Abstract
Background
Diabetic macular oedema (DMO) is a common cause of vision loss and blindness. To inform the 2024 UK NICE guideline for treating people with centre-involving DMO (CI-DMO) and central retinal thickness (CRT) of ≥400 µm, the cost-effectiveness of various anti-vascular endothelial growth factor (anti-VEGF) and macular laser treatments was evaluated.
Methods
A de novo Markov model evaluated the lifetime costs and quality-adjusted life-years (QALYs) of various anti-VEGFs (aflibercept, bevacizumab, brolucizumab, faricimab, ranibizumab and ranibizumab biosimilar), macular lasers (standard threshold laser and subthreshold micropulse laser), and some treatment combinations from the perspective of the UK NHS. The model included eight health states defined by best-corrected visual acuity ranging between >85 and ≤25 letters. The model’s inputs were derived from published literature, while an original network meta-analysis of several clinical trials informed visual outcomes.
Results
All anti-VEGFs demonstrated greater clinical effectiveness and produced more QALYs (ranging from 9.211 to 9.271) than both types of macular lasers (8.928 and 8.944), but lasers were the most cost-effective due to their substantially lower costs. Using confidential price discounts, ranibizumab biosimilar (Ongavia) and brolucizumab had ICERs below £20,000 per QALY, while aflibercept, ranibizumab (Lucentis) and faricimab had ICERs below £25,000 per QALY, compared to no treatment. Bevacizumab was the most cost-effective anti-VEGF treatment due to its significantly lower cost.
Conclusions
Given their clinical and cost-effectiveness at confidential prices, NICE recommends offering a licensed cost-effective anti-VEGF as first-line treatment for people with CI-DMO and CRT ≥ 400 µm. The use of bevacizumab for this population is not licensed in the UK and would be considered off-label.
Similar content being viewed by others
Introduction
Diabetic macular oedema (DMO) is a leading cause of vision loss and blindness worldwide [1]. Approximately 7.5% of people living with diabetes develop DMO [2], which can profoundly affect their quality of life (QoL) and ability to work [3, 4]. People with DMO use significantly more healthcare resources than those with diabetes who do not have retinal complications [5]. DMO can be classified as non-centre-involving DMO and centre-involving DMO (CI-DMO); the latter defined by the presence of fluid in the central 1 mm of the retina—referred to as the central subfield thickness or central retinal thickness (CRT). CI-DMO can be mild (CRT < 400 µm) or severe ( ≥ 400 µm) [6].
For people with CI-DMO and CRT < 400 µm, macular laser treatment is considered a highly cost-effective option [6]. However, in the study by Mansouri et al. [7], all eyes with an initial CRT≥400μm did not respond to laser treatment and subsequently required rescue therapy with anti-vascular endothelial growth factor (anti-VEGF) injections. During the past years, several intravitreal anti-VEGF and steroid therapies were successfully introduced for the management of DMO. To manage people with CI-DMO and CRT ≥ 400 µm, the National Institute for Health and Care Excellence (NICE) in the UK supports the use of aflibercept [8], brolucizumab [9], faricimab [10] and ranibizumab [11]. Although anti-VEGFs can be administered by various healthcare professionals, they are generally expensive, require prolonged treatment courses, and raise significant concerns regarding cost-effectiveness [12, 13].
While randomised controlled trials (RCTs) have demonstrated the clinical effectiveness of anti-VEGFs in treating visual impairment due to CI-DMO and CRT ≥ 400 µm, no RCT has yet compared all available anti-VEGFs, nor has any assessed their cost-effectiveness in a single analysis. There is a compelling public health argument for selecting safe treatments that enhance vision and improve health outcomes, while ensuring an acceptable balance between health benefits and costs. Informing the 2024 UK NICE guideline [14], this study aimed to evaluate the cost-effectiveness of various anti-VEGFs, macular lasers, and some treatment combinations in a single analysis for treating people with CI-DMO and CRT ≥ 400 µm within a UK NHS setting.
Methods
Model overview and structure
A cohort Markov model with a 3-monthly cycle length and a half-cycle correction was developed in Microsoft® Excel® to evaluate the lifetime costs and quality-adjusted life-years (QALYs) of eleven treatment strategies for people with CI-DMO and CRT ≥ 400 µm, from the perspective of UK NHS. The strategies evaluated included: aflibercept (Eyela) 2 mg, bevacizumab (Avastin) (off-label) 1.25 mg, brolucizumab (Beovu) 6 mg, faricimab (Vabysmo) 6 mg, ranibizumab (Lucentis) 500 µg, ranibizumab biosimilar (Ongavia) 500 µg, standard threshold laser, subthreshold micropulse laser, bevacizumab plus standard threshold laser, ranibizumab plus standard threshold laser, and no treatment. It was assumed that the ranibizumab biosimilar (Ongavia) would have the same efficacy, safety and resource use as ranibizumab (Lucentis). All costs and QALYs were discounted at an annual rate of 3.5% [15].
Macular laser treatment, delivered via either standard threshold or subthreshold micropulse laser, was included in the analysis to allow comparison with other treatment options, as most RCTs comparing anti-VEGF therapy with macular laser involved participants with CRT well over 400 µm [16]. Although laser treatments are predominantly used in people with CI-DMO and CRT < 400 µm, including them as a comparator for the CRT ≥ 400 µm subgroup allowed for a more complete network and enabled the inclusion of additional studies. Intravitreal steroids, such as dexamethasone [17] and fluocinolone [18], were excluded, as steroids are primarily used as second-line treatments and are only considered first-line treatments for people who are not suitable for other first-line treatments. This study focused solely on first-line treatments.
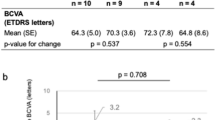
As shown in Fig. 1, the Markov model consisted of nine health states, including eight levels of best-corrected visual acuity (BCVA) ranging between >85 and ≤25 letters and a death state. Based on clinical input, the model allowed people to transition by up to one BCVA state in each 3-monthly cycle (i.e., a BCVA change of 5 to 15 letters; a 10-letter range), reflecting the average eye rather than using the midpoint. The rationale for adopting a BCVA-based model (even though it may not fully capture all outcomes related to disease progression) was to incorporate the primary outcome data from RCTs and to validate the results against previously published cost-effectiveness analyses.
Baseline data
The cohort in the model began at the age of 63 years, with 58% being male [19]. The model assumed an initial distribution of people across BCVA-based health states at baseline (Table 1). In line with previous BCVA-based models [19,20,21,22], the model structure was based on one eye only. However, as in NICE TA799 [10] and NICE TA820 [9], the costs for treating the fellow eye were also included, assuming that 22% of people received treatment in both eyes at baseline [22]. Of those treated in one eye, 67.2% received treatment in their worst seeing eye (WSE) and 32.8% in their best seeing eye (BSE) [19]. The probability of developing DMO in the fellow eye was 5.4% per 3-monthly cycle [21]. The parameters used in the model are shown in Table 1.
Natural history and treatment efficacy
The natural history of DMO was informed by Mitchell et al. [19], who used data from the Wisconsin epidemiologic study of diabetic retinopathy (WESDR) to create a transition matrix for the natural progression of DMO. This was recalibrated with data from the RESTORE trial, yielding 3-monthly probabilities of 3.5% for moving up and 4.5% for moving down one health state (Table 1). The natural history was applied to the no treatment arm and other interventions when treatment efficacy was assumed to have ceased in scenario analyses.
Mean treatment effects with 95% confidence intervals, measured as changes in BCVA at 12 months, were derived from an original network meta-analysis (NMA), described elsewhere [23], which compared each treatment to no treatment. The NMA included 32 RCTs with a total sample size of 7,721 participants; a forest plot is shown in Fig. S1 in Supplementary material. Although several RCTs enroled participants with CRT both above and below 400 µm, only those with CRT ≥ 400 µm were included in the NMA. Using these data, 3-monthly probabilities of transitioning between different health states were calculated (Table S1; Supplementary material), assuming that changes in BCVA follow a normal distribution [24]. The efficacy of the subthreshold laser was assumed to be equivalent to that of the standard threshold laser given their similar performance in the DIAMONDS clinical trial [25]. In the absence of data, it was assumed that the efficacy and resource use of bevacizumab plus standard threshold laser would be equal to those of ranibizumab plus standard threshold laser.
In the base-case analysis, the model assumed that the treatment effects observed in the NMA would continue throughout the model’s lifetime. However, scenario analyses assessed the impact of including natural history after 5, 10 and 20 years (i.e., treatment effects ceased at 5, 10 and 20 years, after which natural disease progression resumed).
Treatment discontinuation
The model assumed that everyone would continue treatment during the first year to assess their response (clinical consensus). The published treatment-specific discontinuation rates were applied between years 1 and 3 (Table S2; Supplementary material). From years 3 to 5, the model assumed that 75% of people would continue treatment [26], and from year 5 onward, 50% would continue treatment [10]. The treatment-specific discontinuation rates were applied alongside the fixed treatment discontinuation starting from year 3 onward.
Adverse events (AEs)
AEs related to each treatment were included in the model. In the absence of data, it was assumed that the frequency of AEs for subthreshold micropulse laser would be the same as that for standard threshold laser, ranibizumab plus standard threshold laser would be the same as for ranibizumab, and bevacizumab plus standard threshold laser would be the same as for bevacizumab. The proportion of each AE for each treatment strategy is shown in Table S3 (Supplementary material), while the associated cost of each AE is provided in Table S4 (Supplementary material).
Mortality
Mortality was modelled using age- and gender-specific national life tables for England and Wales (2018–2020) [27]. It was anticipated that the main mortality risk associated with poor vision would be reflected in the diabetes population rather than in the diabetic retinopathy population. Therefore, following the approach in NICE TA346 [8], a hazard ratio of 1.95 [28] was applied to account for the increased mortality risk associated with diabetes relative to the general population.
Resource use and costs
The costs associated with treatment (list prices), administration, monitoring and low vision (BCVA ≤ 35 letters) are shown in Table 2. The cost year 2019-20 was chosen to represent standard care in the NHS and to avoid any cost outliers caused by the COVID-19 outbreak.
Aflibercept, brolucizumab, faricimab, ranibizumab (Lucentis) and ranibizumab biosimilar (Ongavia) have confidential patient access scheme prices to NICE; these prices cannot be disclosed due to confidentiality agreements. A weighted cost of £257.98 was applied for administering an anti-VEGF (Table 2), while the cost of laser administration was assumed to be included within the overall laser treatment cost. It was also assumed that treatment would be administered during the same visit as monitoring, and that both eyes would receive the same treatment during that visit for those who require it. Treatment monitoring costs covered the cost of an optical coherence tomography scan, as well as the cost of a post-treatment monitoring visit (Table 2). The number of monitoring visits for each treatment and the annual number of anti-VEGF injections are shown in Tables S5 and S6 (Supplementary Material), respectively. The number of laser treatments is shown in Table S7 (Supplementary material), with the assumption that all combination treatment options involved the same number of laser treatments. The total cost of AEs for each treatment was calculated by multiplying the proportion of people experiencing an AE (Table S3; Supplementary material) by the cost of that AE (Table S4; Supplementary material).
Some people may switch to a different treatment after discontinuing the previous one due to lack of response. However, there is significant variability in how subsequent treatments are reported in the literature, and no data were available on the duration of these treatments. The model allowed some people to receive a subsequent treatment (Table S8; Supplementary material), but this was applied to costs for only two years to prevent overestimating the cost of first-line treatment by including the additional cost of the subsequent treatment, for which evidence is limited. The total cost of subsequent treatment expected for each first-line treatment strategy is shown in Table S9 (Supplementary material). The costs were calculated based on the weightings shown in Table S8 (Supplementary material), including the costs of treatment itself, administration and treatment-specific AEs.
Health state utility values
Utility values were obtained from a review of QoL literature and are summarised in Table 2. Although several studies estimated utility values based on BCVA, the utility values for BSE from Czoski-Murray et al. [29] were considered the most relevant and were also used in NICE technology appraisals (TAs) for DMO [8, 17, 18]. Consistent with Haig et al. [20], the utility values for BSE were applied to people receiving treatment in both eyes, as BSE is identified as the primary factor influencing overall QoL and functioning [19, 30]. QALYs were calculated by multiplying the time spent in each BCVA-based health state by its corresponding utility value, with adjustments made for utility losses due to treatment-related adverse events. In addition to the health state utility values, the model also included utility losses associated with presumed AEs (Table S10; Supplementary material).
Deterministic and probabilistic sensitivity analyses
The following deterministic scenario analyses were performed:
-
Treatment efficacy ceased and natural history starts from 20 years.
-
Treatment efficacy ceased and natural history starts from 10 years.
-
Treatment efficacy ceased and natural history starts from 5 years.
-
25% of people continue treatment after 5 years.
-
75% of people continue treatment after 5 years.
-
Treatment occurs in a separate visit from monitoring.
-
Monitoring and treatment assumption (minimum).
-
Monitoring and treatment assumption (maximum).
The probabilistic sensitivity analysis was performed to assess the uncertainty in the true values of the input parameters. Probability distributions were assigned to the input parameters, with the distribution type chosen based on the properties of the data. Where possible, dispersion data from the original source were used to parameterise each distribution. In the absence of such data, expert judgement was used to apply reasonable ranges.
Results
The probabilistic lifetime cost-effectiveness results, based on list prices, are shown in Table 3. All anti-VEGF treatments generated more QALYs (ranging from 9.211 to 9.271) than either type of macular laser (8.928 and 8.944), but the lasers were substantially less expensive (£4,458 and £4,919) compared to the anti-VEGF treatments (ranging from £9,308 to £34,522). When evaluating cost-effectiveness relative to no treatment using the incremental cost-effectiveness ratio (ICER), lasers were found to be the most cost-effective treatment option due to their significantly lower costs. Bevacizumab (off-label) became the most cost-effective anti-VEGF treatment due to its lower cost compared to all other anti-VEGFs, despite producing fewer QALYs than the others. However, with confidential price discounts, ranibizumab biosimilar (Ongavia) and brolucizumab had ICERs below NICE’s lower threshold of £20,000 per QALY, while aflibercept, ranibizumab (Lucentis) and faricimab had ICERs below £25,000 per QALY, compared to no treatment. The ICER was calculated by dividing the difference in costs by the difference in QALYs, indicating that the high ICERs for anti-VEGF therapies were primarily driven by their higher incremental costs. Each strategy was compared to no treatment rather than using a full incremental analysis, as laser treatment is not clinically recommended for this population.
Based on net monetary benefit (NMB) estimates at the £20,000 per QALY gained threshold, bevacizumab had the highest NMB (£174,916), followed by subthreshold micropulse laser with the second-highest NMB (£174,414) and standard threshold laser with the third-highest NMB (£173,646) in the base-case analysis using list prices. The results involving confidential prices cannot be disclosed due to confidentiality agreements. However, the NMB rankings of the interventions, calculated using both list and confidential prices, are shown in Table 3.
Table 4 shows a summary of the probabilistic cost-effectiveness results from various scenario analyses, including the base-case. In all the scenarios explored, bevacizumab, subthreshold micropulse laser and standard threshold laser consistently ranked among the top three interventions. While the use of confidential prices did not allow other interventions to enter the top three across all scenarios, it did cause some changes in the rankings compared to using list prices. The model results were robust to most parameters explored in the scenario analyses. However, all anti-VEGF options were somewhat impacted by changes in assumptions about the frequency of treatment and monitoring visits, as these factors affected the associated costs.
Discussion
The cost-effectiveness of various anti-VEGFs and macular lasers was evaluated to inform the 2024 NICE guideline update for treating people with CI-DMO and CRT ≥ 400 µm. Although anti-VEGFs were more clinically effective (Table S1; Supplementary material) and produced more QALYs (Table 3) than either type of laser, both lasers emerged as the most cost-effective treatment option due to their very low costs. However, with confidential price discounts, all anti-VEGFs were cost-effective compared to no treatment, with ICERs either below £20,000 or £25,000 per QALY. NICE considers interventions costing between £20,000 and £30,000 per additional QALY gained to represent good value for money for the NHS. Bevacizumab was identified as the most cost-effective anti-VEGF treatment; however, its use in the UK would be considered off-label as it is not covered by its marketing authorisation.
A key strength of this study is that the mean difference in BCVA used to inform the model was derived from data across multiple RCTs included in the NMA. However, several of these RCTs reported only aggregated outcomes for the overall study population, without stratifying results by CRT subgroups. For this analysis, RCTs were categorised based on whether their mean baseline CRT was above or below 400 µm. A limitation of this approach is that individuals with a CRT below 400 µm may have been included in the ‘above 400 µm’ subgroup if the RCT’s mean CRT exceeded that threshold (and vice versa). Fewer RCTs assessed treatment strategies for individuals with CRT < 400 µm, resulting in insufficient data to perform a comparable analysis for this subgroup.
Another key strength of this study is that the model compared all relevant treatments in a single analysis, an aspect that had not been addressed in the published literature previously. All parameters used in the model were informed by RCTs, published literature or assumptions, and were verified by clinical experts. The model included people treated in their WSE, BSE or both eyes, along with any subsequent treatments and treatment-related AEs. The model was developed in close collaboration with clinical experts, with meetings held at various stages of the development process to review the model structure and input parameters for clinical relevance.
The model’s base case assumed that the treatment effects observed in the NMA would persist throughout the model’s lifetime, as the two primary reasons for discontinuation were reported to be death and early discharge due to stable disease [31]. This assumption may have led to an overestimation of the treatment effect, as a small number of individuals were reported to have discontinued due to treatment being considered futile, and treatment effect typically declines when assessments or retreatments are delayed [32, 33]. However, assuming that treatment effect would cease immediately upon discontinuation is problematic, as the risk of vision loss in those who discontinue would likely develop gradually over time, and people may return to the same treatment if signs of vision loss appear. Scenario analyses assessed the impact of treatment effects being ceased after 5, 10 and 20 years.
Although the analysis benefited from incorporating AEs, there is inconsistency in how AEs are reported in the literature. Since the overall costs and disutilities associated with AEs included in the model were applied to a very small proportion of people, it is unlikely to impact the conclusions drawn. Mitchell et al. [19] excluded AEs from their analysis based on the assumption that they had a negligible impact on cost-effectiveness. The model allowed people to switch between standard threshold laser and some anti-VEGF treatments, but no switches within the therapeutic class were permitted. However, limited or poorly reported data were available to determine the frequency and distributions of these subsequent treatments, and no data were available on the distribution of treatments for those switching from combination regimens. The duration of subsequent treatment was restricted to two years to avoid overestimating the costs associated with it, given the uncertainty surrounding the true distribution and duration of subsequent treatments. As a result, it is likely that the costs of subsequent treatment were underestimated. However, the analysis benefitted from being more representative of clinical practice by incorporating subsequent treatment at all. The model did not include a separate risk for proliferative diabetic retinopathy (PDR), as most clinical studies report data on PDR and DMO separately. However, the impact of PDR was expected to be captured within the BCVA-based transition probabilities. This approach is consistent with previously published cost-effectiveness models [19, 20, 22].
Patient time and productivity losses were excluded from the analysis to align with the NICE reference case [15], which adopts the NHS perspective in the UK. Although these costs can place a significant burden on patients, aggregating individual patient preferences into a societal preference remains both a theoretical and practical challenge [12]. Future clinical trials may consider collecting these cost data to help address this issue. However, direct healthcare-related costs associated with low vision were considered for people with BCVA ≤ 35 ETDRS letters, including the costs of residential care, community care and low vision rehabilitation.
Given the nature of DMO, both eyes may be affected over time and might require treatment. Since most clinical studies focus on the study eye, the data needed to construct a two-eye model could not be obtained. Consistent with prior research, the BCVA-based health states were based on the study eye, though treatment costs for the fellow eye were included in the model. While this may not fully capture the disease profile, the Markov model structure is widely used in the literature. A discrete event simulation model would have been better suited to capture heterogeneity in disease profile [34, 35]; however, the lack of publicly available patient-level data prevented its development. Instead, a simple and flexible Markov model structure was adopted to enhance transparency and accessibility, ensuring the model remained uncomplicated and enabling a broad range of stakeholders to critically appraise it.
The current analysis aligns with the findings of Lois et al. [25], who found that both standard and subthreshold macular laser were equally effective in people with CI-DMO and CRT < 400 µm, and that both were considered cost-effective. The current analysis also aligns with Sharma et al. [36], who found that laser treatment alone was cost-effective compared to no treatment. Consistent with Mitchell et al. [19], the current analysis found that combined ranibizumab and laser treatment was less cost-effective than ranibizumab alone, primarily because ranibizumab alone produced a higher QALY gain than the combination treatment.
Hutton et al. [37] found that aflibercept was less cost-effective than bevacizumab, which is in agreement with the current analysis. Brown et al. [38] found that ranibizumab was cost-effective over sham, with an ICER of $4,587 per QALY. In contrast, the current analysis found that ranibizumab (Lucentis) was cost-effective over no treatment only when the ICER was below £25,000 per QALY, but the ranibizumab biosimilar (Ongavia) was cost-effective with an ICER below £20,000 per QALY. Holekamp et al. [39] found that aflibercept was less cost-effective than ranibizumab. While the current analysis, based on list prices, aligns with this, the same conclusion does not hold when confidential prices are used—unless the ranibizumab biosimilar replaces ranibizumab. This difference is likely due to variations in the drug prices used in the analysis. Régnier et al. [22] found that ranibizumab was dominant (less expensive and more effective) over aflibercept. In contrast, the current analysis found aflibercept to be more effective. This discrepancy may be due to variations in mean BCVA gain, as the Régnier et al. [22] analysis was based solely on the RESTORE trial, while the current analysis included data from several RCTs. Similar to Stein et al. [40], the current analysis found that bevacizumab was the most cost-effective option among the anti-VEGF treatments examined, primarily due to its substantially lower drug cost.
All anti-VEGFs produced higher QALYs than macular lasers. Off-label bevacizumab (QALYs: 9.204) emerged as a highly cost-effective option, yielding nearly as many QALYs as ranibizumab (QALYs: 9.218), due to its lower cost than all other anti-VEGFs. Faricimab (9.267), brolucizumab (9.262) and aflibercept (9.258) achieved the highest QALYs among all interventions. When confidential prices were factored in, all anti-VEGFs had ICERs either below £20,000 or £25,000 per QALY compared to no treatment. Recommendations for the use of anti-VEGFs referenced the relevant NICE TAs for aflibercept [8], brolucizumab [9], faricimab [10] and ranibizumab [11] in people with CI-DMO and CRT ≥ 400 µm, aligning with the current analysis and ensuring consistency with the evidence base. Although anti-VEGFs require more clinic visits and frequent retreatment compared to either type of macular laser, the benefits of improved vision and the relatively low risk of AEs are expected to outweigh this factor. The introduction of additional biosimilar agents, as patents expire, may further enhance the cost-effectiveness of newer anti-VEGF treatments. Notably, for people with CI-DMO and CRT≥400μm, laser treatment was not as clinically effective as anti-VEGFs and ultimately required rescue treatment with anti-VEGF injections [7].
This study incorporated a substantial body of the best available evidence and offers value to both clinicians and health service planners. However, there is still a need for discussions between clinicians and patients regarding the advantages and disadvantages of the available anti-VEGF treatments. A clear understanding of the available options, their potential benefits, and associated trade-offs can help guide and enrich such discussions within the context of a broad care plan. Since the dosage and timing guidance vary among anti-VEGFs, the information outlined in the summary of product characteristics should be followed by clinicians.
Conclusions
Macular laser treatment (either standard threshold laser or subthreshold micropulse laser) emerged as the most cost-effective option due to its substantially lower costs. However, all anti-VEGF treatments showed greater clinical effectiveness and produced more QALYs than either type of macular laser in people with CI-DMO and CRT ≥ 400 µm. Furthermore, with confidential price discounts, ranibizumab biosimilar (Ongavia) and brolucizumab had ICERs below NICE’s lower threshold of £20,000 per QALY, while aflibercept, ranibizumab (Lucentis) and faricimab had ICERs below £25,000 per QALY (middle of NICE’s acceptable lower and upper cost-effectiveness thresholds), compared to no treatment. Given their clinical and cost-effectiveness at confidential prices, the 2024 UK NICE guideline recommends offering a licensed cost-effective anti-VEGF as the first-line treatment for people with CI-DMO and CRT ≥ 400 µm. Among the licensed anti-VEGF treatments analysed, ranibizumab biosimilar (Ongavia) and brolucizumab were the two most cost-effective options. Although bevacizumab was the most cost-effective anti-VEGF treatment, its use in the UK would be considered off-label as it is not covered by its marketing authorisation for ophthalmic conditions.
Summary
What was known before
-
Multiple anti-VEGF agents are approved by NICE technology appraisals for the treatment of people with CI-DMO and CRT of at least 400 micrometres.
What this study adds
-
For people with CI-DMO and CRT of at least 400 micrometres, macular laser (standard or subthreshold) emerged as the most cost-effective treatment option due to its significantly lower costs, although all anti-VEGF treatments produced higher QALYs than either type of macular laser.
-
Anti-VEGFs were found to be cost-effective compared to no treatment for people with CI-DMO and CRT of at least 400 micrometres, in reducing the risk of visual impairment, when confidential drug prices were applied.
References
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:1–25.
Amoaku WM, Ghanchi F, Bailey C, Banerjee S, Banerjee S, Downey L, et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye. 2020;34:1–51.
Davidov E, Breitscheidel L, Clouth J, Reips M, Happich M. Diabetic retinopathy and health-related quality of life. Graefe’s Arch Clin Exp Ophthalmol. 2009;247:267–72.
Hariprasad SM, Mieler W, Grassi M, Green J, Jager R, Miller L. Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol. 2008;92:89–92.
Chen E, Looman M, Laouri M, Gallagher M, Van Nuys K, Lakdawalla D, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin. 2010;26:1587–97.
Lois N, Campbell C, Waugh N, Azuara-Blanco A, Maredza M, Mistry H, et al. Diabetic macular edema and diode subthreshold micropulse laser: a randomized double-masked noninferiority clinical trial. Ophthalmology. 2023;130:14–27.
Mansouri A, Sampat K, Malik K, Steiner J, Glaser B. Efficacy of subthreshold micropulse laser in the treatment of diabetic macular edema is influenced by pre-treatment central foveal thickness. Eye. 2014;28:1418–24.
National Institute for Health and Care Excellence (NICE). Aflibercept for treating diabetic macular oedema. Technology appraisal guidance TA346. 2015 [Available from: https://www.nice.org.uk/guidance/ta346.
National Institute for Health and Care Excellence (NICE). Brolucizumab for treating diabetic macular oedema. Technology appraisal guidance TA820. 2022 [Available from: https://www.nice.org.uk/guidance/ta820.
National Institute for Health and Care Excellence (NICE). Faricimab for treating diabetic macular oedema. Technology appraisal guidance TA799. 2022 [Available from: https://www.nice.org.uk/guidance/ta799.
National Institute for Health and Care Excellence (NICE). Ranibizumab for treating diabetic macular oedema. Technology appraisal guidance TA274. 2013 [Available from: https://www.nice.org.uk/guidance/ta274.
Luckham K, Tebbs H, Claxton L, Burgess P, Dinah C, Lois N, et al. A Markov model assessing the cost-effectiveness of various anti-vascular endothelial growth factor drugs and panretinal photocoagulation for the treatment of proliferative diabetic retinopathy. Eye. 2025;39:1364–72.
Simmonds M, Llewellyn A, Walker R, Fulbright H, Walton M, Hodgson R, et al. Anti-VEGF drugs compared with laser photocoagulation for the treatment of diabetic retinopathy: a systematic review and meta-analysis. Health Technol Assess. 2024;23:1–71.
National Institute for Health and Care Excellence (NICE). Diabetic retinopathy: management and monitoring. NICE guideline NG242. 2024 [Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ng10256.
National Institute for Health and Clinical Excellence (NICE). Developing NICE guidelines: the manual. NICE process and methods PMG20. 2024 [Available from: https://www.nice.org.uk/process/pmg20.
Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Systemat Rev. 2014;10. https://doi.org/10.1002/14651858.CD007419.pub4.
National Institute for Health and Care Excellence (NICE). Dexamethasone intravitreal implant for treating diabetic macular oedema. Technology appraisal guidance TA824. 2022 [Available from: https://www.nice.org.uk/guidance/ta824.
National Institute for Health and Care Excellence (NICE). Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy. Technology appraisal guidance TA301. 2013 [Available from: https://www.nice.org.uk/guidance/ta301.
Mitchell P, Annemans L, Gallagher M, Hasan R, Thomas S, Gairy K, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol. 2012;96:688–93.
Haig J, Barbeau M, Ferreira A. Cost-effectiveness of ranibizumab in the treatment of visual impairment due to diabetic macular edema. J Med Econ. 2016;19:663–71.
Pochopien M, Beiderbeck A, McEwan P, Zur R, Toumi M, Aballéa S. Cost-effectiveness of fluocinolone acetonide implant (ILUVIEN®) in UK patients with chronic diabetic macular oedema considered insufficiently responsive to available therapies. BMC Health Serv Res. 2019;19:1–14.
Régnier SA, Malcolm W, Haig J, Xue W. Cost-effectiveness of ranibizumab versus aflibercept in the treatment of visual impairment due to diabetic macular edema: a UK healthcare perspective. Clinicoecon Outcomes Res. 2015;7:235–47.
National Institute for Health and Care Excellence (NICE). Evidence reviews for the effectiveness and acceptability of intravitreal steroids, macular laser and anti-vascular endothelial growth factor agents for treating diabetic macular oedema. NICE guideline NG242. 2024 [Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/ https://www.nice.org.uk/guidance/ng242/evidence/g-effectiveness-and-acceptability-of-intravitreal-steroids-macular-laser-and-antivascular-endothelial-growth-factor-agents-for-treating-diabetic-macular-oedema-pdf-13490775475.
Claxton L, Hodgson R, Taylor M, Malcolm B, Pulikottil Jacob R. Simulation modelling in ophthalmology: application to cost effectiveness of ranibizumab and aflibercept for the treatment of wet age-related macular degeneration in the United Kingdom. Pharmacoeconomics. 2017;35:237–48.
Lois N, Campbell C, Waugh N, Azuara-Blanco A, Maredza M, Mistry H, et al. Standard threshold laser versus subthreshold micropulse laser for adults with diabetic macular oedema: the DIAMONDS non-inferiority RCT. Health Technol Assess. 2022;26:1.
Wykoff CC, Ou WC, Khurana RN, Brown DM, Clark WL, Boyer DS. Long-term outcomes with as-needed aflibercept in diabetic macular oedema: 2-year outcomes of the ENDURANCE extension study. Br J Ophthalmol. 2018;102:631–6.
Office for National Statistics. National life tables–life expectancy in the UK: 2018 to 2020 2021 [Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2018to2020.
Preis SR, Hwang S-J, Coady S, Pencina MJ, D’Agostino SrRB, Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–35.
Czoski-Murray C, Carlton J, Brazier J, Young T, Papo NL, Kang HK. Valuing condition-specific health states using simulation contact lenses. Value Health. 2009;12:793–9.
Bressler NM, Chang TS, Suñer IJ, Fine JT, Dolan CM, Ward J, et al. Vision-related function after ranibizumab treatment by better-or worse-seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology. 2010;117:747–56. e4.
Dhingra N, Upasani D, Ghanchi FD. Patterns of treatment discontinuation in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Indian J Ophthalmol. 2022;70:2065–70.
Ciulla TA, Pollack JS, Williams DF. Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol. 2021;105:216–21.
Kern C, Fu DJ, Huemer J, Faes L, Wagner SK, Kortuem K, et al. An open-source data set of anti-VEGF therapy in diabetic macular oedema patients over 4 years and their visual acuity outcomes. Eye. 2021;35:1354–64.
Mohiuddin S. A systematic and critical review of model-based economic evaluations of pharmacotherapeutics in patients with bipolar disorder. Appl Health Econ Health Policy. 2014;12:359–72.
Mohiuddin S, Busby J, Savović J, Richards A, Northstone K, Hollingworth W, et al. Patient flow within UK emergency departments: a systematic review of the use of computer simulation modelling methods. BMJ Open. 2017;7:e015007.
Sharma S, Brown GC, Brown MM, Hollands H, Shah GK. The cost-effectiveness of grid laser photocoagulation for the treatment of diabetic macular edema: results of a patient-based cost-utility analysis. Curr Opin Ophthalmol. 2000;11:175–9.
Hutton DW, Glassman AR, Liu D, Sun JK, Network DR, Sneath M, et al. Cost-effectiveness of aflibercept monotherapy vs bevacizumab first followed by aflibercept if needed for diabetic macular edema. JAMA Ophthalmol. 2023;141:268–74.
Brown GC, Brown MM, Turpcu A, Rajput Y. The cost-effectiveness of ranibizumab for the treatment of diabetic macular edema. Ophthalmology. 2015;122:1416–25.
Holekamp N, Duff SB, Rajput Y, Garmo V. Cost-effectiveness of ranibizumab and aflibercept to treat diabetic macular edema from a US perspective: analysis of 2-year Protocol T data. J Med Econ. 2020;23:287–96.
Stein JD, Newman-Casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013;120:1835–42.
National Institute for Health and Clinical Excellence (NICE). Aflibercept solution for injection for treating wet age‑related macular degeneration. Technology appraisal guidance TA294. 2013 [Available from: https://www.nice.org.uk/guidance/ta294.
Scanlon PH, Aldington SJ, Leal J, Luengo-Fernandez R, Oke J, Sivaprasad S, et al. Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Health Technol Assess. 2015;19:1–116.
National Institute for Health and Care Excellence (NICE). Age-related macular degeneration. NICE guideline NG82. 2018 [Available from: https://www.nice.org.uk/guidance/ng82.
Acknowledgements
We thank the NICE guideline committee members for their contributions to the development of “Diabetic retinopathy: management and monitoring”. Members of the committee are listed here: https://www.nice.org.uk/guidance/NG242/documents/committee-member-list-2.
Author information
Authors and Affiliations
Contributions
KL and HT developed the model and performed the model analysis under the supervision of SM who led the economic model development team. SM and LC conducted quality assurance of the model. CD and AY conducted the network meta-analysis, and KK and NT performed quality check. NL, CD and PB are consultant ophthalmologists who contributed equally by agreeing on the clinical validity of the model structure and its parameters and assumptions. SM drafted the manuscript, and all authors commented on the draft and read and approved the final manuscript. The authors alone are responsible for the content and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luckham, K., Tebbs, H., Dadswell, C. et al. Cost-effectiveness of anti-vascular endothelial growth factor and macular laser treatments for people with centre-involving diabetic macular oedema and central retinal thickness of at least 400 micrometres. Eye (2025). https://doi.org/10.1038/s41433-025-04015-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-025-04015-6