Abstract
The ability to sense cellular temperature and induce physiological changes is pivotal for plants to cope with warming climate. Biomolecular condensation is emerging as a thermo-sensing mechanism, but the underlying molecular basis remains elusive. Here we show that an intrinsically disordered protein FUST1 senses heat via its condensation in Arabidopsis thaliana. Heat-dependent condensation of FUST1 is primarily determined by its prion-like domain (PrLD). All-atom molecular dynamics simulation and experimental validation reveal that PrLD encodes a thermo-switch, experiencing lock-to-open conformational changes that control the intermolecular contacts. FUST1 interacts with integral stress granule (SG) components and localizes in the SGs. Importantly, FUST1 condensation is autonomous and precedes condensation of several known SG markers and is indispensable for SG assembly. Loss of FUST1 significantly delays SG assembly and impairs both basal and acquired heat tolerance. These findings illuminate the molecular basis for thermo-sensing by biomolecular condensation and shed light on the molecular mechanism of heat stress granule assembly.
Similar content being viewed by others
Introduction
Heat stress is one of the most challenging environmental risks in the coming decades due to climate change.1,2 Unlike mammals, plants are unable to maintain their body temperature at a constant level. Heat alters the structure and function of macromolecules, induces protein misfolding and triggers the production of reactive oxygen species (ROS), which can cause plant cell death.3 Therefore, understanding how plants sense and respond to high temperature stress is instrumental to crop engineering or breeding via optimal tilt of the balance between defense and growth.
The sensing of heat by plant cells is widely distributed throughout subcellular compartments and regulatory nodes.4,5,6,7 Recently, the reversible condensation of proteins is emerging as a crucial mechanism of thermosensing both in plants8,9,10 and yeast.11,12,13 In these cases, the condensation involves weak and multivalent interactions from intrinsically disordered regions (IDRs)8,9,10,12,13 as well as stronger interactions provided by the modular domains.10,11,12,13 Conformational rearrangements were reported to be a key feature during temperature sensing.10,11 Nevertheless, the atomic basis underlying temperature sensing by condensation remains largely elusive.
Stress granules (SGs) are one prominent type of conserved cytoplasmic biomolecular condensates that form in response to a variety of stressors.14,15 SGs assemble via liquid‒liquid phase separation (LLPS) driven by dynamic and promiscuous protein‒protein and protein‒RNA interactions.14 These interactions are distributed unevenly, with G3BP as the central node.16 The current model in mammalian cells holds that G3BP is a molecular switch: stalled translation leads to a rise in cellular free RNA concentrations, which, above a certain threshold, triggers G3BP conformational change that favors G3BP‒RNA interaction. This then results in RNA-dependent LLPS that seeds SG assembly.16,17,18,19 In plants, the general composition of heat-induced SGs has been determined, but how heat signal is transmitted to SG assembly is yet unknown.
Here, we discover a novel protein, FUST1, that directly senses heat and drives heat-induced SG assembly. We employ all-atom molecular dynamics simulation (DM) in combination with biophysical studies to show that FUST1 contains a thermo-switch that undergoes lock-to-open conformational change upon temperature increase. Imaging analyses reveal that FUST1 condensation in vivo is autonomous, precedes the condensation of several known SG markers and is indispensable for SG assembly. Ablation of FUST1 function significantly delays SG formation. We conclude that FUST1 is a molecular thermosensor that drives heat-induced SG assembly in plants.
Results
FUST1 is an uncharacterized protein that undergoes heat-dependent condensation
We have previously characterized the potential phase-separation proteins from plants.20 These proteins were expressed in tobacco epidermal cells and fission yeast cells and exposed to stress conditions. This allowed us to identify stress-responsive biomolecular condensates in Arabidopsis.21 AT3G07660 is an uncharacterized protein that we named FUST1, standing for a sacred FuSang Tree in ancient Chinese legend on which ten suns rest. FUST1 was highly enriched as potential phase-separation proteins20 and formed cytoplasmic condensates in tobacco epidermal cells in response to heat treatment (Supplementary information, Fig. S1a). FUST1 also formed heat-dependent condensates when heterologously expressed in yeast cells (Supplementary information, Fig. S1b). We obtained two mutant alleles: fust1-1 harbors a T-DNA insertion within the ninth exon, producing a truncated protein lacking the C-terminal fragment (Supplementary information, Fig. S1c); fust1-2 is a CRISPR allele that has a deletion of 603-bp fragment harboring promoter, 5’UTR and first exon (Supplementary information, Fig. S1c, e), therefore representing a complete knockout allele (Supplementary information, Fig. S1c‒e). To confirm heat-dependent condensation of FUST1 in vivo, we generated Arabidopsis complementation plants expressing the full-length genomic sequence of FUST1 fused with the coding sequence of a yellow fluorescent protein in fust1 mutants (Supplementary information, Fig. S1c). The transgenes were expressed at comparable levels to that of endogenous FUST1 (Supplementary information, Fig. S1d). FUST1-mVenus formed cytoplasmic condensates as quickly as 2 min upon heat stress treatment at 37 °C in the root tip cells (Fig. 1a; Supplementary information, Fig. S1f and Video S1). FUST1 condensates were quickly dissolved by 1,6-hexanediol (Supplementary information, Fig. S1g), dynamically exchanged with the surrounding milieu as probed by fluorescence recovery after photobleaching (FRAP; Fig. 1b, c; Supplementary information, Video S2). FUST1 was ubiquitously expressed in shoot and root tissues as well as in early embryos of imbibed seeds (Supplementary information, Fig. S1h, i). Heat-dependent condensation was also observed in imbibed seeds and leaf epidermal cells (Supplementary information, Fig. S1i, j). We conclude that FUST1 undergoes heat-dependent condensation in vivo.
a Time-lapse imaging of FUST1-mVenus condensation in Arabidopsis root tip cells. Scale bar, 5 μm. b FRAP of FUST1 condensates in Arabidopsis root tip cells. Time 0 s indicates the time of the photobleaching pulse. White dashed circles indicate the bleached condensates. Scale bar, 1 μm. c Plot showing the fluorescence recovery of FUST1 condensates after photobleaching in Arabidopsis root tip cells. Error bars indicate mean ± SD (n = 13). d In vitro phase separation of 2.5 μM FUST1-GFP at varying temperatures in 40 mM Tris-HCl, pH 7.4 and 100 mM NaCl. Scale bars, 5 μm. e Time-lapse imaging of 2.5 μM FUST1-GFP phase separation at 4 °C and 37 °C in 40 mM Tris-HCl, pH 7.4 and 100 mM NaCl in vitro. Scale bars, 5 μm. f FRAP of FUST1-GFP droplet. Time 0 s indicates the time of the photobleaching pulse. Scale bar, 2 μm. g Plot showing the recovery after photobleaching of FUST1-GFP droplets. Error bars indicate mean ± SD (n = 6). h Relaxation of two FUST1-GFP droplets into one droplet. Time points are indicated above. Scale bar, 2 μm.
FUST1 condensation is intrinsically sensitive to temperature increase
FUST1 is largely unfolded (Supplementary information, Fig. S2a) and contains long IDRs (Fig. 2a), promoting us to test whether it undergoes autonomous phase separation. We purified FUST1 that is fused to monomeric GFP from prokaryotic cells (Supplementary information, Fig. S2b) and assessed the phase behavior in vitro. FUST1-GFP formed very few droplets at 4 °C (Fig. 1d, e). Elevating the temperature to 30 °C and 37 °C gradually increased both the size and number of FUST1-GFP droplets (Fig. 1d, e). FUST1-GFP molecules dynamically exchanged between the dense phase and the dilute phase (Fig. 1f, g). Two FUST1-GFP droplets rapidly fused upon contacting each other (Fig. 1h). These features are consistent with the liquid-like property of FUST1 condensates in vivo. Untagged FUST1 also formed more droplets at higher temperature (Supplementary information, Fig. S2c). Temperature-dependent turbidity analysis of FUST1 protein solution confirmed the temperature sensitivity of FUST1 phase separation (Supplementary information, Fig. S2d). To obtain more sensitive measurements, we used dynamic light scattering (DLS) and monitored the apparent hydration radius (Rh), which is a proxy of droplet size, of FUST1 protein solution during a slow temperature ramp. The droplet size of FUST1 grew rapidly between 35 °C and 45 °C (Supplementary information, Fig. S2e), suggesting that FUST1 undergoes a highly cooperative assembly transition when exposed to higher temperatures. To exclude the possibility that FUST1 protein was denatured at elevated temperature, we used far-UV circular dichroism (CD) spectroscopy22 to evaluate the secondary structure content. FUST1 protein showed slightly increased CD spectra between 205 nm and 230 nm at higher temperature (Supplementary information, Fig. S2f), which is suggestive of a mild conformational change but not global denaturation. Taken together, these results indicate that FUST1 phase separation is enhanced by heat.
a Top, illustration of the amino acid composition of PrLD. Middle, protein domain structure of FUST1. Bottom, prediction of the IDRs and prion-like domain by PONDR and PLACC algorithms. b Representative confocal microscopic images of tobacco epidermal cells expressing FUST1 and its variants. The cells were treated at 37 °C for 30 min and 22 °C as control. Scale bars, 10 μm. c In vitro phase separation assay of Cy5-labeled PrLDWT at different temperatures and concentrations. Scale bar, 5 μm. d Phase diagram of PrLDWT as a function of temperature. Red dots, with detectable condensates. Empty circles, without detectable condensates. e, f Temperature-dependent turbidity of indicated proteins with indicated protein concentrations. In vitro phase separation assay in c and turbidity measurements in e, f were conducted in 40 mM Tris-HCl pH, 7.4 and 500 mM NaCl. Error bars indicate mean ± SD (n = 3). g Representative confocal microscopic images of Arabidopsis root tip cells expressing FUST1, FUST1∆PrLD and FUST1PrLD Y>S. The roots were treated at 35 °C for 30 min. Scale bars, 10 μm. h Statistical analysis of number of condensates per cell in each genotype in g at 35 °C. Error bars indicate mean ± SD (n = 15). P values were calculated using two-sided Student’s t-test. ****P < 0.0001.
The heat-dependent condensation of FUST1 is primarily determined by a prion-like domain
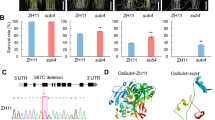
FUST1 harbors an N-terminal ubiquitin-associated (UBA) domain, four predicted IDRs and one prion-like domain (PrLD) (Fig. 2a). To define which part(s) of FUST1 is responsible for its condensation, we generated deletion variants and assessed their condensation in tobacco epidermal cells and yeast cells. While variants lacking UBA or IDR still retained condensation to some extent, deletion of PrLD completely abolished FUST1 condensation in both systems (Fig. 2b; Supplementary information, Fig. S3a). Consistently, purified FUST1∆PrLD failed to form condensates in vitro while other variants still did (Supplementary information, Fig. S3b). Furthermore, FUST1∆PrLD lost temperature responsiveness as revealed by the measurement of turbidity and Rh (Supplementary information, Fig. S2d, e).
PrLD contains few positive charges and no negative charge, and is enriched with aromatic and hydrophobic amino acids (Supplementary information, Fig. S3c‒e), consistent with features of lower critical solution temperature (LCST) behavior.23 Furthermore, the regularly spaced aromatic tyrosine residues in PrLD may act as attractive stickers.24 To test whether PrLD indeed possesses LCST behavior, we purified PrLD without tag fusion to avoid any impact of tagging on its phase behavior (Supplementary information, Fig. S4a) and labeled it with Cy5 for microscopic observation. We found that PrLD formed droplets, which are not destabilized by higher salt concentration (Supplementary information, Fig. S4b). We then characterized the PrLD phase boundary in detail (Fig. 2c, d). At 4 °C, phase separation was observed at protein concentrations above 5.0 μM. As temperature increased, phase separation occurred at lower concentrations, with droplets being observed at concentrations as low as 0.625 μM at 43 °C. These results indicate that the saturation concentration (Csat) of PrLD is lowered by increasing temperature, agreeing with an LCST behavior. Consistent with this result, the temperature-dependent turbidity curve of PrLD closely resembled that of a typical LCST protein (Fig. 2e). Conversely, PrLDY>S lost the temperature-dependent phase separation in vitro (Fig. 2f; Supplementary information, Fig. S4c). In the context of full-length FUST1, neither FUST1∆PrLD nor FUST1PrLD Y>S was able to form condensates, both in vitro and in Arabidopsis (Fig. 2g, h; Supplementary information, Fig. S2d). These results indicate that the PrLD of FUST1 is the main driver for heat sensing and condensation.
PrLD encodes a thermo-switch
To understand the molecular basis underlying how PrLD senses temperature, we carried out MD simulations on PrLD. We used all-atom simulation with explicit water model (Supplementary information, Fig. S5a) to accurately account for the entropy effects of both the solute and the solvent that are important in temperature-responsiveness. The simulation was performed under the CHARMM36m force field, which has improved accuracy in generating conformational ensembles for IDRs.25 Further, replica-exchange MD (REMD) was applied with a total simulation time of 62 µs to sample sufficient conformations of the PrLD and its mutant variant (see Methods for details). Our simulations revealed that the radius of gyration (Rg), as a metric of the physical size of the PrLD, decreased as the temperature increased (Fig. 3a), which is a hallmark of LCST behavior.23 The same trend was observed for the solvent accessible surface area (SASA) (Supplementary information, Fig. S5b). To investigate the molecular driving force underlying the change of Rg, we analyzed the interactions between amino acids and found that intra-molecular hydrogen bonds (H-bonds, Fig. 3b) and the side chain‒side chain contacts between amino acid residues (Fig. 3c) increased as temperature elevates. It has been reported that the intramolecular contacts at the monomeric level can reflect multivalent intermolecular interactions during phase separation of IDRs.26,27 The increase of intramolecular interactions in PrLD therefore suggests enhanced intermolecular affinity. Indeed, we observed more intermolecular contacts (Fig. 3d, e) and intermolecular H-bonds (Supplementary information, Fig. S5c, d) at higher temperature when two PrLD molecules were simulated. As a comparison, the Rg and SASA of PrLDY>S were much bigger than that of PrLDWT (Fig. 3a; Supplementary information, Fig. S5b) while the number of intramolecular H-bonds and side chain contacts was much less (Fig. 3b, c).
a–c The gyration of radius (Rg, a), the number of intramolecular H-bonds (b), and the side chain-side chain contacts between amino acid residues (c) of PrLDWT or PrLDY>S at elevating temperatures as revealed by MD simulation. d The conformation of two PrLD molecules at 310 K or 280 K as revealed by MD simulation. e The structure prediction shows the intermolecular contacts between two PrLD molecules increase at 310 K or 280 K as revealed by MD simulation. f Diagram showing the change of contacts for each amino acid with other residues in PrLD at 310 K compared to 280 K. Positive and negative values indicate gain and loss of contacts, respectively. g Heat map showing the strength of contacts between indicated residues at 280 K or 310 K. h Diagram showing the β-strand probability across PrLD at 280 K or 310 K. i Schematic diagram showing the lock-to-open conformational switch of PrLD at elevating temperatures. The orange region indicates the β-strand lock. j Illustration of the FRET assay. k–m Quantification of mGFP-lifetime in mGFP-PrLDWT or mGFP-PrLDWT-mCherry (k) mGFP-PrLDM1 or mGFP-PrLDM1-mCherry (l) and mGFP-PrLDM2 or mGFP-PrLDM2-mCherry (m). Error bars indicate mean ± SD (n ≥ 20).
However, when we plotted the difference of contact number between 310 K and 280 K (∆contact) for each residue, we found that while the majority of residues exhibited increased intramolecular contacts, two specific groups of residues (25‒30 and 77‒82, highlighted with blue boxes) exhibited reduced contacts (Fig. 3f). Intriguingly, these residues had strong contacts with each other (Fig. 3g) and together formed high-confident β-strand at 280 K (Fig. 3h; Supplementary information, Fig. S5e). Both the contacts and the β-strand probability reduced at 310 K (Fig. 3g, h). These observations led to the hypothesis that PrLD adopts a locked conformation to prevent intermolecular interactions, which is unlocked by heat, enabling intermolecular interactions thereby phase separation (Fig. 3i).
To test this possibility, we performed Förster resonance energy transfer–fluorescence lifetime imaging (FLIM–FRET) by fusing donor fluorophore mGFP and acceptor fluorophore mCherry to the N and C terminus of PrLD, respectively (Fig. 3j). The lifetime of mGFP can serve as an indicator of lock-to-open conformational switch, as two groups of residues (25‒30 and 77‒82) are positioned near the two ends of the PrLD. The lifetime of mGFP will be short at low temperature and increase upon heating if the proposed conformational switch exists (Fig. 3j). To avoid any impact of protein concentration on FRET efficiency, we quantified donor lifetime only in the dense phases. We found that the lifetime of mGFP in mGFP-PrLDWT-mCherry is much lower than the no acceptor control before heating (Fig. 3k), in agreement with a locked conformation. Post treatment at 37 °C, the lifetime of mGFP rapidly increased (Fig. 3k; Supplementary information, Fig. S5f), suggesting open conformations. It should be noted that the reduction of Rg in simulation only indicates the increase of interactions but not the actual conformational change. We then mutated the residues that are involved in the “lock” formation, resulting M1 (Y27A, L28A, S29A, N30A) and M2 (G77A, Y78A, G79A, P80A) variants. FRET analysis showed that both M1 and M2 exhibited higher mGFP lifetime than PrLDWT and showed only minor increase in response to heat (Fig. 3i, m; Supplementary information, Fig. S5f). Consistently, the phase separation of M1 and M2 showed diminished heat responsiveness as observed in both microscopy and turbidity measurements (Supplementary information, Fig. S5g‒i). Taken together, these results indicate that a lock-to-open conformational switch is essential for PrLD of FUST1 to sense temperature.
FUST1 interacts with and localizes in SGs
To investigate the function of FUST1 condensates, we sought out to characterize the composition. Given the dynamic property of FUST1 condensates (Fig. 1b), we chose TurboID-based proximity labeling as it allows to capture transient interactions.28 pFUST1::FUST1-TurboID/fust1-1 seedlings were labeled at 37 °C for 1 h (Supplementary information, Fig. S6a). YFP-TurboID/Col-0 seedlings were treated and labeled in parallel for background control (Supplementary information, Fig. S6a). With four biological replicates, we identified 438 proteins that were enriched at least two-fold by FUST1-TurboID compared to YFP-TurboID (Supplementary information, Fig. S6a and Table S1). Gene Ontology (GO) analysis of these proteins revealed biological functions related to heat stress response and ribonucleoprotein complex biogenesis (Supplementary information, Fig. S6b). Amongst, known integral components of Arabidopsis SGs, including G3BPs,29 RBP47b,30 PABs,31 UBP1s,31 TSNs,32 ECTs,33 and ALBA434 were highly enriched (Fig. 4a; Supplementary information, Tables S1, S2). In addition, translation factors, ribosomal proteins, tRNA ligases and chaperons, which were reported to be SG constituents in mammalian cells,35 were also highly enriched by FUST1-TurboID (Supplementary information, Tables S1, S2).
a The integral SG components enriched by FUST1-TurboID proximity labeling. Error bars indicate mean ± SD (n = 4). b Volcano plot showing the enrichment of proteins by IP-MS of FUST1 in Arabidopsis. The values were calculated from 4 biological replicates. c Co-immunoprecipitation of FUST1 with indicated proteins expressed in tobacco epidermal cells. Cells were treated at 37 °C for 1 h and crosslinked with UV. Empty vector was included as a negative control. d Representative confocal microscopic images of Arabidopsis root tip cells co-expressing FUST1-mScarlet with G3BP5-mVenus or PAB2-GFP under their native promoters. Roots were treated at 37 °C for 30 min. Scale bars, 10 μm. e FISH with oligodT probes in pFUST1::FUST1-mVenus/fust1-1 roots treated at 37 °C for 30 min. Scale bar, 10 μm. f Partitioning of 2.5 μM indicated proteins by 2.5 μM FUST1 droplets in 40 mM Tris-HCl pH 7.4 and 100 mM NaCl in vitro. Scale bars, 5 μm.
To confirm the interactors of FUST1, we performed immunoprecipitation and mass spectrometry (IP-MS) of FUST1 in vivo before and after heat treatment. UV crosslinking was utilized to capture transient protein‒RNA interactions. The results showed that 412 proteins were identified from heat-treated seedlings, including most of the SG components (Fig. 4b; Supplementary information, Table S3). These proteins exhibited similar GO terms to those enriched in TurboID-based proximity labeling assay (Supplementary information, Fig. S6c). The overlapped proteins between FUST1 proximity labeling and IP-MS were mainly involved in stress granule assembly and heat signaling pathway (Supplementary information, Fig. S6e, f). As a comparison of FUST1 IP-MS, fewer SG components were identified in untreated seedlings (Supplementary information, Fig. S6d), suggesting that the interactions with FUST1 are heat-dependent. Some of these interactions were validated by co-immunoprecipitation assay in tobacco epidermal cells (Fig. 4c). Consistent with their interactions, G3BP5 and PAB2 colocalized with FUST1 condensates in stable transgenic Arabidopsis (Fig. 4d) and all selected SG markers colocalized with FUST1 when co-expressed in tobacco cells (Supplementary information, Fig. S6g). SGs are cytoplasmic mRNP granules that form from mRNA stalled in translation initiation.14 Therefore, polyA mRNA condensates were used as a proxy to represent SGs.36 In line with this, fluorescence in situ hybridization (FISH) assay using Cy5-oligo deoxythymidine (dT) probe revealed complete colocalization of FUST1 condensates with mRNAs (Fig. 4e). Additionally, cycloheximide (CHX), which blocks the assembly of SGs,15 inhibited FUST1 condensation (Supplementary information, Fig. S6h). Together, these results indicate that FUST1 associates with SGs.
The colocalization of FUST1 condensates with mRNAs promoted us to interrogate the influence of RNA on FUST1 condensation. Addition of low amount of total RNA purified from Arabidopsis seedlings strongly promoted the degree of FUST1 condensation in vitro (Supplementary information, Fig. S7a). Both oligodT-enriched mRNA (polyA+) and oligodT-depleted RNA (polyA‒) enhanced FUST1 condensation as potently as total RNA (Supplementary information, Fig. S7b). Consistent with the in vivo colocalization, FUST1 condensates partitioned RNAs in vitro (Supplementary information, Fig. S7c). We next tested whether FUST1 possesses RNA-binding activity. In the electrophoretic mobility shift assay (EMSA), a 118-nt long RNA of random sequence formed high molecular weight complexes with FUST1 (Supplementary information, Fig. S7e), suggesting non-specificity for FUST1 RNA-binding. A patch towards the N-terminus of FUST1 had a net positive charge density (Supplementary information, Fig. S7d). Indeed, FUST1 with the positive patch deleted (FUST1∆PP) failed to form RNA‒protein complexes in the EMSA assay (Supplementary information, Fig. S7e). These results indicate that FUST1 condensates partition mRNAs via electrostatic interactions.
FUST1 condensation is indispensable for SG assembly
To establish the relationship between FUST1 and SG proteins, we carried out in vitro partitioning assay. We prepared recombinant SG markers including G3BP5, PAB2, RBP47b, TSN2 and ECT2 and found that none of them alone formed condensates in vitro at either low or high temperature (Supplementary information, Fig. S8a). Instead, all of the selected SG proteins were partitioned by FUST1 condensates (Fig. 4f). As negative controls, an RNA-binding protein KH22, which was not enriched by FUST1 proximity labeling, and mCherry were not partitioned by FUST1 condensates (Supplementary information, Fig. S8b, c). Intriguingly, the partitioning of selected SG proteins was dramatically enhanced at 37 °C compared to 4 °C (Supplementary information, Fig. S8b, c). These results suggest that FUST1 could be a driver for SG assembly.
We next sought to determine the hierarchy relationship between FUST1 and SG markers in vivo. In the literature, temperatures at or above 37 °C and duration of treatment longer than 10 min were used to induce G3BP5-,37 PAB2-,30 RPB47b-,38 TSN2-,32 and ECT2-labeled33 SGs. We tested this in stable transgenic plants expressing each of the SG markers under the corresponding native promoter. We confirmed that condensation of SG markers required temperatures above 35 °C and at least 10 min (Fig. 5a; Supplementary information, Fig. S9). In contrast, FUST1 condensation occurred below 35 °C and as quickly as 2 min at 37 °C (Fig. 5a; Supplementary information, Fig. S9), suggesting that condensation of FUST1 and SGs is a sequential event with FUST1 being earlier than SG formation.
a Confocal microscopy images of Arabidopsis root tip cells expressing indicated proteins under the corresponding native promoters. The roots were treated as indicated. Scale bars, 10 μm. b Representative confocal microscopic images of Arabidopsis root tip cells co-expressing FUST1-mScarlet with G3BP5-mVenus or PAB2-GFP under their native promoters. Roots were treated at 33 °C for 90 min. Scale bars, 10 μm. c Time-lapse imaging of Arabidopsis root tip cells co-expressing G3BP5-mVenus or PAB2-GFP with FUST1-mScarlet under their corresponding native promoters. The plants were treated at 37 °C for the indicated time. Scale bars, 1 μm. d, e Left, Confocal microscopic images of Arabidopsis root tip cells expressing indicated proteins under corresponding native promoters in FUST1 wild type background or fust1-2 mutants. The roots were treated at 37 °C for the indicated time. Scale bars, 10 μm. Right, Statistical analysis of the number of condensates per cell in the indicated genotypes. Error bars indicate mean ± SD (n = 15). P values were calculated using two-sided Student’s t-test. ****P < 0.0001. f FISH with Cy5-oligodT probes in Col-0 and fust1 mutants treated at 37 °C for 30 min. Scale bar, 5 μm. P values were calculated using two-sided Student’s t-test. ****P < 0.0001.
To more accurately compare the dynamics of FUST1 with SG components during condensation, we co-expressed FUST1-mScarlet transgene with G3BP5-mVenus or PAB2-GFP transgene and performed double-colored time-lapse live imaging in the same root cells. The results revealed that below SG threshold temperature, FUST1 condensation occurred autonomously without G3BP5 or PAB2 (Fig. 5b), and above SG threshold temperature, FUST1 condensates formed first and G3BP5 or PAB2 was subsequently condensed into FUST1 condensates (Fig. 5c). These observations prompted us to test whether FUST1 is required for heat-induced SG assembly. Herein, we crossed fust1-2 mutant into the SG marker lines and found that the formation of PAB2, and G3BP5-labeled SGs was drastically delayed in fust1-2 background (Fig. 5d, e). In line with this, FISH assay revealed that polyA RNA condensates were drastically reduced in fust1 mutants (Fig. 5f). Together, these data demonstrate that FUST1 condensation precedes condensation of several known SG markers and is indispensable for SG assembly.
FUST1 confers both basal and acquired heat tolerance
To assess the functional significance of FUST1 condensation, we first examined the basal thermotolerance (Fig. 6a). Five-day-old seedlings were subjected to heat shock at 45 °C for 2 h and stained with propidium iodide (PI) to assess cell viability. While the majority of Col-0 plants showed no PI signal, fust1 mutants had a much higher frequency of PI staining and this defect was fully recovered by FUST1 re-expression (Fig. 6b; Supplementary information, Fig. S10a), suggesting that FUST1 is necessary for early heat signaling events that confer cell survivability. And we observed the germination ability of fust1 mutant seeds. Seeds of fust1-2 showed decreased germination rate upon heat treatment, whereas we rescued this phenotype by expressing FUST1, while the germination rate was similar to Col-0 under normal condition (Fig. 6c; Supplementary information, Fig. S10b). To assess the phenotypes at tissue level, seedlings were recovered at normal growth temperature for 7 days after heat treatment to allow cell division and elongation. Compared to Col-0, fust1 mutants had significantly higher fraction of pale or white sectors on leaves that are indicative of cell death and exhibited retarded growth and less fresh weight (Fig. 6d, e; Supplementary information, Fig. S10c). We next performed prolonged heat stress by placing five-day-old seedlings under 37 °C for 2 days. Post recovery for 5 days, we observed that root growth of fust1-1 seedlings was much slower than Col-0 (Supplementary information, Fig. S10d). The defect of fust1 mutant in heat tolerance can be fully rescued by expressing full-length FUST1 but not by the variant lacking PrLD (Fig. 6b‒e; Supplementary information, Fig. S10d), further supporting the importance of PrLD for FUST1 function. These data indicate that FUST1 is required for basal thermotolerance.
a Scheme of heat exposures in basal and acquired tolerance assays. b Left, cell death staining of Arabidopsis roots upon heat stress treatment at 42 °C for 2 h. Scale bars, 100 μm. Right, the percentage of seedlings containing cell death. Error bars indicate mean ± SD (n = 3). Twenty seedlings were assayed in each replicate. P values were calculated using two-sided Student’s t-test. ***P < 0.001, ****P < 0.0001. ns no significance. c Germination rate of Arabidopsis seeds after heat treatment at 50 °C for 60 min. Error bars indicate mean ± SD (n = 4). Each replicate contains 49 seeds. P values were calculated using two-sided Student’s t-test. **P < 0.01. d, f Phenotypes of indicated seedlings assayed for basal (d) or acquired (f) heat tolerance. Pictures were taken at seven days (d) or four days (f) of recovery after treatment as indicated. Scale bars, 0.5 cm. e, g Quantification of the damage rate and fresh weight of seedlings shown in d, f. Error bars indicate mean ± SD (n = 3). At least 50 seedlings were assayed for each replicate. P values were calculated using two-sided Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Plants acquire enhanced tolerance to stress through prior exposure to a milder challenge, a process known as defense priming.39 Given that FUST1 formed condensates in response to mild temperatures, we tested whether FUST1 is necessary for acquired heat tolerance. Seedlings were exposed to a priming period at 33 °C for 2 h followed by a recovery phase for 1 h (Fig. 6a) and subsequent heat shock at 45 °C for 4 h. While almost all seedlings of both wild type and mutant died without priming, over 90% of wild-type Col-0 seedlings survived after priming (Fig. 6f, g), indicating that wild-type seedlings acquired increased thermotolerance upon priming at 33 °C. In contrast, more than 70% of the fust1-2 seedlings either developed severely damaged leaves or completely died even after priming (Fig. 6f, g), indicating that the thermotolerance acquired by pre-treatment at 33 °C is FUST1-dependent. Consistent with the essential role of molecular chaperone HSP101 in acquired thermotolerance,40 the majority of hsp101 mutant plants died after priming (Fig. 6f, g). Measurement of the fresh weight of those seedlings confirmed this trend (Fig. 6g). These results demonstrate that FUST1 is indispensable for acquired thermotolerance.
Temperature-dependent condensation of FUST1 is conserved in plants
To assess whether the condensation of FUST1 is conserved in plants, we explored the diversity of this protein in other plant species. FUST1 homologs were found in all land plants but not in green algae (Supplementary information, Fig. S11a). Multiple sequence alignment of FUST1 homologs from angiosperms revealed that conservation score of the PrLD was much higher than other IDRs (Supplementary information, Fig. S11b). In particular, the tyrosine residues, which are key for heat-dependent phase separation, were highly conserved (Supplementary information, Fig. S11c). The sequence comprising the β-strand lock is also conserved (Supplementary information, Fig. S11b, c), suggesting that “lock-to-open” mechanism for heat sensing in PrLD is preserved during evolution. We expressed FUST1 of maize, soybean and Chinese cabbage in tobacco epidermal cells and found that they all exhibited heat-dependent condensation (Supplementary information, Fig. S11d). Like Arabidopsis FUST1, in vitro phase separation of all homologs was heat-dependent as revealed by DLS assay (Supplementary information, Fig. S11e). These results suggest that FUST1 is functionally conserved in plants.
Discussion
In summary, our findings demonstrate that an uncharacterized protein, FUST1, directly senses elevated temperature, giving rise to heat-dependent condensation in Arabidopsis. Notably, FUST1 condensation precedes condensation of several known SG markers, priming the assembly of SGs thereby supporting cell survival under heat stress (Fig. 7). The combination of all-atom MD simulations and biophysical analyses allow us to uncover the mechanism of thermosensing by FUST1. The PrLD has a strong ability to drive phase separation which is autoinhibited via a β-strand lock under low temperature. Increasing temperature tends to break the β-strand, allowing intermolecular interactions to occur and releasing phase separation. Our data also indicate that the sticker tyrosine residues are crucial for both phase separation and heat-dependent conformational rearrangements, but the spacer residues, in particular the hydrophobic ones, also make key contributions. Future work should pinpoint the sequence grammar for thermosensing. Heat-induced conformational changes were reported recently for a transcriptional co-regulator TWA1 in Arabidopsis10 and translation initiation factor eIF4G in yeast,11 resulting in changes of interaction partners and condensate formation. Arabidopsis EARLY FLOWERING 3 (ELF3), a component of the evening complex, senses heat via its PrLD8 with the mechanism being obscure. The atomic simulation and biophysical approaches established in this study will shed light on understanding the molecular basis of heat sensing by both globular and disordered proteins.
Further, autoinhibition mechanism was also described for G3BP phase separation during mammalian SG formation. In this scenario, two IDRs within G3BP interact, giving rise to a more closed conformation that reduces its valency. When RNA reaches a certain threshold, it competes with IDR for interactions, disrupting intramolecular interactions and allowing the protein to undergo RNA-dependent phase separation.16,18 We envisage that autoinhibition could be a general strategy in the regulation of phase separation. While extensive studies have focused on the electrostatics and multivalent weak interactions at the amino-acidic level, our work suggests that dynamic secondary structure, such as β-strand, could be an overlooked yet crucial feature for specific IDR sequences in driving and modulating phase separation.
According to the studies in mammalian cells, SGs are further separated into internal structures including cores and shells.35,41 The cores are more dense and solid while the shell is less concentrated and more dynamic. It has been under debate how the substructures in SGs are assembled. Two models, “core first” model and LLPS first model, were postulated.14 We propose that FUST1 forms the shell network that allows the “cores” to assemble. The interactions between FUST1 and the “cores” are weak and transient, making the SG dynamic. Indeed, biochemical purification of the solid-like “cores” of SG from Arabidopsis identified proteins including SG markers we used in this study but not FUST1.38 Future investigation of FUST1 should help us to understand the exact mechanism of SG assembly.
Arabidopsis acquires thermotolerance mostly by priming at 37 °C or higher,10,42 which induces the production of signals including but not limited to heat shock proteins,39 epigenetic factors,43 metabolites44 that can last for short period of time. We show that a milder temperature at 33 °C also acquires increased heat stress tolerance in a FUST1-dependent manner, implying that biomolecular condensates could be a novel mechanism for short-term memory that enables plant acclimation to stress. Due to its dynamicity and reversibility, we propose that FUST1 condensates act as an alarm system that warns the plants of upcoming severe heat stress.
Materials and methods
Plant materials and growth conditions
The T-DNA insertion mutants fust1-1 (SALK_116148C) and hsp101 (SALK_099583C) were obtained from the AraShare. The pPAB2::PAB2-GFP/pab2/8 transgenic line was described previously.45 Seeds were sterilized and sown on half-strength Murashige and Skoog (MS, PhytoTech, M519) medium with 1.0% sucrose, 0.4% phytagel (Sigma, P8169) at pH 5.8. Plate media were stratified at 4 °C for 2 d and transferred to a growth chamber under long-day condition (16-h light at 22 °C and 8-h darkness at 18 °C).
DNA constructs
To generate pFUST1::FUST1-mVenus, pRBP47B::RBP47B-mVenus, pG3BP5::G3BP5-mVenus, pECT2::ECT2-mVenus and pTSN2::TSN2-mVenus constructs, genomic DNA fragments including the promoter regions of approximately 2 kb were amplified from wild-type Col-0 genomic DNA and cloned into the pCAMBIA1300-N1-mVenus vector (digested with HindIII). To generate pFUST1::FUST1∆PrLD-mVenus constructs, FUST1 genomic sequence lacking PrLD-coding region was generated and inserted into pCAMBIA1300-N1-mVenus vector (digested with HindIII). All the constructs were introduced into Agrobacterium tumefaciens strain GV3101 and transformed into fust1 mutants or wild-type Col-0 plants via the standard floral dipping method. Positive transformants were selected on half-strength MS plates containing 30 mg/L hygromycin (AMRESCO, K547). Homozygous transgenic lines were used for experiments.
The construct for generating fust1-2 CRISPR mutant was based on CRISPR-Cas9 system reported previously.46 Two sgRNAs were designed for FUST1, and inserted into the BbsI sites of the pAtU6-26-M vector. The primers used for annealing are listed (Supplementary information, Table S4). The Cas9 cassettes were subcloned into KpnI and EcoRI sites of pCambia1300-UBQ: Cas9-P2A-GFP-rbcS-E9t.46
The constructs for expression in tobacco epidermal leaf cells were cloned by inserting the coding sequences into the pCAMBIA1300-35S-N1-mVenus, pCAMBIA1300-35S-N1-Flag or pCAMBIA1300-35S-N1-mScarlet vector (digested with KpnI).
To generate the constructs used for heterologous expression in yeast cells, the coding sequences of FUST1 and its variants were amplified and inserted into the pDUAL-Pnmt1-yeGFP vector (digested with NheI and BamHI).
To generate the constructs used for in vitro protein expression, coding sequences were amplified and inserted into the pET11-6× His-GFP, pET11-6× His-mCherry (digested with NheI and BamHI) or pET11-6× His expression vector (digested with NheI and XhoI). Where necessary, a maltose-binding protein (MBP) solubility tag was placed at the N-terminus of the construct, followed by a tobacco etch virus (TEV) protease cleavage site.
All cloning was performed using the ClonExpress II One Step Cloning kit (Vazyme, C112). Primer sequences are provided (Supplementary information, Table S4).
Quantitative RT-PCR analysis
Total RNA was extracted from Arabidopsis seedlings using TRIzol reagent (Invitrogen, 15596018) according to the manufacturer’s protocol. Contaminating DNA was removed using DNase I (Promega, M6101). Reverse transcription was performed by M-MLV reverse transcriptase (Invitrogen, 28025013) and by oligo(dT) primer. Quantitative PCR reactions were performed using the Applied Biosystems® 7500 fast with 2× M5 HiPer SYBR Premix EsTaq (Mei5 Biotechnology, MF787-T) in a final volume of 20 μL. Tubulin was used as an internal control. The primers used for PCR are listed (Supplementary information, Table S4).
GUS staining
Ten-day-old pFUST1::GUS/Col-0 seedlings were immersed in the GUS staining solution (0.5 mg/mL X-glucuronide in 100 mM sodium phosphate pH 7.0, 0.5 mM ferricyanide, 0.05 mM ferrocyanide, 1 mM EDTA, and 0.1% Triton X-100) for 12 h at 37 °C. After staining, the seedlings were washed several times in 75% (v/v) ethanol to clear chlorophyll.
Yeast transformation for heterologous expression
The plasmids were linearized with NotI and the fragments were gel-purified and transformed into the fission yeast strain LD328 (genotype his3-D1 leu1-32) as described previously.47 Briefly, yeast cells were cultured until the OD600 reached 0.4‒0.8. For each reaction, 500 µL cultured cells were collected, washed three times with sterilized water and resuspended in buffer I (240 µL of 50% PEG3350, 36 µL of 1.0 M LiAc and 50 µL of 2.0 mg/mL carrier DNA). The linearized DNA (34 µL, up to 1 µg) was added to the resuspended cells, mixed vigorously and incubated at 42 °C for 40 min. The cells were collected and resuspended in 100 µL water and plated on EMM + HT (EMM medium supplemented with 45 mg/L histidine and 15 µM thiamine) plates. After incubation at 30 °C for 2‒3 days, individual colonies were selected on EMM + H (EMM medium supplemented with 45 mg/L histidine) plates. The cells were used for subsequent imaging analyses.
Fluorescent imaging of cells and tissues
Vertically grown Arabidopsis seedlings or a tobacco leaf were soaked in liquid half-strength MS medium and treated at the indicated temperatures for the indicated time. The root tip or a small leaf disc was mounted on a slide, covered with a coverslip and immediately imaged under a Zeiss LSM880 confocal microscope. GFP was excited at 488 nm and detected at 491‒535 nm. mVenus was excited at 514 nm and detected at 529‒570 nm, mScarlet or mCherry was excited at 561 nm and detected at 575‒625 nm. The signal of mVenus and mScarlet was acquired sequentially to avoid cross contamination.
For time-lapse imaging of FUST1 condensation, four-day-old vertically grown Arabidopsis seedlings were embedded with solid half-strength MS medium in 15 mm Glass Bottom Cell Culture Dish (NEST, 801002). The dish was imaged at 37 °C on Nikon AX R confocal microscope equipped with Okolab microscope incubator using 40× 0.95-NA objective. Excitation and emission of mVenus and mScarlet were as mentioned above. The interval of image acquisition is 1 min.
For imaging of yeast cells, a colony grown on a medium plate was inoculated into liquid medium and cultured overnight (OD600 < 1.5). Ten microliters of cells were collected by centrifugation and resuspended in liquid medium with or without heat treatment. The cells were sprayed onto a slide and covered with a coverslip. Imaging was performed on a Zeiss LSM880 with Airyscan (AxioObserver 7) mode confocal laser microscope. GFP was excited as mentioned above.
Protein expression and purification
All proteins were expressed in the Escherichia coli Rosetta strain. Cells were cultured at 37 °C until the OD600 reached 0.4 and induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 16 °C for 16‒20 h. Cells were collected, resuspended in lysis buffer (40 mM Tris-HCl pH 7.4, 500 mM NaCl, 10% glycerol) and sonicated for lysis. The lysates were centrifuged at 15,000 rpm for 60 min at 4 °C and the supernatants were passed through Ni-NTA column (Smart-Lifescience, SA004100). The bound proteins were washed with wash buffer (40 mM Tris-HCl pH 7.4, 500 mM NaCl, 20 mM imidazole), eluted with elution buffer (40 mM Tris-HCl pH 7.4, 500 mM NaCl, 500 mM imidazole) and further purified on a Superdex 200 Increase 10/300 column (GE Healthcare, 28990944). Proteins were stored in storage buffer (40 mM Tris-HCl pH 7.4, 500 mM NaCl, 1 mM DTT). The quality of purified proteins was determined by SDS-PAGE and Coomassie blue staining.
Fluorophore labeling of proteins
Fluorescent labeling dye Cy5 NHS esters (AAT Bioquest) were dissolved in DMSO as a stock at the concentration of 10 mg/mL. The dye was added to the purified protein in 20 mM HEPES, pH 7.5, 500 mM NaCl, 1 mM DTT to the final concentration of 10 µg/mL. The labeling was carried out with gentle rotation at 4 °C overnight in the dark, and then quenched by adding 200 mM Tris-HCl. The unbound dye was removed using three concatenated 5 mL HiTrap desalting columns (GE Healthcare, GE17-1408-01).
In vitro phase separation assay
For in vitro phase separation, the solubility tag MBP was removed by TEV protease. TEV cleavage efficiency was confirmed by SDS-PAGE analysis. The proteins were diluted to desired concentrations with indicated ionic strengths. The protein samples were incubated at indicated temperatures for indicated time before imaging. For the in vitro phase separation assay of Cy5-labeled PrLDWT at different temperatures (Fig. 2c), proteins were diluted to desired concentrations in 40 mM Tris-HCl pH 7.4 and 500 mM NaCl. Each image represented an independent experimental result, and the protein was incubated at the indicated temperature for 15 min before imaging. To analyze the effect of RNA on FUST1 phase separation, various concentrations of Arabidopsis total RNA, polyA+ RNA or polyA‒ RNA were added to the protein samples in 40 mM Tris-HCl pH 7.4, 100 mM NaCl. RNAs were visualized by GelRed (Mei5 Biotechnology, MF079-plus-01). Droplets were imaged in a 384-well, low-binding multiwell 0.17-mm microscopy plate (Greiner bio-one, 781090) using a Zeiss LSM880 confocal microscope equipped with a 63× 1.40-NA oil objective. The quantification of mean intensity was performed in ImageJ.
FRAP assay
FRAP of FUST1 condensates in Arabidopsis root cells was performed on a Zeiss LSM880 confocal laser microscope. After 5 acquisitions, ROI(s) corresponding to FUST1 condensate(s) was bleached with 5 iterations using a laser intensity of 100% at 488 nm. mVenus fluorescence was detected using a 63× 1.40-NA oil objective. Recovery was recorded for every second for a total of 30 s after bleaching.
In vitro FRAP of FUST1 droplet was carried out with protein samples in 384-well microscopy plates using a Zeiss LSM880 confocal laser microscope equipped with 100× oil immersion objective. A small area of the droplet was bleached with 5 iterations of 100% laser intensity at 488 nm. After bleaching, recovery was recorded for every second for a total of 150 seconds.
Turbidity measurements
The proteins were diluted to desired concentrations in 40 mM Tris-HCl pH 7.4, 100 mM NaCl or 500 mM NaCl. The protein samples of 100 µL were transferred into flat bottom 96-well plates (Corning, 3364) on ice. Temperature-dependent turbidity measurements were performed on VARIOSKAN FLASH (Thermo) with temperature increasing from 22 °C to 45 °C at a rate of 0.5 °C/min. The absorbance at 600 nm or 350 nm was recorded at each temperature point.
DLS measurements
The proteins were diluted to desired concentrations in 40 mM Tris-HCl pH 7.4, 100 mM NaCl. The protein samples of 200 µL were transferred into quartz cuvette (WYATT, JC-0247). DLS measurements were performed using DynaPro NanoStar (Wyatt). For data collection, each time point was the average of three 0.25 s acquisitions filtering out samples with a baseline higher than 1.003. The temperature was set to increase at the speed of 1 °C/min. Measurements were recorded every minute. Data were analyzed in the DYNAMICS software with a cumulant fit to the autocorrelation function. Protein concentrations are specified in the Figure legend.
CD spectroscopy
CD measurements were performed on a Chirascan plus (Aimil) using 1 mm path length cuvette. The FUST1 protein was diluted to a final concentration of 0.1 mg/mL (1.07 µM) in 40 mM Tris-HCl pH 7.4, 50 mM NaCl. CD spectra were recorded from 260 nm to 200 nm with a bandwidth of 1 nm, a 0.5 s integration time with data collected every 1 nm. Each data point was the average of 3 scans. Spectra were baseline corrected using buffer.
FLIM-FRET
Protein samples were incubated with 6% PEG6000 in 40 mM Tris-HCl, pH 7.4 and 100 mM NaCl to facilitate droplet formation in 384-well microscopy plates. FLIM-FRET was performed using a Leica TCS SP8 laser-scanning confocal microscope equipped with a 100×/1.40-NA oil immersion objective. Samples were scanned with a slow speed of 100 Hz with a repetition rate of 80 MHz. mGFP fluorescence was excited at 488 nm and detected at 500‒540 nm using the in-built hybrid detector. A time-correlated single photon counting (TCSPC) system was used for recording photon events.
All FLIM data analysis was performed using Leica LAS X FLIM FCS software. The minimum threshold count of recorded photons for modeling was 100. The recorded TCSPC photon arrival time histogram of mGFP-PrLDWT-mCherry or mGFP-PrLDM1/2-mCherry showed multi-exponential decay. Therefore, the photon arrival times were fitted to a double-exponential reconvolution function, allowing the calculation of mean lifetime by intensity weight. A minimum of 20 ROIs were selected for analysis.
All-atom MD simulation
The initial structures of the wild-type and the mutant (Y > S) PrLD were predicted by the AlphaFold2 program48 based on their amino acid sequence. The protein was put into a simulation box with the dimensions of 7 × 7 × 7 nm solvated with explicit water molecules. Chloride ions were added to make the simulation system electrostatically neutralized. The final simulation system contains approximately 34,000 atoms. The protein was described by the CHARMM36m force field. The TIP3P model49 was used to represent water molecules. After energy minimization with the steepest descent method, a 200 ns MD simulation at a pressure of 1 bar and temperature of 323 K was performed. From the last 100 ns trajectory, 62 simulation systems with different protein conformations were randomly extracted for the REMD simulations50 with the temperature ranging from 280 K to 316 K. 500 ns MD sampling simulations under NPT ensemble were subsequently performed for each replica. Exchanges between two replicas were attempted every 2 ps, resulting in a mean acceptance ratio of 20%. In addition, 2 µs conventional MD simulation was carried out to investigate the intermolecular interactions between two PrLD at 280 K and 310 K, respectively.
All simulations were performed using the Gromacs 2019 package.51 The pressure was maintained at 1 bar using the Parrinello-Raham barostat52 with a relaxation time of 2.0 ps, the temperatures were kept at the chosen values using the velocity rescaling method53 with a coupling constant of 0.1 ps. Bonds containing hydrogen were constrained by the LINCS algorithm.54 The non-bonded interactions were calculated at a cutoff distance of 1.0 nm, the particle mesh Ewald (PME) method55 with a cutoff radius of 1.0 nm was employed to treat the long-range electrostatic interactions. The equations of motion were integrated using a leap-frog algorithm with a time step of 2 fs. 3D periodic boundary conditions were applied to all the simulations.
The protein structures sampled by REMD simulations were characterized by Rg, intramolecular H-bond, side chain-side chain contacts, solvent accessible surface areas and beta-strand probability. The data analysis was based on simulations from 100 to 500 ns, with the first 100 ns of REMD trajectories discarded to avoid bias from the starting conformation. The formation of side chain‒side chain contact was defined if the minimum distance between two residue side chains is less than 0.6 nm. Intramolecular contacts were ignored between three adjacent residues. The interactions between two PrLD molecules were characterized by intermolecular H-bonds and contacts, and the last 1 µs simulation data were used as statistical averages. H-bonds were defined based on distance and angle with the acceptor-donor distance being within 0.35 nm and the acceptor-donor-hydrogen angle being less than 30°. The beta-strand potential was calculated with the DSSP method.56 All trajectory analyses were accomplished using our in house developed scripts and tools implemented in the Gromacs and MDTraj packages.57 For structural visualization and animation production, the Visual Molecular Dynamics (VMD) program was used.58
Assays for heat stress tolerance
For basal tolerance analysis, five-day-old Arabidopsis seedlings grown horizontally on half-strength MS plates were treated at 45 °C for 3 h and recovered at 22 °C for 7 days in the growth chamber. Photographs were taken and the root lengths were measured.
For the acquired heat tolerance response, five-day-old Arabidopsis seedlings grown either horizontally or vertically on half-strength MS plates were treated at 33 °C for 2 h, recovered at 22 °C for 1 h and then treated at 45 °C for 4 h in the growth chamber. As no priming control, seedlings from the same batch were treated in parallel except for 33 °C treatment. The seedlings were then assayed for survival rate, fresh weight or root length. Photographs were taken and the damage rates were analyzed after recovery at 22 °C for 4 days.
Seed germination assays
For germination analysis, about 50 sterilized seeds were treated at 50 °C or 22 °C for 1 h in distilled water, placed onto half-strength MS medium plates and incubated in a growth chamber under long-day condition (16-h light at 22 °C and 8-h darkness at 18 °C). Testa and endosperm rupture were photographed over time through a Stereo Microscope (Nikon, SMZ18).
PI staining
To detect cell death of Arabidopsis seedlings after heat treatment, PI staining was performed. Five-day-old seedlings were treated at 42 °C for 2 h in ½ MS liquid medium. Roots were subsequently stained with 10 μg/mL PI (MedChemExpress, HY-D0815) for 5 min at room temperature, washed once in distilled water and imaged under Zeiss LSM880 confocal microscope. The excitation and emission wavelengths are 561 nm and 591‒635 nm, respectively. The ratio of seedlings with PI staining was calculated.
Immunoprecipitation with UV crosslinking
Seven-day-old pFUST::FUST1-mVenus/fust1 and Col-0 seedlings grown on half-strength MS plates were treated with or without heat stress at 37 °C for 1 h. Seedlings were immediately crosslinked by 600 mJ/cm2 UV in cold phosphate buffer saline (PBS) buffer (pH 7.4). After crosslinking, 2 g seedlings were fast-frozen and ground in liquid nitrogen. The resulting fine powder was resuspended in 2 mL of lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 4 mM MgCl2, 0.5% NP-40, 5 mM DTT, 1× Protease Inhibitor Cocktail) and incubated at 4 °C for 30 min with rotation. After filtering by Miracloth (Millipore, 475855-1RCN), the supernatant was incubated with 10 μL of GFP-Nanoab-Magnetic Beads (LABLEAD, GNM-25-1000) for 30 min at 4 °C. The beads were washed four times with lysis buffer and then boiled in SDS loading buffer. Four biological replicates were performed for this experiment.
Proximity labeling with Turbo-ID
Ten-day-old seedlings of 35S::YFP-TurboID/Col-0 and pFUST1::FUST1-TurboID/fust1-1 were treated at 37 °C for 1 h in half-strength liquid MS medium containing the final concentration of 50 μM biotin (Invitrogen, B1595). The seedlings were washed three times with distilled water and flash-frozen with liquid nitrogen. The tissues were ground to fine powder and resuspended with 2 mL ice-cold extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 1× Protease Inhibitor Cocktail) per gram powder. Lysates were centrifuged twice at 4 °C for 10 min and the supernatant was passed through 0.22 μm filter (Millipore, SLGVR33RB).
To remove free biotin, extract was run through three concatenated 5 mL HiTrap desalting columns (GE Healthcare, GE17-1408-01) with desalting buffer (50 mM Tris-HCl, pH 7.5, 0.05% Triton X-100) on AKTA protein purification system (GE Healthcare). Protein fractions were collected based on UV280 peak and fractions with high conductivity were discarded.
A final concentration of 150 mM NaCl was added to the desalted extract. The extract was incubated with 100 μL streptavidin magnetic beads (MCE, HY-K0208) on a rotor at 4 °C overnight. The beads were washed with wash buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Triton X-100, 1 mM PMSF) three times and boiled in SDS loading buffer. Four biological replicates were performed for this experiment.
Mass spectrometry and data analysis
Mass spectrometry was performed as described previously.20 Briefly, protein samples were separated by SDS-PAGE and digested in-gel with trypsin (0.5 ng/μL). The peptides were extracted from gel slices, separated by HPLC and sprayed into an LTQ Orbitrap Elite System mass spectrometer (Thermo Fisher Scientific). A database search was performed on the MASCOT server (Matrix Science Ltd) against the IPI (International Protein Index) Arabidopsis protein database. The relative amount of each protein was determined by label-free quantification as described.59 Briefly, the area of each protein was calculated by averaging the mean area of the three highest peptides from each replicate. The missing values were replaced by the average of minimum values from all replicates.
For proximity labeling data, fold change was calculated by dividing the area of each protein in FUST1-TurboID with the corresponding value in background control YFP-TurboID. The proteins with log2Fold-change ≥1 were considered as enriched proteins.
For IP-MS data, fold change was calculated by dividing the area of each protein in FUST1-mVenus with the corresponding value in background control Col-0. P value was calculated with the limma package.60 Proteins with an adjusted P < 0.05 and log2Fold-change ≥1 were considered as being enriched by FUST1.
Co-immunoprecipitation
Approximately 0.5 g of Nicotiana benthamiana leaves co-expressing FUST1 with other proteins were ground to a fine powder in liquid nitrogen. The powder was lysed with 2 mL lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 4 mM MgCl2, 0.5% NP-40, 5 mM DTT, 1× Protease Inhibitor Cocktail) at 4 °C for 30 min. After filtration through Miracloth (Millipore, 475855-1RCN) and a brief centrifugation, the supernatant was incubated with 10 μL of FLAG-Nanoab-Magnetic Beads (LABLEAD, FNM-25-1000) for 30 min at 4 °C. The beads were washed four times with lysis buffer and boiled in SDS loading buffer. The immunoprecipitates were subjected to Western blot analyses.
Western blot analysis
Protein samples were resolved by SDS-PAGE and transferred to PVDF membranes. Antibodies against GFP (Roche, 11814460001) and Flag (Sigma, F1804) were used as primary antibodies. After the primary antibody incubation, horseradish peroxidase (HRP)-conjugated secondary antibodies (GE Healthcare) were used for protein detection by chemiluminescence (Thermo FIsher Scientific, 34095).
FISH
RNA FISH was performed as previously described.61 Roots from seven-day-old seedlings were treated at 37 °C for 1 h, fixed in 4% paraformaldehyde for 30 min at room temperature with gentle vacuum. The roots were washed twice with 1× PBS and arranged onto a slide and covered with a coverslip. The samples were squashed, flash-frozen for ~5 s in liquid nitrogen and air-dried at room temperature for 30 min. The samples were then permeabilized in 70% ethanol for 2 h and washed twice with wash buffer (10% formamide, 2× SSC). 100 µL of hybridization buffer (100 mg/mL dextran sulphate, 10% formamide, 2× SSC) containing 1 µM 5’-Cy5-T35 probe was added to each slide and incubated at 45 °C chamber overnight in the dark. After hybridization, the samples were washed twice in wash buffer (10% formamide, 2× SSC) in the dark and imaged by Nikon AX R with NSPARC confocal microscope system using a 100×/1.45 oil objective.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this manuscript.
Data availability
All data are available in the main text or the supplementary materials. The plasmids that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
29 May 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41422-025-01134-3
References
Battisti, D. S. & Naylor, R. L. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 323, 240–244 (2009).
Verslues, P. E. et al. Burning questions for a warming and changing world: 15 unknowns in plant abiotic stress. Plant Cell 35, 67–108 (2023).
Guihur, A., Rebeaud, M. E. & Goloubinoff, P. How do plants feel the heat and survive? Trends Biochem. Sci. 47, 824–838 (2022).
Kerbler, S. M. & Wigge, P. A. Temperature sensing in plants. Annu. Rev. Plant Biol. 74, 341–366 (2023).
Vu, L. D., Gevaert, K. & De Smet, I. Feeling the heat: searching for plant thermosensors. Trends Plant Sci. 24, 210–219 (2019).
Jung, J. H., Seo, P. J., Oh, E. & Kim, J. Temperature perception by plants. Trends Plant Sci. 28, 924–940 (2023).
Liu, X., Zhu, J. K. & Zhao, C. Liquid-liquid phase separation as a major mechanism of plant abiotic stress sensing and responses. Stress Biol. 3, 56 (2023).
Jung, J. H. et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 585, 256–260 (2020).
Chen, D. et al. Integration of light and temperature sensing by liquid-liquid phase separation of phytochrome B. Mol. Cell 82, 3015–3029 (2022).
Bohn, L. et al. The temperature sensor TWA1 is required for thermotolerance in Arabidopsis. Nature 629, 1126–1132 (2024).
Desroches Altamirano, C. et al. eIF4F is a thermo-sensing regulatory node in the translational heat shock response. Mol. Cell 84, 1727–1741 (2024).
Iserman, C. et al. Condensation of Ded1p promotes a translational switch from housekeeping to stress protein production. Cell 181, 818–831 (2020).
Riback, J. A. et al. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168, 1028–1040 (2017).
Protter, D. S. W. & Parker, R. Principles and properties of stress granules. Trends Cell Biol. 26, 668–679 (2016).
Ivanov, P., Kedersha, N. & Anderson, P. Stress granules and processing bodies in translational control. Cold Spring Harb. Perspect. Biol. 11, a032813 (2019).
Yang, P. et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345 (2020).
Deniz, A. A. Networking and dynamic switches in biological condensates. Cell 181, 228–230 (2020).
Guillén-Boixet, J. et al. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181, 346–361 (2020).
Sanders, D. W. et al. Competing protein-RNA interaction networks control multiphase intracellular organization. Cell 181, 306–324 (2020).
Zhang, H. Large-scale identification of potential phase separation proteins from plants using a cell-free system. Mol. Plant 16, 310–313 (2023).
Wang, B. et al. Condensation of SEUSS promotes hyperosmotic stress tolerance in Arabidopsis. Nat. Chem. Biol. 18, 1361–1369 (2022).
Greenfield, N. J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1, 2876–2890 (2006).
Ruff, K. M., Roberts, S., Chilkoti, A. & Pappu, R. V. Advances in understanding stimulus-responsive phase behavior of intrinsically disordered protein polymers. J. Mol. Biol. 430, 4619–4635 (2018).
Martin, E. W. et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 367, 694–699 (2020).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Dignon, G. L., Zheng, W., Best, R. B., Kim, Y. C. & Mittal, J. Relation between single-molecule properties and phase behavior of intrinsically disordered proteins. Proc. Natl. Acad. Sci. USA 115, 9929–9934 (2018).
Dong, X. et al. Liquid-liquid phase separation of Tau protein is encoded at the monomeric level. J. Phys. Chem. Lett. 12, 2576–2586 (2021).
Dragwidge, J. M. et al. Biomolecular condensation orchestrates clathrin-mediated endocytosis in plants. Nat. Cell Biol. 26, 438–449 (2024).
Abulfaraj, A. A. et al. The Arabidopsis homolog of human G3BP1 is a key regulator of stomatal and apoplastic immunity. Life Sci. Alliance 1, e201800046 (2018).
Weber, C., Nover, L. & Fauth, M. Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J. 56, 517–530 (2008).
Sorenson, R. & Bailey-Serres, J. Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA 111, 2373–2378 (2014).
Gutierrez-Beltran, E., Moschou, P. N., Smertenko, A. P. & Bozhkov, P. V. Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis. Plant Cell 27, 926–943 (2015).
Scutenaire, J. et al. The YTH domain protein ECT2 is an m6A reader required for normal trichome branching in Arabidopsis. Plant Cell 30, 986–1005 (2018).
Tong, J. et al. ALBA proteins confer thermotolerance through stabilizing HSF messenger RNAs in cytoplasmic granules. Nat. Plants 8, 778–791 (2022).
Jain, S. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164, 487–498 (2016).
Xie, Z. et al. Phenolic acid-induced phase separation and translation inhibition mediate plant interspecific competition. Nat. Plants 9, 1481–1499 (2023).
Reuper, H., Amari, K. & Krenz, B. Analyzing the G3BP-like gene family of Arabidopsis thaliana in early turnip mosaic virus infection. Sci. Rep. 11, 2187 (2021).
Kosmacz, M. et al. Protein and metabolite composition of Arabidopsis stress granules. New Phytol. 222, 1420–1433 (2019).
Charng, Y. Y., Mitra, S. & Yu, S. J. Maintenance of abiotic stress memory in plants: lessons learned from heat acclimation. Plant Cell 35, 187–200 (2023).
McLoughlin, F. et al. Class I and II small heat shock proteins together with HSP101 protect protein translation factors during heat stress. Plant Physiol. 172, 1221–1236 (2016).
Souquere, S. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells. J. Cell Sci. 122, 3619–3626 (2009).
Charng, Y. Y., Liu, H. C., Liu, N. Y., Hsu, F. C. & Ko, S. S. Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol. 140, 1297–1305 (2006).
Oberkofler, V., Pratx, L. & Baurle, I. Epigenetic regulation of abiotic stress memory: maintaining the good things while they last. Curr. Opin. Plant Biol. 61, 102007 (2021).
Serrano, N., Ling, Y., Bahieldin, A. & Mahfouz, M. M. Thermopriming reprograms metabolic homeostasis to confer heat tolerance. Sci. Rep. 9, 181 (2019).
Zhao, T., Huan, Q. & Sun, J. et al. Impact of poly(A)-tail G-content on Arabidopsis PAB binding and their role in enhancing translational efficiency. Genome Biol. 20, 189 (2019).
Wang, J. & Chen, H. A novel CRISPR/Cas9 system for efficiently generating Cas9-free multiplex mutants in Arabidopsis. aBIOTECH 1, 6–14 (2020).
Gietz, R. D. Yeast transformation by the LiAc/SS carrier DNA/PEG method. Methods Mol. Biol. 1163, 33–44 (2014).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Sugita, Y. & Okamoto, Y. Replica-exchange molecular dynamics method for protein folding. Chem. Phys. Lett. 314, 141–151 (1999).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
McGibbon, R.T. et al. MDTraj: a modern open library for the analysis of molecular dynamics trajectories. Biophys. J. 109, 1528–1532 (2015).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Silva, J. C., Gorenstein, M. V., Li, G. Z., Vissers, J. P. & Geromanos, S. J. Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Mol. Cell Proteom. 5, 144–156 (2006).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Duncan, S., Olsson, T. S. G., Hartley, M., Dean, C. & Rosa, S. Single molecule RNA FISH in Arabidopsis root cells. Bio Protoc. 7, e2240 (2017).
Acknowledgements
We thank Dr. Pilong Li for helpful discussion and suggestions and Zitong Yang for illustrations. This work was funded by grants from the National Natural Science Foundation of China (32222015, 32450060 and 22203055) to X.F. and K.H., the Ministry of Science and Technology of China (2022YFA1303400 and 2024YFA130810) to X.F., the Major Program of Shenzhen Bay Laboratory (S241101001) to K.H., the Shenzhen Bay Laboratory Open Fund Project (SZBL2021080601013) to K.H. and the Guangdong Pearl River Talent Program (grant no. 2021QN02Y495) to K.H.
Author information
Authors and Affiliations
Contributions
X.F. and K.H. conceived, guided and supervised the project. P.G. and C.L. designed and performed all in planta experiments. X.Q. and M.Y. developed and performed computer simulations. The simulations were carried out at the Shenzhen Bay Laboratory High Performance Computing and Informatics Core. J.P. and Yanning W. performed the in vitro experiments. Y.J. analysed mass spectrometry data. Yunhe W. did the FISH experiment. Z.Y. and P.Y. participated in the imaging of SGs. Y.X. and H.L. helped with the analyses of stress phenotypes. X.F. wrote the manuscript. Y.Q. provided support for the writing of the manuscript. All authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Figure 5 has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geng, P., Li, C., Quan, X. et al. A thermosensor FUST1 primes heat-induced stress granule formation via biomolecular condensation in Arabidopsis. Cell Res 35, 483–496 (2025). https://doi.org/10.1038/s41422-025-01125-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41422-025-01125-4
This article is cited by
-
FUSTer than stress granules: a prion-like domain warns plants of heat
Cell Research (2025)