Abstract
Background
Inherited retinal dystrophies (IRDs) are a genetically heterogeneous group of conditions, with approximately 40% of cases remaining unresolved after initial genetic testing. This study aimed to assess the impact of a personalised genomic approach integrating whole-exome sequencing (WES) reanalysis, whole-genome sequencing (WGS), customised gene panels and functional assays to improve diagnostic yield in unresolved cases.
Subjects/Methods
We retrospectively reviewed a cohort of 597 individuals with IRDs, including 525 probands and 72 affected relatives. Among the 221 genetically unresolved cases, a subset of 101 was selected for stepwise re-evaluation. This included WES reanalysis with updated virtual panels, WGS in selected cases and targeted sequencing of complex regions. Variant interpretation was refined using updated classification criteria, segregation analysis and functional assays such as mRNA and minigene/midigene studies.
Results
An initial diagnostic yield of 59.6% (313/525) was achieved through first-tier genetic testing. Re-evaluation of the 101 prioritised cases resulted in 42 new diagnoses in probands and resolution of 7 more familial cases, yielding 49 additional diagnoses among previously unresolved patients (48.5%). This increased the overall diagnostic rate for probands to 67.6% (355/525). Functional assays confirmed pathogenicity of variants in ABCA4, ATF6, REEP6, and TULP1, while WGS enabled the detection of structural and deep intronic variants, further enhancing diagnostic accuracy.
Conclusions
A patient-centred, stepwise genomic approach significantly improved the molecular diagnosis of IRDs. This strategy supports the clinical utility of periodic WES reanalysis and targeted use of customised panels, WGS and functional assays. The proposed workflow is scalable and applicable to routine clinical practice, contributing to precision medicine in IRDs.
Similar content being viewed by others
Introduction
Inherited retinal dystrophies (IRDs) are a leading cause of blindness worldwide, characterised by extensive genetic heterogeneity that complicates molecular diagnosis [1,2,3]. To date, pathogenic variants in over 300 genes have been implicated in IRDs (RetNet, https://web.sph.uth.edu/RetNet/; accessed on 3 February 2025), affecting both coding and non-coding regions. These include deep intronic variants (e.g., ABCA4, CEP290 and USH2A), GC-rich regions (RPGR-ORF15) and structural variants (SVs) such as large deletions and complex rearrangements [4,5,6,7,8,9,10].
Next-generation sequencing (NGS), particularly whole-exome sequencing (WES) and gene panels, has transformed IRD diagnostics by enabling simultaneous analysis of multiple genes [11,12,13]. However, despite these advancements, a significant proportion of cases remain unresolved, with diagnostic yields ranging from 49% to 75%, leaving nearly 40% of patients without a molecular diagnosis [3, 12,13,14]. This highlights key limitations of current NGS approaches: gene panels fail to capture non-targeted regions and WES has limited sensitivity for deep intronic variants [15]. Additionally, both methods struggle with SVs detection and pathogenic variants in repetitive or homologous regions [15]. Whole-genome sequencing (WGS) provides a more comprehensive analysis, covering both coding and non-coding regions and allowing the identification of complex genomic rearrangements often missed by WES [16,17,18,19,20].
Beyond sequencing, variant interpretation remains a challenge, with many variants classified as variants of uncertain significance (VUS), complicating clinical decision-making [2, 21]. Emerging research continues to refine variant classification, revealing that synonymous and hypomorphic variants, previously considered benign, can contribute to IRD pathogenesis [22, 23]. This highlights the need for periodic WES reanalysis and refinements to American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG-AMP) guidelines to improve diagnostic accuracy [24,25,26].
Functional validation is critical for confirming variant pathogenicity, particularly in non-coding regions. However, the inaccessibility of retinal tissue remains a key challenge [27]. In vitro assays, such as mRNA analysis and minigene/midigene assays, have emerged as powerful tools to elucidate IRD mechanisms and improve variant interpretation [28, 29].
This study focuses on a subset of 101 unresolved cases, selected from a larger diagnostic cohort, to assess the impact of a personalised, case-by-case re-evaluation strategy. By integrating WES reanalysis, customised gene panels, WGS, functional assays and updated classification frameworks, this approach aims to enhance the diagnostic yield for IRDs. Beyond improving molecular diagnosis, this strategy contributes to a better understanding of IRD pathogenesis, facilitates access to gene-targeted therapies and enables more accurate genetic counselling.
Subjects and methods
Cohort description
We retrospectively reviewed the clinical and genetic records of 597 adult patients with a confirmed clinical diagnosis of IRD, all monitored at the Hereditary Retinal Dystrophies Unit of Bellvitge University Hospital. This cohort included 525 probands from unrelated families and 72 affected relatives (familial cases). Clinical diagnoses were based on comprehensive ophthalmological assessments, including fundus examination, optical coherence tomography, autofluorescence imaging and electrophysiology when indicated. All individuals underwent genetic testing between 2021 and 2024, primarily through targeted gene panels or WES, both of which included copy number variant (CNV) analysis as part of standard diagnostic protocols.
Among the 525 probands, 221 remained without a conclusive molecular diagnosis after initial testing. Based on clinical presentation, family history and previous genetic findings, a subset of 101 unresolved cases were selected for further re-evaluation. This subgroup constitutes the primary study population in which the personalised genomic approach described in this study was applied.
Genetic testing workflow
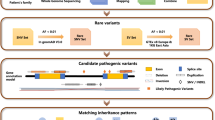
Initially, cases were classified as resolved or unresolved based on prior genetic results. From the 221 unresolved cases, a subset of 101 cases was selected for stepwise, case-by-case genetic re-evaluation. This re-assessment involved one or more of the following approaches: variant reinterpretation and reclassification, WES reanalysis with updated virtual panels, WGS, customised gene panels or functional studies, depending on the specific characteristics of each case. This additional testing was performed between 6 months and 3 years after the initial analysis, depending on the clinical course, newly available evidence and the implementation of updated sequencing tools.
Case prioritisation was based on both clinical and genetic criteria, including reproductive planning, family history of IRDs, the presence of a single pathogenic variant in recessive genes with a consistent phenotype, and cases with no candidate variants in which the initial study had been performed over a year earlier—especially when the original panel might not have included recently associated IRD genes. Notably, timing alone was not the sole determinant; in several cases, prioritisation was driven more by the nature of preliminary findings than by the time elapsed since the first analysis. Exclusion criteria included patient mortality, lack of clinical follow-up or reclassification of the phenotype as non-IRD.
WES reanalysis
Unresolved cases underwent WES reanalysis using an updated IRD gene panel for non-syndromic cases (Supplementary Data) and a phenotype-driven approach with Human Phenotype Ontology (HPO)-guided analysis for syndromic IRDs [30]. Updated annotation tools were applied in both analyses, with bioinformatics performed using Datagenomics software (versions 19.1 and 22.4.0) and CNV detection was carried out via the VarSeq platform (Golden Helix).
Whole-genome sequencing analysis and customised gene panel sequencing
WGS was performed using the KAPA HyperPrep Kit (Roche) and the xGen DNA Library Prep EZ Kit (Integrated DNA Technologies), with sequencing conducted on the Illumina NovaSeq 6000 platform. Bioinformatics analysis was carried out using the CNAG (Centro Nacional de Análisis Genómico) GPAP (Genome-Phenome Analysis Platform, hg19) and Emedgene (Illumina, hg19) platforms. Variants were filtered using an expanded IRD panel that also included candidate IRD genes (Supplementary Data) [17, 31]. A customised gene panel targeting ABCA4 deep intronic regions and RPGR-ORF15 repetitive region was processed using the Agilent SureSelect XT HS2 and the Magnis NGS Prep system (Agilent Technologies, CA, USA), sequenced on the Illumina MiSeq platform and analysed using the Datagenomics software.
Variant filtering and classification
Variants with read depth >20x and an allele frequency ≥20% were considered, except for RPGR-ORF15, where all variants were retained. A minor allele frequency threshold of 0.05 in gnomAD v2.1.1 [32] was applied, prioritising deleterious variants, including nonsense, frameshift, splice site and missense variants. Pathogenicity was assessed using REVEL [33] for missense variants and SpliceAI [34] for splicing impact. Variants were classified according to the ACMG-AMP classifications standards [24], the latest recommendations from the Sequence Variant Interpretation Working Group (SVI-WG) [35] and gene-specific adaptations, such as those from Cornelis et al. (2023) for ABCA4 gene [36].
Validation of variants and splice site assays
Variants were validated using Sanger sequencing, digital PCR, array-CGH or MLPA, depending on variant type, following standard protocols. Splicing impact was assessed through mRNA analysis and minigene/midigene assays.
To evaluate the impact of REEP6 c.349-4G>T and c.349-1G>A variants, as well as the ATF6 c.160-8A>G variant, RNA was extracted from nasal ciliary cells (REEP6) and whole blood (ATF6) using the RNeasy Mini Kit (Qiagen) and Maxwell® RSC SimplyRNA Blood Kit (Promega), respectively. cDNA synthesis was performed using the PrimeScript RT Reagent Kit (TaKaRa), followed by PCR amplification with primers listed in Supplementary Table S1. PCR products were purified (ExoSAP-IT, Applied Biosystems) and analysed by Sanger sequencing (BigDye Terminator v3.1, Applied Biosystems). Electropherograms were analysed using Mutation Surveyor v5.1.2 (for ATF6) and FinchTV (for REEP6) software. The potential protein impact of these variants was assessed using Expasy translate tool [37].
The splicing effect of ABCA4 c.859-442C>T variant was investigated using an in vitro splice assay based on a previously established wild-type midigene (BA7) containing ABCA4 exons 7 to 11 [38]. The variant was introduced via site-directed mutagenesis using oligonucleotides listed in Supplementary Table S2. Wild-type and mutant constructs were transfected into HEK293T cells, followed by RNA extraction (Nucleospin RNA, Machery-Nagel) and cDNA synthesis (iScript, Bio-Rad). RT-PCR was performed using ACTB and RHO exon 5 as controls. Splicing defects were analysed via electrophoresis, Sanger sequencing and semi-quantitative mRNA analysis using Fiji software. Further details on the midigene assay are provided in the Supplementary Material.
Additionally, a minigene splice assay for TULP1 c.822G>T was conducted as previously described [39].
Ethical considerations
This study was approved by the Research Ethics Committee of Bellvitge University Hospital (reference number PR014/22) and conducted in accordance with the Declaration of Helsinki [40]. Informed consent was obtained from all participants and biological samples were sourced from the Biobank HUB-ICO-IDIBELL, part of the ISCIII Biobanks and Biomodels Platform when needed.
Results
Cohort characterisation
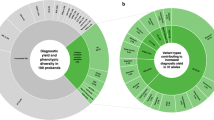
Of the 597 cases, 376 were classified as genetically resolved (P1-P376 in Supplementary Table S3), including 313 probands and 63 familial cases. This cohort exhibited a near-equal sex distribution (189 females and 187 males). The mean age of symptom onset was 23.3 years (range: 1 to 75 years), with 51.1% (192/376) of patients reporting a family history of IRD. Pathogenic variants were identified in 70 genes across 24 IRD subtypes (Fig. 1). Among the 525 probands tested, first-tier genetic testing achieved a diagnostic yield of 59.6% (313/525).
A The most common clinical diagnoses in the genetically resolved cohort (n = 376) were non-syndromic retinitis pigmentosa (nsRP, 40.4%, 152/376), Stargardt disease (STGD, 11.4%, 43/376) and Usher syndrome (USH, 6.3%, 24/376). B Pathogenic variants were identified in 70 genes across 24 IRD subtypes. Only genes implicated in ≥5 cases are shown; the remaining 49 genes not shown were found in fewer than 5 cases. The most frequently affected genes were ABCA4 (18.1%, 68/376), USH2A (8.5%, 32/376), and RPGR (6.7%, 25/376). CD cone dystrophy, BMD best macular dystrophy, LCA leber congenital amaurosis, sRP syndromic retinitis pigmentosa, CRD cone-rod dystrophy, BBS Bardet-Biedl syndrome, CHM choroideremia, CSNB congenital stationary night blindness, ACHM achromatopsia, AOVMD adult-onset vitelliform macular dystrophy, PXE pseudoxanthoma elasticum.
From the 221 unresolved cases, a subset of 101 was selected for personalised reanalysis based on clinical and genetic prioritisation criteria. This group constitutes the primary study population.
Diagnostic improvement
The 101 selected cases underwent further analysis through a stepwise, case-by-case strategy. Variant re-evaluation and reclassification were conducted for 41 cases with VUS that matched the clinical phenotype (P377–P417, Table 1), resolving 18 cases through VUS reclassification to likely pathogenic or pathogenic (Fig. 2). WES reanalysis identified 16 additional diagnoses, while WGS and customised gene panels provided molecular diagnoses for 15 more cases from a subset of 60 patients (P418–P477, Table 2).
Among the 101 unresolved cases prioritised for further assessment, 41 cases (37 probands and 4 familial) with phenotype-associated VUS underwent reclassification, leading to 18 new diagnoses (15 probands and 3 familial). The remaining 23 cases were considered partially resolved due to phenotype-matching VUS. WES reanalysis identified 16 additional diagnoses (14 probands and 2 familial), while targeted ABCA4/RPGR panel testing and WGS contributed to 5 and 10 new diagnoses, respectively. However, 29 cases (28 probands and 1 familial) remained inconclusive due to insufficient evidence of pathogenicity or genotype-phenotype discordance.
In total, 49 new molecular diagnoses were established, comprising 42 probands and 7 familial cases. This personalised approach achieved a diagnostic rate of 48.5% (49/101) in reassessed cases and increased the overall diagnostic rate for probands to 67.6% (355/525), reflecting a 13.4% relative improvement in diagnostic yield.
Reclassification of candidate variants
Family co-segregation and functional studies played crucial roles in reclassifying VUS. In patient P394, the REEP6 c.349-4G>T variant, initially classified as a VUS, was upgraded to likely pathogenic following co-segregation and mRNA analysis, which revealed a 32 nt deletion in exon 4 resulting in a frameshift (Supplementary Fig. S1). Computational predictions (SpliceAI) predicted minimal splicing impact (acceptor loss: 0.07; cryptic acceptor activation: 0.16) (Supplementary Table S4), but cDNA sequencing demonstrated loss-of-function, supporting pathogenicity. Conversely, despite a strong genotype-phenotype correlation, the AIPL1 c.767T>G variant identified in case P395 remained classified as a VUS due to insufficient functional evidence.
In addition to the 18 resolved cases, the reclassification of variants also contributed to the partial resolution of 23 additional cases, for which future evidence may provide further insights leading to conclusive classification.
Non-coding variants
Pathogenic non-coding variants were identified in ABCA4, ATF6, NPHP4, RPGRIP1 and USH2A genes through WES reanalysis, a customised ABCA4 panel and WGS (Table 2). Seven deep intronic variants in ABCA4 were detected, including six previously reported [36] (c.4539+2064C>T in P420, P429 and P432; c.5196+1137G>A in P423 and P430; and c.4253+43G>A in P428) and a novel c.859-442C>T variant in patient P434. Segregation analysis clarified cases initially classified as resolved. For example, in patients P423 and P430, it confirmed that ABCA4 variants were in cis, leading to the identification of deep intronic variants in trans, which resolved both cases.
WGS identified the novel ABCA4 c.859-442C>T variant in P434. SpliceAI predictions indicated acceptor and donor gain (acceptor gain: 0.28; donor gain: 0.23) (Supplementary Table S4). In vitro splice assays revealed three splicing alterations: inclusion of a 238 nt pseudoexon (37%), exons 8–10 skipping (35%) and exon 8 skipping (7%) (Supplementary Table S5), which resulted in frameshifts and premature stop codons, likely disrupting ABCA4 function (Fig. 3). Consequently, the c.859-442C>T variant was classified as moderately-severe in line with previous severity ABCA4 variants classifications [28, 38, 41].
A Wild-type and mutant midigenes assay results. Rhodopsin exon 5 (RHO ex5) RT-PCR was used as a control for transfection efficiency. To the right, schematic representation of WT midigene (BA7_WT), in which the position of the variant is indicated with an arrow and the forward (fwd) and reverse (rev) primers used for PCR amplification are depicted as triangles. Beneath, schematic representation of the four RT-PCR products identified in panel, heteroduplex bands are labelled with an asterisk. ABCA4 c.859-442C>T variant leads to the inclusion of a 238 nt long pseudoexon (PE) in intron 7 (Fragment 1), exon 8 skipping (Fragment 3), exon 8 to 10 skipping (Fragment 4) and WT product (Fragment 2). B The chromatograms show the exact exonic and intronic breakpoints in the four fragments as confirmed by Sanger sequencing.
In patient P448, diagnosed with non-progressive cone-rod dystrophy at age 10, WES reanalysis identified a homozygous ATF6 c.160-8A>G variant with a strong genotype-phenotype correlation. SpliceAI predicted a highly impactful acceptor gain (score: 0.99) and a minor acceptor loss (score 0.11) (Supplementary Table S4). mRNA analysis confirmed the inclusion of 7 nt of intron 2 into de coding sequence, leading to a frameshift and introducing a premature stop codon (Supplementary Fig. S2). This led to the reclassification of the variant as likely pathogenic.
In siblings P435 and P436, biallelic splice-site NPHP4 variants (c.2485+2T>C and c.2611+1G>A) were identified, which had been missed due to the absence of NPHP4 from the original virtual panel. This finding confirmed the diagnosis of Senior-Løken syndrome and revealed previously unrecognised kidney involvement in one sibling.
WES reanalysis also identified a second RPGRIP1 c.2367+23del variant in trans with a previously detected c.1111C>T pathogenic variant in patient P445, confirming the molecular diagnosis. Additionally, WGS identified a novel deep intronic USH2A c.11048-1055A>G variant in siblings P446 and P447, reinforcing their clinical diagnosis.
Structural and copy number variants
WGS detected previously overlooked SVs (Table 2), including a partial exon 6 deletion in ABCA4 in patient P433 and a homozygous deletion affecting three genes, including NPHP1, in patient P440, who presented with retinitis pigmentosa and renal disease. WES reanalysis identified a homozygous intragenic PDE6B deletion in P443 and a likely pathogenic deletion involving ARSG exon 2 in P418, which had been missed due to limitations in the original analysis pipeline for detecting CNVs.
Coding variants and emerging gene associations
WES reanalysis identified previously overlooked coding variants in ABCA4, CERKL, HK1, RPGR, and TULP1 (Table 2). Emerging functional evidence, newly reported gene-disease associations, and advances in bioinformatics facilitated the detection and reclassification of variants. For example, biallelic ABCA4 variants, including the c.5603A>T hypomorphic variant, were identified in several cases (P421, P422, P424, P425, P426, P427 and P431). Additionally, a pathogenic homozygous CERKL c.769C>T variant was detected in patient P441, previously missed due to outdated transcript annotation.
In patient P444, a de novo pathogenic HK1 c.1334C>G variant was identified through WES reanalysis, guided by HPO terms. In patients P438, P439 and P442, variants in RPGR-ORF15 were detected using a customised RPGR panel, which facilitated the detection of variants in low-coverage regions. Finally, a novel splice-site TULP1 c.822G>T variant was identified in patient P437, with its pathogenicity validated through a minigene assay previously reported by our group [39].
Discussion
This study demonstrates a significant improvement in the molecular diagnostic yield for IRDs through a patient-centred, multi-step genomic approach. By integrating variant reclassification, WES reanalysis, WGS, customised gene panels and functional assays, we resolved previously undiagnosed cases, providing deeper insights into IRD pathogenesis. These findings highlight the diagnostic challenges posed by the genetic heterogeneity of IRDs, particularly the presence of SVs and variants in GC-rich and non-coding regions, which are often missed by conventional methods [42].
The initial WES diagnostic yield in our cohort was 59.6%, aligning with previously reported rates for IRDs [3, 12,13,14]. However, incorporating variant reclassification, additional sequencing and functional validation increased the yield to 48.5% among the prioritised cases that were re-evaluated. This surpasses diagnostic rates reported in large cohort studies where WGS alone was used as a second-tier method (33.3% [19], 24% [27] and 13% [18]), emphasising the value of a personalised approach.
While WES and WGS significantly contributed to variant identification, the interpretation of VUS remains challenging. Cases P394 and P448 exemplify how segregation analysis and functional assays can refine variant classification and resolve previously inconclusive cases. In contrast, patient P395 remained unresolved despite a strong genotype-phenotype correlation, underscoring the need for periodic reassessment and functional validation beyond in silico predictions.
The ABCA4 c.5603A>T hypomorphic variant, now recognised as pathogenic [22], was initially not reported due to limited evidence of pathogenicity. This variant is estimated to account for approximately 50% of unresolved cases in individuals carrying only one ABCA4 pathogenic variant [43], reinforcing the need for WES reanalysis as new evidence emerges [44]. However, its interpretation requires caution, as it is only considered pathogenic when in trans with severe variants. For instance, in cases P449, P459 and P472, c.5603A>T was found in trans with non-loss-of-function variants, limiting resolution of these cases.
Additionally, updates to transcript annotation were crucial, as demonstrated by case P441, where a CERKL variant was initially undetected. Similarly, in other cases, the detection of causative variants was hindered by the absence of certain genes in the applied virtual panels, highlighting the need for continuous updates to gene lists, transcript-aware analysis and periodic WES reanalysis using updated bioinformatics pipelines [44, 45].
One case was resolved through HPO-driven reanalysis, demonstrating its utility in syndromic cases, though its impact on non-syndromic IRDs remains limited [46]. Furthermore, the use of a customised RPGR panel enriched for low-coverage regions proved particularly effective in detecting variants within the ORF15 region, providing a cost-effective alternative to WGS and long-read sequencing technologies for sequencing this hotspot [47, 5].
Our findings reinforce the role of non-coding variants in IRD pathogenesis [18]. The identification of pathogenic intronic variants in ABCA4, ATF6, NPHP4, RPGRIP1 and USH2A further validate their significance in disease development [4, 25, 48]. Notable examples include the novel deep intronic variants ABCA4 c.859-442C>T and USH2A c.11048-1055A>G, both classified as pathogenic following segregation and/or functional analyses. The acceptor gain position at ABCA4 c.859-685 (243 nt upstream of -442) appears to be recurrently activated, as shown in Khan et al. (2020) and Corradi et al. (2022) [49, 50], where it coincided with pseudo-exon inclusion and the largest exon elongation for the -25A>G variant of intron 7. Another variant using this splice acceptor site has also been reported [22], reinforcing its functional relevance. These findings highlight the need for routine non-coding region screening, particularly in ABCA4 and USH2A. Cases P423 and P430 further emphasise the importance of segregation analysis to prevent misinterpretation when pathogenic variants are inherited in cis.
The identification of SVs—including a previously overlooked homozygous intragenic PDE6B deletion, a partial exon 6 deletion in ABCA4, which represents the second most frequently reported SV in ABCA4, particularly prevalent in the Spanish population [51] and a large deletion involving NPHP1—, reinforces the need to integrate WGS as a second-tier test in unresolved cases. These findings highlight the limitations of WES in detecting complex genomic rearrangements and emphasise the need for complementary approaches to improve diagnostic accuracy [18, 19].
Beyond diagnostics, these findings have direct clinical implications. Establishing a molecular diagnosis enables tailored genetic counselling, informed clinical decision-making and eligibility for emerging gene-specific therapies [52]. In addition to the 49 new diagnoses, segregation analysis in asymptomatic individuals identified carriers, facilitating reproductive counselling in at-risk couples, some of whom opted for preimplantation genetic diagnosis, directly impacting the next generation. Moreover, the identification of previously undetected variants enhances our understanding of disease mechanisms, which is crucial for developing more precise molecular diagnostic protocols for IRDs [41, 53].
Based on our findings, we propose a flexible and scalable diagnostic workflow for IRDs that integrates reanalysis, WGS and functional assays as complementary tools. Targeted gene panels and WES remain cost-effective and reliable first-line options in many healthcare settings, especially when their design is periodically updated to include newly associated IRD genes. However, clinicians must be aware of their limitations in detecting non-coding, structural and complex variants. Current literature supports systematic reanalysis of WES data every 18 to 24 months, due to ongoing advances in gene discovery and variant interpretation [54]. Nonetheless, timing should remain flexible and adapted to individual clinical contexts, particularly when preliminary findings suggest the presence of deep intronic or SVs, or when reproductive planning is a priority [16]. In selected cases with strong genotype–phenotype correlation, such as individuals carrying a single pathogenic variant in ABCA4 or USH2A, re-evaluation should not be delayed, even if the initial study was recent, as deep intronic or SVs may have been missed. In this context, the increasing implementation of genome sequencing as a first-tier test in certain countries may further accelerate and streamline the diagnostic process [55]. Overall, this patient-centred, stepwise approach proved effective and can be adapted to routine clinical practice to optimise IRD diagnostics.
In conclusion, our case-by-case genomic approach significantly improved the diagnostic yield for IRDs. These findings support the routine integration of advanced sequencing methodologies, variant reclassification and functional validation in IRD diagnostics to optimise patient outcomes and expand the role of precision medicine in ophthalmic genetics. Future efforts should prioritise refining diagnostic workflows, identifying novel candidate genes, improving variant classification systems and incorporating emerging technologies such as long-read sequencing to further enhance diagnostic accuracy and patient care [47, 56].
Summary
What was known before:
-
Whole-exome sequencing (WES) is the primary diagnostic tool for inherited retinal dystrophies (IRDs), yet approximately 40% of cases remain unresolved.
-
Whole-genome sequencing (WGS) and functional assays have demonstrated potential to improve the diagnostic yield.
-
Variants of uncertain significance (VUS) complicate clinical interpretation and limit patient access to gene therapies.
What this study adds:
-
A personalised genomic approach integrating WES reanalysis, WGS and customised gene panels improves IRD diagnosis.
-
Deep intronic, non-coding and structural variants were identified, broadening the spectrum of IRD-related variants.
-
Functional assays and systematic variant reclassification resolved previously undiagnosed cases.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
García Bohórquez B, Aller E, Rodríguez Muñoz A, Jaijo T, García García G, Millán JM. Updating the genetic landscape of inherited retinal dystrophies. Front Cell Dev Biol. 2021;9:695435.
Murro V, Banfi S, Testa F, Iarossi G, Falsini B, Sodi A, et al. A multidisciplinary approach to inherited retinal dystrophies from diagnosis to initial care: a narrative review with inputs from clinical practice. Orphanet J Rare Dis. 2023;18:223.
Perea-Romero I, Gordo G, Iancu IF, Del Pozo-Valero M, Almoguera B, Blanco-Kelly F, et al. Genetic landscape of 6089 inherited retinal dystrophies affected cases in Spain and their therapeutic and extended epidemiological implications. Sci Rep. 2021;11:1526.
González-del Pozo M, Martín-Sánchez M, Bravo-Gil N, Méndez-Vidal C, Chimenea Á, Rodríguez-de la Rúa E, et al. Searching the second hit in patients with inherited retinal dystrophies and monoallelic variants in ABCA4, USH2A and CEP290 by whole-gene targeted sequencing. Sci Rep. 2018;8:13312.
Li J, Tang J, Feng Y, Xu M, Chen R, Zou X, et al. Improved diagnosis of inherited retinal dystrophies by high-fidelity PCR of ORF15 followed by next-generation sequencing. J Mol Diagn. 2016;18:817–24.
Lin S, Vermeirsch S, Pontikos N, Martin-Gutierrez MP, Daich Varela M, Malka S, et al. Spectrum of genetic variants in the most common genes causing inherited retinal disease in a large molecularly characterized United Kingdom cohort. Ophthalmol Retina 2024;8:699–709.
Broadgate S, Yu J, Downes SM, Halford S. Unravelling the genetics of inherited retinal dystrophies: past, present and future. Prog Retin Eye Res. 2017;59:53–96.
Karali M, Testa F, Di Iorio V, Torella A, Zeuli R, Scarpato M, et al. Genetic epidemiology of inherited retinal diseases in a large patient cohort followed at a single center in Italy. Sci Rep. 2022;12:20815.
Rodríguez-Muñoz A, Aller E, Jaijo T, González-García E, Cabrera-Peset A, Gallego-Pinazo R. et al.Expanding the clinical and molecular heterogeneity of nonsyndromic inherited retinal dystrophies.J Mol Diagn. 2020;22:532–43.
Zampaglione E, Kinde B, Place EM, Navarro-Gomez D, Maher M, Jamshidi F, et al. Copy-number variation contributes 9% of pathogenicity in the inherited retinal degenerations. Genet Med. 2020;22:1079–87.
Ganapathi M, Thomas-Wilson A, Buchovecky C, Dharmadhikari A, Barua S, Lee W, et al. Clinical exome sequencing for inherited retinal degenerations at a tertiary care center. Sci Rep. 2022;12:9358.
Matczyńska E, Beć-Gajowniczek M, Sivitskaya L, Gregorczyk E, Łyszkiewicz P, Szymańczak R, et al. Optimised, broad NGS panel for inherited eye diseases to diagnose 1000 patients in Poland. Biomedicines 2024;12:1355.
Mihalich A, Cammarata G, Tremolada G, Manfredini E, Bianchi Marzoli S, Di Blasio AM. Genetic characterization of 191 probands with inherited retinal dystrophy by targeted NGS analysis. Genes (Basel). 2024;15:766.
Britten-Jones AC, Gocuk SA, Goh KL, Huq A, Edwards TL, Ayton LN. The diagnostic yield of next generation sequencing in inherited retinal diseases: a systematic review and meta-analysis. Am J Ophthalmol. 2023;249:57–73.
Burdick KJ, Cogan JD, Rives LC, Robertson AK, Koziura ME, Brokamp E, et al. Limitations of exome sequencing in detecting rare and undiagnosed diseases. Am J Med Genet A 2020;182:1400–6.
Daich Varela M, Bellingham J, Motta F, Jurkute N, Ellingford JM, Quinodoz M, et al. Multidisciplinary team directed analysis of whole genome sequencing reveals pathogenic non-coding variants in molecularly undiagnosed inherited retinal dystrophies. Hum Mol Genet. 2023;32:595–607.
González-del Pozo M, Fernández-Suárez E, Bravo-Gil N, Méndez-Vidal C, Martín-Sánchez M, Rodríguez-de la Rúa E, et al. A comprehensive WGS-based pipeline for the identification of new candidate genes in inherited retinal dystrophies. NPJ Genom Med. 2022;7:17.
Liu X, Hu F, Zhang D, Li Z, He J, Zhang S, et al. Whole genome sequencing enables new genetic diagnosis for inherited retinal diseases by identifying pathogenic variants. NPJ Genom Med. 2024;9:6.
Zeuli R, Karali M, de Bruijn SE, Rodenburg K, Scarpato M, Capasso D, et al. Whole genome sequencing identifies elusive variants in genetically unsolved Italian inherited retinal disease patients. Hum Genet Genomics Adv. 2024;5:100314.
Wojcik MH, Lemire G, Berger E, Zaki MS, Wissmann M, Win W, et al. Genome sequencing for diagnosing rare diseases. N Engl J Med. 2024;390:1985–97.
Walsh N, Cooper A, Dockery A, O’Byrne JJ. Variant reclassification and clinical implications. J Med Genet. 2024;61:207–11.
Bauwens M, Garanto A, Sangermano R, Naessens S, Weisschuh N, De Zaeytijd J, et al. ABCA4-associated disease as a model for missing heritability in autosomal recessive disorders: novel noncoding splice, cis-regulatory, structural, and recurrent hypomorphic variants. Genet Med. 2019;21:1761–71.
Kaltak M, Corradi Z, Collin RWJ, Swildens J, Cremers FPM. Stargardt disease-associated missense and synonymous ABCA4 variants result in aberrant splicing. Hum Mol Genet. 2023;32:3078–89.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Runhart EH, Valkenburg D, Cornelis SS, Khan M, Sangermano R, Albert S, et al. Late-onset Stargardt disease due to mild, deep-intronic ABCA4 alleles. Investig Opthalmol Vis Sci. 2019;60:4249.
Schulz HL, Grassmann F, Kellner U, Spital G, Rüther K, Jägle H, et al. Mutation spectrum of the ABCA4 Gene in 335 Stargardt disease patients from a multicenter german cohort—impact of selected deep intronic variants and common SNPs. Investig Opthalmol Vis Sci. 2017;58:394.
Fadaie Z, Whelan L, Ben-Yosef T, Dockery A, Corradi Z, Gilissen C, et al. Whole genome sequencing and in vitro splice assays reveal genetic causes for inherited retinal diseases. NPJ Genom Med. 2021;6:97.
Corradi Z, Khan M, Hitti-Malin R, Mishra K, Whelan L, Cornelis SS, et al. Targeted sequencing and in vitro splice assays shed light on ABCA4-associated retinopathies missing heritability. Hum Genet Genomics Adv. 2023;4:100237.
Rodriguez-Muñoz A, Liquori A, García-Bohorquez B, Jaijo T, Aller E, Millán JM, et al. Functional assays of non-canonical splice-site variants in inherited retinal dystrophies genes. Sci Rep. 2022;12:68.
Robinson PN, Köhler S, Bauer S, Seelow D, Horn D, Mundlos S. The human phenotype ontology: a tool for annotating and analyzing human hereditary disease. Am J Hum Genet. 2008;83:610–5.
Carss KJ, Arno G, Erwood M, Stephens J, Sanchis-Juan A, Hull S, et al. Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease. Am J Hum Genet. 2017;100:75–90.
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020;581:434–43.
Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99:877–85.
Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, et al. Predicting splicing from primary sequence with deep learning. Cell 2019;176:535–48.e24.
Walker LC, Hoya M, de la, Wiggins GAR, Lindy A, Vincent LM, Parsons MT, et al. Using the ACMG/AMP framework to capture evidence related to predicted and observed impact on splicing: Recommendations from the ClinGen SVI Splicing Subgroup. Am J Hum Genet. 2023;110:1046–67.
Cornelis SS, Bauwens M, Haer-Wigman L, De Bruyne M, Pantrangi M, De Baere E, et al. Compendium of clinical variant classification for 2,246 unique ABCA4 variants to clarify variant pathogenicity in stargardt disease using a modified ACMG/AMP framework. Hum Mutat. 2023;2023:1–12.
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–8.
Sangermano R, Khan M, Cornelis SS, Richelle V, Albert S, Garanto A, et al. ABCA4 midigenes reveal the full splice spectrum of all reported noncanonical splice site variants in Stargardt disease. Genome Res. 2018;28:100–10.
Esteve-Garcia A, Cobos E, Sau C, Padró-Miquel A, Català-Mora J, Barberán-Martínez P, et al. Deciphering complexity: TULP1 variants linked to an atypical retinal dystrophy phenotype. Front Genet. 2024;15:1378105.
World Medical Association Declaration of Helsinki. JAMA. 2013;310:2191.
Cremers FPM, Lee W, Collin RWJ, Allikmets R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog Retin Eye Res. 2020;79:100861.
Wen S, Wang M, Qian X, Li Y, Wang K, Choi J, et al. Systematic assessment of the contribution of structural variants to inherited retinal diseases. bioRxiv. 2023.
Zernant J, Lee W, Collison FT, Fishman GA, Sergeev YV, Schuerch K, et al. Frequent hypomorphic alleles account for a significant fraction of ABCA4 disease and distinguish it from age-related macular degeneration. J Med Genet. 2017;54:404–12.
Surl D, Won D, Lee ST, Lee CS, Lee J, Lim HT, et al. Clinician-driven reanalysis of exome sequencing data from patients with inherited retinal diseases. JAMA Netw Open. 2024;7:e2414198.
Romero R, de la Fuente L, Del Pozo-Valero M, Riveiro-Álvarez R, Trujillo-Tiebas MJ, Martín-Mérida I, et al. An evaluation of pipelines for DNA variant detection can guide a reanalysis protocol to increase the diagnostic ratio of genetic diseases. NPJ Genom Med. 2022;7:7.
Perea-Romero I, Blanco-Kelly F, Sanchez-Navarro I, Lorda-Sanchez I, Tahsin-Swafiri S, Avila-Fernandez A, et al. NGS and phenotypic ontology-based approaches increase the diagnostic yield in syndromic retinal diseases. Hum Genet. 2021;140:1665–78.
Bonetti G, Cozza W, Bernini A, Kaftalli J, Mareso C, Cristofoli F, et al. Towards a long-read sequencing approach for the molecular diagnosis of RPGRORF15 genetic variants. Int J Mol Sci. 2023;24:16881.
Nassisi M, Mohand-Saïd S, Andrieu C, Antonio A, Condroyer C, Méjécase C, et al. Prevalence of ABCA4 deep-intronic variants and related phenotype in an unsolved “One-Hit” Cohort with Stargardt disease. Int J Mol Sci. 2019;20:5053.
Khan M, Cornelis SS, De Pozo-Valero ML, Whelan L, Runhart EH, Mishra K, et al. Resolving the dark matter of ABCA4 for 1054 Stargardt disease probands through integrated genomics and transcriptomics. Genet Med. 2020;22:1235–46.
Corradi Z, Salameh M, Khan M, Héon E, Mishra K, Hitti-Malin RJ, et al. ABCA4 c.859-25 A > G, a frequent palestinian founder mutation affecting the intron 7 branchpoint, is associated with early-onset Stargardt disease. Investig Opthalmol Vis Sci. 2022;63:20.
Corradi Z, Dhaenens CM, Grunewald O, Kocabaş IS, Meunier I, Banfi S, et al. Novel and recurrent copy number variants in ABCA4-associated retinopathy. Int J Mol Sci. 2024;25:5940.
Georgiou M, Fujinami K, Michaelides M. Inherited retinal diseases: therapeutics, clinical trials and end points-A review. Clin Exp Ophthalmol. 2021;49:270–88.
Brar AS, Parameswarappa DC, Takkar B, Narayanan R, Jalali S, Mandal S, et al. Gene therapy for inherited retinal diseases: from laboratory bench to patient bedside and beyond. Ophthalmol Ther. 2024;13:21–50.
Areblom M, Kjellström S, Andréasson S, Öhberg A, Gränse L, Kjellström U. A description of the yield of genetic reinvestigation in patients with inherited retinal dystrophies and previous inconclusive genetic testing. Genes (Basel). 2023;14:1413.
Nisar H, Wajid B, Shahid S, Anwar F, Wajid I, Khatoon A, et al. Whole-genome sequencing as a first-tier diagnostic framework for rare genetic diseases. Exp Biol Med. 2021;246:2610–7.
Gupta P, Nakamichi K, Bonnell AC, Yanagihara R, Radulovich N, Hisama FM, et al. Familial co-segregation and the emerging role of long-read sequencing to re-classify variants of uncertain significance in inherited retinal diseases. NPJ Genom Med. 2023;8:20.
Acknowledgements
We thank the patients and their families for their participation in this study. This work was supported by Bellvitge University Hospital (Grant No. PUB22015) and the Macula Society with the International Retinal Research Foundation Award (DON23020). We also acknowledge the valuable contributions of Health in Code and the Biobank HUB-ICO-IDIBELL (PT20/00171), which is integrated in the ISCIII Biobanks and Biomodels Platform. Additionally, we thank CERCA Programme/Generalitat de Catalunya for their institutional support.
Funding
LA was supported by the Macula Society (grant number: DON23020). EC received funding from Bellvitge University Hospital (grant number: PUB22015). ZC was supported by the Stichting tot Verbetering van het Lot der Blinden, Stichting voor Ooglijders and the Stichting Blindenhulp. GG-G acknowledges two grants from Instituto de Salud Carlos III (ISCIII), CP22/00028 and PI22/01371, co-funded by the European Union. PB-M had a grant from Ministerio de Universidades FPU20/04736. JM has a grant from Instituto de Salud Carlos III (ISCIII), PI22/00213, co-funded by the European Union.
Author information
Authors and Affiliations
Contributions
AE-G designed the study, performed the genetic analyses and drafted the manuscript. CA contributed to data analysis and interpretation and conducted mRNA analysis. JC-M, II, LA and EC reviewed the clinical diagnoses. DY reanalysed WES data. CS and AP-M provided critical feedback on the manuscript. ZC and FC designed the ABCA4 midigene assay. PB-M, JM and GG-G performed the REEP6 mRNA analysis. EC and CA supervised the study. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esteve-Garcia, A., Padró-Miquel, A., Català-Mora, J. et al. Personalised genomic strategies improve diagnostic yield in inherited retinal dystrophies: a stepwise, patient-centred approach. Eye 39, 2899–2911 (2025). https://doi.org/10.1038/s41433-025-03981-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03981-1