Abstract
Demethylase Tet2 plays a vital role in the immune response. Acute kidney injury (AKI) initiation and maintenance phases are marked by inflammatory responses and leukocyte recruitment in endothelial and tubular cell injury processes. However, the role of Tet2 in AKI is poorly defined. Our study determined the degree of renal tissue damage associated with Tet2 gene expression levels in a cisplatin-induced AKI mice model. Tet2-knockout (KO) mice with cisplatin treatment experienced severe tubular necrosis and dilatation, inflammation, and AKI markers’ expression levels than the wild-type mice. In addition, the administration of Tet2 plasmid protected Tet2-KO mice from cisplatin-induced nephrotoxicity, but not Tet2-catalytic-dead mutant. Tet2 KO was associated with a change in metabolic pathways like retinol, arachidonic acid, linolenic acid metabolism, and PPAR signaling pathway in the cisplatin-induced mice model. Tet2 expression is also downregulated in other AKI mice models and clinical samples. Thus, our results indicate that Tet2 has a renal protective effect during AKI by regulating metabolic and inflammatory responses through the PPAR signaling pathway.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is a global public health concern impacting ∼13.3 million patients per year [1]. Apparently, direct nephrotoxin is believed to be the reason for AKI development in about 20% of patients [2]. Cisplatin is an effective chemotherapeutic drug used for various solid tumors; however, AKI associated with cisplatin treatment limits its clinical usage [3]. High concentration of cisplatin accumulation has toxic effects in the proximal tubule segment [4]. Although multiple mechanisms such as ferroptosis oxidative stress injury, dysfunction of autophagy, necrosis, and apoptosis contribute to the pathogenesis of cisplatin-induced AKI, more and more evidence suggests inflammation to play a crucial role [5,6,7,8,9]. Cisplatin-induced AKI demonstrated an increased concentration of various pro-inflammatory cytokines such as IL-1β, IL-6, IL-18, tumor necrosis factor (TNF)-α, monocyte chemotactic protein-1 (MCP-1), and transforming growth factor-β1 (TGF-β1) [3, 10, 11].
Ten eleven translocation (Tet) methylcytosine dioxygenase family members are known to catalyze 5-methylcytosine (5mc) to 5-hydroxymethylcytosine (5hmc). Besides, Tet proteins play a role in regulating immunity and inflammation through the DNA methylation-independent way. It has also been reported that in dendritic cells and macrophages, Tet2 can suppress inflammation by recruiting histone deacetylases 2 (Hdac2) to repress IL-6 transcription specifically and downregulate other inflammatory mediators [12]. Tet2 KO will increase the IL-1β/NLRP3 inflammasome production resulting in accelerated development of atherosclerosis and heart failure [13, 14]. Tet2 is essential for various pathological processes, including leukemia, atherosclerotic cardiovascular diseases, and inflammation [12, 14, 15]. However, the role of Tet2 in AKI remains largely unknown. Our studies demonstrated the protective role of Tet2 in cisplatin-induced AKI by modulating metabolic and inflammatory responses. As known, cisplatin exacerbated AKI in Tet2-KO mice, but administration of Tet2 plasmid protected Tet2-KO mice from cisplatin-induced nephrotoxicity. In addition, renal RNA-seq results showed that Tet2 deletion correlated with downregulation of the PPAR pathway genes’ expression in metabolism regulation and increased the expression of inflammatory cytokines.
Results
Tet2 is highly expressed in mice kidney, kidney cell line and decreased in cisplatin-induced AKI
We first explored the in vitro and in vivo expression of the Tet2 gene under healthy and disease conditions. We performed immunofluorescence staining of Tet2 in HK-2 cells to examine the site and Tet2 expression. Significant Tet2 protein enrichment was observed in the nucleus, and siRNA knockdown significantly decreased the immunofluorescence intensity (Fig. 1A). Real-time quantitative PCR (qPCR) results showed a high expression of Tet2 mRNA in WT-mice kidney compared to Tet1 and Tet3 genes (Fig. 1B). Normal HK-2 cells showed similar results (Fig. 1C). To investigate the Tet2 expression in cisplatin-induced AKI, we established an AKI mice model by intraperitoneal injection of 22 mg/kg cisplatin. The mice-kidney immunohistochemistry revealed a marked reduction in Tet2 protein after 48 and 72 h of cisplatin administration (Fig. 1D). Similar results were observed in the in vitro experiments. HK-2 cells treated with 10 µM cisplatin (6, 12, 24, and 48 h) showed ~50% Tet2 mRNA level reduction after 6 h of cisplatin treatment. Western blot analyses also showed a dramatic reduction of cellular Tet2 protein in cisplatin-treated cells compared to the controls (Fig. 1E, F). These observations indicated that Tet2 might play a predominant role in cisplatin-induced AKI than the other two family members (Tet1 and Tet3).
A Immunofluorescence in HK-2 cells showing the location of Tet2 (green, orange arrows) predominantly in the nucleus (DAPI, blue), while the signal of Tet2 was attenuated by RNAi. Scale bar, 15 μm. B, C Both in vivo and in vitro qPCR analyses showing high expression of Tet2. ***P < 0.001 versus Tet1 gene; +++P < 0.001 versus Tet3 gene (n = 3). D Male C57BL/6 mice intraperitoneally injected with 0.9% saline or 22 mg/kg cisplatin (n = 6). Immunohistochemistry revealed a reduced Tet2 expression in the AKI model (original magnification, ×200). E, F HK-2 cells were treated with 10 µM cisplatin to mimic acute injury. E qPCR analyses showing a significant downregulation of Tet2 mRNA in the cisplatin-treated HK-2 cells compared to control cells. F Western blot analyses demonstrate a decreased Tet2 protein in the HK-2 cells after cisplatin treatment compared to the control cells.
Characterization of Tet2-knockout mice
To further delineate the function of Tet2 in cisplatin-induced AKI, we generated Tet2-KO mice by using the Cre-loxP system. Homozygous Tet2-KO mice were created by mating with transgenic mice expressing Cre recombinase (EIIA-Cre)—their Cre was expressed widely in the early embryo. The exon3 between the two LoxP sites in the target gene was deleted after mating with LoxP mice (Fig. 2A). Figure 2B shows the various genotypes of individual mice genomic DNA through PCR analyses. Mice with Tet2 ablation were designated as KO (Tet2f/f Cre+/−) (Fig. 2B, lane 1), whereas Tet2-floxed mice were designated WT (Tet2f/f Cre−/−) (Fig. 2B, lane 2). The qPCR experiment results showed that the Tet2 mRNA level reduced by 90% or more in the kidney and spleen isolated from KO mice, whereas it reduced by 40–50% in heterozygous-KO (het KO) mice compared to control-WT littermate mice. Furthermore, we also found that Tet1 and Tet3 mRNA levels were not affected by Tet2 ablation, and there were hardly any alterations in Tet1 and Tet3 mRNA expression (Fig. 2C, D). Moreover, there was no significant difference in the mice body weight, kidney weight, serum creatinine (Scr), and blood urea nitrogen (BUN) between KO and their control littermates at 2 and 4 months after birth (Fig. 2E–H). Therefore, we excluded the phenotypic variance caused by Tet2 gene deletion.
A The schematic diagram showing the crossbreeding strategy of Tet2-floxed mice (Tet2f/f) with EIIa-Cre transgenic mice. B Mice genotyping by PCR analyses of genomic DNA. Lane 1: Tet2f/f, Cre+/−, designated as KO, Lane 2: Tet2f/f, Cre−/−, designated as WT. C Real-time PCR analyses demonstrated a substantial reduction of the spleen and renal Tet2 mRNA in KO mice compared to WT mice. ***P < 0.001 versus WT mice. E–H Tet2 gene deletion had no impact on phenotype. There was no significant difference in the body weight (E), kidney weight (F), Scr (G), BUN (H) between KO and WT mice at 2 and 4 months (n = 6).
Tet2 deletion exacerbates cisplatin-induced AKI
A cisplatin-induced AKI mice model was established by administering 22 mg/kg cisplatin to both WT and Tet2-KO mice to investigate the role of Tet2. It was observed that the Scr and BUN levels (indicative of renal function) were markedly elevated in the KO and WT mice at 48 and 72 h after cisplatin injection (Fig. 3A, B). Furthermore, it was noticed that the Scr and BUN levels increased dramatically in the KO mice than the WT mice. Histological analysis with hematoxylin–eosin (HE) staining showed an intensified cast formation, tubular necrosis, and dilation in both mice after cisplatin treatment; pathological phenotype was more severe in KO mice than WT mice (Fig. 3C, D). There was a significant increase in the expression level of kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) in the kidneys of cisplatin-injected mice; the increase was higher in the KO mice than the WT mice (Fig. 3E, F). Thus, our data confirmed that Tet2 ablation accelerated renal function loss and aggravated kidney injury after cisplatin administration.
A, B Cispaltin treatment (22 mg/kg) led to a marked elevation of both Scr and BUN in KO + CIS mice compared to the KO and WT + CIS mice (n = 6). C Representative images of HE-stained kidney sections (200×). D Histologic damage in HE-stained kidney sections (n = 6) was scored by the criterion used in previous research. Ten randomly selected fields (original magnification, ×200) per kidney were required for counting the percentage of tubules that displayed cast formation, tubular necrosis, and dilation as follows: 0 = normal; 1 ≤ 10%; 2 = 10–25%; 3 = 26–50%; 4 = 51–75%; 5 ≥ 75%. E, F qRT-PCR analyses revealed a remarkable increase in KIM-1 and NGAL expression in KO + CIS mice, compared to KO and WT + CIS mice. *P < 0.05, **P < 0.01, and ***P < 0.001 represent statistical significance between WT + CIS mice or KO + CIS mice versus KO mice; #P < 0.05, ##P < 0.01, and ###P < 0.001 represent statistical significance between WT + CIS mice or KO + CIS mice versus WT mice; +P < 0.05, ++P < 0.01, and +++P < 0.001 represent statistical significance between KO + CIS mice versus WT + CIS mice.
In vivo expression of Tet2 attenuates cisplatin-induced AKI
We performed in vivo introduction of Tet2 in the kidneys during the AKI progression to further confirm the role of Tet2 in cisplatin-induced AKI. A small amount (2 mg/kg) of mouse Tet2 catalytic domain expression vector (Tet2 Normal), Tet2 catalytic domain vector with non-catalytic function (Tet2 Mutant), or empty vector (pcDNA3) were administered intravenously through hydrodynamic gene transfer technique for 3 days before cisplatin treatment (Fig. 4A) [16, 17]. Quantitative analyses demonstrated Tet2 induction in kidneys after continuous injection of Tet2-expressing plasmids (Fig. 4B). Immunohistochemistry analysis also showed similar results (Fig. 4C).
A The diagram shows the experimental design. Black arrows indicate the time point of when pcDNA3 or Tet2 plasmids were injected. The green arrowhead represents the time point of cisplatin administration (n = 3). B, C qRT-PCR analyses and immunohistochemistry of mouse kidney reveal an increased Tet2 expression after plasmids injection. D, E Overexpression of Tet2 Normal plasmid can significantly reduce the marked elevation of Scr and BUN caused by cisplatin injection. F, G Representative images of HE-stained kidney sections (n = 6) (200×) and histologic damage in the section were scored by the same evaluation method as shown in Fig. 4. H, I Graphic presentation shows the levels of KIM-1 and NGAL in different groups. ***P < 0.001 versus Control; ###P < 0.001 versus pcDNA3 group; +++P < 0.001 versus Tet2 Mutant group.
We further investigated if overexpression of Tet2 could positively impact cisplatin-induced AKI. The Scr and BUN elevation was significantly inhibited in Tet2-overexpressed mice after cisplatin administration (Fig. 4D, E). The HE-stained kidney sections revealed that Tet2-overexpression dramatically reduced tubular injury and necrosis in cisplatin-administered treatment, while the Tet2 Mutant or control plasmids could not (Fig. 4F, G). In addition, the qRT-PCR assessment confirmed KIM-1 and NGAL downregulation in the Tet2 Normal plasmid injection group compared to the injection groups of pcDNA3 or Tet2 Mutant plasmid (Fig. 4H, I).
Deletion of Tet2 contributes to aggravated metabolic pathway disorders and inflammation induced by cisplatin
We conducted RNA-seq of the kidneys from cisplatin-injected KO and WT mice to explore the Tet2 repression mechanism for cisplatin-induced renal injury. We observed 198 suppressed and 119 upregulated genes in the KO mice and compared them with the WT mice (Fig. 5A). A GO enrichment analysis in RNA-seq data showed highly enriched metabolic processes for organic acid, oxoacid, carboxylic acid, and the small molecule (Fig. 5B). The KEGG pathway analyses showed differential expressions of genes associated with metabolic pathways like arachidonic acid (AA) metabolism, xenobiotics cytochrome P450 metabolism, retinol metabolism, and PPAR signaling pathway (Fig. 5C). We then identified 13 downregulated genes (P ≤ 0.05; |fold change)| ≥1.5) related to the PPAR signaling pathway, PPARGC1B, CYP4A14, APOC3, and so forth (Fig. 5D). Among these genes, the transcription of CYP4a14, APOC3, ACOX2, ACOX3, and Gyk reduced at least two folds (Fig. 5E). Besides, a series of inflammation genes were upregulated in Tet2-KO mice (Fig. 5E). The qPCR analyses indicated an increased transcript of inflammatory cytokine genes (Ccl2, IL-6, CHil3, TNF-α, and IL-1β) and a decreased PPAR signaling pathway-related genes (CYP4a14, APOC3, ACOX2, and ACOX3) in the conditional KO mice compared to the WT mice after cisplatin injection (Fig. 6A, B). Besides, overexpression of Tet2 in vivo could markedly suppress the transcription of inflammation genes (Ccl2, IL-6, CHil3, TNF-α, and IL-1β) and restore the reduced expression of CYP4a14, APOC3, ACOX2, and ACOX3 in cisplatin-treated KO mice compared to overexpression of mutant Tet2 or the control plasmid (Fig. 6C, D).
A Different expression genes heatmap. Cisplatin treatment group: CWT vs. CKO (n = 3). B, C Enrichment pathways in the cisplatin treatment group. D, E mRNA variations of indicated genes in RNA-seq analysis of mice stimulated with cisplatin. RPKM of each of the genes in the CKO group were compared with the CWT group, and calculated to log2 ratio.
Aggravation of cisplatin-induced AKI by deletion of Tet2 gene is independent of tubular cell apoptosis
Cell apoptosis is a characteristic feature of cisplatin-induced AKI [18, 19], with the pro-apoptotic gene Bax playing a vital role [20, 21]. Therefore, we investigated if Tet2 KO could affect the level of cisplatin-induced apoptosis in mouse kidney sections by conducting terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay. Both KO and WT-mouse kidneys showed a similar apoptosis degree, although TUNEL-positive nuclei increased under cisplatin treatment (Fig. S1A, B). Immunohistochemistry analysis revealed no significant differences in BAX protein between cisplatin-administered WT and KO mice (Fig. S1C). Western blot results further indicated that Tet2 KO did not affect the protein level of active caspase-3 (Fig. S2). Thus, we can infer that aggravation of cisplatin-induced AKI by Tet2 gene deletion is independent of tubular apoptosis.
Decreased Tet2 expression is detected in both other mice AKI models and clinical patients
We observed reduced Tet2 expression in IR, renal transplant, and sepsis mice models at different time points, suggesting a possible important role of Tet2 in AKI caused by other reasons (Fig. 7A–C). Immunohistochemistry of clinical renal biopsy tissues also confirmed lesser Tet2 proteins in AKI patients (Fig. 7D). Decreased Tet2 expression in various AKI models was a common observation; therefore, Tet2 might be a novel marker protein for predicting AKI in the future.
Discussion
Tet methylcytosine dioxygenase (TET)’s primary function is its catalytic capability in oxidizing DNA 5mC to 5hmC through complete removal of methylated cytosine [22, 23]. DNA methylation changes have been implicated in diabetic nephropathy, kidney fibrosis, and chronic kidney disease [24]. However, very little research has been done on DNA hydroxymethylation and AKI relationship. Ning Huang and colleagues reported reduced levels of 5hmC and dramatically downregulated mRNA expression of Tet2 in IR-injured kidneys [25]. The research indicated Tet2 involvement in IR development, but the specific function of Tet2 in this pathological process and its underlying mechanisms remained unclear. Therefore, in our study, we aimed to investigate the role of Tet2 in cisplatin-induced AKI.
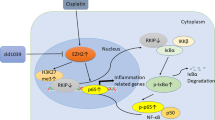
Tet2-KO increases cisplatin-induced tubular cell damage in vivo as evidenced by increased BUN, SCr, and pathological indexes. In addition, RNA-seq data suggested the protective role of Tet2 by influencing multiple metabolic pathways such as arachidonic acid (AA) metabolism, cytochrome P450, other metabolic pathways, and the PPAR signaling pathway. Metabolic disorders impact the pathogenesis of renal dysfunction [26, 27]. Cytochrome P450 enzymes metabolize AA to four bioactive regioisomeric epoxyeicosatrienoic acids (EETs) and 20-hydroxyeicosatetraenoic acid (20-HETE) that mediate renal microcirculation, possess anti-inflammatory, and other renoprotective effects [28, 29]. The PPAR signaling pathway also plays a crucial protective role in acute renal tubular injury [30, 31]. PPARs are the primary regulators of fatty acid metabolism, and their derangement results in kidney injury [32,33,34]. Atf6α KO maintained expression of PPARα, and therefore reduced the apoptosis and lipid accumulation of proximal tubular cells following unilateral IR injury [33]. In addition, synthetic PPARα ligands, and PPAR-γ activation were proven to limit the expression of pro-inflammatory cytokines by attenuating NF-kB activity in cisplatin nephrotoxicity [35, 36]. Li et al. reported the anti-inflammatory effects of PPARα ligand in cisplatin-induced AKI by reducing renal endonuclease G [37]. Also, activation of PPAR-γ significantly contributed to protection against the IR-induced AKI because of its anti-inflammatory and anti-oxidant effects [38,39,40].
Our results showed that Tet2 could regulate the PPAR signaling pathway, metabolism pathway, and inflammation response, thereby alleviating cisplatin-induced kidney injury. Renal tubular cell injury and necrosis, infiltration of inflammatory cells, especially macrophages, are the primary reasons for the occurrence and progression of AKI [41,42,43]. Therefore, in future experiments, Tet2-loxp mice should be mated with Kap-cre to dissect the functions of Tet2 specifically in proximal tubule in cisplatin-induced AKI. Kap-cre expression is mainly detected in the proximal tubule cells [44]. Nakamura et al. explored the roles of transcription factor EB (TFEB) in AKI using proximal tubule-specific TFEB-KO mouse [44]. Suzuki et al. also demonstrated that Atg7 was essential for suppressing cell injury and apotosis using proximal tubule-specific Atg7-KO mouse [45]. The detailed roles of Tet2 of renal tubular cells in cisplatin-induced AKI are worthy of further study. Overall, this study explored the function of Tet2 in cisplatin-induced AKI for the first time; Tet2 and DNA methylation may provide the potential therapeutic cues to treat cisplatin-induced AKI as well as other types of AKI.
Materials and methods
Animals
Tet2-floxed mice and EIIa-Cre mice were kindly provided by Dr. Ye Dan (Fudan University). The mice were housed in the Zhejiang University laboratory animal center. All experiments were approved by the Laboratory Animal Management and Ethics Committee of Zhejiang University per the Chinese Guidelines on the Care and Use of Laboratory Animals. Tet2-KO mice were generated by the Cre-loxP (Cre recombinase-locus of x-over, P1) system as described earlier (Fig. 2A). Mice genotypes were confirmed by tail-snip PCR amplification. Littermate control mice were used as controls in all animal-related experiments.
Mouse model of cisplatin-induced AKI
An earlier research protocol was used to construct the cisplatin-induced AKI model [46]. Cisplatin (Selleck, S1166) was dissolved in 0.9% of saline at a concentration of 1 mg/mL. Male mice (8–10 weeks old, 20–25 g) were intraperitoneally injected with a single dose of cisplatin (22 mg/kg body wt) or an equal volume of saline. This dose was based on earlier studies [4, 47], and our preliminary experiments also proved that a lower dose did not produce a stable and significant acute tubular injury, while a higher dose was associated with more unpredictable mortality before 72 h after cisplatin injection in C57BL/6 mice. Mice ocular blood extraction was done at 0, 24, 48, and 72 h for BUN and creatinine measurements. The mice were sacrificed at 72 h after cisplatin administration, and the kidneys were either frozen with liquid nitrogen for qRT-PCR and Western blotting or fixed in 10% buffered formalin for histology/immunohistochemistry (IHC).
Ischemia–reperfusion (IR) model
First, 50–60 mg/kg pentobarbital (5 mg/mL) was used to anesthetize the mouse by intraperitoneal (i.p.) injection [46]. The body temperature was maintained at 36.5–37 °C during the surgery. Second, the renal artery and vein were clamped by micro-aneurysm clips for a variable length of time to induce kidney injuries at varying severities. Successful ischemia was confirmed by a gradual darkening of the kidney (from red to dark purple). After ischemia, the clamp was removed at the desired time to achieve reperfusion; the kidney color immediately reverted to red. The mice were sacrificed at 5 and 10 days after IR, and the kidneys were fixed in 10% buffered formalin for further experimentation.
Renal transplant model
Ectopic kidney transplantation was carried out as detailed previously [48, 49]. The kidneys from BALB/c mice were transplanted into C57BL/6 recipients. In brief, the renal artery and vein from BALB/c mice were anastomosed to the abdominal aorta and vena cava of the recipient mice, respectively. In addition, the donated ureter was attached to the recipient’s bladder. The transplanted kidneys were collected at 3 and 5 days after kidney transplantation.
Mouse model of sepsis-associated AKI
The cecal ligation and puncture (CLP) model is the most frequently used model for its simplicity [46]. Intraperitoneal anesthesia of the mice was done as described in an earlier section. Ligation of the cecum from the distal to the ileocecal valve was made, followed by two needle-punctures to extrude the stool into the abdominal cavity. The CLP mice could produce typical bacterial peritonitis symptoms after 6 h. The mice were sacrificed at 24 and 72 h after CLP operations, and the kidneys were fixed in 10% buffered formalin for IHC detection.
Overexpression of Tet2 in vivo
Male C57BL/6 mice (8–10 weeks old) were given a continuous intravenous injection of WT and catalytic domain catalytic-dead Tet2 overexpression plasmids (pScalps_Puro_mTet2 catalytic domain (#79554); pScalps_Puro_mTet2 catalytic domain HxD (#79611); Addgene) at 2 mg/kg body wt for 3 days (Fig. 5A) by using the hydrodynamic gene transfer technique [16, 50]. The control group was injected with an equal volume of saline. After plasmids administration, the mice were subjected to the mouse model of cisplatin-induced AKI as described earlier and were euthanized at 72 h after the model. The serum and the kidneys were collected for further experiments.
Serum creatinine and BUN assay
Serum creatinine and BUN levels were detected by 7000i Automatic biochemical analyzer (FUJI). Serum creatinine was expressed as μmol/L and the BUN was expressed as mg/dL.
Cell culture
HK-2 cells were obtained from ATCC, and the cells were cultured in DMEM medium supplemented with 10% heat-inactivated FBS, 1% penicillin, and streptomycin mixture. The cells plated in a 6-well plate overnight were treated with 10 µM cisplatin for 6–48 h to mimic acute injury. After the treatment, the cells were collected at different time points and subjected to various analyses.
RNA-sequencing and bioinformatics analysis
RNA-sequencing (RNA-seq) was performed as described previously [41]. The total RNA was subjected to RNA-seq to determine mRNA expression patterns. Bioinformatics analyses including raw data process, differential gene expression analysis, gene ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were conducted by Novogene Genomic Center.
qPCR analysis
Total RNA was isolated from the kidneys or HK-2 cells using TRIzol Reagent (Invitrogen), and cDNA was synthesized using Reverse Transcription System Kit (Takara). The mRNA levels of various genes were quantified using CFX-96 Sequence Detection System (BIO-RAD). The detailed sequence of qPCR primers are listed in Table S1.
Western blot analysis
Western blotting was performed as described in earlier research [51]. The primary antibodies used were as follows: anti-Tet2 (ab94580; Abcam), anti-GAPDH (10494-1-AP; Proteintech), and anti-caspase-3 antibody, active (cleaved) form (AB3623; Merk).
Immunofluorescence staining
Immunofluorescence staining was performed as previously described [51]. The antibody used was anti-Tet2 (21207-1-AP; Proteintech).
TUNEL staining assay
Apoptotic cell death in kidney tissue was determined by In Situ Cell Apoptosis Detection Kit (BBI Life Science), and the manufacturer’s instructions were followed for experimental operations.
Histology and immunohistochemical staining
Immunohistochemical (IHC) staining was prepared in a traditional way described in earlier research [51], and human kidney biopsy samples were obtained from Kidney Disease Center at the First Affiliated Hospital, Zhejiang University. The kidney sections for IHC staining were prepared following the routine protocol. Antibodies used were anti-Tet2 (ab94580; Abcam) and anti-Bax (Sc-493; Santa Cruz Biotechnology).
Statistical analyses
All data were expressed as mean ± SEM for statistical analyses. An unpaired t-test was used to analyze the group differences. A P value of ≤0.05 was considered statistically significant.
References
Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–43.
Martin M, Wilson FP. Utility of electronic medical record alerts to prevent drug nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2019;14:115–23.
Peres LA, da Cunha AD,Jr. Acute nephrotoxicity of cisplatin: molecular mechanisms. Braz. J. Nephrol.2013;35:332–40.
Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, et al. A role for tubular necroptosis in cisplatin-induced AKI. J. Am. Soc. Nephrol. 2015;26:2647–58.
Deng F, Sharma I, Dai Y, Yang M, Kanwar YS. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J. Clin. Investig. 2019;129:5033–49.
Mishima E, Sato E, Ito J, Yamada KI, Suzuki C, Oikawa Y, et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 2020;31:280–96.
Guo Q, Wang J. Effect of combination of vitamin E and umbilical cord-derived mesenchymal stem cells on inflammation in mice with acute kidney injury. Immunopharmacol. Immunotoxicol. 2018;40:168–72.
Kumar P, Sulakhiya K, Barua CC, Mundhe N. TNF-alpha, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats. Mol. Cell Biochem. 2017;431:113–22.
Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 2019;20:E3011.
Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Therapeut. 2007;322:8–15.
Amirshahrokhi K, Khalili AR. Thalidomide ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in an experimental model. Inflammation. 2015;38:476–84.
Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–93.
Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J. Am. Coll. Cardiol. 2018;71:875–86.
Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–7.
Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell. 2017;170:1079–95.
Dai C, Yang J, Liu Y. Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J. Am. Soc. Nephrol. 2002;13:411–22.
Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, et al. Sustained activation of wnt/beta-catenin signaling drives AKI to CKD progression. J. Am. Soc. Nephrol. 2016;27:1727–40.
Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40.
Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J. Am. Soc. Nephrol. 2014;25:2689–701.
Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, et al. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 2019;29:1261–73.
Benedetti G, Fredriksson L, Herpers B, Meerman J, van de Water B, de Graauw M. TNF-α-mediated NF-κB survival signaling impairment by cisplatin enhances JNK activation allowing synergistic apoptosis of renal proximal tubular cells. Biochemical Pharmacol. 2013;85:274–86.
Koivunen P, Laukka T. The TET enzymes. Cell Mol. Life Sci. 2018;75:1339–48.
Hon GC, Song CX, Du T, Jin F, Selvaraj S, Lee AY, et al. 5mC oxidation by Tet2 modulates enhancer activity and timing of transcriptome reprogramming during differentiation. Mol. Cell. 2014;56:286–97.
Guo C, Pei L, Xiao X, Wei Q, Chen JK, Ding HF, et al. DNA methylation protects against cisplatin-induced kidney injury by regulating specific genes, including interferon regulatory factor 8. Kidney Int. 2017;92:1194–205.
Huang N, Tan L, Xue Z, Cang J, Wang H. Reduction of DNA hydroxymethylation in the mouse kidney insulted by ischemia reperfusion. Biochem. Biophys. Res. Commun. 2012;422:697–702.
Yeboah MM, Hye Khan MA, Chesnik MA, Skibba M, Kolb LL, Imig JD. Role of the cytochrome P-450/epoxyeicosatrienoic acids pathway in the pathogenesis of renal dysfunction in cirrhosis. Nephrol. Dial. Transpl. 2018;33:1333–43.
Capdevila JH, Wang W, Falck JR. Arachidonic acid monooxygenase: genetic and biochemical approaches to physiological/pathophysiological relevance. Prostaglandins Other Lipid Mediat. 2015;120:40–49.
Sato Y, Sato W, Maruyama S, Wilcox CS, Falck JR, Masuda T, et al. Midkine regulates BP through cytochrome P450-derived eicosanoids. J. Am. Soc. Nephrol. 2015;26:1806–15.
Yeboah MM, Hye Khan MA, Chesnik MA, Sharma A, Paudyal MP, Falck JR, et al. The epoxyeicosatrienoic acid analog PVPA ameliorates cyclosporine-induced hypertension and renal injury in rats. Am. J. Physiol. Ren. Physiol. 2016;311:F576–585.
Guan Y, Breyer MD. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int. 2001;60:14–30.
Zhou TB, Drummen GP, Jiang ZP, Long YB, Qin YH. Association of peroxisome proliferator-activated receptors/retinoic acid receptors with renal diseases. J. Receptor Signal Transduct. Res. 2013;33:349–52.
Parikh SM. Metabolic stress resistance in acute kidney injury: evidence for a PPAR-gamma-coactivator-1 alpha-nicotinamide adenine dinucleotide pathway. Nephron. 2019;143:184–7.
Jao TM, Nangaku M, Wu CH, Sugahara M, Saito H, Maekawa H, et al. ATF6α downregulation of PPARα promotes lipotoxicity-induced tubulointerstitial fibrosis. Kidney Int. 2019;95:577–89.
Console L, Scalise M, Giangregorio N, Tonazzi A, Barile M, Indiveri C. The link between the mitochondrial fatty acid oxidation derangement and kidney injury. Front. Physiol. 2020;11:794.
Zhang J, Zhang Y, Xiao F, Liu Y, Wang J, Gao H, et al. The peroxisome proliferator-activated receptor γ agonist pioglitazone prevents NF-κB activation in cisplatin nephrotoxicity through the reduction of p65 acetylation via the AMPK-SIRT1/p300 pathway. Biochem. Pharmacol. 2016;101:100–11.
Baud L, Letavernier E. PPARalpha contributes to tubular protection. J. Am. Soc. Nephrol. 2007;18:3017–8.
Li S, Basnakian A, Bhatt R, Megyesi J, Gokden N, Shah SV, et al. PPAR-alpha ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. Am. J. Physiol. Ren. Physiol. 2004;287:F990–998.
Kapil A, Singh JP, Kaur T, Singh B, Singh AP. Involvement of peroxisome proliferator-activated receptor gamma in vitamin D-mediated protection against acute kidney injury in rats. J. Surgical Res. 2013;185:774–83.
Reel B, Guzeloglu M, Bagriyanik A, Atmaca S, Aykut K, Albayrak G, et al. The effects of PPAR-γ agonist pioglitazone on renal ischemia/reperfusion injury in rats. J. Surgical Res. 2013;182:176–84.
Singh AP, Singh N, Pathak D, Bedi PMS. Estradiol attenuates ischemia reperfusion-induced acute kidney injury through PPAR-γ stimulated eNOS activation in rats. Mol. Cell. Biochem. 2019;453:1–9.
Wang J, Nie W, Xie X, Bai M, Ma Y, Jin L, et al. MicroRNA-874-3p/ADAM (a disintegrin and metalloprotease) 19 mediates macrophage activation and renal fibrosis after acute kidney injury. Hypertension. 2021;77:1613–26.
Okubo K, Kurosawa M, Kamiya M, Urano Y, Suzuki A, Yamamoto K, et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat. Med. 2018;24:232–8.
Singbartl K, Formeck CL, Kellum JA. Kidney-immune system crosstalk in AKI. Semin. Nephrol. 2019;39:96–106.
Nakamura S, Shigeyama S, Minami S, Shima T, Akayama S, Matsuda T, et al. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat. cell Biol. 2020;22:1252–63.
Suzuki C, Tanida I, Oliva Trejo JA, Kakuta S, Uchiyama Y. Autophagy deficiency in renal proximal tubular cells leads to an increase in cellular injury and apoptosis under normal fed conditions. Int. J. Mol. Sci. 2019;21:155
Bao YW, Yuan Y, Chen JH, Lin WQ. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool. Res. 2018;39:72–86.
Zhang J, Rudemiller NP, Patel MB, Wei Q, Karlovich NS, Jeffs AD, et al. Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J. Am. Soc. Nephrol. 2016;27:2257–64.
Kalina SL, Mottram PL. A microsurgical technique for renal transplantation in mice. Aust. N.Z. J. Surg. 1993;63:213–6.
Wang YY, Jiang H, Pan J, Huang XR, Wang YC, Huang HF, et al. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J. Am. Soc. Nephrol. 2017;28:2053–67.
Dai C, Saleem MA, Holzman LB, Mathieson P, Liu Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 2010;77:962–73.
Zhu X, Ye Y, Xu C, Gao C, Zhang Y, Zhou J, et al. Protein phosphatase 2A modulates podocyte maturation and glomerular functional integrity in mice. Cell Commun. Signal. 2019;17:91.
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2018YFC2000400), National Natural Science Foundation of China (81670651, 81970573, 82000637, and 81770752), and Medical and Health Science and Technology in Zhejiang Province (2020KY538). We also thank Shenghui Hong and Ping Liu in the Laboratory Animal Center of Zhejiang University for their technical assistance in the biological purification of mice.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Maria Victoria Niklison Chirou.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bao, Y., Bai, M., Zhu, H. et al. DNA demethylase Tet2 suppresses cisplatin-induced acute kidney injury. Cell Death Discov. 7, 167 (2021). https://doi.org/10.1038/s41420-021-00528-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-021-00528-7
This article is cited by
-
DNA dioxygenase TET2 deficiency aggravates sepsis-induced acute lung injury by targeting ITGA10 via the PI3K/AKT signaling pathway
Cellular & Molecular Biology Letters (2025)
-
TET2 germline variants promote kidney disease by impairing DNA repair and activating cytosolic nucleotide sensors
Nature Communications (2024)
-
Cisplatin nephrotoxicity: new insights and therapeutic implications
Nature Reviews Nephrology (2023)
-
ROS attenuates TET2-dependent ZO-1 epigenetic expression in cerebral vascular endothelial cells
Fluids and Barriers of the CNS (2022)
-
Targeting iron metabolism using gallium nanoparticles to suppress ferroptosis and effectively mitigate acute kidney injury
Nano Research (2022)