Abstract
Soybean is a photoperiod-sensitive staple crop. Its photoperiodic flowering has major consequences for latitudinal adaptation and grain yield. Here, we identify and characterise a flowering locus named Time of flower 4b (Tof4b), which encodes E1-Like b (E1Lb), a homologue of the key soybean floral repressor E1. Tof4b protein physically associates with the promoters of two FLOWERING LOCUS T (FT) genes to repress their transcription and delay flowering to impart soybean adaptation to high latitudes. Three E1 homologues undergo subfunctionalisation and show differential subcellular localisation. Moreover, they all possess self-repression capability and each suppresses the two homologous counterparts. Subfunctionalisation and the transcriptional regulation of E1 genes collectively finetune flowering time and high-latitude adaptation in soybean. We propose a model for the functional fate of the three E1 genes after the soybean whole-genome duplication events, refine the molecular mechanisms underlying high-latitude adaption, and provide a potential molecular-breeding resource.
Similar content being viewed by others
Introduction
Soybean (Glycine max (L.) Merr.) is a major source of vegetable oil and the largest source of protein for animal feed1,2. As a short-day plant, soybean is extremely sensitive to photoperiod, such that its photoperiodic response and induction of flowering greatly influence its latitudinal adaptation and grain yield3. It is widely believed that modern cultivated soybean was domesticated from wild soybean (Glycine soja) in the Huanghuai region in Central China around 5000 years ago4. Reduced photoperiod sensitivity and early flowering were target traits during domestication, which enabled soybean to adapt to higher latitudes. The underlying genetic mechanism was the stepwise selection for loss-of-function alleles of two PSEUDO-RESPONSE-REGULATOR (PRR) genes, Tof11 and Tof125. Following domestication, photoperiodic flowering time remains a major target of artificial selection during soybean improvement, thus enabling the gradual extension of soybean cultivation to a wide latitudinal range from 53° N to 35° S6,7.
During the expansion of cultivated soybean around the world, components of the legume-specific E1-family photoperiodic flowering pathway accumulated polymorphisms3,6,8,9,10,11,12,13,14,15,16,17,18,19. Among this unique flowering pathway, E1 (Glyma.06G207800) is a core component that encodes a transcription factor containing a B3 domain and a putative bipartite nuclear-localisation signal (NLS)20. E1 has four major recessive alleles: e1-as (a single missense point mutation), e1-fs (a 1-bp deletion leading to frame-shift), e1-nl (~130 kb deletion comprising the E1 gene) and e1-b3a (three SNPs and a 2-bp deletion in the middle of the B3 domain)21. Due to two rounds of whole-genome duplication in soybean, E1 has two homologues, E1 LIKE a (E1La, Glyma.04G156400) and E1 LIKE b (E1Lb, Glyma.04G143300)22. However, the functional and evolutionary relationships amongst the three soybean E1 homologues are poorly characterised.
The impairments in the E1 family itself and its positive regulators, such as E3 and E4 (two PHYTOCHROME A homologues), E2 (GIGANTEA), Tof11/Tof12 (PRR3a/b), FKFs (Flavin-binding, Kelch repeat, F-box) contributed to cultivated soybean adapting to high latitudes5,20,23,24,25,26. However, the genetic basis for high-latitude adaption for wild soybean accessions remain poorly understood. So far, only three genes have been reported to contribute to the expansion of wild soybean to higher latitudes, namely an enhancer of E1 family (E3), an E1 family member (E1La) and an element regulated by the E1 family (Tof5/FRUITFULL)8,27. The genetic basis behind wild soybean adapting to high latitudes merits further investigation.
In this study, we identify a flowering-time locus that we name Tof4b and confirm that its causal gene is E1Lb, a homologue of E1. We provide mechanistic insight into how Tof4b affects soybean flowering time, trace the evolutionary trajectory of Tof4b and explore its role in high-latitude adaptation of both cultivated and wild soybean. The E1 genes all possess the ability for self-repression and suppression of homologous gene and have undergone subfunctionalisation during evolution to form a rare genetic module participating in the photoperiodic flowering pathway. These results refine the molecular mechanisms underlying high-latitude adaption of soybean.
Results
Identification of the Tof4b locus
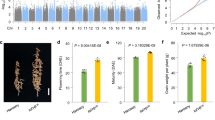
In a QTL mapping study using a population developed from a cross between Dongnong 50 (DN50) and Williams 82 (Wm82), a QTL named Tof4 was initially mapped to an interval at a physical position of 15,526,145–44,508,948 bp on chromosome 428. E1La was identified as a candidate gene8. However, Tof4/E1La alone cannot fully explain the flowering-time differences caused by this chromosomal region28. Further inspection revealed a secondary population with homozygosity at the interval including E1La, but heterozygosity for the interval between 15,526,145–29,675,002 bp showed a difference in flowering time. These observations suggest the presence of another locus (Tof4b). We generated a large (n = 5764) inbred F8 segregating population from the cross between DN50 and Wm82 by continually selecting for a residual-heterozygous line for Tof4b. Because this interval is located at the centromere, we could only refine the Tof4b locus within the 21,162,447–29,675,002-bp interval on chromosome 4 (Fig. 1a; Supplementary Data 1). This region harbours a member of the E1 family, E1Lb (Glyma.04G143300), a floral repressor controlling night-break responses that is associated with late flowering under non-inductive long-day photoperiods26,29. The E1Lb coding sequence was identical in Wm82 and DN50, but three variants were found in the E1Lb promoter of the parents: a 50-bp deletion and two SNPs in DN50 (Fig. 1b). The 50-bp deletion harbours a Box-4 element, a light-responsive element that plays a role in light-controlled transcriptional activity30. Expression of E1Lb in NILs of Tof4b from Wm82 was statistically significantly higher than from DN50 (Fig. 1c), implying that the effect of Tof4b might be caused by differential promoter activity. To determine which promoter polymorphism/s exerts this, we designed four different reporter-gene vectors and found that the 50-bp deletion, but not the other SNPs, decreased the activity of the E1Lb promoter (Fig. 1d, e and Supplementary Fig. 1). We therefore concluded E1Lb underlies Tof4b and the 50-bp promoter deletion is the causative mutation.
a Delimitation of the Tof4b locus to an 8512-kb region on chromosome 4 in a segregating, heterozygous inbred family from a cross between Williams 82 (Wm82) and Dongnong 50 (DN50). Flowering time was recorded in 2020 in Harbin under natural long-day conditions. Segregation of flowering time is shown in box-plot format, where the interquartile region, median and range are represented by the box, the bold vertical line and the horizontal line, respectively. n represents the number of plants. b Schematic of allelic variation in the Tof4b candidate gene E1Lb in Wm82 and DN50. Numbers refer to the nucleotide-residue position relative to the annotated translational start site. c Expression of E1Lb in the Tof4b NILs (Near Isogenic Lines). Data are the mean ± S.D. of n = 3 biological replicates and relative expression was normalised to Tubulin (Glyma.05G157300). ZT: Zeitgeber time. d Schematic of the constructs used for a transient reporter-gene assay to validate the causal mutations of E1Lb. P1 represents the promoter of Wm82, P4 represents the promoter of DN50, P2 and P3 are the transitional form. e Relative LUC activity driven by the promoters in (d). Data are the mean ± S.D. of n = 5 biological replicates. The two-sided Student’s t-test was used to generate the P values. f Phenotypes of wild-type c.v. Wm82 and tof4bCR-Wm82 plants cultivated under long-day conditions in a growth chamber. Scale bar = 10 cm. g Flowering time of Wm82 and tof4bCR-Wm82 plants grown as described in (f). Data are the mean ± S.D. of n = 10 plants. DAE Days after emergence. The two-sided Student’s t-test was used to generate the P values. h Phenotypes of wild-type c.v. DN50 and tof4bCR-DN50 plants cultivated under long-day conditions in a growth chamber. Scale bar = 10 cm. i Flowering time of DN50 and tof4bCR-DN50 plants grown as described in (h). Data are the mean ± S.D. of n = 10 plants. The two-sided Student’s t-test was used to generate the P values. Source data are provided as a Source Data file.
Tof4 encodes an E1Lb protein
To investigate Tof4b function in soybean, we generated tof4b null mutants by CRISPR–Cas9 gene editing in both Wm82 and DN50 backgrounds, designated tof4bCR-DN50 and tof4bCR-Wm82. DNA sequencing identified 2-bp and 4-bp deletions in the E1Lb coding sequence in the Wm82 and DN50 mutants, respectively. The two deletions resulted in frameshifts at 369 bp and 370 bp in the 579-nucleotide coding sequence, respectively. (Supplementary Fig. 2a, b). Both tof4bCR lines flowered early under long-day photoperiods relative to their respective wild types (Fig. 1f–i). We developed stable-transgenic lines in the Wm82 background over-expressing the Tof4b coding sequence fused to a triple FLAG tag (Tof4b–3xFLAG, designated Tof4b-OE) (Supplementary Fig. 3a). Two genetically independent T3 homozygous lines had delayed flowering under long-day photoperiods relative to Wm82 controls (Supplementary Fig. 3b, c). Taken together, these data confirmed that E1Lb functions as a flowering inhibitor and is the causative gene underlying the Tof4b locus.
Tof4b delays soybean flowering under long days by repressing FT2a and FT5a
E2, E3 and E4 are upstream regulators of E1 in the E1-dependent soybean photoperiodic pathway and play key roles in adaptation to high latitudes10,14,31,32. To understand their influence on Tof4b, we compared Tof4b expression in near-isogenic line (NIL) pairs for E2 and E3/E4. Tof4b expression was higher in NIL-E3/E4 compared with in NIL-e3/e4 under long-day photoperiods (Fig. 2a), suggesting that these two PHYTOCHROME A (PHYA) homologues, E3 (PHYA3) and E4 (PHYA2) up-regulate the expression of Tof4b, consistent with a previous study31,33. In addition, Tof4b is up-regulated in NIL-E2 plants versus NIL-e2 plants, indicating that E2 also promotes the expression of Tof4b (Fig. 2b).
Diurnal variation in Tof4b expression levels in NILs of E3/E4 (a) and NILs of E2 (b) under long-day photoperiod in a growth chamber. Data are the mean ± S.D. of n = 3 biological replicates and relative expression was normalised to Tubulin. Diurnal variation in FT2a (c) and FT5a (d) expression levels in Wm82 and Wm82-tof4bCR-Wm82 under long-day photoperiod in a growth chamber. Data are the mean ± S.D. of n = 3 biological replicates and expression was normalised to Tubulin. ChIP–qPCR assay of Tof4b–3xFLAG enrichment at the promoter region of FT2a (e) and FT5a (f). ELF1b was used as negative control. Data are the mean ± S.D. of n = 4 biological replicates. The two-sided Student’s t-test was used to generate the P values. ns indicate no significant difference (P > 0.05). Source data are provided as a Source Data file.
Virus-induced silencing of E1Lb up-regulated the expression of FT2a and FT5a29—the two major soybean florigens. Here, we observed a similar trend in that FT2a and FT5a were up-regulated in wild-type Wm82 relative to tof4bCR-Wm82, confirming that E1Lb is a repressor of FT2a and FT5a (Fig. 2c, d). To determine whether E1lb protein directly binds to the promoters of FT2a and FT5a, ChIP–qPCR assays using the Tof4b-OE transgenic lines and wild-type Wm82 were performed. Like its homologues E1 and E1La8, Tof4b–3xFLAG directly binds to the P2 and P4 fragments in the FT2a promoter and the P1 and P4 fragments in the FT5a promoter (Fig. 2e, f). These results suggested that Tof4b–3xFLAG delays flowering by directly binding to the promoters of FT2a and FT5a to suppress their expression. Taken together, Tof4b encodes a E1-family protein, E1Lb and Tof4b have a similar genetic mechanism to its homologues: it is enhanced by E2 and E3/E4 and directly suppresses the expression of FT2a and FT5a to delay soybean flowering under long-day conditions.
Soybean E1-family members have subfunctionalised
To further understand the relationship amongst E1, E1La (Tof4) and E1Lb (Tof4b), we scored the flowering times of a series of mutants in the Wm82 background described previously: e1 E1La E1Lb (a null e1 mutant), e1-as e1la E1Lb, e1-as E1La e1lb, e1-as e1la e1lb and e1 e1la e1lb31 grown under long-day conditions. The Wm82 background is designated as ‘e1-as E1La E1Lb’ and carries a weak e1 mutant allele previously designated ‘e1-as’ containing an amino-acid substitution in the nuclear-localisation sequence (NLS)19. e1 mutants generated here are null mutants (Supplementary Fig. 4). Although e1-as (Wm82) is a partial loss-of-function allele, the difference in days to flowering between e1-as E1La E1Lb and e1 E1La E1Lb (14 days) is much greater than that between e1-as E1La E1Lb and e1-as e1la E1Lb (4 days), e1-as E1la E1Lb and e1-as E1La e1lb (4.6 days), respectively. Moreover, the flowering-time difference between E1La E1Lb and e1la e1lb also depended on the strength of the E1 allele:17.8 days and 14.3 days for e1-as and e1, respectively. These results suggested that E1 has a much stronger effect on delaying flowering than either E1La or E1Lb, and there might be a regulatory relationship between E1 and two E1-LIKE genes. To explain the relationship between the three E1 genes, we characterised them at the protein and transcript levels as follows.
E1 encodes a 174-amino acid (aa) protein, while E1La and E1Lb both encode 192-aa proteins20,29. We found a potential translation-initiation site upstream of the previously annotated translation initiation site, extending the length of E1 to 193-aa, similar to E1La and E1Lb (Supplementary Fig. 5a). By individually replacing the E1 ATG start codons with ATT codons, both proteins are indeed expressed and do not markedly affect their corresponding nuclear-localisation (Supplementary Fig. 5b–d). Using transient-reporter assays, we found that the longer E1 protein has much stronger transcriptional inhibition on the FT2a promoter than the previously characterised E1, suggesting that the longer E1 isoform possesses greater biological activity (Supplementary Fig. 5e). In subsequent experiments, we use the longer 193-aa E1 isoform.
An alignment of the three E1 amino-acid sequences found four substitutions (position 7 S→I, position 12 T→K, position 13 T→I and position 14 L→I) among E1 and E1La/E1Lb in the N-terminus near the putative bipartiteNLS20 (Fig. 3a). To test whether these substitutions affect the nuclear targeting of two E1-Like proteins, we developed a series of E1/E1L–GFP fusion constructs (Fig. 3b) and tracked their subcellular distribution by transient expression in N. benthamiana leaves. Confocal imaging showed that E1–GFP was distributed primarily in the nucleus. E1La–GFP and E1Lb–GFP were distributed in both the nucleus and the cytoplasm, exhibiting a distribution pattern similar to that of e1-as whose NLS has undergone mutation. When we replaced the N-terminal peptide of E1La/E1Lb with the N-terminual peptide of E1 (designated ‘E1–E1La–GFP’ and ‘E1Ω–E1Lb–GFP’); both proteins were exclusively localised to the nucleus, indicating the N-terminal peptide influences nuclear-localisation. We therefore shifted the N-terminal peptide of E1La/E1Lb to E1 (designated ‘E1La/bΩ–E1–GFP’) and the fusion localised to both the nucleus and cytoplasm, like E1La–GFP and E1Lb–GFP (Fig. 3c, Supplementary Fig. 6a–c).
a Alignment of full-length E1 orthologues from Wm82-E1. NLS represents nuclear-localisation signal. The numbers indicate every 20 residues. ‘Ω’ depicts the N-terminus peptide of E1 family proteins. b Schematic of the constructs used to assay subcellular localisation of soybean E1 proteins by confocal microscopy in transiently transgenic N. benthamiana leaves. GFP (Green Fluorescent Protein) was fused to C-termini and constructs were under the control of the CaMV35S (Cauliflower Mosaic Virus 35S) promoter. ‘Ω’ depicts the N-terminus peptide of E1 family proteins. c Confocal-microscopy analysis of subcellular localisation of soybean E1 proteins and their variants as summarised in (b). Scale bar = 25 μm. Three independent biological replicates were performed. d ddPCR comparison of E1, E1La and E1Lb expression in Wm82-E1 at ZT 4 under long-day conditions at 20 DAE in a growth chamber. Data are the mean ± S.D. of n = 3 biological replicates. The two-sided Student’s t-test was used to generate the P values. e ddPCR comparison of E1La and E1Lb expression in Wm82-E1 and Wm82 (e1-as) backgrounds at ZT 4 under long-day conditions at 20 DAE in a growth chamber. Data are the mean ± S.D. of n = 3 biological replicates. The two-sided Student’s t-test was used to generate the P values. f ddPCR comparison of E1 expression in Wm82-E1 and Wm82 (e1-as) backgrounds at ZT 4 under long-day conditions at 20 DAE in a growth chamber. Data are the mean ± S.D. of n = 3 biological replicates. The two-sided Student’s t-test was used to generate the P values. ChIP–qPCR assay for enrichment of E1–3xFLAG at the promoter of E1 (g), E1La (h) and E1Lb (i). ELF1b served as a negative control. Schematics of promoters and coding regions are shown on the left with the amplified regions depicted (P1–3). ‘N’ depicts negative control. Data are the mean ± S.D. of n = 4 biological replicates. The two-sided Student’s t-test was used to generate the P values. ns indicate no significant difference (P > 0.05). Source data are provided as a Source Data file.
To investigate which substitution/s caused the difference in nuclear localisation, we fused eGFP to four E1 genes with different amino-acid substitutions in their N-terminal sequences. Confocal microscopy showed that only T12K led E1–GFP to localise in both the nucleus and cytoplasm, while the other substitutions did not affect nuclear localisation (Supplementary Fig. 7a–d). These results suggested that mutations at position 12 in the N-terminus of E1La and E1Lb affect nuclear localisation and reduce their regulatory activity, while E1 localises exclusively to the nucleus to strongly suppress FT2a and FT5a. Consistent with this, transient reporter-expression assay showed that E1 imparted stronger inhibition on the FT2a promoter activity than E1La and E1Lb (Supplementary Fig. 7e), and E1T12K more weakly inhibited FT2a promoter activity compared to E1 and isoforms with substitutions in the N-terminus (Supplementary Fig. 7f). These results therefore partly explain the difference in flowering time governed by E1 and two E1-LIKE genes.
In conclusion, the E1La and E1Lb amino-acid sequences have changed compared with E1, thereby affecting their nuclear-localisation. E1 maintains full protein activity while E1La and E1Lb have weakened activity. The partially compromised ability to inhibit the FT2a gene by mutation of E1La and E1Lb suggested that the E1 family has undergone subfunctionalisation.
Transcriptional regulation amongst E1 family members is complex
E1 and its homologues display a genetic-compensation response34. Here, to block the interference caused by genetic compensation, we conducted droplet digital PCR (ddPCR) analysis on the three E1 genes using Wm82 (e1-as) and its isogenic line Wm82-E1. E1 was expressed more strongly than E1La and E1Lb (Fig. 3d) in Wm82-E1. Copy numbers for E1La and E1Lb transcripts in Wm82 (e1-as) were greater than those in Wm82-E1 (Fig. 3e). E1 copy numbers were higher in Wm82 (e1-as) than in Wm82-E1 (Fig. 3f). These observations prompted us to investigate whether E1 exerts transcriptional inhibition on E1La, E1Lb and even on itself. To address this, we conducted a transient reporter-expression assay. As an effector, E1–GFP could suppress the promoter activity of E1, E1La and E1Lb (Supplementary Fig. 8a). ChIP–qPCR suggested that E1–3xFLAG associates with the promoters of E1 and its two homologues (Fig. 3g–i). These results indicate that E1 possesses self-repression capability and could also suppress its two homologous genes.
Given that E1-family proteins might bind to the same promoter elements and are functionally conserved, it is conceivable that E1La and E1Lb might also possess similar inhibitory capabilities like E1. E1La and E1Lb could indeed suppress promoter activities of E1, E1La and E1Lb, and bind to the promoters of three E1 genes using transient reporter-expression and ChIP–qPCR assays (Supplementary Fig. 8a–g). These experiments suggested that E1, E1La and E1Lb all bear self-repression capability and the ability to suppress their homologous genes. Whereas the results of qRT–PCR assays indicate that in the E1La8 and E1Lb NILs (E1Lb-Wm82 and E1Lb-DN50), E1La and E1Lb do not have the same strong inhibitory capability towards their homologous genes as E1 does (Supplementary Fig. 8h). The statistically significantly higher expression levels of E1, coupled with the weaker nuclear-localisation ability of E1La and E1Lb, contribute to a dominant inhibitory effect of E1 on both E1La and E1Lb.
These results also suggest the presence of a rare regulatory module composed of the E1 homologues: E1 maintains a central role and directly suppresses its homologues E1La and E1Lb. With the addition of this module, the model of soybean photoperiodic flowering pathway under long-day photoperiods has expanded: expression of E1, E1La and E1Lb is promoted by E3/E4 and E2. In the middle layer, E1 suppresses the transcription of E1La and E1Lb. Subsequently, three E1 homologues inhibit FT expression and flowering time in a hierarchical manner. When E1 is impaired, two relatively weak E1 like genes (E1La and E1Lb) are derepressed, resulting in earlier flowering. If all three E1 homologues are impaired simultaneously, soybean will exhibit a remarkable early-flowering phenotype (Supplementary Fig. 9).
The evolutionary trajectory of Tof4b
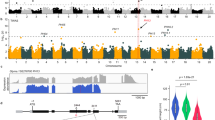
Analysis of polymorphisms in the promoter and coding regions of Tof4b identified four high-confidence haplotypes, designated H1–H4 (Fig. 4a, b). Haplotype H1 is the functional Tof4b allele and the most abundant haplotype, H2 (tof4b-1) is the tof4b allele characterised above with the 50-bp promoter deletion, and H3 (tof4b-2) is a known loss-of-function haplotype with a 1-bp deletion causing a frameshift resulting in a truncation to 73 amino acids in the 192-amino-acid for Tof4b protein in addition to the aforementioned 50-bp promoter deletion26. H4 has a base substitution at nucleotide position 159 in the coding region, causing a substitution from serine to arginine at residue 53. Alignment of E1 homologues revealed that the conserved amino acid at residue 53 is serine, indicating that H4 constitutes a mutant allele (Supplementary Fig. 10a). A transient reporter-expression assay with FT promoters suggested that Tof4b-H4 is a weak allele, consequently, it was designated tof4b-3 (Supplementary Fig. 10b–d). Median-joining network analysis indicated that tof4b-1 and tof4b-3 might be derived from Tof4b, and that tof4b-2 is derived from tof4b-1 (Fig. 4b).
a Summary of Tof4b haplotypes based on polymorphisms in the promoter and coding region. Dark blue represents a deletion in the promoter or coding sequence. b Haplotype origins of Tof4b. Grey represents wild soybean accessions, green represents landraces, blue represents improved cultivars. Triangles represent mutations that lead to weakened or loss of function. H1–H4 correspond to the haplotypes described in (a). c Allelic distribution of Tof4b in wild soybeans, landraces and cultivars in China according to region of origin. d Comparison of FST values for chromosome 4 from cultivated soybean with Tof4b vs cultivated soybean with tof4b-1/2, and cultivated soybean with tof4b-1/2 vs wild soybean with tof4b-1. The red arrow represents the location of Tof4b. e High-confidence mutations on chromosome 4 for wild soybeans, landraces and cultivars. Coloured cells represent SNPs or indels based on comparison with the Wm82 reference genome. The y-axis indicates six subgroups. The orange lines correspond to the introgression segment.
Alleles with the 50-bp promoter deletion (tof4b-1 or tof4b-2) are present in cultivated soybeans (landraces and cultivars) widely across China, and in wild soybeans in northern China, but are absent from wild soybean accessions originating from the core domestication region of Huanghuai at middle latitudes (Fig. 4c). This implies that alleles with this mutation in cultivated soybean originated from an introgression from wild soybeans in northern China, rather than occurring later during domestication and improvement. A phylogenetic tree was constructed using re-sequencing data for the Tof4b region (2 Mb) from wild and cultivated soybeans in northern China. Cultivated accessions carrying the 50-bp promoter deletion clustered together with wild accessions carrying the deletion, and they collectively cluster with wild rather than with cultivated accessions (Supplementary Fig. 11, Supplementary Data 2). These observations suggested that the 50-bp deletion mutation in cultivated accessions originated from an introgression from wild soybeans.
To further confirm and demarcate the segment carrying the promoter deletion of Tof4b introgressed into cultivated soybean, we calculated genetic diversity (FST values) across chromosome 4 between cultivated soybean with the tof4b-1/2 alleles and wild soybean with tof4b-1, cultivated soybeans with tof4b-1/2 and cultivated soybean with Tof4b, respectively. The FST value curve of cultivated soybeans with tof4b-1/2 and wild soybeans with the tof4b-1 allele has a conspicuous trough between 16.1–26.3 Mb, suggesting that in this region, cultivated soybeans with tof4b-1/2 and wild soybeans with tof4b-1 have extremely low genetic differentiation. In the same region, differentiation between cultivated soybeans with tof4b-1/2 and cultivated soybeans with Tof4b is much higher (Fig. 4d). A map of SNPs on chromosome 4 corroborates the pairwise FST comparisons (Fig. 4e, Supplementary Data 3). This indicates that cultivated soybeans with the Tof4b promoter deletion originated from an introgression, during which the 10.2-Mb segment spanning from 16.1–26.3 Mb on chromosome 4 was introgressed from wild soybeans carrying the deletion. Moreover, we investigated nucleotide diversity (Pi) on chromosome 4 and observed that wild and cultivated soybeans carrying tof4b1/2 exhibit significantly lower nucleotide diversity in this region compared to those carrying Tof4b, implying that tof4b1/2 is a relatively recent mutation (Supplementary Fig. 12). In summary, we propose the evolutionary trajectory of the Tof4b gene was as follows: in high-latitude regions, wild soybeans have two mutations, a 50-bp promoter deletion and amino-acid substitution, corresponding to tof4b-1 and tof4b-3, respectively. Of these, the tof4b-1 allele was introgressed first into cultivated soybeans in these regions, wherein it subsequently evolved tof4b-2 (Fig. 4b).
Mutations in Tof4b drove the spread of soybean to extremely high latitudes
Recent studies have identified and characterised a series of genes related to soybean adaption to high latitudes3,5,19,23,24,35,36,37. Our findings show that Tof4b may also play essential roles in the adaptation of cultivated soybean to high latitudes. To verify the effects of the introduction of tof4b, we examined its association with flowering time at Harbin (45° N) for 2 years. In a genetic background suited to high latitudes (e1 e2), cultivated soybean accessions carrying tof4b-1 alleles flowered statistically significantly earlier than cultivated soybeans carrying the tof4b allele. Cultivated soybeans carrying tof4b-2 showed distinctly early flowering out of the three alleles (Supplementary Fig. 13a, b). These phenotypes suggest that certain tof4b alleles, especially tof4b-2, can further improve adaptation to extremely high latitudes in an e1 e2 genetic background, which is already adapted to high latitudes to a certain extent.
Next, we examined the global distribution of Tof4b alleles in cultivated soybeans (Fig. 5a, Supplementary Data 4). tof4b alleles are almost absent in countries and regions at low latitudes, including southeast Asia, south Asia, Africa, Australia and southern China. The only exception is Brazil, where the tof4b-1 allele has a considerable degree of enrichment (allele frequency of 32%). A potential reason might be that the accessions in Brazil are all in the E1 background, and E1 could largely mask the effect of e1lb, thus soybeans in this region have no selection pressure on E1Lb. In contrast to the situation at low latitudes, the frequency of tof4b alleles increases with increasing latitude at middle and high latitudes. Nearly half of the surveyed Russian soybeans carry tof4b alleles, and tof4b-1 accounted for 24% of varieties in Europe. A considerable percentage of tof4b alleles are also present in northern China, Canada, Europe and Kazakhstan. Furthermore, the least-functional allele tof4b-2 is enriched in extremely high latitudes, such as Russia, Kazakhstan and Canada. Notably, 15% of Russian soybeans carry the tof4b-2 allele. Taken together, tof4b alleles were subjected to strong artificial selection because they greatly improve high-latitude adaptation of cultivated soybeans, thus driving the spread of cultivated soybean to these latitudes.
a Geographic distribution of Tof4b genetic diversity in cultivated soybean. Pie charts depict the relative abundance of each haplotype at each location. The map was drawn using ArcGIS v.10.3 software for desktop (https://desktop.arcgis.com/en/). Geographic distribution of genetic diversity of Tof4b (b), Tof4 (c), Tof5 (d), E3 (e), E1 (f) and Tof12 (g) in wild soybeans. Numbers of accessions in each group are 34 (Russia), 333 (northern China), 39 (Huanghuai region, China), 61 (southern China), 65 (Japan), 23 (Korean peninsula). h Geographical distribution of genetic diversity of E1, Tof4, Tof4b, Tof5 and Tof12 in wild soybeans.
The genetic basis of wild soybean adapting to high latitudes
To identify whether the Tof4b allele contributes to the adaptation of wild soybeans to high latitudes, we examined the association between Tof4b genotype and flowering time using a panel of 254 wild soybean accessions grown in Harbin. Similar to cultivated accessions, wild accessions with tof4b-1 or tof4b-3 alleles flowered statistically significantly earlier than accessions carrying Tof4b (Supplementary Fig. 13c, d).
We then investigated the geographic distribution of the major Tof4b alleles in a panel of 591 sequenced wild soybean accessions found in northern China, Russia, Japan and the Korean peninsula. As expected, all the sampled wild soybeans from Japan and mid- and low-latitude regions of China carry the functional Tof4b allele. In northern China, 55% of the sampled accessions carry weak tof4b alleles, of which 45% are tof4b-1 and the remaining 10% is tof4b-2. Moreover, the proportion of tof4b-1 allele carriers in wild soybeans in Russia reaches 66%. These analyses confirm that tof4b alleles have been subjected to natural selection and thus contribute to the adaptation of wild soybean to high latitudes (Fig. 5b).
Tof4, Tof5 and E3 also contribute to the adaptation of wild soybeans to high latitudes8,25,27. To gain further insight into the genetic basis of wild soybean adapting to these latitudes, we simultaneously examined the allelic distributions of a series of known high-latitude-adaption genes of cultivated and wild soybean. The geographic distribution of Tof4, Tof5 and E3 is consistent with the present study and validates the proposed roles of tof4, e3 mutants and the Tof5H2 allele in expansion toward higher latitudes for wild soybeans (Fig. 5c–e)8,27.
Although most wild soybeans carry E1 with a normal coding sequence27, we still consider E1 to play a major role in high-latitude adaption of wild soybean because of its strong effect on flowering time20. We, therefore, evaluated the E1 promoter in the 591-accession panel and found the promoters could be roughly assigned based on the nucleotide polymorphisms (6 SNPs and 4 InDels) between two groups, Group I and Group II (Supplementary Fig. 14a). Using transient-reporter assays, we found that Group-I promoter activity is stronger than Group II (Supplementary Fig. 14b, c). We also examined the expression levels of E1 in ten wild soybeans carrying the E1 and e1-wp alleles. We found differences in the mean E1 expression levels between accessions carrying the E1 and those carrying e1-wp (Supplementary Fig. 15a, Supplementary Table 1). This suggests that the E1 allele with a Group-II promoter is another weak e1 allele in addition to e1-as, e1-fs, e1-nl and e1-b3a21, which we have assigned e1-wp (e1-weak promoter).
We then explored the latitudinal distribution of different E1 promoters. In high latitudes, the Group-II promoter is present at a frequency of about 50% in accessions from Russia and northern China, 20% in Korea and Japan, and is almost absent in the core domestication region of Huanghuai and southern China (Fig. 5f). These results suggest that alleles with mutations in the E1 promoter (e1-wp) are also important for high-latitude adaption for wild soybean, and the e1-wp allele can be further introgressed into elite cultivars from wild soybean for enhanced adaptability. We also found an abundant loss-of-function allele in the Tof12 gene. This allele involves a C→T mutation at nucleotide position 43 that results in a premature stop codon, referred to as tof12-25. The tof12-2 allele is distinct from the tof12-1 allele (a stop-gain mutation at 1879 bp) fixed in cultivated soybeans during domestication5, and only presents in wild soybeans found at high latitudes and is completely absent in cultivated soybeans. tof12-2 carriers occupy a large percentage of the wild soybeans from northern China and Russia, suggesting that the loss-of-function of Tof12 also contributed to the adaptation of wild soybean to high-latitude regions (Fig. 5g). Taken together, more than 92% of wild soybean accessions harbour at least one mutant allele in E1, Tof4 (E1La), Tof4b (E1Lb), Tof5 (FUL) and Tof12 in the high-latitude regions of northern China and Russia. Mutations in the three E1 genes themselves, their enhancer (Tof12) and downstream gene (Tof5), were selected and incorporated to condition wild soybean for high latitudes (Fig. 5h, Supplementary Data 5).
Discussion
As an extremely photoperiod-sensitive plant, soybean’s latitudinal expansion necessitates the loss of photoperiod sensitivity38,39. Reducing photoperiod sensitivity and reshaping flowering time have always been a major focus during soybean domestication, improvement and diversification. Previous studies have characterised a series of genes conferring adaptation of cultivated soybean to high latitudes3. However, the genetic basis for the expansion of wild soybean towards high-latitude regions remained poorly understood. Here, we have identified the Tof4b gene and demonstrated that its impairment facilitates adaptation to high latitudes for both wild and cultivated soybean. The early-flowering tof4b alleles, especially tof4b-2, have potential in molecular breeding to improve adaptation in extremely high latitude regions. In addition, the coding region of Tof4b/E1Lb, together with its homologues E1 and E1La, have undergone subfunctionalisation during soybean genome duplication and evolution. Additionally, three E1 genes all possess self-repression capability and can suppress their two homologous genes. These features form a rare regulatory module and participate in the soybean photoperiodic flowering pathway. Mutations in this pathway facilitated the expansion of wild soybean to high latitudes.
Cultivated soybean was domesticated from its wild progenitor G. soja and most genes/alleles were sourced from wild soybean in the domestication region or appeared during subsequent improvement and diversification40. While genetic bottlenecks during domestication lost approximately half of the genetic diversity41, this inevitably led to missing many potential allelic variants in breeding that control key agronomic traits, including flowering time. Previous work found Tof5H2 and Tof4H1 facilitate high-latitude adaptation for wild soybean, but these alleles have rarely been applied to cultivated soybean8,25. Here, we found a weak E1 allele (e1-wp), and a loss-of-function allele of Tof12 (tof12-2) from wild soybean, which have not been used in breeding programmes, based on our analyses. We also identified an allele named tof4b-1 that confers adaptation to wild soybean at high latitude and has been introgressed to cultivated soybean and thereby drove the spread of cultivated varieties. This phenomenon is not uncommon, as evidenced by instances of gene introgression from corresponding wild species into the genomes of cultivated varieties of other plants, such as maize, sunflower and rice42,43,44. Such genetic introgression serves to enhance the genetic diversity of cultivated varieties, equipping them with the ability to adapt to diverse environments. Further exploration is needed to identify other introgressed genes in the soybean genome and understand their biological functions.
Whole-genome duplications are widespread in plants and tend to generate gene duplications. Evolutionary processes suggest three alternative fates for the duplicate homologues: non-functionalisation, neofunctionalisation and subfunctionalisation45,46. Duplicated genes can be preserved by neofunctionalisation and subfunctionalisation, thus generating biological novelty and diversity45,46,47. In soybean, two whole-genome duplications occurred at ~59 and 13 million years ago (Mya), resulting in nearly 75% of the genes being present in multiple copies48. One such example is that E1 has two homologues, namely E1La and E1Lb. The divergence time between E1 and E1La/E1Lb can be traced back to 10.6 and 8.8 Mya, respectively22. However, there is scant understanding of the relationship and evolutionary fate of E1 homologues. In plants with CRISPR–Cas9-mediated E1 mutations, E1La and E1Lb were up-regulated, indicating a common genetic-compensation response34. Such compensation phenomena are common in knockout mutations and are caused by H3 lysine 4 trimethylation (H3K4me3) triggered by mutant mRNA degradation49, thus not reflecting the bona fide regulatory relationship between homologous genes. In this study, we quantified the expression of E1, E1La and E1Lb in Wm82 (e1-as) and Wm82-E1 NIL lines instead of CRISPR–Cas9-mediated E1 mutant alleles to avoid the interference from genetic compensation and performed transient assays and ChIP–qPCR to confirm the direct inhibitory effect of three E1 genes on themselves, rather than from genetic compensation. Such self-repression has been reported for REDUCED COMPLEXITY (RCO)50 and some MYB transcription factors51. A possible outcome of the self-repression of E1 genes is to balance the total activity of the E1 homologues to an ideal level to finetune flowering and adaptation.
Together with the subcellular-localisation analysis, we uncovered a rare evolutionary fate for E1 homologues. Firstly, the three E1 genes all possess self-repression capability and can suppress their two homologous genes. While this may appear consistent with subfunctionalisation, defining it as such would be inappropriate if E1 possessed self-suppression capability before genomic duplication. Determining whether this suppression capability emerged when E1 first appeared in soybeans or after E1 was expanded to three copies is for future studies. Secondly, the E1 homologues have undergone subfunctionalisation by altering their subcellular localisation. The driving force of the evolution of the E1 homologues might be avoiding extreme late flowering caused by the strong flowering suppressor, E1. Based on this model, E1La and E1Lb will be released from repression when E1 function is impaired. Many cultivars adapted to high latitudes possess loss-of-function E1 mutations. Moreover, in addition to mutations in E1, further variation in E1La or E1Lb will help enhance soybean’s capacity to adapt to even higher latitudes. (Supplementary Fig. 16a, b).
The past few decades have seen substantial progress in understanding high-latitude adaptation of cultivated soybeans, while attention has seldom been paid to this for their wild progenitors. Tof4-1, Tof5H2 and loss-of-function alleles of E3 all contribute to wild soybean high-latitude adaptation8,25,27. These genes alone, however, cannot fully explain high-latitude adaptation. Here, we identified tof4b-1/3, two contributors to high-latitude adaptation and further assessed the significance of several genes relating to cultivated-soybean adaptation, including E1, E3, Tof4, Tof5 and Tof12. Among them, Tof4 is located close to Tof4b on chromosome 4. We found that besides carriers of double-gene mutations, carriers of Tof4/tof4b-1/3 are significantly fewer than those carrying tof4/Tof4b, hinting at the possibility that mutations in tof4b occurred later than mutations in tof4. Furthermore, the fact that 71.9 % of tof4b-1/3 mutations occur together with tof4-2, rather than the 50% expected if the two genes were completely unlinked, indicates a degree of linkage between tof4-2 and tof4b-1/3. Although E1 negatively regulates E1La and E1lb, there is no obvious enrichment in mutations between e1-wp and e1la or e1lb or both (48.1% e1la carries are under e1-wp background, and for e1la is 46.8%) (Supplementary Fig. 17). Besides, it is intriguing to note that the expression level of E1 in wild soybeans carrying e1-wp is indeed lower. Conversely, the expression level of E1Lb is elevated, while no statistically significant difference was seen for E1La expression (Supplementary Fig. 15b, c, Supplementary Table 1). This could possibly be attributed to the complexity of the genetic background.
In addition, we found that wild and cultivated soybeans generally selected different early-flowering alleles at E1 and Tof12. Cultivated soybeans enriched tof12-1 alleles and several e1 alleles with mutations in the protein-coding region, meanwhile wild soybean selected the tof12-2 allele, which has a frameshift at 45 bp and the e1-wp allele with a weaker promoter. These two alleles from wild soybean have been lost during genetic bottlenecks associated with domestication and therefore have great potential for the molecular breeding of elite cultivars adapted to high-latitude regions.
In conclusion, we found that the E1 gene family, the legume-specific transcription factor and core regulator of flowering in soybeans, has undergone subfunctionalisation and capable of self-suppression and mutual inhibition, shaping the regulatory ability of the E1 family in flowering. These findings provide new insights into the fate of duplicated genes and the control of flowering time in soybeans. Additionally, we discovered that mutations in the E1 gene family, as well as in the genes Tof5 and Tof12, enhance the adaptability of wild soybeans in high-latitude regions. These early-flowering alleles will hopefully contribute to advancements in soybean breeding for high-latitude regions.
Methods
Plant materials and growth conditions
The soybean diversity panel used in this study was grown from May to October under natural long-day conditions in 2020 and 2021 in Harbin, China (45° 750’ N, 126° 630’ E). Each row was 2 m long, with 60 cm spacing of between rows. 20 plants were sown in each row. Flowering time was recorded in 2020 and 2021. NILs of Tof4b were selected from F8 progeny of this same cross using molecular markers for Tof4.
The NILs, CRISPR–Cas9 knockout mutants and overexpression lines were grown under long-day conditions (16 h light/8 h dark at 25 °C) in growth cabinets (Conviron Adaptis A1000) with a light intensity of 500 μmol photons m−2 s−1.
Resequencing, mapping, variation calling and haplotype-origin analysis
We used several published resequencing data or VCF files5,8,18,27. For each of the new accessions in this work, at least 5 µg of DNA was used to construct a sequencing library with an Illumina TruSeq DNA Sample Prep Kit, according to the manufacturer’s instructions. Paired-end sequencing of each library was performed on an Illumina HiSeq X Ten system. Paired-end resequencing reads of the new accessions in this study were mapped to the reference genome (Gmax_Wm82_a2_v1) with BWA software with default parameters52. Filtration of duplicates of the sequencing reads, SNP and InDel calling was performed by VariantFiltration tools in GATK (4.1.1.0)53. The new VCF file in this study and published VCF files are merged by vcftools (0.1.16)54. Annotation was achieved by ANNOVAR (−0400)55. High-confidence mutations (MAF > 0.05, max missing <0.01) on chromosome 4 were called by vcftools to estimate the introgress interval of Tof4b. Mutations called by vcftools on the ~4-kb region upstream of E1 were used for grouping the promoter of E1 in wild soybeans from high latitudes. Haplotype-origin analysis was performed by Network 10.2 with median-joining method (https://www.fluxus-engineering.com/sharenet.htm).
Construction of phylogenetic trees
To conduct the phylogenetic analysis, SNPs of selected accessions (wild and cultivated soybeans from high latitudes) were filtered with MAF = 0.05. These SNPs were used to construct an approximately-maximum-likelihood tree with FastTree (2.1) software56 and were visualised with iTOL (https://itol.embl.de).
DNA isolation and map-based cloning
Genomic DNA was extracted from fresh trifoliate leaves at 20 DAE with NuClean Plant Genomic DNA Kit (CWBIO) in accordance with the manufacturer’s instructions. The primer sequences used to amplify the markers for mapping are listed in Supplementary Data 1. Markers were developed in the regions of Tof4b based on the resequencing data of the two parents, Wm82 and DN50. Recombinants were identified in the Tof4b fine-mapping population using seven markers. The flowering time of the progeny of these recombinants was evaluated to delimit the genomic interval containing Tof4b.
Soybean transformation
tof4b mutants was created by CRISPR–Cas9 gene editing. Small-guide RNA sequences were cloned into pYLCRISPR/Cas9-DB vector to generate Tof4b CRISPR–Cas9 construct, which was then transformed into Agrobacterium tumefaciens EHA101 and used to transformed Williams 82 and DN50 plants using the cotyledonary-node method37. Material was selected on 8 mg/L Basta. To identify edited transformants, genomic DNA was first extracted from the leaves and specific primers were used to amplify then sequence the target sites to genotype the mutants. Primers used to construct the editing vector and identify the mutants are listed in Supplementary Data 1.
The CDS of Tof4b were amplified from Williams 82 and ligated into modified pTF101-3xFLAG vector under the control of the cauliflower mosaic virus CaMV35S promoter. The recombinant constructs were introduced into Williams 82 using the cotyledonary-node method as followed: First, isolate and sterilise soybean embryos. Second, infect them with Agrobacterium carrying target vector. Third, grow them on selective media to remove non-transformed cells. Fourth, cultivate surviving cells to develop shoots and roots. Fifth, transfer plantlets to soil and grow until mature. Transfer plantlets was selected on 8 mg/L Basta. To validate transgenic Tof4b overexpression plants, total protein from Wm82 and transgenic 35Spro:Tof4b–3xFLAG lines were extracted for immunoblot analysis in extraction buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.1% v/v Triton X-100 and protease inhibitor cocktail). The anti-FLAG antibody (Sigma, M8823) was used in Immunoblot analysis was to detect transgene expression in the transgenic lines.
RNA isolation and RT–qPCR
Trifoliate leaves were collected at 20 DAE at ZT 4 under long-day (16 h light/8 h dark) conditions. Each sample has three independent replicates. Total RNA was extracted using an Ultrapure RNA kit (CWBIO) and 0.5 μg was reverse transcribed using a Super Script First-strand cDNA Synthesis System (Takara, Dalian, China), all according to the manufacturers’ instructions, respectively. Quantitative PCR (qPCR) was performed using SYBR Green Real-Time PCR Master Mix (Roche), according to the manufacturer’s instructions. Three independent RNA samples were prepared for biological replicates. Tubulin (Glyma.05G157300) was used as the reference gene to normalise relative expression using the 2-ΔΔCT method57. All qPCR primers are listed in Supplementary Data 1.
Droplet digital PCR
Droplet digital PCR (ddPCR) was used to analyse the absolute expression levels of E1, E1La and E1Lb in leaves of Wm82 and Wm82-E1 under long-day conditions. The ddPCR was conducted in a 20 mL reaction mixture containing 10 mM of each target-gene primer and probe, 5 mL of ddPCR Supermix (dUTP), 10 ng cDNA and distilled water. The generation of nanoliter-sized droplets and PCR amplification were carried out using a MicroDrop-100A instrument (Forevergen, Guangzhou, China), following the manufacturer’s protocol. The generation of nanoliter-sized droplets and PCR were performed in a MicroDrop-100A instrument (Forevergen, Guangzhou, China) following manufacturer’s instruction. Each sample was obtained from three individuals and the data were analysed with three technical replicates.
ChIP–qPCR
Leaf samples were collected at 20 DAE at ZT 4 under long-day conditions from Wm82 and 35Spro:E1–3xFLAG transgenic lines. Samples were fixed for 20 min in 1% v/v formaldehyde under vacuum using ice to cool samples down. The immunoprecipitation of soluble chromatin was done using anti-FLAG M2 magnetic beads (Sigma, M8823). The coimmunoprecipitated DNA was recovered and used for qPCR in triplicate. Relative fold enrichment was quantified by normalising the amount of a target DNA fragment against that of a genomic fragment of a reference gene, ELONGATION FACTOR 1B (ELF1B, Glyma.02G276600). Enriched of the ELF1b fragment was used as a negative control. Primers used for amplification are listed in Supplementary Data 1.
Transient dual-reporter assays to assess promoter activity
The ~3 kb E1 promoter regions were amplified from the wild soybean accessions GDW061 and GDW099 (referred to Group I), ZKW0175 and ZKW0126 (referred to Group II). The four fragments were introduced into the pGreen0800-LUC/REN vector to generate the 35Spro-REN-pE1-LUC reporter. ~3-kb promoter sequences for E1Lb from Wm82 (P1 in Fig. 1d), ZKW0146 (P2 in Fig. 1d), GDW097 (P3 in Fig. 1d), DN50 (P4 in Fig. 1d) were also introduced into pGreen0800-LUC/REN to generate the 35Spro-REN-pE1Lb-LUC reporters. Correctly assembled and sequenced vectors were introduced into A. tumefaciens GV3101 to grow overnight to OD600 nm = 0.4–0.6. Cell suspensions from effector and reporter constructs were added in equal amounts to infiltrate into fresh N. benthamiana leaves. At least three leaves from different N. benthamiana plants were infiltrated. LUC and REN activities were measured using a Luciferase 1000 Assay System (cat. no. E4550; Promega) and a Renilla Luciferase Assay System (cat. no. E2820; Promega), respectively. The final activity was expressed as the ratio between LUC and REN activities.
Transient dual-reporter assays to assess transcriptional-regulatory activity
The ~3 kb promoter sequences for E1La and E1Lb, FT2a and FT5a were amplified from Wm82. The four fragments were introduced into pGreen0800-LUC/REN vector to generate the 35Spro-REN-promoter-LUC reporter58. E1, and different alleles of Tof4b (Tof4b–H1, Tof4b–H3 and Tof4b–H4) were introduced into the pTF101–3Flag vector to generate the constructs 35Spro:Tof4b–H1–3xFLAG, 35Spro:Tof4b–H3–3xFLAG and 35Spro:Tof4b–H4–3xFLAG and were used as the effectors in the N. benthamiana transient-expression system59. Subsequent processing and testing are described above (Transient dual-reporter assays to assess promoter activity).
Subcellular localisation and confocal microscopy
Coding sequences of all E1-family members without respective stop codons were fused with GFP in the pTF101–GFP vector driven by the cauliflower mosaic virus CaMV35S promoter59. The plasmid was transformed into A. tumefaciens GV3101, which was then infiltrated into N. benthamiana leaves for transient expression. The infiltrated plants were grown at 26 °C under long-day conditions (16 h light/8 h dark) for another 2 d before imaging. A Zeiss LSM 800 confocal laser-scanning microscope (Zeiss, Germany) was used to observe green fluorescent protein (GFP) fluorescence. CaMV35S:GFP was infiltrated as a localisation control.
DAPI was used to stain nuclei in 1x PBS at a working concentration of 100 ng/mL. N. benthamiana leaf samples were placed on a microscope slide to which a few drops of DAPI staining solution were and allowed to incubate for 10 min prior to imaging. Samples were observed under a confocal laser-scanning microscope with an excitation wavelength of 405 nm and emission wavelength of 460–500 nm.
Cell-fractionation assays
Nucleus–cytosolic protein-fraction assays were performed as followed. Briefly, 200 mg N. benthamiana leaf samples were infiltrated with various E1/E1La/E1Lb expression plasmids for 48 h then frozen and ground in liquid nitrogen. Powdered leaf tissue was mixed with 500 μL of fraction lysis buffer (20 mM Tris-HCl pH 7.5, 25% v/v glycerol, 2 mM EDTA, 2.5 mM MgCl2, 20 mM KCl, 250 mM sucrose, with the addition of 5 mM DTT with 1×protease inhibitor cocktail added fresh). The mixture was vortexed and filtered through a double layer of Miracloth (Millipore), and 40 μL sample was set aside as total protein. The flowthrough was centrifuged at 1500 g for 10 min at 4 °C, after which the supernatant was centrifuged at 10,000 g for 10 min at 4 °C, and the supernatant was collected as the cytosolic fraction. The pellet from the first centrifugation was washed five times with NRBT buffer (20 mM Tris-HCl pH 7.5, 25% v/v glycerol, 2.5 mM MgCl2, 0.2% v/v TritonX-100) until it turned white in colour. The pellet was resuspended in 500 μL NRB2 buffer (20 mM Tris-HCl pH 7.5, 10 mM MgCl2, 0.25 M sucrose, 0.5% v/v TritonX-100 and 5 mM β-mercaptoethanol supplemented with 1×protease inhibitor cocktail) and added on top of a layer of 500 μL NRB3 buffer (20 mM Tris-HCl pH 7.5, 10 mM MgCl2, 1.7 M sucrose, 0.5% v/v Triton X-100 and 5 mM β-mercaptoethanol supplemented with 1×protease inhibitor cocktail added fresh). The suspension was centrifuged at 16,000 g for 45 min at 4 °C, and the pellet was collected as the nuclear fraction. Total protein, cytosolic proteins and nuclear fraction were resuspended in 2× SDS loading buffer and boiled for 5 min for immunoblot assays. E1–GFP fusion proteins were detected using anti-GFP antibodies (HT801-01; TransGen). PEPC was detected with anti-PEPC antibodies as the cytoplasmic marker (AS09458; Agrisera). Histone H3 was detected using anti-H3 antibodies as the nuclear marker (HL102-01; TransGen). All immunoblot bands were analysed for band greyscale values using ImageJ to estimate intensity as a measure of protein abundance. The calculation method for the proportion of proteins in nucleus and cytoplasm involves dividing the nuclear-protein intensity by the total-protein intensity and comparing it with the ratio of cytoplasmic-protein intensity to total-protein intensity, finally presenting it in the form of a percentage chart.
Quantification and statistical analysis
All values were presented as the mean ± standard deviation (S.D.) and numbers (n) of samples or replicates are indicated in the respective figure legends. Data were analysed with GraphPad Prism 8 (ver. 8.0.1). Significance levels were calculated by the two-sided Student’s t-tests or one-way ANOVA and presented by GraphPad Prism 8. To evaluate the phenotypes of the various accessions or lines, at least 10 individual plants of each accession were analysed. The n-values of statistical samples and the exact P-values are indicated in the figures
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing data used in this study have been deposited in the NCBI SRA database under accession code SRP469369. The previously reported sequence data used in this study are available on the NCBI SRA database under accession codes SRP114890, SRP250886, SRP387668, SRP326758, SRP344122, and SRP387668. Source data are provided with this paper.
References
Graham, P. H. & Vance, C. P. Legumes: importance and constraints to greater use. Plant Physiol. 131, 872–877 (2003).
Fang, C. & Kong, F. Soybean. Curr. Biol. 32, R902–R904 (2022).
Du, H., Fang, C., Li, Y., Kong, F. & Liu, B. Understandings and future challenges in soybean functional genomics and molecular breeding. J. Integr. Plant Biol. 65, 468–495 (2023).
Hymowitz, T. On the domestication of the soybean. Econ. Bot. 24, 408–421 (1970).
Lu, S. et al. Stepwise selection on homeologous PRR genes controlling flowering and maturity during soybean domestication. Nat. Genet. 52, 428–436 (2020).
Lu, S. et al. Natural variation at the soybean J locus improves adaptation to the tropics and enhances yield. Nat. Genet. 49, 773–779 (2017).
Zhang, L. et al. Principles and practices of the photo-thermal adaptability improvement in soybean. J. Integr. Agr 19, 295–310 (2020).
Dong, L. et al. The genetic basis of high-latitude adaptation in wild soybean. Curr. Biol. 33, 252–262 e4 (2023).
Kong, F. et al. Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiol. 154, 1220–1231 (2010).
Watanabe, S. et al. A map-based cloning strategy employing a residual heterozygous line reveals that the GIGANTEA gene is involved in soybean maturity and flowering. Genetics 188, 395–407 (2011).
Watanabe, S. et al. Map-based cloning of the gene associated with the soybean maturity locus E3. Genetics 182, 1251–1262 (2009).
Samanfar, B. et al. Mapping and identification of a potential candidate gene for a novel maturity locus, E10, in soybean. Theor. Appl. Genet. 130, 377–390 (2017).
Cober, E. R., Molnar, S. J., Charette, M. & Voldeng, H. D. A new locus for early maturity in soybean. Crop Sci. 50, 524–527 (2010).
Wang, L. et al. GIGANTEA orthologs, E2 members, redundantly determine photoperiodic flowering and yield in soybean. J. Integr. Plant Biol. 65, 188–202 (2023).
Liu, Z. et al. Integrated single-nucleus and spatial transcriptomics captures transitional states in soybean nodule maturation. Nat. Plants 9, 515–524 (2023).
Kong, F. et al. A new dominant gene E9 conditions early flowering and maturity in soybean. Crop Sci. 54, 2529–2535 (2014).
Li, X. et al. Overcoming the genetic compensation response of soybean florigens to improve adaptation and yield at low latitudes. Curr. Biol. 31, 3755–3767 e4 (2021).
Dong, L. et al. Genetic basis and adaptation trajectory of soybean from its temperate origin to tropics. Nat. Commun. 12, 5445 (2021).
Qin, C. et al. GmEID1 modulates light signaling through the evening complex to control flowering time and yield in soybean. Proc. Natl. Acad. Sci. USA 120, e2212468120 (2023).
Xia, Z. et al. Positional cloning and characterization reveal the molecular basis for soybean maturity locus E1 that regulates photoperiodic flowering. Proc. Natl. Acad. Sci. USA 109, E2155–E2164 (2012).
Zhai, H. et al. Allelic variations at four major maturity E genes and transcriptional abundance of the E1 gene are associated with flowering time and maturity of soybean cultivars. PloS ONE 9, e97636 (2014).
Zhang, X. et al. Functional conservation and diversification of the soybean maturity gene E1 and its homologs in legumes. Sci. Rep. 6, 29548 (2016).
Li, H. et al. Natural variation of FKF1 controls flowering and adaptation during soybean domestication and improvement. New Phytol. 238, 1671–1684 (2023).
Jiang, B. et al. Allelic combinations of soybean maturity loci E1, E2, E3 and E4 result in diversity of maturity and adaptation to different latitudes. PLoS ONE 9, e106042 (2014).
Dietz, N. et al. Geographic distribution of the E1 family of genes and their effects on reproductive timing in soybean. BMC Plant Biol. 21, 441 (2021).
Zhu, J. et al. Loss of function of the E1-Like-b gene associates with early flowering under long-day conditions in soybean. Front. Plant Sci. 9, 1867 (2018).
Dong, L. et al. Parallel selection of distinct Tof5 alleles drove the adaptation of cultivated and wild soybean to high latitudes. Mol. Plant 15, 308–321 (2022).
Kong, L. et al. Quantitative trait locus mapping of flowering time and maturity in soybean using next-generation sequencing-based analysis. Front. Plant Sci. 9, 995 (2018).
Xu, M. et al. The soybean-specific maturity gene E1 family of floral repressors controls night-break responses through down-regulation of FLOWERING LOCUS T orthologs. Plant Physiol. 168, 1735–1746 (2015).
Yin, G. et al. The large soybean (Glycine max) WRKY TF family expanded by segmental duplication events and subsequent divergent selection among subgroups. BMC Plant Biol. 13, 148 (2013).
Lin, X. et al. Novel and multifaceted regulations of photoperiodic flowering by phytochrome A in soybean. Proc. Natl. Acad. Sci. USA 119, e2208708119 (2022).
Liu, B. et al. Genetic redundancy in soybean photoresponses associated with duplication of the phytochrome A gene. Genetics 180, 995–1007 (2008).
Fang, C. et al. Mechanisms underlying key agronomic traits and implications for molecular breeding in soybean. J. Genet. Genom. 151, 379–393 (2024).
Wan, Z. et al. CRISPR/Cas9-mediated targeted mutation of the E1 decreases photoperiod sensitivity, alters stem growth habits, and decreases branch number in soybean. Front. Plant Sci. 13, 1066820 (2022).
Tsubokura, Y. et al. Natural variation in the genes responsible for maturity loci E1, E2, E3 and E4 in soybean. Ann. Bot. 113, 429–441 (2014).
Lin, X., Liu, B., Weller, J. L., Abe, J. & Kong, F. Molecular mechanisms for the photoperiodic regulation of flowering in soybean. J. Integr. Plant Biol. 63, 981–994 (2021).
Kou, K. et al. A functionally divergent SOC1 homolog improves soybean yield and latitudinal adaptation. Curr. Biol. 32, 1728–1742 e6 (2022).
Kim, M. Y., Shin, J. H., Kang, Y., Shim, S. R. & Lee, S. H. Divergence of flowering genes in soybean. J. Biosci. 37, 857–870 (2012).
Cober, E. R. & Morrison, M. J. Regulation of seed yield and agronomic characters by photoperiod sensitivity and growth habit genes in soybean. Theor. Appl. Genet. 120, 1005–1012 (2009).
Sedivy, E. J., Wu, F. & Hanzawa, Y. Soybean domestication: the origin, genetic architecture and molecular bases. New Phytol. 214, 539–553 (2017).
Zhou, Z. et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 33, 408–414 (2015).
Baute, G. J., Kane, N. C., Grassa, C. J., Lai, Z. & Rieseberg, L. H. Genome scans reveal candidate domestication and improvement genes in cultivated sunflower, as well as post-domestication introgression with wild relatives. New Phytol. 206, 830–838 (2015).
Pusadee, T., Schaal, B. A., Rerkasem, B. & Jamjod, S. Population structure of the primary gene pool of in Thailand. Genet. Resour. Crop Evol. 60, 335–353 (2013).
Doebley, J. The genetics of maize evolution. Annu. Rev. Genet. 38, 37–59 (2004).
Lynch, M. & Conery, J. S. The evolutionary fate and consequences of duplicate genes. Science 290, 1151–1155 (2000).
Conant, G. C., Birchler, J. A. & Pires, J. C. Dosage, duplication, and diploidization: clarifying the interplay of multiple models for duplicate gene evolution over time. Curr. Opin. Plant Biol. 19, 91–98 (2014).
Van De Peer, Y., Mizrachi, E. & Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 18, 411–424 (2017).
Schmutz, J. et al. Genome sequence of the palaeopolyploid soybean. Nature 463, 178–183 (2010).
El-Brolosy, M. A. et al. Genetic compensation triggered by mutant mRNA degradation. Nature 568, 193–197 (2019).
Hajheidari, M. et al. Autoregulation of RCO by low-affinity binding modulates cytokinin action and shapes leaf diversity. Curr. Biol. 29, 4183–4192 e6 (2019).
Ma, D. W. & Constabel, C. P. MYB repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci. 24, 275–289 (2019).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2-approximately maximum-likelihood trees for large alignments. PloS ONE 5, e9490 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408 (2001).
Hellens, R. P. et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13 (2005).
Paz, M. M. et al. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 136, 167–179 (2004).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32022062 and 32372112 to S. Lu, 31771815 to B.L., 32090064 to F.K., 32001503 to C.F., 32201865 to S. Li), the National Key Research and Development Program (2022YFD1201501 to F.K.), National Key Research and Development Program (2021YFF1001100 to S. Lu), and the Major Program of Guangdong Basic and Applied Research 2019B030302006 to F.K. and B.L., and the open-competition program of top-ten critical priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2022SDZG05) to F.K. and B.L. Science and Technology Plan of Guangzhou, China (2023A04J1500 to C.F.).
Author information
Authors and Affiliations
Contributions
B. Liu, F. Kong and S. Lu designed and supervised the experiments and managed the projects. C. Fang, Z. Sun, S. Li, T. Su, L. Dong, L. Wang, H. Li, L. Kong, L Li, Z. Yang, F. He, Q. Cheng, F. Wang, M. He, X. Pei, X. Lin performed the experiments. S. Li, C. Fang, A. Zatybekov, and S. Lu performed the data analysis. C. Fang and S. Lu drafted the manuscript. B. Liu, F. Kong and S. Lu revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Feng Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, C., Sun, Z., Li, S. et al. Subfunctionalisation and self-repression of duplicated E1 homologues finetunes soybean flowering and adaptation. Nat Commun 15, 6184 (2024). https://doi.org/10.1038/s41467-024-50623-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-50623-3
This article is cited by
-
Uncovering the genetic landscape of soybean accessions from Kazakhstan in comparison with global germplasm using whole genome resequencing
BMC Genomics (2025)
-
Identification of new genomic loci for seed protein and oil content in the soybean pangenome using genome-wide association and haplotype analyses
Theoretical and Applied Genetics (2025)
-
Silicon alleviates aluminum-induced programmed cell death through regulating the antioxidant defenses in soybean roots
Biologia (2024)