newman test results. On the following day (T2), patients or caregivers were interviewed to assess amnesic effects, clinical effects, subjective anxiolysis and pain (see online Supplementary Information Table 5). The specific operating protocol is shown in Table 1.
Sample size
To calculate the sample size based on the primary endpoint of ‘assessing the success rate of procedural sedation using remimazolam besylate', the following formula was used:

Where ‘Z' is the test statistic for a chosen confidence level, ‘P' is the expected proportion, and ‘d' is the margin of error or precision.
Considering that 95%14 of subjects in the population completed the dental procedure without the use of rescue sedatives, 91 patients were needed to estimate the expected proportion, with a margin of error of 4.5% and a confidence level of 95%. Assuming an expected response rate of 90%, the study required a sample size of 101 patients.
Statistical methods
Descriptive statistics were used for all outcomes. Continuous variables were presented as means and standard deviations or as medians and interquartile ranges, depending on their distribution. Binary or categorical variables were described with counts and percentages. The primary outcome success rate was calculated by dividing the number of patients who completed the procedure without rescue sedatives by the total number of enrolled patients and multiplying by 100. For secondary outcomes involving categorical or binary data, proportions were calculated similarly.
For comparisons of continuous variables across different phases, the Kruskal-Wallis test was used. In the case of significant differences, Dunn's post-hoc test was applied for pairwise comparisons. To analyse the time from the first dose of remimazolam to patient discharge, the Kaplan-Meier estimator was applied and the survival curve was displayed. Statistical significance was set at p <0.05 for all tests and all analyses were conducted using R statistical software (v4.1.2; R Foundation for Statistical Computing, Vienna, Austria).
The study followed 2020 IACSD recommendations21 for rigorous record-keeping, including documentation of sedation dosages, patient responses and any adverse events. Continuous audit of outcomes was conducted to ensure compliance with sedation practice standards in the United Kingdom.
Results
Demographic and clinical characteristics
The study included 101 patients, with a sex distribution of 42% men and 58% women, and a mean age of 43.15 ± 19.26 years (range: 18-86; median: 37). Most participants were born in Europe (82% from Italy), with smaller groups from Bulgaria, Ukraine, Germany, Hungary, Egypt, El Salvador and Bangladesh. Average weight and height were 70.27 ± 17.10 kg and 167.44 ± 9.78 cm, resulting in a mean body mass index (BMI) of 27.22 ± 4.66, indicating a generally overweight population per World Health Organization criteria. Tobacco use was reported by 9% of participants, with ASA classifications showing 4% as ASA 1, 56% as ASA 2, and 40% as ASA 3. MDAS had a mean score of 16.87 ± 4.80, while the VAS-A indicated high general anxiety levels with a mean of 7.07 ± 2.25 (Table 2).
Patient categorisation and scheduled dental procedures
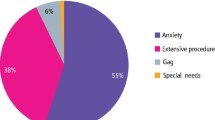
Categorisation indicated that 19% of patients were anxious, 46% odontophobic and 25% had cognitive disabilities. A low perioperative risk was observed in 34% of patients, but 40% underwent invasive or prolonged procedures, with extractions being the most common. More specific breakdowns of procedures and complexities are available in Table 3.
Prevalence of medical conditions in the study population
The study group presented a range of medical conditions, with hypertension being the most common (31%), followed by Class 1 obesity (12%). Neurological and psychiatric conditions included epilepsy (19%) and cognitive disabilities (23%). Additionally, asthma was the most frequent respiratory disease (7%), while other conditions included allergies (6%) and gastrointestinal disorders. A detailed summary is in online Supplementary Information Table 6.
Medication in the study population
Commonly prescribed medications were anticonvulsants (22%), proton pump inhibitors (20%) and benzodiazepines (15%), with other notable drugs including antihypertensives and antidepressants. Medications with lower prevalence included anticoagulants and bronchodilators (see online Supplementary Information Table 7).
Vital signs during different phases of the procedure
Distinctive patterns were observed in vital signs across phases, with notable changes in SBP and DBP, MAP, HR, RR, SpO2, and EtCO2. Kruskal-Wallis tests were conducted to assess significant differences across phases, yielding significant findings for DBP (p <0.0001), MAP (p = 0.0209), HR (p <0.0001) and SpO2 (p <0.0001), while SBP (p = 0.0693), RR (p = 0.0537) and EtCO2 (p = 0.381) were not statistically significant. Detailed results for each phase, including observed trends and values, are summarised in Table 4. Dunn's post-hoc test revealed specific differences, such as a decrease in DBP from the ‘recruitment phase' to ‘maintenance phases I to IV' and the ‘end of procedure', with p-values between 0.0198 and 0.0033. MAP significantly dropped between the ‘pre-operative phase' and ‘maintenance II' (p = 0.0229), while HR showed marked declines, particularly from ‘recruitment' to ‘maintenance III' (p = 0.0235) and ‘pre-operative' to ‘maintenance III' (p = 0.0116). SpO2 also showed specific phase differences, particularly between ‘recruitment' and certain ‘maintenance' phases (Table 5).
Remimazolam dosage and sedation monitoring across procedural phases
Doses of remimazolam varied, typically 2.5 mg or 7 mg in induction phases and 2.5 mg for maintenance. Sedation levels were monitored through VAS-T, showing an increase during induction with stable values in maintenance. The Ramsay sedation scores remained predominantly at 2, indicating moderate sedation, while PADSS scores suggested readiness for discharge by the procedure's end, with details in Table 6. Kruskal-Wallis tests showed significant VAS-T differences across phases (p <0.0001), and Dunn's post-hoc analysis identified significant differences between the pre-operative, induction and end phases (Table 7).
Procedure outcomes and sedation metrics
All procedures were completed successfully without rescue sedatives. The average total remimazolam dose was 15.52 ± 6.21 mg. The procedure duration averaged 26.17 ± 16.90 minutes, with a mean discharge time of 86.04 ± 22.38 minutes. Patient satisfaction was high, with VAS Comfort and VAS Satisfaction scores of 9.69 ± 1.12 and 9.73 ± 1.09, respectively (Table 8).
Psychomotor recovery was evaluated using the Newman test, showing significant improvement (pre-operative score: 6.62 ± 6.16 versus post-operative score: 3.52 ± 4.07; p <0.0001 via Kruskal-Wallis test). Dunn's post-hoc test further supported significant differences between pre- and post-sedation Newman scores (Table 9).
Discharge timeline and recovery pattern following remimazolam sedation in dental procedures
The discharge timeline illustrates recovery efficiency after sedation with remimazolam in dental procedures. All 101 patients remained sedated initially, with the first discharge occurring at 30 minutes. Discharges gradually increased, peaking between 60 and 90 minutes, when the number of sedated patients dropped significantly from 80 to 27. After 90 minutes, discharges slowed, with the last patient discharged at 140 minutes, completing recovery in 150 minutes. The modified Kaplan-Meier curve (Fig. 2) highlights this peak discharge period, providing a detailed view of patient recovery times.
Patient experience and adverse effects of remimazolam sedation
A low percentage of patients (35%) recalled the final part of the procedure, while most (65%) reported complete anterograde amnesia and being asleep the entire time. Most patients preferred a sleep-inducing experience during the procedure (74%), while 26% preferred to feel calm. Patient comfort was high, with 69% reporting ‘high' anxiolytic effects and minimal adverse effects, including occasional headaches and mild syncope, demonstrating remimazolam's favourable safety profile. The subjective assessment of sedation was highly favourable, with 69% rating the anxiolytic effect as ‘high' and 15% as ‘very high'. These findings are summarised in Table 10.
Discussion
This observational study comprehensively examined patient demographics, clinical characteristics, procedural outcomes and physiological responses in a population undergoing dental procedures with remimazolam sedation. The findings highlight remimazolam's efficacy, safety and patient experience, underscoring its potential advantages in procedural sedation, especially within dental practice. Notably, to the best of our knowledge, this research represents the first investigation of remimazolam besylate for dental sedation in Europe. This lack of European data underscores the novelty and clinical significance of our findings, providing a foundational evidence base for the integration of remimazolam in dental sedation practices.
Our study cohort consisted of 101 patients, predominantly of European origin, with a mean age of 43.15 years and a mean BMI of 27.22, classifying them as overweight. This demographic reflects a typical clinical population requiring sedation, often presenting with comorbidities that complicate sedation. In our cohort, 56% were classified as ASA 2 and 40% as ASA 3, indicating a moderate-risk group. This distribution is consistent with findings by Cowell et al.30 and supported by Sun et al.,15 who also noted similar ASA distributions in sedated patient populations. Furthermore, we agree with Kim et al.16 that remimazolam's short action profile, lack of accumulation and minimal cardiovascular impact make it a highly suitable option for these higher-risk patients.16 Finally, our study primarily involved a white population, with 89% of participants being of European ethnicity, whereas most of the available literature evaluates the use of remimazolam in patients of Asian ethnicity.31 This distinction provides valuable insights into the efficacy and safety of remimazolam in a different ethnic group, broadening the understanding of its clinical application across diverse populations.
The primary outcome of this study was the procedural success rate with remimazolam besylate (Byfavo 20 mg), defined as successful procedure completion without the need for rescue sedatives. We achieved a 100% procedural success rate, with all patients reaching adequate sedation levels and no need for alternative sedative interventions. This aligns with findings from Guo et al.14 and Sun et al.,15 which documented high success rates with remimazolam in dental and oral surgery procedures. In contrast, studies on midazolam, such as those by Cowell et al.,30 report lower success rates due to higher rates of adverse events or suboptimal sedation in patients with significant comorbidities, highlighting remimazolam's reliability in complex cases. Additionally, Swart et al.,18 in a pilot study on office-based dental sedation, also reported a high procedural success rate with remimazolam, though with some minor adverse events, which are manageable in a clinical setting. This further supports remimazolam's suitability for dental sedation, where its efficacy in various settings is validated.
Our study confirmed the high efficacy and safety of remimazolam in moderate- to high-risk dental patients (ASA II and III), demonstrating its predictable recovery profile with a mean recovery time of 49 minutes. These findings align with Stöhr et al.,32 who reported remimazolam's stable pharmacokinetic profile even in patients with hepatic or renal impairment. Although severe hepatic impairment increased exposure by 38.1%, recovery time was only modestly prolonged (16.7 minutes versus 8 minutes in healthy individuals). Our results similarly showed no significant delay in recovery or adverse pharmacokinetic effects, further supporting remimazolam's reliability across diverse patient profiles.
Pre-operative anxiety was measured using the MDAS and the VAS-A. The cohort had a mean MDAS score of 16.87 and a mean VAS-A score of 7.07, reflecting high general anxiety. With 46% of patients classified as odontophobic and 11% with needle phobia, effective anxiolytic control was essential. Studies on midazolam, such as those by Lawler et al.,33 highlight challenges in managing high dental anxiety, often requiring higher doses, which can increase adverse effects. Remimazolam, with comparable anxiolytic efficacy to midazolam but a superior safety profile, according to Li et al.,9 presents a safer alternative for highly anxious or respiratory-sensitive patients. Swart et al.18 observed high patient satisfaction and a reduction in pre-operative anxiety with remimazolam, corroborating its effectiveness in managing dental anxiety - a critical factor in successful sedation outcomes.
Tranquility levels were assessed pre-, intra- and post-sedation using the VAS-T, targeting pre-sedation scores of <8 and intra- and post-sedation scores of ≥8. Remimazolam consistently maintained high tranquility levels throughout the procedure, which is crucial for patient cooperation in dental settings. These findings align with results by Guo et al.14 and Sun et al.15 Compared to traditional agents like midazolam, remimazolam offers stable anxiolytic effects without prolonged sedation.17,34
Sedation depth was evaluated using the Ramsay Sedation Scale, with optimal scores of 2/6 or 3/6. Most patients achieved a score of 2, indicating moderate sedation suitable for dental procedures. These findings are consistent with the literature on remimazolam's efficacy in achieving sedation levels appropriate for conscious sedation in dentistry,35 as outlined by IACSD guidelines.21 Unlike midazolam, which sometimes necessitates additional medications for enhanced anxiolysis, remimazolam has demonstrated predictable pharmacokinetics while maintaining conscious sedation parameters.
Psychomotor recovery was assessed using the Newman test,27 comparing the number of points omitted before and after sedation. The mean recovery time was 49 minutes, underscoring remimazolam's suitability for outpatient settings. Chen et al.36 observed similar findings, noting faster recovery with remimazolam compared to midazolam, enhancing efficiency in busy clinical environments. Oue et al.17 also documented rapid recovery times with remimazolam, reinforcing its value for outpatient dental procedures where quick discharge is essential.
Physiological recovery was measured with the PADSS, with scores of 9 or 10 indicating readiness for discharge. Most patients were discharged within 60-90 minutes, reinforcing remimazolam's effectiveness for rapid recovery, as supported by studies from Chen et al.36 and Ito et al.37
Key vital parameters, including SBP, DBP, MAP, HR, RR, SpO2, and EtCO2, remained clinically stable throughout procedures, despite statistically significant variations, with changes consistently <20% from pre-operative values, as outlined in the PADSS criteria. SBP and DBP stabilised after initial fluctuations, consistent with studies reporting cardiovascular stability with remimazolam.14 MAP and HR increased during induction but quickly stabilised, contrasting with midazolam, which often has prolonged cardiovascular effects. RR, SpO2 and EtCO2 remained stable, supporting remimazolam's advantage in minimising respiratory depression.38 Swart et al.18 also observed stability in cardiovascular parameters with remimazolam, further supporting its safe use in outpatient dental sedation.
Anaesthesiologist intervention was not needed, demonstrating remimazolam's reliability in maintaining stable sedation without the need for additional support, especially compared to midazolam.30 This is advantageous in outpatient dental practices, where stable sedation with minimal oversight is desirable.
Anterograde amnesia was observed in 65% of patients, who were unable to recall the time when the dental procedure ended, highlighting the ability of remimazolam to effectively induce amnesia while minimising post-sedation recollection. This balance is particularly beneficial for reducing procedural anxiety, as reflected by the 74% of patients who preferred to sleep during the procedure. These findings are consistent with the observations of Dahiya et al.,39 further highlighting remimazolam's suitability for outpatient dental sedation.
Adverse effects were minimal, with only minor headache and vomiting cases reported, consistent with findings from Kim et al.,16 who observed fewer post-operative nausea and vomiting events with remimazolam compared to propofol. Our results reinforce remimazolam's favourable safety profile, particularly in outpatient settings where quick recovery and minimal post-sedation symptoms are essential.
Subjective anxiolytic effects were rated highly by most patients, supporting remimazolam's effectiveness in managing pre-operative anxiety and ensuring a comfortable experience. These outcomes align with findings by Guo et al.,14 highlighting remimazolam's stable anxiolytic profile, which benefits procedural comfort and cooperation in dental settings.
Most patients rated intra-operative pain as low to moderate, suggesting that remimazolam provides adequate analgesic support for dental procedures. This aligns with Sun et al.,15 who reported that remimazolam maintains patient comfort without needing additional analgesics, supporting its use in procedures involving moderate discomfort.
The study highlights key limitations and future directions in the use of remimazolam for procedural sedation, particularly in dental practice. One significant limitation noted is the subjective nature of the Ramsay Sedation Scale, which could lead to variability in the assessment of sedation depth. Future studies could benefit from using objective measures like the Bispectral Index to improve sedation assessment accuracy. This study did not account for weight-related dosing variations, which could impact remimazolam distribution and clearance. While we found no significant correlation between weight and Ramsay scores, larger studies could explore this relationship further. Future research should also consider randomised controlled trials for a more robust comparison with other sedatives. In addition, we will plan to conduct a study to refine the current EMA-recommended dosing protocol. Specifically, in the context of conscious sedation in dentistry - where anxiolysis and careful titration to achieve the minimum effective concentration of the drug are priorities - we intend to demonstrate that the induction dose of remimazolam should start with lower doses (e.g., 2.5-5.0 mg) rather than the 7 mg currently recommended for patients younger than 65 years, weighing ≥50 kg and classified as ASA <III. This approach aligns more closely with the US Food and Drug Administration (FDA)-recommended dosing guidelines and may reduce unnecessary drug exposure and adverse effects. However, we do not agree with the FDA's limitation of remimazolam use to procedures lasting a maximum of 30 minutes, as our study completed interventions lasting up to 104 minutes with excellent safety and efficacy outcomes. Additionally, further studies will investigate the application of remimazolam in paediatric populations, as well as in individuals with disabilities. For the latter group, the approval and testing of intranasal administration of remimazolam will be pursued to provide a more effective and less invasive approach for non-cooperative patients. Finally, a dedicated infusion pump tailored for remimazolam delivery will be developed to enhance the precision and adaptability of its use in longer and more complex procedures.
Conclusion
This prospective observational study provides important insights into the use of remimazolam for procedural sedation in dental practice, particularly within a European cohort, where its application is still relatively novel. The findings demonstrate remimazolam's strong efficacy, safety and positive impact on patient experience, highlighted by a 100% procedural success rate and minimal adverse events. The sedative's ability to maintain cardiovascular stability and minimise respiratory depression makes it a suitable choice for patients with complex health profiles, particularly those classified as ASA II and III. Remimazolam consistently achieved sedation and tranquility levels that supported procedural success, while enabling rapid recovery and discharge. These advantages, including enhanced patient comfort, cooperation and quick recovery, underscore remimazolam as a preferable option in outpatient dental settings compared to traditional agents like midazolam.
Data availability
The data collected and presented in this study is not publicly available. This is to protect the study participants' privacy. General Data Protection Regulations were strictly adhered to during data collection, including informed consent and maintaining participants' confidentiality.
References
Mesut E, Ülkü Şermet E, Emine K, Özlem Peyman K. Effects of photobiomodulation with different application parameters on injection pain in children: a randomized clinical trial. J Clin Pediatr Dent 2023; 47: 54-62.
Vitale M C, Gallo S, Pascadopoli M, Alcozer R, Ciuffreda C, Scribante A. Local anesthesia with SleeperOne S4 computerized device vs traditional syringe and perceived pain in pediatric patients: a randomized clinical trial. J Clin Pediatr Dent 2023; 47: 82-90.
Zanette G, Manani G, Favero L et al. Conscious sedation with diazepam and midazolam for dental patient: priority to diazepam. Minerva Stomatol 2013; 62: 355-374.
Wang L, Jing Q, Pei L et al. Efficacy of continuous intravenous remimazolam versus midazolam in the extraction of impacted wisdom teeth: protocol of a randomised controlled trial. BMJ Open 2023; DOI: 10.1136/bmjopen-2022-067908.
Yang R, Zhao R, Chaudry F et al. Modern sedative agents and techniques used in dentistry for patients with special needs: a review. J Taibah Univ Med Sci 2024; 19: 153-163.
Gonzalez Castro L N, Mehta J H, Brayanov J B, Mullen G J. Quantification of respiratory depression during pre-operative administration of midazolam using a non-invasive respiratory volume monitor. PLos One 2017; DOI: 10.1371/journal.pone.0172750.
Wandel C, Böcker R, Böhrer H, Browne A, Rügheimer E, Martin E. Midazolam is metabolized by at least three different cytochrome P450 enzymes. Br J Anaesth 1994; 73: 658-661.
European Medicines Agency. Byfavo (remimazolam). 2023. Available at https://www.ema.europa.eu/en/documents/overview/byfavo-epar-medicine-overview_en.pdf (accessed 17 March 2025).
Li X, Tian M, Deng Y, She T, Li K. Advantages of sedation with remimazolam compared to midazolam for the removal of impacted tooth in patients with dental anxiety. J Oral Maxillofac Surg 2023; 81: 536-545.
Sneyd J. Remimazolam - current status, opportunities and challenges. Anesthesiol Perioper Sci 2023; 1: 25.
Lee A, Shirley M. Remimazolam: a review in procedural sedation. Drugs 2021; 81: 1193-1201.
Jin N, Xue Z. Benefits of remimazolam as an anesthetic sedative for older patients: a review. Heliyon 2024; DOI: 10.1016/j.heliyon.2024.e25399.
Oka S, Satomi H, Sekino R et al. Sedation outcomes for remimazolam, a new benzodiazepine. J Oral Sci 2021; 63: 209-211.
Guo Z, Wang X, Wang L, Liu Y, Yang X. Can remimazolam be a new sedative option for outpatients undergoing ambulatory oral and maxillofacial surgery? J Oral Maxillofac Surg 2023; 81: 8-16.
Sun Y, Li Q. Evaluation of the efficacy and safety of remimazolam in tooth extraction surgery: a randomized, single-blind, multi-center clinical trial. Technol Health Care 2024; 32: 3473-3484.
Kim E-J, Kim C-H, Yoon J-Y, Byeon G-J, Kim H Y, Choi E-J. Comparison of postoperative nausea and vomiting between remimazolam and propofol in patients undergoing oral and maxillofacial surgery: a prospective randomized controlled trial. BMC Anesthesiol 2023; 23: 132.
Oue K, Oda A, Shimizu Y et al. Efficacy and safety of remimazolam besilate for sedation in outpatients undergoing impacted third molar extraction: a prospective exploratory study. BMC Oral Health 2023; 23: 774.
Swart R, Maes S S A, Cavanaugh D, Mason K P. Remimazolam pilot for office-based dental sedation: adverse events, awareness and outcomes. J Clin Med 2023; 12: 7308.
Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; DOI: 10.4103/sja.SJA_543_18.
Intercollegiate Advisory Committee for Sedation in Dentistry. Remimazolam for intravenous conscious sedation for dental procedures. 2023. Available at https://www.rcseng.ac.uk/-/media/fds/iacsd/iacsd-remimazolam-statement-130623.pdf (accessed 17 March 2025).
Intercollegiate Advisory Committee for Sedation in Dentistry. Standards for conscious sedation in the provision of dental care. 2020. Available at https://www.saad.org.uk/IACSD%202020.pdf (accessed 17 March 2025).
European Medicines Agency. Byfavo: EPAR - summary of product characteristics. 2023. Available at https://www.ema.europa.eu/en/documents/product-information/byfavo-epar-product-information_en.pdf (accessed 17 March 2025).
Facco E, Gumirato E, Humphris G et al. Modified dental anxiety scale: validation of the Italian version. Minerva Stomatol 2015; 64: 295-307.
Facco E, Stellini E, Bacci C et al. Validation of visual analogue scale for anxiety (VAS-A) in preanesthesia evaluation. Minerva Anestesiol 2013; 79: 1389-1395.
Ducoulombier V, Chiquet R, Graf S et al. Usefulness of a visual analog scale for measuring anxiety in hospitalized patients experiencing pain: a multicenter cross-sectional study. Pain Manag Nurs 2020; 21: 572-578.
Dawson R, von Fintel N, Nairn S. Sedation assessment using the Ramsay scale. Emerg Nurse 2010; 18: 18-20.
Newman M G, Trieger N, Miller J C. Measuring recovery from anesthesia - a simple test. Anesth Analg 1969; 48: 136-140.
Palumbo P, Tellan G, Perotti B, Pacilè M A, Vietri F, Illuminati G. Modified PADSS (Post Anaesthetic Discharge Scoring System) for monitoring outpatients discharge. Ann Ital Chir 2013; 84: 661-665.
American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology 2017; 126: 376-393.
Cowell A, Hare K, Campbell C. Adult intravenous sedation in general dental practice: indication, effectiveness and patient experiences. Br Dent J 2024; DOI: 10.1038/s41415-024-7562-x.
Teixeira M T, Goyal A. Remimazolam. Adv Anesth 2024; 42: 131-150.
Stöhr T, Colin P J, Ossig J et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth 2021; 127: 415-423.
Lawler H, Walker P. Evaluation of maximum dose intravenous midazolam used in dental intravenous sedation: a West of Scotland regional audit. Br Dent J 2022; 233: 135-138.
Kim H-J, Kim H, Park H-S, Kim H J, Ro Y-J, Koh W U. Effective remimazolam loading dose for adequate sedation in regional anesthesia. Can J Anaesth 2024; 71: 818-825.
Hu Q, Liu X, Wen C, Li D, Lei X. Remimazolam: an updated review of a new sedative and anaesthetic. Drug Des Devel Ther 2022; 16: 3957-3974.
Chen X, Sang N, Song K et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther 2020; 42: 614-624.
Ito T, Utsumi N, Baba Y, Matsumura T, Wakita R, Maeda S. Considerations for satisfactory sedation during dental implant surgery. J Pers Med 2023; 13: 461.
Zhu X, Wang H, Yuan S et al. Efficacy and safety of remimazolam in endoscopic sedation-a systematic review and meta-analysis. Front Med 2021; 8: 655042.
Dahiya D S, Kumar G, Parsa S et al. Remimazolam for sedation in gastrointestinal endoscopy: a comprehensive review. World J Gastrointest Endosc 2024; 16: 385-395.
Funding
We acknowledge Associazione Italiana Sedazionisti Odontoiatri (AISOD) for their unconditional contribution.
Author information
Authors and Affiliations
Contributions
GBG conducted the project, collected the data, and revised the manuscript. GM performed all dental procedures. MP collected the data and drafted the manuscript. MC collected the data. AG revised the manuscript and English. AS performed the statistical analysis. AP and SL performed the peripheral venous accesses. GS reviewed the manuscript and prompted anaesthesiological availability. GBG, GM, CM, MP, MC, AG, AS, AP, SL, GS commented on previous versions of the manuscript and have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest. This study was approved by the local ethical committee (Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Territorial Ethics Committee Lombardy 3) (Trial ID 4996) and complied with the fundamental principles of the 1964 Helsinki Declaration for clinical research involving human subjects (amended by the 75th World Medical Association General Assembly held in Helsinki, Finland, from October 16-19, 2024). Written informed consent was obtained from all participants.
Supplementary Information
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0.© The Author(s) 2025.
About this article
Cite this article
Grossi, G., Menozzi, G., Maiorana, C. et al. Remimazolam besylate in intravenous conscious sedation for dental treatment: a prospective cohort study. Br Dent J (2025). https://doi.org/10.1038/s41415-025-8664-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41415-025-8664-9